Abstract
Background
Allosensitization is associated with inferior waitlist outcomes in pediatric heart transplant candidates, presumably because of the requirement for a negative prospective crossmatch. However, there are no reports of heart transplant candidate outcomes according to prospective crossmatch requirements.
Methods
We analyzed data on all children listed for isolated heart transplantation from 1995 to 2009 in the USA according to prospective crossmatch requirement (PXMR). Primary objectives were to describe the prevalence of PXMR at and during listing and to compare waitlist and post-transplant survival for patients based on PXMR. Patients with a PXMR during listing include those with a PXMR at the time of listing as well as those who were designated by the listing center as needing a prospective crossmatch at some point after being placed onto the waitlist.
Results
Among 6,343 listed children, 7.7% had a requirement for a prospective crossmatch at the time of listing and 11.8% had a requirement for a prospective crossmatch during listing. After controlling for risk factors associated with inferior survival, PXMR at listing was associated with increased waitlist mortality (HR 1.32, 95% CI 1.10 to 1.56; p = 0.003). Recipients with a PXMR during listing more commonly had a positive DSXM (22.1% vs 10.3%, p < 0.0001), as did recipients who carried a PXMR throughout listing (21.7% vs 11.3%, p = 0.004). However, there was no significant difference in post-transplant survival on the basis of a PXMR during listing (HR 1.04, 95% CI 0.87 to 1.25; p = 0.67). Nearly 30% of recipients with a PXMR during listing had a peak pre-transplant PRA ≤ 10%.
Conclusions
PXMR increases the likelihood of death while awaiting, but not after, pediatric heart transplantation. Further study is necessary to understand how PXMR is applied, and changes, after listing for pediatric heart transplantation.
Keywords: pediatric, heart transplantation, allosensitization, crossmatch
In retrospective cohort studies, allosensitization has been associated with longer waitlist duration and increased pre-transplant mortality among pediatric heart transplant candidates.1,2 Presumably, this is because many of these children were listed with a requirement for a negative prospective crossmatch and thus endured an additional burden in order to receive a suitable donor organ. However, there are no reports that have examined heart transplant candidate outcomes on the basis of prospective crossmatch requirements. Because there is no threshold panel-reactive antibody (PRA) level that necessitates listing with a requirement for a prospective crossmatch, decisions about listing with a requirement for a prospective crossmatch may vary across centers, or over time within a center, potentially resulting in differences in outcomes between these indicators of allosensitization.
Using national registry data from the Organ Procurement Transplantation Network (OPTN), we sought to address this important topic. The main objectives of our analysis were to describe the prevalence of the prospective crossmatch requirement (PXMR) among pediatric heart transplant candidates at listing and at any time during listing and to compare waitlist outcomes and post-transplant survival for patients based on the requirement for a prospective crossmatch. Other objectives were to compare donor-related transplant characteristics for recipients with and without a requirement for a prospective crossmatch as well as to examine pre-transplant PRA data and post-transplant donorspecific crossmatch (DSXM) results for the groups.
Methods
Data source and study population
The OPTN is an internally audited, mandatory, government-sponsored registry that collects listing and transplantation information on all solid-organ registrations in the USA. We received a data file of all listings for heart transplantation in children < 18 years of age between January 1, 1993 and December 31, 2009 (8,049 listings / 7,382 children). We excluded listings before April 1, 1995, when data on PXMR were first collected (907 children). We also excluded the following: primary listings for multiple-organ transplant procedures (106 children); listings in which the record depicting changes in the specification for a prospective crossmatch could not be reconciled with listing data in the candidate registration file (9 children); listings in which the candidate was listed as “inactive” for the entire duration of the listing (12 children); listings in which the reported death date preceded the listing date (4 children); and all simultaneous listings for patients listed at multiple transplant centers if the earliest listing date predated the availability of prospective crossmatch requirement data (1 child).
The final cohort included 6,343 children listed for transplantation. This cohort was followed from time of listing until death, loss to follow-up or date of data extraction (March 4, 2011). Death dates were based on the Social Security death master file data that were included with the data set.
