Abstract
Recent studies suggest a link between mitochondria and proinflammatory cytokine generation. We previously demonstrated that overexpression of mitochondrial chaperone glucose-regulated protein75 (Grp75/mortalin) protects mitochondria. In this study we investigated the modulation of the lipopolisaccharide (LPS)-induced inflammatory response of microglial BV-2 cells by Grp75. We demonstrate that LPS-induced activation promotes significant metabolic changes suppressing mitochondrial function and increasing glycolysis. Overexpression of Grp75 attenuates the LPS-induced oxidative and metabolic responses, and suppresses proinflammatory activation, which depends on both NF-κB activation and lactate. Thus overexpression of Grp75 provides a novel strategy to modulate proinflammatory cytokine production of relevance to inflammation-associated pathologies.
Keywords: mitochondria, metabolism, inflammation, cytokine
Introduction
Many chronic human diseases, including neurodegenerative disorders, are associated with inflammation. These diseases are characterized by excessive reactive oxygen species (ROS) production [1–3]. Mitochondrial impairment has also been implicated in these disorders [4, 5], with mitochondria both a source of ROS and a critical organelle that is the target of ROS damage. Mitochondrial ROS can act as a major trigger of proinflammatory cytokine production. Nakahira et al. (2011) and Zhou et al. (2011) used antioxidants, genetic models, and inhibitors of mitochondrial function to demonstrate that inflammatory stimuli lead to increased mitochondrial ROS production, that in turn induces inflammasome activation [6, 7]. ROS generated by mitochondrial function were shown to be important for lipopolysaccharide (LPS)-induced production of proinflammatory cytokines [8]. Mitochondrial density and/or respiratory activity were found to contribute to LPS-induced proinflammatory cytokine release from macrophages in a recent study that used a ρ0 macrophage cell line that lacks mitochondria [9].
While the connection between mitochondrial ROS production and proinflammatory cytokine generation is now well established [10], there is also evidence that mitochondrial metabolism is important for the anti-inflammatory program of macrophage activation [11]. In their study Vats et al. suggested that glycolysis promotes proinflammatory activation of macrophages, whereas oxidative metabolism primes macrophages for a less-inflammatory mode of activation [11]. The importance of a metabolic switch to glycolysis, through HIF-1-associated mechanisms, during the inflammatory responses of myeloid cells has been demonstrated in earlier studies [12, 13]. Glycolytic metabolism is also associated with increased levels of lactate production [14]. It has been reported that lactate enhances LPS-stimulated proinflammatory macrophage activation [15]. This lactate-associated proinflammatory boost is mediated through nuclear factor-κB (NF-κB) [16], a pivotal transcription factor in inflammatory response [17]. Overall, the involvement of mitochondrial metabolism per se in pro-inflammatory cytokine production has not been extensively investigated.
In our previous studies we showed that overexpression of mitochondrial glucose-regulated protein 75 (Grp75/mtHsp70/mortalin) leads to decreased ROS production, preservation of mitochondrial membrane potential, and preservation of ATP production in the face of ischemic injury both in vivo and in vitro [18, 19]. This is consistent with a previous study that demonstrated protection of ATP levels, mitochondrial function, and reduced ROS accumulation during glucose deprivation in neuronal cells overexpressing Grp75 [20]. The purpose of this study was to investigate whether Grp75 overexpression downregulates LPS-induced mitochondrial ROS production in microglial BV-2 cells, and whether this downregulation reduces the pro-inflammatory response. We investigated, for the first time, metabolic shifts induced during LPS-stimulation, their modulation by Grp75 overexpression, and the importance of these metabolic changes in the proinflammatory response of BV-2 cells.
