Abstract
Purpose of Review
The need for better drugs to treat patients with Chagas disease remains urgent. This review summarizes the advancements in drug development over the past two years.
Recent Findings
Drug development efforts are almost exclusively occurring as preclinical research. The exceptions being Phase I safety studies for the cruzain inhibitor, K-777, and potential Phase II studies for the antifungal drug, posaconazole, and a prodrug of ravuconazole. Several recent laboratory investigations demonstrate anti-T. cruzi activity of novel small molecules in animal models. These include nonpeptidic cruzain inhibitors, novel inhibitors of the sterol 14α-demethylase enzyme, new compounds (arylimidamides) related to pentamidine, derivatives of nifurtimox, compounds using ruthenium complexes, and several natural products. The recent implementation of a high-throughput screen of >300,000 compounds against intracellular T. cruzi amastigotes done at the Broad Institute is an important development, yielding ~300 selective inhibitors, many of which may serve as leads for medicinal chemistry efforts.
Summary
Progress is slow, but recent advancements in both drug development and advocacy for research on neglected diseases are encouraging. Efforts to define a target product profile and to harmonize methodologies for testing drugs for Chagas disease are described herein.
Keywords: Chagas disease, Trypanosoma cruzi, drug development
Introduction
After a period of doubt in the 1980s – ‘90s, there is growing consensus that etiologic treatment is beneficial for patients with chronic Chagas disease [1-3, 4**]. Unfortunately, the available drugs to treat Trypanosoma cruzi infection (benznidazole and nifurtimox) have significant side effects. The need for long treatment courses combined with poor tolerability has translated to low utilization of these medicines. Better drugs have the potential to jump-start public health efforts to address the problem of Chagas disease, one of the most neglected of tropical diseases. The academic research community has shown increased activity in recent years in drug discovery efforts for Chagas disease, and a most welcome interest by some drug companies brightens future prospects for new drugs [5]. In this review, drug discovery activities for Chagas disease reported in the literature between January 2009 and June 2010 are summarized.
Target product profile for Chagas drugs
The reported compounds are reviewed with a target product profile for Chagas disease in mind [6*]. Specifically, new compounds with characteristics that make them amenable for successful treatment of Chagas disease in endemic countries are given the most emphasis. To show the most promise, compounds must have activity against the most clinically relevant mammalian stages of T. cruzi (intracellular amastigotes or trypomastigotes) and preferably activity in an animal model. Activity against multiple, diverse T. cruzi strains is desirable to ensure the potential for applicability in different disease endemic regions. Low potential for toxicity (less than that of benznidazole and nifurtimox) is essential. This should include low potential for genotoxicity and teratogenicity given the potential use in young persons as well as low risk for cardiotoxicity such as prolonged QT interval since the heart is the primary organ affected in Chagas disease. Low risk for interactions with hepatic cytochrome P450s is important to avoid drug-drug interactions, particularly because many patients may be taking antiarrhythmic drugs, anticoagulants, and other medications. The drug that will finally be used in the field will need to be orally administered, so oral bioavailability is a very important characteristic. Lastly, a stable compound with low cost of goods is critical to ensure that a future compound will be available in areas where it is most needed.
The compounds that appear to have the most promise for future progression in the Chagas drug development pipeline are discussed below.
Compounds acting on defined drug targets
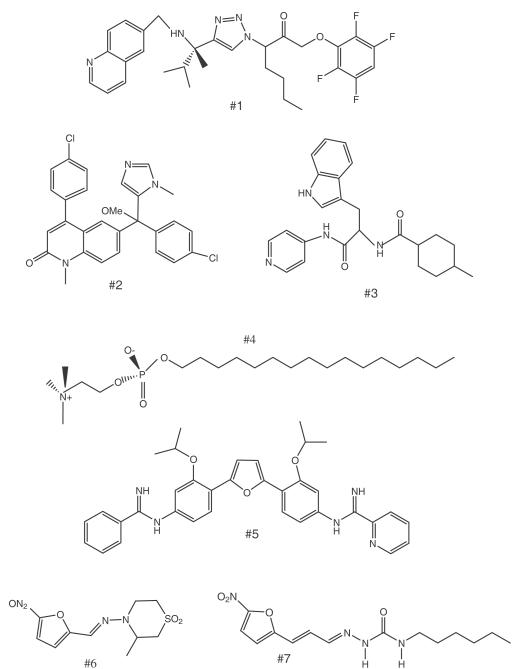
Cruzain is the primary cysteine protease expressed during T. cruzi infection, and is believed to be essential for infectivity in the mammalian host [7]. Inhibitors targeting cruzain have been the focus of intense investigation for over a decade with the compound K-777 entering advanced preclinical studies [8]. To improve upon the peptidic vinyl sulfone series (i.e. K-777), new nonpeptidic inhibitors were synthesized and tested. The best candidate, a 1,2,3-triazole-based tetrafluoropheno-xymethyl ketone (#1, Fig 1), has suppressive activity in the mouse T. cruzi model when administered intraperitoneally (IP) at 20 mg/kg [9]. The compound did not exhibit any apparent toxicity. The non-peptidic nature of the new series may help improve pharmacological properties of cruzain inhibitors. Drug design using the crystal structure of cruzain bound to the inhibitor is being used to further optimize this series.