Study definitions and outcome measures
Our primary hypothesis was that, after adjusting for risk factors known to decrease survival (e.g., congenital heart disease, urgency status, etc.), children with the requirement for a prospective (actual or virtual) crossmatch have inferior waitlist outcomes but similar post-transplant survival compared to patients without such a requirement. Specifically, we hypothesized that patients with a PXMR at listing would have longer waitlist durations, a higher cumulative incidence of death on the waiting list, and a lower cumulative incidence of transplantation. We also hypothesized that, among recipients, a PXMR during listing would not be associated with post-transplant survival. This last hypothesis was made under the assumption that patients with a PXMR during listing who achieved transplantation would almost exclusively be transplanted across a negative DSXM.
We identified patients with a PXMR in the OPTN data set based on either direct measurement (“Preliminary crossmatch required?”) or designation of one or more “unacceptable antigens” during their earliest listing in the data set. Because we observed some changes in PXMR were recorded within minutes of the original entry or of a prior change in PXMR, we assumed such changes were corrections to clerical errors. Thus, patients who maintained the PXMR requirement for the first 24 hours after listing were designated as having a PXMR at listing and patients who had a PXMR for at least 24 hours at any point during listing (including the first 24 hours after listing) were designated as having a PXMR during listing.
In the OPTN database, PRA data are only collected at the time of transplantation. Both “peak” and “most recent” PRA are recorded without data on methodology. After June 2004, HLA Class I and Class II PRA data were collected and in these cases we used the highest recorded value. We considered patients to have had a positive DSXM if they had ≥ 1 “weak positive” or “positive” result by any methodology (including flow cytometry), cell type (T-cell, B-cell or unseparated lymphocytes) and antibody class (IgG only, IgG and IgM or not reported).
Our analyses of waitlist outcomes were censored at 2 years after listing, on the last day of observation (March 4, 2011), or upon delisting if not relisted within 14 days. Patients who were delisted for reasons other than transplantation and then relisted at the same center within 14 days were considered to have a single listing comprised of waitlist time from both listings.
Statistical analysis
Summary statistics are presented as mean ± standard deviation or number (percent). Cohort characteristics were compared using Student's t-test, chi-square test or Fisher's exact test, as appropriate. For the analysis of waitlist outcomes, the proportion of patients who died, received a transplant, were delisted, and still awaiting transplant are depicted as competing outcomes plots and compared using Gray's test.3 A multivariate Cox model of waitlist survival to 2 years after listing, censored at transplantation and delisting, was also fitted to assess the independent association of a PXMR at the time of listing. Post-transplant survival was assessed by Kaplan–Meier plot with log-rank test. Multivariate Cox proportional hazard modeling of this outcome was performed using covariables associated with post-transplant survival.4 All tests were 2-sided with p < 0.05 considered statistically significant. Data were analyzed with SAS version 9.2 (SAS Institute, Inc., Cary, NC), STATA version 10.1 (StataCorp LP, College Station, TX) and R (R Foundation for Statistical Computing, Vienna, Austria). The study was conducted with approval from the institutional review board of the University of Pittsburgh and the OPTN.
Results
Of the 6,343 children in the study cohort, 488 (7.7%) had a PXMR at the time of listing. Whereas most children (68.2%) were identified solely on the basis of the “preliminary crossmatch required” indicator, 12.1% were identified on the basis of specified unacceptable antigens alone and 19.7% were flagged with both designations. A total of 751 patients (11.8%) had a PXMR during the waitlist period and 236 patients (3.7%) had PXMR throughout their entire listing.
Table 1 depicts cohort characteristics stratified on the basis of PXMR at the time of listing. The PXMR group was older and more commonly had a diagnosis of congenital heart disease and a prior history of solid-organ transplantation. In terms of status at listing, a greater proportion of patients in the crossmatch required group were listed as Status 1B and fewer as historical Status 1; however, when analyzed as Status 1/1A/1B vs Status 2/7 at listing, there was no statistical difference between the groups. Likewise, assisted ventilation, inotropic support and extracorporeal membrane oxygenation (ECMO) support at listing were also similar between the groups, whereas VAD at listing was more common in those listed with a requirement for prospective crossmatch.