Materials and Methods
BV-2 cells and Grp75 overexpression
BV-2 murine microglia were plated on uncoated plastic tissue culture plates and grown in DMEM supplemented with 10% FBS, and 100 U/ml penicillin and 100 µg/ml streptomycin. The macrophage specific mouse LysM promoter was cloned from C57BL/J6 mouse genomic DNA by PCR using the following primers: CTGAGAGGTCCCAAGTTCAATC and GGTGACTGGAGGCTGGGTCAGC. The product was cloned into pCR2.1 using a TOPO-TA kit (Invitrogen, Carlsbad, CA) and excised by HindIII and XhoI. It was subcloned into pAcGFP1-1 (Clontech, Mountain View, CA). The resulting construct LysM-GFP was used as a control plasmid in all transfection experiments. The human Grp75 coding sequence in pBluescript [21] was a generous gift from R. Morimoto at Northwestern University. To produce LysM-Grp75 the Grp75 coding sequence was excised by NotI and SalI, and inserted into the control LysM-GFP plasmid after removing the GFP sequence with the same enzymes. BV-2 cells were plated in 24-well plates one day before transfection with LysM-Grp75 or LysM-GFP (control) plasmids, using Lipofectamine reagent (Invitrogen). Successfully transfected BV-2 cells were selected in 400 µg/mL G418 (Sigma) for 4 weeks, then experiments were performed on essentially confluent cultures of BV-2 cells in which essentially all cells stably expressed Grp75, or control GFP protein. Three independently derived stable cell lines were used in the study. For LPS treatment BV-2 cells were rinsed once with DMEM then incubated for 3 h in DMEM with added LPS (1µg/ml, Sigma, St Louis, Missouri). When added, Trolox+Edaravone (100 µM each) were applied simultaneously with LPS for the duration of treatment.
Live Imaging
Cells were incubated with the mitochondrial ROS sensitive dye MitoSox (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions beginning at 2 h of LPS treatment for 1 h (total 3 h LPS treatment) before fluorescence measurements. We found that MitoSox fluorescence measurements need to be performed within a short time after the initial dye incubation to avoid migration of the MitoSox signal to other cellular, particularly nuclear, locations. Cells were illuminated at 535 nm, and fluorescence emission was observed at 590 nm using an Axiovert 200M fluorescence microscope (Carl Zeiss, Jena, Germany). Several fields/condition were selected at random using phase-contrast optics, and the intensity of six field of view areas (approximately 100 cells/area) were analyzed to produce the average intensity reading for each well.
Western Blot Analysis
Western blot analysis was performed as previously described [18]. The Grp75 band intensity was normalized to the actin band intensity in the same lane.
TNF-α, IL-6, and lactate measurements
After 3 h of LPS treatment (1 µg/mL) the media from LPS-treated or control DMEM-treated BV-2 cells was collected. Levels of proinflammatory cytokines were determined by ELISA TNF-α and IL-6 kits (Invitrogen, Carlsbad, CA).
Measurements of oxygen consumption rates, lactate and ATP levels
Real-time measurements of oxygen consumption rates, and extracellular acidification rates, a measure of lactate production, were performed on an XF24 Seahorse extracellular flux analyzer (Seahorse Biosciences) according to manufacturer’s instructions and as previously described [22]. Lactate levels were also independently estimated using the Lactate Assay Kit (Biovision Research, Mountain View, CA). Cell lysate protein concentrations were measured with the BCA protein assay reagent kit (Pierce, Rockford, IL). Cellular ATP concentrations after 3 h of LPS treatment were measured using the CellTiter-Glo luminescent ATP assay kit (Promega, Madison, WI), based on the luciferase/luciferin reaction. A Veritas luminescence counter (Turner BioSystems, Sunnyvale, CA) was used to measure the luminescence signal.
In vitro immunocytochemistry and functional assay of NF-κ B translocation
Fluorescence immunocytochemistry was performed using primary anti-Grp75 antibody (1:300, Stressgen, Ann Arbor, MI) and secondary Alexa Fluo 488-conjugated secondary antibodies (1:200, Invitrogen) as previously described [18]. Co-staining with the mitochondrial specific dye MitoTracker Red (Invitrogen, Carlsbad, CA) was used to confirm mitochondrial localization of Grp75. To assess the accumulation of NF-κB in 4’6’-diamidino-2-phenylindole (DAPI)-stained nuclei we used antibody to Rel A (p65) subunit (sc-109, Santa Cruz Biotechnology, Santa Cruz, CA) followed by staining with secondary AlexaFluo 488-conjugated secondary antibody (1:200, Invitrogen). NF-κB consists of two subunits, p65 (Rel A) and p50 (NF-κ B1), and is present in the cytoplasm in an inactive form, complexed with inhibitor of NF-κB (IκB). In response to pro-inflammatory activators, NF-κB dissociates from IκB and translocates from the cytoplasm to the nucleus, with subsequent binding to genes containing the κB site [23]. We evaluated the intensity of NF-κB fluorescent staining within the area of the cell nuclei in comparison to cytoplasmic staining using the DAPI nuclei images of the same area. In addition, NF-kB activation was quantitated using the TransAM NF-kB Activation Assay (Active Motif, Carlsbad, CA), according to the manufacturer’s instructions. We also used a specific NF-κB inhibitor BAY11-7085 (Santa Cruz Biotechnology, Santa Cruz, CA).