Figure 1. Compounds with anti-T. cruzi activity in mice.
#1, non-peptidic cruzain inhibitor; #2, tipifarnib analog; #3, non-azole 14α-demethylase inhibitor; #4, miltefosine; #5, DB766; #6, nifurtimox; and #7, nifurtimox analog.
The sterol biosynthesis enzyme, 14α-demethylase (CYP51), has also been the subject of extensive investigation for many years with several significant advances in the past 1-2 years. This enzyme is the target of azole antifungal drugs and efforts to “repurpose” clinically available antifungal drugs such as posaconazole have potential to introduce a new drug class into the arena of anti-Chagas chemotherapy [10;11]. Recent studies describe new highly active chemical scaffolds that may have lower costs and/or better anti-T. cruzi activity than clinically available antifungals. Compounds derived from the anti-cancer drug candidate, tipifarnib, have extremely potent in vitro and in vivo anti-T. cruzi activity [12*]. The parent compound (tipifarnib) targets the mammalian protein farnesyltransferase enzyme, but this activity was deliberately engineered out of the molecule to reduce toxicity while simultaneously improving activity against the parasite 14α-demethylase. The lead compound (#2, Fig. 1) has oral bioavailability and weak inhibitory activity on hepatic CYP450 enzymes, which is unusual for CYP51 inhibitors. Another compound series derived from protein farnesyltransferase inhibitors are dialkylimidazoles [13]. These were also shown to have subnanomolar activity on intracellular amastigotes and partially curative activity in a stringent mouse model of T. cruzi infection. They do demonstrate fairly potent inhibitory activity on the hepatic CYP3A4 enzyme, thus making them somewhat less attractive than the tipifarnib analogs at this time. Finally, a non-azole 14α-demethylase inhibitor (#3, Fig. 1) has recently been described with curative activity (4 of 5 mice) when administered by IP route for 30 days [14]. This new chemical scaffold presents an important advance in the field, although analogs with oral bioavailability and demonstrated low CYP450 inhibition remain necessary for the new chemical scaffold’s ultimate success.
Another target specific compound that has been researched for repurposing against Chagas disease is miltefosine (#4, Fig. 1). This clinically used anti-leishmanial drug acts by interfering with phosphatidylcholine biosynthesis [15]. In vitro studies show potent activity (0.08 – 0.63 μM) on eleven T. cruzi strains, but in vivo studies were not described [16]. Earlier work has shown that miltefosine (30 mg/kg) had suppressive but not curative activity in T. cruzi infected mice [17].
Glyceraldehyde-3-phosphate dehydrogenase, farnesyl diphosphate synthase, and dihydrofolate reductase are three additional enzyme targets that have been investigated in the past two years in the context of anti-T. cruzi drug development. The studies are limited to enzymatic and structural studies and do not report data on anti-parasitic activities [18-20].
New compounds acting through non-specific targets
The diamidine drug, pentamidine, has been in clinical use for decades against other Kinetoplastid infections: human African trypanosomiasis and leishmaniasis [21;22]. Considerable research has taken place to identify related compounds with better pharmacological and safety properties than pentamidine [23*]. This effort has spilled into the Chagas disease arena with research to identify diamidines or arylimidamides showing activity against T. cruzi. The arylimidamide, DB766 (#5, Fig. 1), given orally to mice suppressed parasitemia and prevented mortality at doses of 25 or 50 mg/kg for ten days [24]. Favorable activity against the relatively resistant Colombian strain was also demonstrated. However, parasitological cures were not demonstrated and the mice treated once daily had weight loss, indicating that the compound has a narrow therapeutic window. In other in vitro studies, DB613A was the best arylimidamide in a series showing a half maximal inhibitory concentration (IC50) of 0.76 μM on intracellular amastigotes [25]. Another study described aromatic diamidines with in vitro activity on amastigotes in the 7-12 μM range and selectivity index >10 [26]. These compounds are DNA minor groove binders and have various other activities [27], thus mediating their cytotoxic effects non-specifically. A persistent challenge has been to identify compounds that have sufficient specificity for parasites, presumably through selective uptake, to avoid toxicities to the host such as were discovered in clinical trials with DB289 for human African trypanosomiasis [28].
The nitroaromatic compounds, such as nifurtimox (#6, Fig. 1) and benznidazole (#9, Fig. 2), have been the cornerstone of Chagas chemotherapy and research groups are making related compounds in hopes of finding molecules with better safety/therapeutic profiles. Noteworthy examples are new 5-nitro-2-furyl derivatives (#7, Fig. 1) that have strong suppressive activity in the mouse model (at 450 mg/kg by mouth for 14 days) and better acute toxicity profiles than nifurtimox [29]. However, achieving maximal antiparasitic activity with minimal toxicity is an ongoing challenge since drugs like nifurtimox mediate their effects inside cells nonspecifically through nitro-radicals. The discovery of a bacterial-like nitroreductase in T. cruzi [30] lends credence to the concept that compounds could be designed that are preferentially activated by parasites instead of host cells, thereby reducing side effects and/or the potentiality for mutagenicity, an important concern with these nitro-containing drugs.