Table 1. Baseline Characteristics at Listing.
| Variable | Category | Prospective crossmatch required at listing | p-value | ||
|---|---|---|---|---|---|
|
| |||||
| Cohort (N = 6343) | No (n = 5,855; 92%) | Yes (n = 488; 8%) | |||
| Age | 5.5 ± 6.1 | 5.4 ± 6.1 | 6.4 ± 6.2 | 0.0011 | |
| Gender | Female | 2,771 (43.7%) | 2,564 (43.8%) | 207 (42.4%) | 0.557 |
| Race/ethnicity | White | 3,732 (58.8%) | 3,453 (59.0%) | 279 (57.2%) | 0.159 |
| Black | 1,246 (19.6%) | 1,134 (19.4%) | 112 (23.0%) | · | |
| Hispanic | 1,052 (16.6%) | 982 (16.8%) | 70 (14.3%) | — | |
| Other | 313 (4.9%) | 286 (4.9%) | 27 (5.5%) | — | |
| Diagnosis | CHD | 3,094 (48.8%) | 2,659 (47.6%) | 435 (57.9%) | <0.0001 |
| DCM | 2,498 (39.4%) | 2,248 (40.2%) | 250 (33.3%) | — | |
| RCM/HCM | 468 (7.4%) | 445 (8.0%) | 23 (3.1%) | — | |
| Retransplant | 174 (2.7%) | 141 (2.5%) | 33 (4.4%) | — | |
| Other | 87 (1.4%) | 79 (1.4%) | 8 (1.1%) | — | |
| Unknown | 22 (0.3%) | 20 (0.4%) | 2 (0.3%) | — | |
| Primary payer | Public/gov’t insurance | 2,724 (43.1%)a | 2,519 (43.2%) | 205 (42.0%) | 0.237 |
| Private insurance | 3,425 (54.1%) | 3,151 (54.0%) | 274 (56.2%) | — | |
| Donation | 93 (1.5%) | 88 (1.5%) | 5 (1.0%) | — | |
| Free care | 9 (0.1%) | 7 (0.1%) | 2 (0.4%) | — | |
| None | 16 (0.3%) | 16 (0.3%) | 2 (0.4%) | — | |
| Other | 59 (0.9%) | 57 (1.0%) | 3 (0.4%) | — | |
| History of a prior Tx UNOS status | Yes | 194 (3.1%) | 165 (2.8%) | 29 (5.9%) | <0.0001 |
| 1 | 1,092 (17.2%) | 1,034 (17.7%) | 87 (11.6%) | 0.002 | |
| 1A | 2,937 (46.3%) | 2,707 (46.2%) | 230 (47.1%) | — | |
| 1B | 613 (9.7%) | 549 (9.4%) | 64 (13.1%) | — | |
| 2 | 1,661 (26.2%) | 1,530 (26.1%) | 131 (26.8%) | — | |
| 7 | 40 (0.6%) | 35 (0.6%) | 5 (1.0%) | — | |
| UNOS status (grouped) | 1/1A/1B | 4,642 (73.2%) | 4,290 (73.3%) | 352 (72.1%) | 0.585 |
| 2/7 | 1,701 (26.8%) | 1,565 (26.7%) | 136 (27.9%) | — | |
| Inotropic support | Yes | 3,104 (48.9%) | 2,867 (49.0%) | 237 (48.6%) | 0.865 |
| Ventilatory support | Yes | 1,712 (27.0%) | 1,593 (27.2%) | 119 (24.4%) | 0.177 |
| ECMO | Yes | 668 (10.5%) | 614 (10.5%) | 54 (11.1%) | 0.689 |
| VAD | Yes | 277 (6.2%)b | 244 (6.0%) | 33 (9.0%) | 0.022 |
Bold indicates statistical significance. ECMO, extracorporeal membrane oxygenation; Tx, transplantation; UNOS, United Network for Organ Sharing; VAD, ventricular assist device.
n = 6,326;
n = 4,456.
Transplant recipients
A total of 4,257 children (67.1%) received at least one transplant. Among these, 417 (9.8%) had a PXMR during listing and 83 (2.0%) carried a PXMR throughout listing. Patients with a PXMR during listing more commonly underwent transplantation with local donors (p = 0.001); however, there was no significant difference in ischemic time between the groups (p = 0.161). Donor-recipient gender mismatch (p = 0.043) and instances with donor weight ≥ 3 times recipient weight (p = 0.018) were less prevalent among those with a PXMR during listing.