Statistics
Statistical differences between two groups were determined using unpaired two-tailed Student’s t-test with Welch’s correction. Comparisons between multiple groups were performed with ANOVA followed by Bonferroni test for selected groups. Data in all plots are pooled from three independent experiments and presented as mean±SD.
Results
Overexpression of Grp75 in BV-2 cells
Previous studies from our laboratory and others revealed endogenous Grp75 expression in various brain cell types [18, 24]. The established Grp75 overexpressing BV-2 cell line demonstrated markedly higher levels of Grp75 expression compared to GFP-overexpressing control cells, as indicated by Western blot (Fig. 1A). Increased Grp75 overexpression and preferential mitochondrial localization were confirmed by immunocytochemistry (Fig. 1B, C).
Fig. 1. Grp75 overexpression in BV-2 cells.
(A) Expression levels of Grp75 in control and Grp75 overexpressing cells as determined by Western blot (*P<0.05). (B) Immunohistochemical Grp75 staining of control and Grp75 overexpressing BV2 cells. The data are representative of three independently derived stable cell lines (C) Co-localization of immunohistochemical Grp75 staining and mitochondrial marker MitoTracker Red.
Mitochondrial ROS levels
We used MitoSox, a fluorescent indicator of mitochondrial ROS production to investigate the effect of LPS treatment on the levels of mitochondrial oxidative stress in control and Grp75-overexpressing cells. As shown in Fig. 2A, B, there was no significant difference in the levels of mitochondrial ROS in non-stimulated control and Grp75-overexpressing BV-2 cells. LPS treatment caused a 3.6-fold increase in the levels of MitoSox fluorescence in control BV-2 cells (Fig. 2C), while Grp75-overexpressing cells demonstrated only a 2.6-fold increase of MitoSox signal (Fig. 2D), significantly lower. We then used a combination of Trolox, a water soluble vitamin E analog and a powerful antioxidant [25], and a free radical scavenger edaravone [26] to suppress LPS-induced mitochondrial ROS generation. As demonstrated in Fig. 2E, F, the trolox+edaravone antioxidant treatment resulted in suppression of LPS-induced mitochondrial ROS down to the basal levels observed in non-stimulated cells. In some of our experiments, in order to investigate the effects of Grp75 overexpression on pro-inflammatory activation of BV-2 cells, we used mitochondrial inhibitors to block mitochondrial function. Among different mitochondrial inhibitors and uncouplers tested (rotenone, antimycin A, oligomycin, FCCP) only oligomycin did not increase mitochondrial ROS levels in LPS-treated cells in the presence of trolox+edaravone (Fig. 2G,H), or under other studied conditions (both with and without LPS), and was therefore used in further experiments. The quantification of MitoSox fluorescence for different conditions is summarized in Fig. 2I.
Fig. 2. Mitochondrial ROS generation induced by LPS and its modulation by antioxidants and oligomycin.
Representative images of control and Grp75 overexpressing BV-2 cells loaded with MitoSox under control conditions (A, B). Images after treatment with LPS (C,D), LPS with trolox+edaravone (100 µM each; TE) cotreatment (E, F), and LPS+TE+oligomycin (10 µM; G,H). Quantification of changes in mitochondrial ROS induced by LPS and co-treatments with TE and oligomycin assessed as MitoSox fluorescence (I). The data show the means of three independent experiments, with at least 150 cells per condition in each experiment (*P<0.05, #P<0.05 compared to all other control treatment groups, and ##P<0.05 compared to all other Grp75 overexpressing treatment groups).