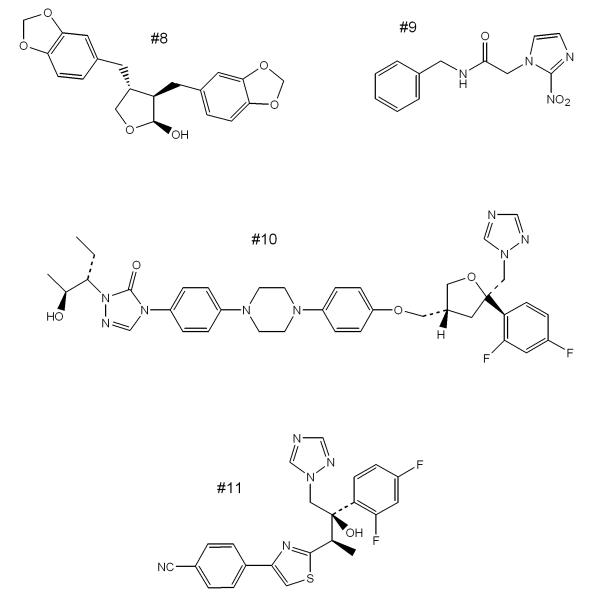
Figure 2. Additional compounds with anti-T. cruzi activity.
#8, natural product cubebin; #9, benznidazole; #10, posaconazole; and #11, ravuconazole.
Interesting work using carrier molecules has been applied in anti-T. cruzi drug research. For example, carrier ruthenium complexes that bind 14α-demethylase inhibitors [31] or benznidazole [32] improve solubility and parasite specificity. The most recent application of this approach employs ruthenium complexes to deliver nitric oxide (NO) to T. cruzi infected cells [33-35]. The complex, trans-[RuCl([15]aneN4)NO]2+, releases NO inside of macrophages and has trypanocidal activity in vitro [34*]. Alone the complex gave parasitological cure rates in mice of 30%, but when combined with benznidazole 80% of mice were cured [33]. There was no toxicity observed at the tested doses. It is likely that there are effects on the host native immune response that contribute to the biological activity. Similar compounds such as [Ru(NO)(NH3)4isn](BF4)3 were also tested in the experimental mouse model of T. cruzi infection and found to eliminate amastigote nests in myocardium tissue and confer survival at 400 nmol/kg for 15 days [35]. A paradoxical increase in tissue parasites was observed at higher doses reinforcing the notion that modulation of the host immune response is at play in addition to any direct antiparasitic activity. Many important issues need to be addressed before this strategy could become clinically applicable, particularly relating to oral bioavailability, safety, and cost of goods.
Another study described suppression of T. cruzi parasitemia in the mouse model using cyclopalladated compounds, however, the nature of these compounds makes them unlikely drug candidates [36].
Other synthetic compounds that won’t be further discussed were reported to have sub- [37], low- [38-42] or mid- [43-46] micromolar activity against T. cruzi cultures.
Natural Products
Previously discovered lignano lactones from the plant Piper cubeba [47] were shown to have in vivo activity in mice infected with T. cruzi (CL strain) [48]. Specifically, tissue parasites were reduced as low as 20% of control levels in mice given cubebin (#8, Fig. 2) or hinokinin for 20 days. The oral route appeared to be at least as effective as IP injection, although further optimization appears necessary to lead to compounds with curative activity. The mechanism of action of these lignans is unknown.
The South American plant, Aristolochia cymbifera, has been used in traditional medicine for fever and other conditions. Leaf extracts from this plant collected in São Paulo State, Brazil yielded the dibenzylbutyrolactone lignan, kusunokinin [49]. This was the most active of the compounds extracted from this plant with an IC50 against T. cruzi amastigotes of 17 μM, although the selectivity index of 2.2 against THP-1 cells indicates that greater selectivity is probably needed to ensure low toxicity to mammals. Lignans are a major class of phytoestrogens, which are estrogen like chemicals.
The natural product (-)-hinokinin was loaded into microparticles and shown to suppress parasitemia in mice when dosed at 40 mg/kg subcutaneously every other day for 20 days [50]. The obvious challenge with this approach will be devising a method for oral dosing using this formulation.
A number of other natural products and synthetic derivatives were observed to have low- [51;52] or mid- [53;54] or high- [55] micromolar activity on T. cruzi cultures.
Compound library screens
The recent implementation of high-throughput compound screening on T. cruzi is accelerating the pace of experiments that are critical to advancing drug discovery. The Rodriguez group utilized a T. cruzi strain expressing the reporter gene β-galactosidase [56] to screen the ChemBridge DIVERSet library of 2000 compounds [57*]. Seventy hits were initially identified against intracellular T. cruzi, but after further selection for high activity on T. cruzi and low toxicity on mammalian cells, the authors selected 3 compounds for further evaluation: (1) PCH1, a hydrazide molecule with an IC50 of 54 nM on T. cruzi, (2) NT1, a nitroazole with an IC50 of 190 nM, and (3) CX1, a chloroxylenol molecule with an IC50 of 23 nM. Several derivatives of these compounds were tested and some were found to have potency in the 2-5 nM range. The paper addresses potential mechanisms of action of these different scaffolds. Work on the pharmacology or in vivo activity of the compounds was not reported.