PRA and donor-specific crossmatch
Among recipients, peak PRA was available for 58.3%, most recent PRA for 86.6% and DSXM data for 80.3%. Peak and most recent PRA were higher in the PXMR group (peak, 44.3 ± 35.7% vs 10.6 ± 23.4%; most recent, 26.3 ± 1.7% vs 6.0 ± 18.2%; p < 0.0001 for both). Also, the proportions with PRA > 10% and > 50% were higher in the PXMR group (> 10%: peak, 71.4% vs 21.0%; most recent, 48.2% vs 12.0%; p < 0.0001 for both; > 50%: peak, 43.1% vs 8.3%; most recent, 23.7% vs 4.6%; p < 0.0001 for both). Recipients who had a PXMR during listing more commonly had a positive DSXM (22.1% vs 10.3%; p < 0.0001), as did recipients who carried a PXMR throughout listing (21.7% vs 11.3%; p = 0.004).
Waitlist outcomes
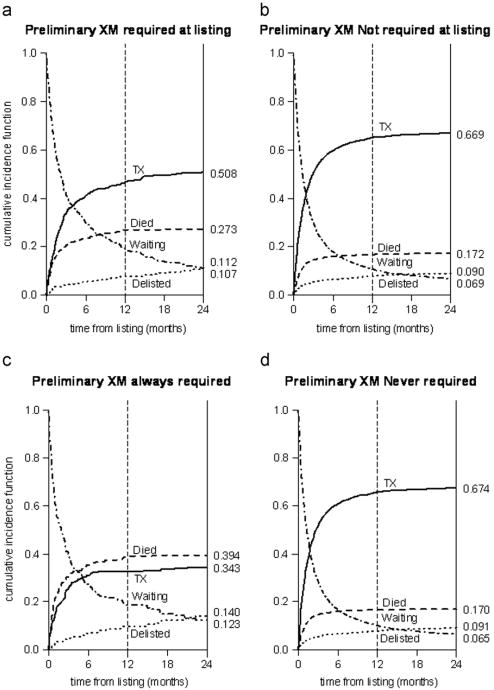
Waitlist duration was greater for patients with a PXMR at listing (total days, 248.7 ± 482.8 vs 186.2 ± 504.0; p = 0.0084; active waitlist days, 115.0 ± 172.9 vs 84.4 ± 161.9; p < 0.0001). PXMR patients were more likely to transfer to other transplant centers (1.9% vs 0.6%; p = 0.007) and to be dual-listed (1.0% vs 0.4%; p = 0.07). Patients with a PXMR at listing less commonly achieved transplantation and more commonly died after listing relative to those not listed with this requirement (Figure 1a and b; p < 0.0001). These differences were even greater among patients who had a PXMR for the entirety of the listing versus those who never had a PXMR (Figure 1c and d; p < 0.0001).
Figure 1.
Waitlist competing outcomes stratified by the requirement for a prospective crossmatch at listing (a, b) and throughout the entirety of listing (c, d).
Factors considered in the multivariate model of waitlist survival are shown in Table 2. After adjusting for these factors, a PXMR at listing remained an independent predictor of waitlist mortality (hazard ratio [HR] 1.32, 95% confidence interval [CI] 1.10 to 1.56; p = 0.003).
Table 2. Multivariate Model at Transplantation, Delisting of Death After Listing, or 2 Years Hazard ratio Censored.
| Variable | Hazard ratio (95% CI) | p-value |
|---|---|---|
| Prospective crossmatch required | 1.32 (1.10–1.56) | 0.003 |
| Agea (years) | ||
| 1–10 | 0.88 (0.76–1.01) | 0.075 |
| 11–17 | 0.91 (0.76–1.09) | 0.296 |
| Gender (male) | 0.93 (0.83–1.05) | 0.246 |
| Raceb | ||
| Black | 1.15 (0.99–1.34) | 0.073 |
| Hispanic | 1.14 (0.97–1.33) | 0.109 |
| Other | 1.13 (0.84–1.52) | 0.409 |
| Listing diagnosis of CHD | 1.87 (1.64–2.13) | <0.0001 |
| History of prior transplant | 2.10 (1.50–2.94) | <0.0001 |
| Status 1/1A/1Bc | 2.21 (1.82–2.68) | <0.0001 |
| Inotropes | 1.26 (1.10–1.45) | 0.0012 |
| Ventilator | 1.82 (1.58–2.10) | <0.0001 |
| ECMO | 2.26 (1.93–2.65) | <0.0001 |
All variables pertain to time of listing. Bold indicates statistical significance. CHD, congenital heart disease; ECMO, extracorporeal membrane oxygenation.