Effect of Grp75 overexpression on TNF-α and IL-6 levels
To determine the effect of Grp75 overexpression on proinflammatory BV-2 activation we measured levels of the inflammatory cytokines TNF-α and IL-6. As demonstrated in Fig. 3A, LPS treatment induced high levels of TNF-α production in both control and Grp75 overexpressing BV-2 cells (6.4 ng/mL and 4.7 ng/mL, respectively), though significantly lower in the Grp75 overexpressors. Elimination of mitochondrial ROS production with trolox+edaravone resulted in a significant decrease in TNF-α production in control cells, consistent with prior literature [10], and a trend to lower levels of TNF-α production in Grp75 overexpressing cells (p=0.054). Importantly, a significant difference in the LPS-induced levels of TNF-α was still observed between control and Grp75-overexpressing cells even when mitochondrial ROS production was eliminated. This suggests that in addition to effects of ROS, other aspects of mitochondrial function contribute to the observed differences in TNF-α production. To assess the importance of mitochondrial metabolic function in determining the ability of Grp75 to exert its anti-inflammatory effect we inhibited mitochondrial metabolism with oligomycin. Elimination of mitochondrial ROS (see Fig. 2G) in combination with inhibition of mitochondrial function by oligomycin equalized LPS induced TNF-α production in control and Grp75-overexpressing BV-2 cells. Thus inhibition of mitochondrial function had the expected pro-inflammatory effect increasing TNFα production in the Grp75 overexpressing cells, and eliminating the difference in the levels of cytokine production between the control and Grp75-overexpressing cells, indicating the contribution of a mitochondrial metabolic mechanism of Grp75-associated modulation of TNF-α production. Similar results were obtained in measurements of IL-6 levels, as demonstrated in Fig. 3B.
Fig. 3. Effects of antioxidants and mitochondrial inhibition with oligomycin on LPS-induced increases in proinflammatory cytokines in control and Grp75 overexpressing cells.
LPS treatment increased levels of TNF-α (A) and IL-6 (B) as assessed by ELISA. These levels were affected by addition of the antioxidant trolox+edravone (TE), and the combined addition of TE and the mitochondrial inhibitor oligomycin. The data show the means of three independent experiments, with two samples per condition in each experiment, for both TNF-α and IL-6 (**P<0.01, ***P<0.001).
Effect of Grp75 overexpression on LPS-induced changes in BV-2 cell metabolism
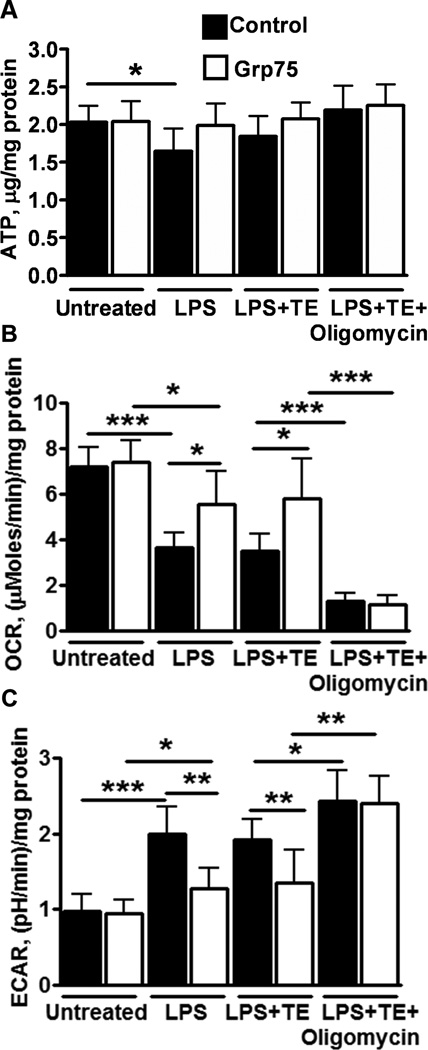
We next investigated metabolic changes in control and Grp75 overexpressing BV-2 cells. Fig. 4A demonstrates that LPS treatment significantly decreased ATP levels in control, but not in Grp75-overexpressing cells. No other treatments resulted in significant changes in ATP. Fig. 4B shows that LPS treatment significantly suppressed oxygen consumption rate, an indicator of mitochondrial respiration, in both control and Grp75 overexpressing cells, but the suppression was significantly higher in control cells. The LPS-induced changes in oxygen consumption were not significantly modulated by trolox+edaravone antioxidant treatment in either cell type. As expected, oligomycin treatment strongly suppressed mitochondrial oxygen consumption rates. Because some cell types can compensate for reduced mitochondrial ATP production by increased glycolytic activity through Pasteur effect-like mechanisms [27], we measured changes in lactate levels as a measure of glycolytic activity [28]. We used both a lactate assay kit (see supplemental figure 1) and made Seahorse real-time measurements of extracellular acidification rates as a measure of lactate levels (see Methods), with similar results. The real-time measurements provided a more pronounced difference in lactate production between the different groups, as we found that lactate differences were attenuated at longer treatment times. Fig. 4C shows that LPS treatment caused a significantly greater increase in lactate levels in control compared to Grp75 overexpressing cells. The reduced rates of oxygen consumption and increased lactate production indicate a metabolic shift to glycolytic ATP production to