Next the Broad Institute conducted a high-throughput screen of 303,221 compounds on T. cruzi cultures (in 384-well format) and posted the results on PubChem (http://pubchem.ncbi.nlm.nih.gov/assay/assay.cgi?aid=1885). The initial screen yielded 4394 active compounds and 4063 of them have since been confirmed. Additional testing yielded a set of 316 compounds with >100-fold selectivity on parasites over host 3T3 fibroblasts. The compounds represent >50 diverse scaffolds and the majority conform to “Lipinski’s rule of 5.” Many of these molecules offer an excellent starting point for medicinal chemistry groups to make new T. cruzi inhibitors.
Summary of clinical developments
The BENEFIT trial is ongoing. It tests the hypothesis that etiologic treatment with benznidazole (#9, Fig. 2) is beneficial for patients with chronic Chagas heart disease [4]. This is the only modern, large-scale drug study that has been performed on Chagas disease. By studying chronic patients, the investigators plan to assess quantifiable endpoints such as cardiovascular outcomes and mortality using a sample of ~3000 patients. The research will not only provide important guidance for clinical management of chronic Chagas patients, but it will also provide experience with diagnostic tools (such as real-time PCR) and infrastructure that will be needed in future studies. More difficult, but perhaps even more important studies that rigorously address the benefit of etiologic treatment in patients with indeterminate disease will need to be done separately.
As indicated above, posaconazole (#10, Fig. 2) is an antifungal drug in clinical use that has extraordinarily potent anti-T. cruzi activity in rodent models [58]. An interesting case-report from Spain described an Argentinean immigrant with reactivated Chagas disease due to immunosuppressive therapy given to treat systemic lupus erythematosis [59*]. Benznidazole induced a reduction of parasitemia, but not elimination. Subsequent treatment with posaconazole led to successful resolution of infection, despite the maintenance of immunosuppressive therapy. It is reported that a Phase II clinical study with posaconazole for Chagas disease sponsored by Merck & Co. is planned in Spain [5], although details are not available at this time.
Similarly, the antifungal drug ravuconazole (#11, Fig. 2) has been shown to have potent (but not curative) activity in the dog model of Chagas disease [60]. Although the plasma half-life in dogs (8.8 hours) is relatively short, ravuconazole’s longer half-life in humans (4-8 days) makes it a good candidate for clinical trial. In fact, it is reported that DNDi and Eisai Pharmaceuticals are partnering for a Phase II trial of a pro-drug of ravuconazole (E1224) possibly in Bolivia and/or Spain [5].
If clinical trials are done with posaconazole and/or E1224, these will be the first such studies testing new drug candidates to be performed in decades for Chagas disease, and they may move the field beyond the era in which poorly tolerated, modestly effective drugs are the only option. Unfortunately, costs associated with these drugs may pose a challenge for delivery to Chagas patients in resource-limited settings. There will need to be plans to make the drugs available through philanthropy from organizations and countries with sufficient resources, or alternative cheaper drugs will be required to fulfill the need.
Coordinating research to accelerate discovery for Chagas disease
The dramatic success in reducing vectorial spread of T. cruzi infection in South America over the past two decades has, perhaps, diminished the sense of urgency for discovering new chemotherapeutics Chagas disease. However, Chagas disease remains an important public health problem, and further research is greatly needed to address this problem. Recent estimates of worldwide prevalence (~10-12 million), incidence (41,200/year), and deaths (12,500/year) from Chagas disease indicate that the disease still impacts a large number of people [2]. Since vector control has not been conducted effectively in all endemic regions, disease transmission unfortunately continues at high rates in many countries such as Bolivia [61*]. The large pool of infected persons maintains the risk of continued dissemination of T. cruzi through blood products, mother to baby transmission, and spread into non-endemic countries through emigration. Since Chagas disease is a lifelong infection, there remains a large pool of individuals who were infected before the era of aggressive domiciliary pesticide spraying and many of these persons may benefit from treatment.
In addition to (or as a result of) low investment into Chagas drug discovery research, some specific factors contribute to the slow progress being made. First, most of the research takes place in small, academic research labs where there tends to be poor coordination or partnering of efforts between the dispersed groups. A second, related problem is the lack of standardization of methodologies, making comparisons of results from various research groups difficult. The establishment of coordinated groups such as the University of Georgia’s Chagas Drug Discovery Consortium (http://www.ctegd.uga.edu/) and private public partnerships such as Drugs for Neglected Diseases Initiative (http://www.dndi.org/) are helping address these issues. Encouragingly, a workshop held in Rio de Janeiro (Nov. 2008) entitled Experimental Models in Drug Screening and Development for Chagas Disease has led to a recent publication recommending protocols for in vitro and in vivo drug screening that will hopefully bring better harmonization to the field [62**].
Conclusions
Except for the occasional case report or small case series of “off-label” use of existing drugs, we have not seen new drugs in use for Chagas patients for ~40 years. Covering the past two years, this review reports on a number of promising compound classes that are in early phases of drug discovery, some demonstrating activity in animal models, and others just showing activity in vitro. The implementation of truly high-throughput compound screening on the T. cruzi is a major advancement, and will hopefully seed productive medicinal chemistry efforts as the field moves forward. Although these discoveries engender optimism, substantial investment and focus will be required to see this preclinical research advance to clinical trials.
Acknowledgements
Buckner receives research support from National Institutes of Health (AI070218), the Consortium for Antiparasitic Drug Development, and Drugs for Neglected Diseases Initiative. Navabi receives research support from the Infectious Diseases Society of America Education and Research Foundation.