Versus age < 1 year;
versus white;
versus Statuses 2 and 7.
Post-transplant survival
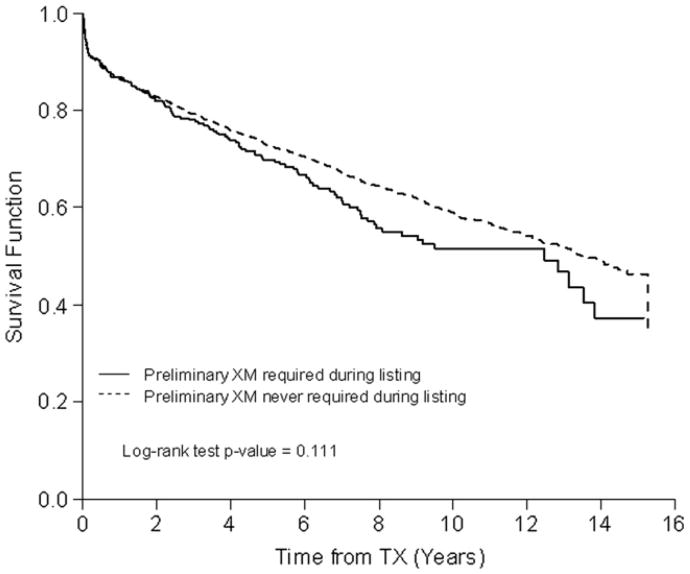
As shown in Figure 2, there was no significant difference in survival after transplantation on the basis of a PXMR during listing (p = 0.11). After adjusting for clinical characteristics at the time of transplantation, including DSXM result, a PXMR during listing was still not significantly associated with post-transplant survival (HR 1.04, 95% CI 0.87 to 1.25; p = 0.67; Table 3).
Figure 2.
Freedom from death after transplantation, stratified by the requirement for a prospective crossmatch during listing.
Table 3. Multivariable Model of Death After Transplantation.
| Variable | Hazard ratio (95% CI) | p-value |
|---|---|---|
| Prospective crossmatch required | 1.04 (0.87–1.25) | 0.670 |
| Agea (years) | ||
| 1–10 | 0.92 (0.79–1.07) | 0.259 |
| 11–17 | 1.47 (1.26–1.71) | <0.0001 |
| Gender (male) | 0.88 (0.78–0.98) | 0.019 |
| Raceb | ||
| Black | 1.84 (1.62–2.10) | <0.0001 |
| Hispanic | 1.06 (0.90–1.25) | 0.510 |
| Other | 1.04 (0.78–1.38) | 0.802 |
| Listing diagnosis of CHD | 1.49 (1.32–1.68) | <0.0001 |
| History of prior heart transplant | 1.76 (1.36–2.28) | <0.0001 |
| Status 1/1A/1Bc | 0.91 (0.77–1.07) | 0.249 |
| Inotropes | 1.08 (0.95–1.23) | 0.232 |
| Ventilator | 1.34 (1.14–1.56) | 0.0003 |
| ECMO | 1.89 (1.53–2.34) | <0.0001 |
| Ischemic time (hours) | 1.02 (0.98–1.07) | 0.307 |
| Donor:recipient gender mismatch | 1.04 (0.93–1.16) | 0.461 |
| Positive donor-specific crossmatchd | 1.24 (1.06–1.46) | 0.009 |
| Unknown donor- specific crossmatchd | 1.15 (1.01–1.32) | 0.042 |
All variables pertain to time of transplant. Bold indicates statistical significance. CHD, congenital heart disease; ECMO, extracorporeal membrane oxygenation.