partially or fully compensate for reduced mitochondrial ATP production. The LPS-induced changes in lactate were not significantly modulated by the trolox+edaravone antioxidant treatment; a significant difference remained in the levels of lactate production between Grp75 overexpressing and control cells. Further, essentially complete inhibition of mitochondrial function by oligomycin promoted a significant increase in lactate levels and eliminated the difference in the rates of lactate production between control and Grp75 overexpressing cells. Since it was proposed that increased lactate levels enhance the LPS-induced production of proinflammatory cytokines [15], we investigated the effect of sodium lactate (5 mM) co-treatment in control and Grp75 overexpressing BV-2 cells exposed to LPS and trolox+edaravone. Sodium lactate addition resulted in a significant increase in both TNF-α and IL-6 levels, comparable to or exceeding those induced by oligomycin co-treatment (see Fig. 4 A,B). The addition of 5 mM sodium lactate, in addition to LPS+trolox+edaravone, resulted in 7.8±0.53 and 8.0±0.6 (ng/mg protein) production of TNF-α (compare to Fig. 4A), and 3.6±0.61 and 3.75±0.67 (ng/mg protein) of IL-6, in control and Grp75 overexpressing cells, respectively (compare to Fig. 4B).
Fig. 4. LPS-induced changes in BV-2 cell metabolism.
(A) ATP levels in control and Grp75 overexpressing cells without and with LPS treatment, and LPS with addition of antioxidant trolox+edravone (TE) treatment and LPS with TE+oligomycin to additionally inhibit mitochondrial metabolism. (B) Oxygen consumption rate (OCR) for the same conditions. (C) Extracellular acidification rates (ECAR), a measure of lactate production, is shown for both cell types for the same conditions. The data show the means of three independent experiments, with two samples per condition in each experiment (*P<0.05,**P<0.01, ***P<0.001).
NF-κB activation
NF-κB activation has been shown to be a major mechanism underlying the proinflammatory response of macrophages. Because it was demonstrated that cellular metabolism, and particularly lactate levels, modulate macrophage activation through the NF-κB pathway [15], we investigated the levels of NF-κB activation in control and Grp75 overexpressing cells. Fig. 5A–D shows that LPS treatment promoted a significant increase in NF-κB activation, as indicated by cytoplasm-to-nucleus translocation, in control and Grp75 overexpressing cells. Quantitative measurements of NF-κB activation are presented in Fig. 5E. The LPS-induced NF-κB activation was attenuated by trolox+edaravone antioxidant treatment, but a significant difference in the levels of NF-κB activation between Grp75 overexpressing and control cells was still observed. As in the case of TNF-α and IL-6 production, this difference persisted despite the elimination of mitochondrial ROS with antioxidant treatment, and is apparently related to significant differences in cellular metabolism, particularly lactate levels (See Fig. 4B). Inhibiting mitochondrial function by addition of oligomycin eliminated the difference in the levels of NF-κB activation between control and Grp75 overexpressing cells. To confirm the involvement of NF-κB activation in the increased levels of proinflammatory cytokine production we used a specific NF-κB inhibitor BAY11-7085, that has been shown to completely block LPS-induced IL-6 secretion by macrophages [15]. Co-treatment with BAY11-7085 (10 µM) suppressed LPS-induced IL-6 and TNF-α levels 90% and 95%, respectively, in control and Grp75 overexpressing cells, thus confirming the crucial role of NF-κB in the observed proinflammatory cytokine production.
Fig. 5. NF-κB activation induced by LPS and its modulation by antioxidants and oligomycin.
Representative images of control and Grp75 overexpressing untreated BV-2 cells (A, B). LPS treatment induced NF-κB activation indicated by nuclear localization in control cells (C) that was attenuated in Grp75 overexpressing cells (D). (E) The level of NF-κB activation induced by LPS and co-treatments with trolox+edaravone (TE) and oligomycin was quantitated using the TransAM NF-κB Activation Assay. The data show the means of three independent experiments (*P<0.05, **P<0.01, ***P<0.001).