Funding sources: National Institutes of Health; Consortium for Parasitic Drug Discovery; Drugs for Neglected Diseases Initiative; Infectious Diseases Society of America Education and Research Foundation
References and Recommended Reading
- (1).Bern C, Montgomery SP, Herwaldt BL, et al. Evaluation and treatment of Chagas disease in the United States: a systematic review. JAMA. 2007;298:2171–2181. doi: 10.1001/jama.298.18.2171. [DOI] [PubMed] [Google Scholar]
- (2).Rassi A, Jr., Rassi A, Marin-Neto JA. Chagas disease. Lancet. 2010;375:1388–1402. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- (3).Urbina JA. Specific chemotherapy of Chagas disease: relevance, current limitations and new approaches. Acta Trop. 2010;115:55–68. doi: 10.1016/j.actatropica.2009.10.023. [DOI] [PubMed] [Google Scholar]
- **(4).Marin-Neto JA, Rassi A, Jr., Avezum A, et al. The BENEFIT trial: testing the hypothesis that trypanocidal therapy is beneficial for patients with chronic Chagas heart disease. Mem Inst Oswaldo Cruz. 2009;104(1 Suppl):319–324. doi: 10.1590/s0074-02762009000900042. This provides an update of the ongoing BENEFIT (Benznidazole Evaluation for Interrupting Trypanosomiasis) trial, which consists of a pilot study and a full-scale trial with 3000 patients. It is the first clinical trial on a large scale to be conducted for Chagas disease.
- (5).Clayton J. Chagas disease: pushing through the pipeline. Nature. 2010;465:S12–S15. doi: 10.1038/nature09224. [DOI] [PubMed] [Google Scholar]
- *(6).Ribeiro I, Sevcsik AM, Alves F, et al. New, improved treatments for Chagas disease: from the R&D pipeline to the patients. PLoS Negl Trop Dis. 2009;3:e484. doi: 10.1371/journal.pntd.0000484. An overview of the challenges relating to Chagas disease drug development and the responses by non-profit product development partnerships, particularly the Drugs for Neglected Diseases Initiative (DNDi). A proposed target product profile for a Chagas drug is outlined.
- (7).Harth G, Andrews N, Mills AA, et al. Peptide-fluoromethyl ketones arrest intracellular replication and intercellular transmission of Trypanosoma cruzi. Mol Biochem Parasitol. 1993;58:17–24. doi: 10.1016/0166-6851(93)90086-d. [DOI] [PubMed] [Google Scholar]
- (8).McKerrow JH, Doyle PS, Engel JC, et al. Two approaches to discovering and developing new drugs for Chagas disease. Mem Inst Oswaldo Cruz. 2009;104(1 Suppl):263–269. doi: 10.1590/s0074-02762009000900034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Brak K, Kerr ID, Barrett KT, et al. Nonpeptidic tetrafluorophenoxymethyl ketone cruzain inhibitors as promising new leads for Chagas disease chemotherapy. J Med Chem. 2010;53:1763–1773. doi: 10.1021/jm901633v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Urbina JA. Ergosterol biosynthesis and drug development for Chagas disease. Mem Inst Oswaldo Cruz. 2009;104(1 Suppl):311–318. doi: 10.1590/s0074-02762009000900041. [DOI] [PubMed] [Google Scholar]
- (11).Olivieri BP, Molina JT, de Castro SL, et al. A comparative study of posaconazole and benznidazole in the prevention of heart damage and promotion of trypanocidal immune response in a murine model of Chagas disease. Int J Antimicrob Agents. 2010;36:79–83. doi: 10.1016/j.ijantimicag.2010.03.006. [DOI] [PubMed] [Google Scholar]
- *(12).Kraus JM, Verlinde CL, Karimi M, et al. Rational modification of a candidate cancer drug for use against Chagas disease. J Med Chem. 2009;52:1639–1647. doi: 10.1021/jm801313t. Novel compounds with potent activity in the murine model of Chagas disease and favorable pharmacokinetic properties are described. The compounds act on the same target as azole drugs (the sterol 14α-demethylase).