Versus age < 1 year;
versus white;
versus Statuses 2 and 7;
versus negative donor-specific crossmatch.
Discussion
Allosensitization has been recognized as a predictor of inferior waitlist survival in pediatric heart transplant candidates, presumably because allosensitized children require a negative prospective crossmatch for transplantation.1,2,5,6 Our analysis supports this assumption by showing that a PXMR at listing is associated with longer waitlist duration and increased waitlist mortality. Moreover, the increased mortality risk persisted even after adjusting for patient demographics; reason for transplantation; and other factors, such as urgency status and use of inotropes, ventilator or ECMO.
With regard to post-transplant survival, we found that a PXMR during listing was not associated with inferior post-transplant survival. This result is of interest because recipients with a PXMR were more commonly transplanted across a positive DSXM. Although it is possible that the magnitude of difference in the proportions transplanted across a positive DSXM between the groups was not sufficient to influence the overall post-transplant survival of the groups, an alternate explanation is that survival after transplantation across a positive DSXM is not unequivocally poor. This is supported by recent data showing that short- and medium-term survival after pediatric heart transplantation across a positive DSXM in small, single-center series (71% to 85%) is only marginally worse than the 1-year survival rate for all children (87% to 89%). 7–9 Based on these data and the poor waitlist outcomes for allosensitized children, some pediatric heart transplant centers have recently adopted a strategy of transplanting highly allosensitized patients with the first suitable donor organ, regardless of the potential for a positive DSXM. In fact, outcomes of this management strategy are being compared with survival for non-allosensitized candidates as part of a multicenter National Institutes of Health (NIH)-funded, prospective study.10
It is notable that 22% of recipients carrying a PXMR throughout the entirety of listing were nonetheless transplanted across a positive DSXM. In some instances, this may have been truly unintended and due to the unforeseen development of anti-HLA antibodies while awaiting transplantation or the low sensitivity of historic anti-HLA antibody detection methods. However, it is also possible that the requirement for a negative prospective crossmatch was ultimately dropped by the listing center and not communicated to the OPTN, or ignored by the listing center so as to proceed to transplantation. Unfortunately, the rationale for changes in listing strategy with respect to PXMR is not captured in the OPTN data set.
Our analysis is unique in that we examined survival after listing on the basis of the PXMR rather than on the basis of pre-transplant PRA. All previous studies that have looked at outcome of allosensitized candidates have done so on the basis of DSXM or PRA, and traditionally these studies used a CDC PRA ≥ 10% to 20% to define significant allosensitization. However, we found nearly 30% of recipients with a requirement for a prospective crossmatch during listing who also had PRA data available had a peak PRA ≤ 10%. This suggests that a sizeable minority of children in whom a prospective crossmatch is required do not meet “traditional” criteria for allosensitization. It is unclear whether other PRA data that were not recorded in this data set may have contributed to this finding, yet future studies to improve our understanding of physician and center-specific factors that drive the decision to list with a prospective crossmatch requirement seem warranted. Some of these factors may include transplant physician knowledge about alloantibody detection testing methods, the availability of histocompatibility laboratory support, and the speed of adoption of newer, more sensitive alloantibody detection methods (i.e., Luminex).
Unfortunately, neither this nor previous studies looking at allosensitization are sufficient to answer the question of whether highly allosensitized patients should be listed with the requirement for a negative prospective crossmatch. From the allosensitized candidate's perspective, our data suggest that survival may be enhanced by listing without a PXMR. However, because our data did not discern between DSXM methodologies and because others have found transplantation across a positive DSXM to be associated with inferior survival, caution is warranted. It is unlikely that transplantation across a strongly positive, cytotoxic DSXM has the same significance as transplantation across a positive, virtual DSXM on the basis of a few, donor-specific antibodies of low or moderate mean fluorescent intensity. Thus, for centers seeking to minimize the possibility of transplantation across a positive DSXM, we suggest utilization of and rigorous adherence to the PXMR for allosensitized candidates. For centers in which transplantation across a positive DSXM is tolerable, we suggest no formal stipulation of unacceptable antigens or a PXMR in the listing registry, but rather careful consideration of the virtual crossmatch on a donor-by-donor basis.