Discussion
Chronically elevated levels of proinflammatory cytokines, particularly TNF-α and IL-6, have been proposed to play a major role in neurodegenerative diseases including Alzheimer’s, Parkinson’s, stroke, dementia, and multiple sclerosis [29, 30]. These neurodegenerative diseases are also characterized by increased levels of oxidative stress and mitochondrial dysfunction [5, 10]. There is growing evidence for the involvement of both mitochondrial ROS and mitochondrial metabolism in proinflammatory macrophage activation [10]. In this study we investigated whether overexpression of mitochondrial Grp75, which was shown previously to improve mitochondrial function and decrease oxidative stress [18–20], was able to modulate the LPS-induced pro-inflammatory response of BV-2 microglial cells.
We observed that LPS treatment promoted significant increases in the proinflammatory cytokines TNF-α and IL-6, and mitochondrial ROS levels, in both control and Grp75 overexpressing cells. However, the LPS-induced increases of both ROS and cytokines were significantly reduced by Grp75 overexpression. We also investigated metabolic changes induced by LPS-treatment in BV-2 cells.
We observed increased lactate production accompanied by decreased cellular ATP levels in control BV-2 cells. These results suggest that increased glycolysis was not sufficient to compensate for decreased mitochondrial ATP production. The LPS-induced lactate increase was significantly attenuated in Grp75 overexpressing cells, with unchanged levels of cellular ATP, indicating a complete compensation for decreased mitochondrial ATP production by glycolysis. Our observations are in line with recent studies demonstrating a switch from oxidative phosphorylation to glycolysis, through inducible NO synthase during inflammatory activation of dendritic cells [31, 32], while anti-inflammatory activation is associated with preserved mitochondrial oxidative metabolism [11].
Since mitochondrial ROS have been shown to play a role in the inflammatory response we compared the effect of eliminating mitochondrial ROS on LPS-induced responses of control and Grp75 overexpressing cells. The combination of trolox+edaravone fully reduced mitochondrial ROS production to baseline levels, and significantly reduced proinflammatory cytokine production in both cell types. Despite this, a significant difference in the levels of proinflammatory cytokine production remained in the absence of elevated mitochondrial ROS between Grp75 and control cells, indicative of an additional mechanism. It should be noted that the trolox+edaravone combination effectively suppresses not only mitochondrial, but overall cellular ROS. The use of more specific mitochondrial ROS inhibitors, like mitoQ [29], would allow a more precise investigation of the contribution of mitochondrial ROS to the inflammatory response.
Metabolic measurements demonstrated a significant difference in the levels of lactate production between control and Grp75 overexpressing cells. These measurements indicate that removal of mitochondrial ROS does not completely eliminate the LPS-induced differences in either metabolic response or cytokine production between control and Grp75 overexpressing cells. The levels of NF-κB activation, a key proinflammatory transcription factor, also remained significantly different between control and Grp75 overexpressing cells, despite ROS elimination. This emphasizes that not only mitochondrial ROS production, but metabolism per se, is involved in the activation of proinflammatory mechanisms. We propose that LPS-induced increased glycolytic activity and lactate levels contribute to the observed pro-inflammatory response, as an earlier study demonstrated a strong enhancement of LPS-induced NF-κB activation and proinflammatory cytokine production by elevated lactate levels [15].
To further confirm the importance of mitochondrial metabolic function we blocked mitochondrial function with oligomycin, the inhibitor of mitochondrial complex V of the respiratory chain, in addition to suppressing mitochondrial ROS, during LPS stimulation. This resulted in increased and essentially equal levels of lactate and proinflammatory cytokine production in Grp75 overexpressing and control cells. This finding confirmed the important contribution of protection of mitochondrial metabolism to the antiinflammatory effect of Grp75 overexpression.
It should be noted that the microglial cell line BV-2, rather than primary microglia, was used in our studies. While we observed similar trends (inflammation-associated increased lactate production, and Grp75-associated decrease in inflammatory cytokine production) in primary isolated microglia, low transfection rates and low post-transfection microglial survival precluded extensive metabolic and Grp75-overexpression studies of proinflammatory activation in primary microglial cells. Another limitation of this study is that Grp75 has been shown to interact with a variety of other mitochondrial and cellular proteins [20], thus Grp75 overexpression might potentially affect cellular function partially through interaction with or via changed levels of these proteins.