- (13).Suryadevara PK, Olepu S, Lockman JW, et al. Structurally simple inhibitors of lanosterol 14alpha-demethylase are efficacious in a rodent model of acute Chagas disease. J Med Chem. 2009;52:3703–3715. doi: 10.1021/jm900030h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Doyle PS, Chen CK, Johnston JB, et al. A nonazole CYP51 inhibitor cures Chagas’ disease in a mouse model of acute infection. Antimicrob Agents Chemother. 2010;54:2480–2488. doi: 10.1128/AAC.00281-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Berman JJ. Treatment of leishmaniasis with miltefosine: 2008 status. Expert Opin Drug Metab Toxicol. 2008;4:1209–1216. doi: 10.1517/17425255.4.9.1209. [DOI] [PubMed] [Google Scholar]
- (16).Luna KP, Hernandez IP, Rueda CM, et al. In vitro susceptibility of Trypanosoma cruzi strains from Santander, Colombia, to hexadecylphosphocholine (miltefosine), nifurtimox and benznidazole. Biomedica. 2009;29:448–455. [PubMed] [Google Scholar]
- (17).Croft SL, Snowdon D, Yardley V. The activities of four anticancer alkyllysophospholipids against Leishmania donovani, Trypanosoma cruzi, and Trypanosoma brucei. J Antimicr Chemoth. 1996;38:1041–1047. doi: 10.1093/jac/38.6.1041. [DOI] [PubMed] [Google Scholar]
- (18).Freitas RF, Prokopczyk IM, Zottis A, et al. Discovery of novel Trypanosoma cruzi glyceraldehyde-3-phosphate dehydrogenase inhibitors. Bioorg Med Chem. 2009;17:2476–2482. doi: 10.1016/j.bmc.2009.01.079. [DOI] [PubMed] [Google Scholar]
- (19).Schormann N, Velu SE, Murugesan S, et al. Synthesis and characterization of potent inhibitors of Trypanosoma cruzi dihydrofolate reductase. Bioorg Med Chem. 2010;18:4056–4066. doi: 10.1016/j.bmc.2010.04.020. [DOI] [PubMed] [Google Scholar]
- (20).Huang CH, Gabelli SB, Oldfield E, Amzel LM. Binding of nitrogen-containing bisphosphonates (N-BPs) to the Trypanosoma cruzi farnesyl diphosphate synthase homodimer. Proteins. 2010;78:888–899. doi: 10.1002/prot.22614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Berman J. Current treatment approaches to leishmaniasis. Curr Opin Infect Dis. 2003;16:397–401. doi: 10.1097/00001432-200310000-00005. [DOI] [PubMed] [Google Scholar]
- (22).Maudlin I. African trypanosomiasis. Ann Trop Med Parasitol. 2006;100:679–701. doi: 10.1179/136485906X112211. [DOI] [PubMed] [Google Scholar]
- *(23).Soeiro MN, de Castro SL, de Souza EM, et al. Diamidine activity against trypanosomes: the state of the art. Curr Mol Pharmacol. 2008;1:151–161. doi: 10.2174/1874467210801020151. This study reports on a pentamidine-like compound with anti-parasitic activity when given orally to T. cruzi infected mice and in vitro activity against diverse strains.
- (24).Batista DG, Batista MM, de Oliveira GM, et al. Arylimidamide DB766, a potential chemotherapeutic candidate for Chagas’ disease treatment. Antimicrob Agents Chemother. 2010;54:2940–2952. doi: 10.1128/AAC.01617-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Pacheco MG, da Silva CF, de Souza EM, et al. Trypanosoma cruzi: activity of heterocyclic cationic molecules in vitro. Exp Parasitol. 2009;123:73–80. doi: 10.1016/j.exppara.2009.06.004. [DOI] [PubMed] [Google Scholar]
- (26).Batista DG, Pacheco MG, Kumar A, et al. Biological, ultrastructural effect and subcellular localization of aromatic diamidines in Trypanosoma cruzi. Parasitology. 2010;137:251–259. doi: 10.1017/S0031182009991223. [DOI] [PubMed] [Google Scholar]
- (27).Nyunt MM, Hendrix CW, Bakshi RP, et al. Phase I/II evaluation of the prophylactic antimalarial activity of pafuramidine in healthy volunteers challenged with Plasmodium falciparum sporozoites. Am J Trop Med Hyg. 2009;80:528–535. [PMC free article] [PubMed] [Google Scholar]
- (28).Yeramian PD, Castagnini LA, Allen JA, et al. Efficacy and safety of DB289, a new oral drug for treatment of Pneumocystis carinii pneumonia (PCP) in AIDS Patients. Presented at the 43rd Annual Interscience Conference on Antimicrobial Agents and Chemotherapy Meeting; Chicago, IL. September 14-17, 2003.2010. [Google Scholar]
- (29).Cabrera E, Murguiondo MG, Arias MG, et al. 5-Nitro-2-furyl derivative actives against Trypanosoma cruzi: preliminary in vivo studies. Eur J Med Chem. 2009;44:3909–3914. doi: 10.1016/j.ejmech.2009.04.015. [DOI] [PubMed] [Google Scholar]
- (30).Wilkinson SR, Taylor MC, Horn D, et al. A mechanism for cross-resistance to nifurtimox and benznidazole in trypanosomes. Proc Natl Acad Sci U S A. 2008;105:5022–5027. doi: 10.1073/pnas.0711014105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Sanchez-Delgado RA, Anzellotti A. Metal complexes as chemotherapeutic agents against tropical diseases: trypanosomiasis, malaria and leishmaniasis. Mini Rev Med Chem. 2004;4:23–30. doi: 10.2174/1389557043487493. [DOI] [PubMed] [Google Scholar]
- (32).Nogueira Silva JJ, Pavanelli WR, Gutierrez FR, et al. Complexation of the anti-Trypanosoma cruzi drug benznidazole improves solubility and efficacy. J Med Chem. 2008;51:4104–4114. doi: 10.1021/jm701306r. [DOI] [PubMed] [Google Scholar]
- (33).Guedes PM, Oliveira FS, Gutierrez FR, et al. Nitric oxide donor trans-[RuCl([15]aneN)NO] as a possible therapeutic approach for Chagas’ disease. Br J Pharmacol. 2010;160:270–282. doi: 10.1111/j.1476-5381.2009.00576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *(34).Silva JJ, Guedes PM, Zottis A, et al. Novel ruthenium complexes as potential drugs for Chagas’s disease: enzyme inhibition and in vitro/in vivo trypanocidal activity. Br J Pharmacol. 2010;160:260–269. doi: 10.1111/j.1476-5381.2009.00524.x. This is another paper in which novel compounds were shown to have activity in the murine model of T. cruzi infection. The compounds appear to have immune modulating effects through nitric oxide induced pathways.