Despite the many advantages of using a data set from a national registry, we encountered some of the disadvantages of large data set research, including missing data. For example, 30% of our listing cohort had missing VAD data, although data on use of ECMO, assisted ventilation and inotropic support, as well as listing status, were present for all candidates. Also, DSXM results were missing in about 20% of recipients, although we were able to include all recipients in our multivariable model of post-transplant survival by analyzing patients without DSXM data as a distinct group. An additional limitation of our analysis was the inability to fully assess the relationship between PRA and PXMR during listing. This was due to the fact that the OPTN only collects pre-transplant PRA data at the time of transplantation. Because a greater proportion of candidates who were listed with a PXMR died, it is unclear whether the PRA findings we observed among recipients are also reflective of candidate PRA levels. The lack of information about methodology by which PRA and crossmatch testing were performed is a further limitation of the data set. Finally, we do not know how many deaths occurred in patients who were delisted or lost to followup post-transplant. To minimize this we used Social Security death master file index data that accompanied the data set. Also, because there were no significant differences in proportions that were delisted or lost to follow-up between the groups, this should not significantly alter our conclusions.
In conclusion, among children listed for heart transplantation in the USA between 1995 and 2009, we found 7.7% had a PXMR at the time of listing and 11.8% had a PXMR during listing. The PXMR requirement was associated with increased waitlist duration and inferior waitlist survival, but not with significant differences in post-transplant survival, although such patients were more commonly transplanted across a positive DSXM. Among recipients who had a PXMR requirement during listing and had PRA data, nearly 30% had a peak pre-transplant PRA ≤ 10%.
Acknowledgments
This project was supported by the National Institutes of Health (KL2RR024154 and KL2TR000146).
Footnotes
Disclosure statement: The authors have no conflicts of interest to disclose.
The content is solely the responsibility of the authors and does not necessarily represent the views of the National Institutes of Health or OPTN.
References
- 1.Feingold B, Bowman P, Zeevi A, et al. Survival in allosensitized children after listing for cardiac transplantation. J Heart Lung Transplant. 2007;26:565–71. doi: 10.1016/j.healun.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 2.Mahle WT, Tresler MA, Edens RE, et al. Allosensitization and outcomes in pediatric heart transplantation. J Heart Lung Transplant. 2011;30:1221–7. doi: 10.1016/j.healun.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 4.Dipchand AI, Naftel DC, Feingold B, et al. Outcomes of children with cardiomyopathy listed for transplant: a multi-institutional study. J Heart Lung Transplant. 2009;28:1312–21. doi: 10.1016/j.healun.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 5.Jacobs JP, Quintessenza JA, Boucek RJ, et al. Pediatric cardiac transplantation in children with high panel reactive antibody. Ann Thorac Surg. 2004;78:1703–9. doi: 10.1016/j.athoracsur.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 6.Rossano JW, Morales DL, Zafar F, et al. Impact of antibodies against human leukocyte antigens on long-term outcome in pediatric heart transplant patients: an analysis of the United Network for Organ Sharing database. J Thorac Cardiovasc Surg. 2010;140:694–9. doi: 10.1016/j.jtcvs.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Holt DB, Lublin DM, Phelan DL, et al. Mortality and morbidity in pre-sensitized pediatric heart transplant recipients with a positive donor crossmatch utilizing peri-operative plasmapheresis and cytolytic therapy. J Heart Lung Transplant. 2007;26:876–82. doi: 10.1016/j.healun.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Pollock-BarZiv SM, den Hollander N, Ngan BY, et al. Pediatric heart transplantation in human leukocyte antigen sensitized patients: evolving management and assessment of intermediate-term outcomes in a high-risk population. Circulation. 2007:I-172–8. doi: 10.1161/CIRCULATIONAHA.107.709022. [DOI] [PubMed] [Google Scholar]
- 9.Kirk R, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: fourteenth pediatric heart transplantation report—2011. J Heart Lung Transplant. 2011;30:1095–103. doi: 10.1016/j.healun.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Project Information: 1U01AI077867-01. [Accessed March 19, 2012];National Institutes of Health Research Portfolio Online Reporting Tools website. Available from: http://projectreporter.nih.gov/project_info_description.cfm?aid=7451546%20&icde=11845357. Updated March 19, 2012.