Overall we demonstrate that LPS-induced activation of BV-2 microglial cells promotes not only excessive production of mitochondrial ROS, as has been reported earlier [7, 8, 10], but also significantly reduces ATP production and increases lactate production. We provide evidence that overexpression of the mitochondrial protective protein Grp75 downregulates mitochondrial ROS production, protects mitochondrial metabolic function, suppresses lactate production and suppresses LPS-induced proinflammatory activation in BV-2 microglial cells. Our results indicate that targeted expression of Grp75 in cells of the macrophage lineage can provide a useful tool for investigation of the effects of suppressing the inflammatory response in a variety of pathologies, without directly affecting other cell types. Because suppression of pro-inflammatory cytokine generation has been suggested to be beneficial in a variety of chronic diseases [33, 34] our study also suggests that Grp75 overexpression can be potentially beneficial in a variety of chronic human diseases with underlying inflammatory pathologies.
Supplementary Material
Highlights.
Mitochondrial metabolism per se contributes to LPS-induced proinflammatory response
LPS activation of microglial cells increases lactate
Mitochondrial protective protein Grp75 downregulates proinflammatory response
Acknowledgements
The work was supported by NIH grants NS014543, NS053898, and GM49831 to RGG. The authors thank Drs. Edward LaGory and Amato Giaccia for help with the Seahorse experiments.
Abbreviations
- Grp75
glucose-regulated protein 75
- LPS
lipopolisaccharide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ludmila A. Voloboueva, Email: ludmilav@stanford.edu.
John F. Emery, Email: johnemery@stanford.edu.
Xiaoyun Sun, Email: xysun@stanford.edu.
Rona G. Giffard, Email: rona.giffard@stanford.edu.
References
- 1.Filippin LI, et al. Redox signalling and the inflammatory response in rheumatoid arthritis. Clin Exp Immunol. 2008;152(3):415–422. doi: 10.1111/j.1365-2249.2008.03634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilgun-Sherki Y, Melamed E, Offen D. The role of oxidative stress in the pathogenesis of multiple sclerosis: the need for effective antioxidant therapy. J Neurol. 2004;251(3):261–268. doi: 10.1007/s00415-004-0348-9. [DOI] [PubMed] [Google Scholar]
- 3.Lee YJ, et al. Inflammation and Alzheimer's disease. Arch Pharm Res. 2010;33(10):1539–1556. doi: 10.1007/s12272-010-1006-7. [DOI] [PubMed] [Google Scholar]
- 4.DiMauro S, Schon EA. Mitochondrial respiratory-chain diseases. N Engl J Med. 2003;348(26):2656–2668. doi: 10.1056/NEJMra022567. [DOI] [PubMed] [Google Scholar]
- 5.Witte ME, et al. Mitochondrial dysfunction: a potential link between neuroinflammation and neurodegeneration? Mitochondrion. 2010;10(5):411–418. doi: 10.1016/j.mito.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Nakahira K, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12(3):222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou R, et al. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469(7329):221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 8.Bulua AC, et al. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS) J Exp Med. 2011;208(3):519–533. doi: 10.1084/jem.20102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kasahara E, et al. Mitochondrial density contributes to the immune response of macrophages to lipopolysaccharide via the MAPK pathway. FEBS Lett. 2011;585(14):2263–2268. doi: 10.1016/j.febslet.2011.05.049. [DOI] [PubMed] [Google Scholar]
- 10.Naik E, Dixit VM. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J Exp Med. 2011;208(3):417–420. doi: 10.1084/jem.20110367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vats D, et al. Oxidative metabolism and PGC-1beta attenuate macrophagemediated inflammation. Cell Metab. 2006;4(1):13–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cramer T, et al. HIF-1α Is Essential for Myeloid Cell-Mediated Inflammation. Cell. 2003;112(5):645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hara T, et al. Deletion of the Mint3/Apba3 Gene in Mice Abrogates Macrophage Functions and Increases Resistance to Lipopolysaccharide-induced Septic Shock. Journal of Biological Chemistry. 2011;286(37):32542–32551. doi: 10.1074/jbc.M111.271726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lenaz G, et al. Role of mitochondria in oxidative stress and aging. Ann N Y Acad Sci. 2002;959:199–213. doi: 10.1111/j.1749-6632.2002.tb02094.x. [DOI] [PubMed] [Google Scholar]