- (35).Silva JJ, Pavanelli WR, Pereira JC, et al. Experimental chemotherapy against Trypanosoma cruzi infection using ruthenium nitric oxide donors. Antimicrob Agents Chemother. 2009;53:4414–4421. doi: 10.1128/AAC.00104-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Matsuo AL, Silva LS, Torrecilhas AC, et al. In vitro and in vivo trypanocidal effects of the cyclopalladated 7a compound: a drug candidate for Chagas’ disease treatment. Antimicrob Agents Chemother. 2010;54:3318–3325. doi: 10.1128/AAC.00323-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Vieites M, Smircich P, Guggeri L, et al. Synthesis and characterization of a pyridine-2-thiol N-oxide gold(I) complex with potent antiproliferative effect against Trypanosoma cruzi and Leishmania sp. insight into its mechanism of action. J Inorg Biochem. 2009;103:1300–1306. doi: 10.1016/j.jinorgbio.2009.02.011. [DOI] [PubMed] [Google Scholar]
- (38).Rodriguez J, Aran VJ, Boiani L, et al. New potent 5-nitroindazole derivatives as inhibitors of Trypanosoma cruzi growth: synthesis, biological evaluation, and mechanism of action studies. Bioorg Med Chem. 2009;17:8186–8196. doi: 10.1016/j.bmc.2009.10.030. [DOI] [PubMed] [Google Scholar]
- (39).Bollini M, Casal JJ, Alvarez DE, et al. New potent imidazoisoquinolinone derivatives as anti-Trypanosoma cruzi agents: biological evaluation and structure-activity relationships. Bioorg Med Chem. 2009;17:1437–1444. doi: 10.1016/j.bmc.2009.01.011. [DOI] [PubMed] [Google Scholar]
- (40).Boiani M, Boiani L, Merlino A, et al. Second generation of 2H-benzimidazole 1,3-dioxide derivatives as anti-trypanosomatid agents: synthesis, biological evaluation, and mode of action studies. Eur J Med Chem. 2009;44:4426–4433. doi: 10.1016/j.ejmech.2009.06.014. [DOI] [PubMed] [Google Scholar]
- (41).Gerpe A, Boiani L, Hernandez P, et al. Naftifine-analogues as anti-Trypanosoma cruzi agents. Eur J Med Chem. 2010;45:2154–2164. doi: 10.1016/j.ejmech.2010.01.052. [DOI] [PubMed] [Google Scholar]
- (42).Gigante F, Kaiser M, Brun R, Gilbert IH. SAR studies on azasterols as potential anti-trypanosomal and anti-leishmanial agents. Bioorg Med Chem. 2009;17:5950–5961. doi: 10.1016/j.bmc.2009.06.062. [DOI] [PubMed] [Google Scholar]
- (43).Pardo Andreu GL, Inada NM, Pellon RF, et al. In vitro effect of a new cinnamic acid derivative against the epimastigote form of Trypanosoma cruzi. Arzneimittelforschung. 2009;59:207–211. doi: 10.1055/s-0031-1296387. [DOI] [PubMed] [Google Scholar]
- (44).Magdaleno A, Ahn IY, Paes LS, Silber AM. Actions of a proline analogue, L-thiazolidine-4-carboxylic acid (T4C), on Trypanosoma cruzi. PLoS ONE. 2009;4:e4534. doi: 10.1371/journal.pone.0004534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Lorente SO, Rodrigues JC, Jimenez JC, et al. Novel azasterols as potential agents for treatment of leishmaniasis and trypanosomiasis. Antimicrob Agents Chemother. 2004;48:2937–2950. doi: 10.1128/AAC.48.8.2937-2950.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Ennes-Vidal V, Menna-Barreto RF, Santos AL, et al. Effects of the calpain inhibitor MDL28170 on the clinically relevant forms of Trypanosoma cruzi in vitro. J Antimicrob Chemother. 2010;65:1395–1398. doi: 10.1093/jac/dkq154. [DOI] [PubMed] [Google Scholar]
- (47).Saraiva J, Vega C, Rolon M, et al. In vitro and in vivo activity of lignan lactones derivatives against Trypanosoma cruzi. Parasitol Res. 2007;100:791–795. doi: 10.1007/s00436-006-0327-4. [DOI] [PubMed] [Google Scholar]
- (48).Esperandim VR, da Silva FD, Saraiva J, et al. Reduction of parasitism tissue by treatment of mice chronically infected with Trypanosoma cruzi with lignano lactones. Parasitol Res. 2010;107:525–530. doi: 10.1007/s00436-010-1885-z. [DOI] [PubMed] [Google Scholar]
- (49).Sartorelli P, Salomone CC, Quero RJ, et al. Antitrypanosomal activity of a diterpene and lignans isolated from Aristolochia cymbifera. Planta Med. 2010 doi: 10.1055/s-0029-1240952. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- (50).Saraiva J, Lira AA, Esperandim VR, et al. (-)-Hinokinin-loaded poly(D,-lactide-co-glycolide) microparticles for Chagas disease. Parasitol Res. 2010;106:703–708. doi: 10.1007/s00436-010-1725-1. [DOI] [PubMed] [Google Scholar]
- (51).Menna-Barreto RF, Goncalves RL, Costa EM, et al. The effects on Trypanosoma cruzi of novel synthetic naphthoquinones are mediated by mitochondrial dysfunction. Free Radic Biol Med. 2009;47:644–653. doi: 10.1016/j.freeradbiomed.2009.06.004. [DOI] [PubMed] [Google Scholar]