- 15.Nareika A, et al. Sodium lactate increases LPS-stimulated MMP and cytokine expression in U937 histiocytes by enhancing AP-1 and NF-kappaB transcriptional activities. Am J Physiol Endocrinol Metab. 2005;289(4):E534–E542. doi: 10.1152/ajpendo.00462.2004. [DOI] [PubMed] [Google Scholar]
- 16.Samuvel DJ, et al. Lactate boosts TLR4 signaling and NF-kappaB pathwaymediated gene transcription in macrophages via monocarboxylate transporters and MD-2 up-regulation. J Immunol. 2009;182(4):2476–2484. doi: 10.4049/jimmunol.0802059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336(15):1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 18.Voloboueva LA, et al. Overexpression of mitochondrial Hsp70/Hsp75 protects astrocytes against ischemic injury in vitro. J Cereb Blood Flow Metab. 2008;28(5):1009–1016. doi: 10.1038/sj.jcbfm.9600600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu L, et al. Overexpression of mitochondrial Hsp70/Hsp75 in rat brain protects mitochondria, reduces oxidative stress, and protects from focal ischemia. J Cereb Blood Flow Metab. 2009;29(2):365–374. doi: 10.1038/jcbfm.2008.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, et al. Effect of GRP75/mthsp70/PBP74/mortalin overexpression on intracellular ATP level, mitochondrial membrane potential and ROS accumulation following glucose deprivation in PC12 cells. Mol Cell Biochem. 2005;268(1–2):45–51. doi: 10.1007/s11010-005-2996-1. [DOI] [PubMed] [Google Scholar]
- 21.Bhattacharyya T, et al. Cloning and Subcellular Localization of Human Mitochondrial hsp70. Journal of Biological Chemistry. 1995;270(4):1705–1710. doi: 10.1074/jbc.270.4.1705. [DOI] [PubMed] [Google Scholar]
- 22.Cairns RA, et al. Pharmacologically Increased Tumor Hypoxia Can Be Measured by 18F-Fluoroazomycin Arabinoside Positron Emission Tomography and Enhances Tumor Response to Hypoxic Cytotoxin PR-104. Clinical Cancer Research. 2009;15(23):7170–7174. doi: 10.1158/1078-0432.CCR-09-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomita K, et al. Functional assay of NF-kappaB translocation into nuclei by laser scanning cytometry: inhibitory effect by dexamethasone or theophylline. Naunyn Schmiedebergs Arch Pharmacol. 1999;359(4):249–255. doi: 10.1007/pl00005349. [DOI] [PubMed] [Google Scholar]
- 24.Massa SM, et al. Cloning of rat grp75, an hsp70-family member, and its expression in normal and ischemic brain. J Neurosci Res. 1995;40(6):807–819. doi: 10.1002/jnr.490400612. [DOI] [PubMed] [Google Scholar]
- 25.Forrest VJ, et al. Oxidative stress-induced apoptosis prevented by Trolox. Free Radic Biol Med. 1994;16(6):675–684. doi: 10.1016/0891-5849(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 26.Tomatsuri N, et al. Edaravone, a newly developed radical scavenger, protects against ischemia-reperfusion injury of the small intestine in rats. Int J Mol Med. 2004;13(1):105–109. [PubMed] [Google Scholar]
- 27.Lenaz G, et al. Mitochondrial bioenergetics in aging. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2000;1459(2–3):397–404. doi: 10.1016/s0005-2728(00)00177-8. [DOI] [PubMed] [Google Scholar]
- 28.Winkler BS, Sauer MW, Starnes CA. Modulation of the Pasteur effect in retinal cells: implications for understanding compensatory metabolic mechanisms. Exp Eye Res. 2003;76(6):715–723. doi: 10.1016/s0014-4835(03)00052-6. [DOI] [PubMed] [Google Scholar]
- 29.Kelso GF, et al. Selective Targeting of a Redox-active Ubiquinone to Mitochondria within Cells: ANTIOXIDANT AND ANTIAPOPTOTIC PROPERTIES. Journal of Biological Chemistry. 2001;276(7):4588–4596. doi: 10.1074/jbc.M009093200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.