- (52).Tonin LT, Panice MR, Nakamura CV, et al. Antitrypanosomal and antileishmanial activities of novel N-alkyl-(1-phenylsubstituted-beta-carboline)-3-carboxamides. Biomed Pharmacother. 2010;64:386–389. doi: 10.1016/j.biopha.2010.02.006. [DOI] [PubMed] [Google Scholar]
- (53).da Silva EN, Jr., Guimaraes TT, Menna-Barreto RF, et al. The evaluation of quinonoid compounds against Trypanosoma cruzi: synthesis of imidazolic anthraquinones, nor-beta-lapachone derivatives and beta-lapachone-based 1,2,3-triazoles. Bioorg Med Chem. 2010;18:3224–3230. doi: 10.1016/j.bmc.2010.03.029. [DOI] [PubMed] [Google Scholar]
- (54).Ferreira DS, Esperandim VR, Toldo MP, et al. Trypanocidal activity and acute toxicity assessment of triterpene acids. Parasitol Res. 2010;106:985–989. doi: 10.1007/s00436-010-1740-2. [DOI] [PubMed] [Google Scholar]
- (55).Cabral MM, Barbosa-Filho JM, Maia GL, et al. Neolignans from plants in northeastern Brazil (Lauraceae) with activity against Trypanosoma cruzi. Exp Parasitol. 2010;124:319–324. doi: 10.1016/j.exppara.2009.11.007. [DOI] [PubMed] [Google Scholar]
- (56).Buckner FS, Verlinde CLMJ, La Flamme AC, Van Voorhis WC. Efficient technique for screening drugs for activity against Trypanosoma cruzi using parasites expressing β-galactosidase. Antimicrob Agents Chemother. 1996;40:2592–2597. doi: 10.1128/aac.40.11.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *(57).Bettiol E, Samanovic M, Murkin AS, et al. Identification of three classes of heteroaromatic compounds with activity against intracellular Trypanosoma cruzi by chemical library screening. PLoS Negl Trop Dis. 2009;3:e384. doi: 10.1371/journal.pntd.0000384. The implementation of a high-throughput screen of a 2000 compound library against T. cruzi cultures is discussed. The same group helped coordinate subsequent screening of a library containing >300,000 compounds at the Broad Institute (data are unpublished, but available in PubChem).
- (58).Molina J, Martins-Filho O, Brener Z, et al. Activities of the triazole derivative SCH 56592 (posaconazole) against drug-resistant strains of the protozoan parasite Trypanosoma (Schizotrypanum) cruzi in immunocompetent and immunosuppressed murine hosts. Antimicrob Agents Chemother. 2000;44:150–155. doi: 10.1128/aac.44.1.150-155.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *(59).Pinazo MJ, Espinosa G, Gallego M, et al. Successful treatment with posaconazole of a patient with chronic Chagas disease and systemic lupus erythematosus. Am J Trop Med Hyg. 2010;82:583–587. doi: 10.4269/ajtmh.2010.09-0620. This paper describes an immunosuppressed patient with a T. cruzi infection that was uncontrolled by benznidazole who then responded completely to posaconazole. Coupled with posaconazole’s known activity in animal models, this case report may help stimulate additional larger scale, controlled studies with posaconazole in Chagas patients.
- (60).Diniz LF, Caldas IS, Guedes PM, Crepalde G, de Lana M, Carneiro CM, et al. Effects of ravuconazole treatment on parasite load and immune response in dogs experimentally infected with Trypanosoma cruzi. Antimicrob Agents Chemother. 2010;54(7):2979–2986. doi: 10.1128/AAC.01742-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *(61).Yun O, Lima MA, Ellman T, et al. Feasibility, drug safety, and effectiveness of etiological treatment programs for chagas disease in Honduras, Guatemala, and Bolivia: 10-year experience of medecins sans frontieres. PLoS Negl Trop Dis. 2009;3:e488. doi: 10.1371/journal.pntd.0000488. A descriptive report of work done by Doctors Without Borders to diagnose and treat patients with T. cruzi infection in Honduras, Guatemala, and Bolivia. It demonstrates feasibility of implementing treatment programs in resource-limited settings, but emphasizes the need for better drugs and diagnostics.
- **(62).Romanha AJ, Castro SL, Soeiro MN, et al. In vitro and in vivo experimental models for drug screening and development for Chagas disease. Mem Inst Oswaldo Cruz. 2010;105:233–238. doi: 10.1590/s0074-02762010000200022. This paper supported by Fiocruz and DNDi summarizes the in vitro and in vivo experimental models for testing compounds for anti-T. cruzi activity. It proposes an algorithm for working up compounds. By facilitating comparison of data from different laboratories, this paper may help bring much needed harmonization to the field.