Abstract
A key function of BRCA1 and BRCA2 is the participation in dsDNAbreak repair via homologous recombination. BRCA1 and BRCA2 mutations, which occur in most hereditary ovarian cancers (OCs) and approximately 10% of all OC cases, are associated with defects in homologous recombination and genomic instability, a phenotype termed ‘BRCAness’. The clinical effects of BRCA1 and BRCA2 mutations have commonly been analyzed together; however, it is becoming increasingly apparent that these mutations do not have the same effects in OC. Recently, three major reports highlighted the unequal clinical characteristics of OCs with BRCA1 and BRCA2 mutations. All studies demonstrated that BRCA2-mutated patients are associated with better survival and therapeutic response than BRCA1-mutated and wild-type patients with serous OC. The differing prognostic effects of the BRCA2 and BRCA1 mutations is likely due to differing roles of BRCA1 and BRCA2 in homologous recombination repair and a stronger association between the BRCA2 mutation and a hypermutator phenotype. These new findings have potentially important implications for clinical management of patients with serous OC.
Keywords: BRCA mutation, drug response, homologous recombination, ovarian cancer, PARP inhibitor, survival
Ovarian cancer (OC) is the second most common gynecological malignancy and the leading cause of death from gynecological cancer. Approximately 75–80% of patients present with advanced disease that is refractory to current therapies; the 5-year survival rate for advanced OC is approximately 30–40%. A family history of OC is one of the strongest risk factors for OC. To date, at least 16 genes have been associated with hereditary OC, and BRCA1 and BRCA2 (BRCA1/2) mutations are beleived to account for the majority of hereditary OC [1]. More than 90% of early-onset cancers in families with breast cancer and OC histories are associated with BRCA1 or BRCA2 mutations. The risk of developing OC by 70 years of age is 40–50% for BRCA1 mutation carriers and 10–20% for BRCA2 mutation carriers [2,3].
The previous studies have demonstrated that BRCA1 and BRCA2 mutation-related OCs have distinct clinicopathological features; they tend to be more sensitive to platinum agents and possibly PARP inhibitors than wild-type BRCA OCs, and secondary BRCA1 and BRCA2 mutations that result in gain of BRCA1 and BRCA2 function are associated with platinum resistance [4,5]. Recently, three independent investigations have shown that among women with serous OC, BRCA2 mutation is more significantly associated with improved patient survival than BRCA1 mutation [6–8]; one study also showed that BRCA2 mutation was more strongly associated with chemotherapy response and genome instability [6]. In addition, two other studies demonstrated the trend of favorable outcomes in BRCA2 mutation patients [9,10] compared with BRCA1 mutation. This article reviews recent progress in our understanding of BRCA1 and BRCA2 mutations in OC and how these emerging findings affect synthetic lethality-based therapeutics in OC.
BRCA1 & BRCA2 mutations are associated with high-grade serous OC
In the general population, an estimated one in 300–800 individuals carries a BRCA1 or BRCA2 mutation [11]. By contrast, 8–13% of women diagnosed with epithelial OC have a germline BRCA1 or BRCA2 mutation [12–14]. In total, 70% of OC are of the serous subtype, 16–21% of which have BRCA1 or BRCA2 mutations [12,14,15]. BRCA1 and BRCA2 mutations can be also found in primary fallopian tube and peritoneal cancers [16]. Many OCs are believed to originate in the fallopian tube [17].
High-grade serous (HGS) OCs are highly genomically unstable, with TP53 gene mutations in up to 96% of cases in addition to mutations in BRCA1, BRCA2, RAD51C and other DNA-repair protein-encoding genes [18–20]. Other OC subtypes, such as clear-cell OCs, have fewer TP53 mutations, but have high-prevalence mutations in the ARID1A and PIK3CA genes [21,22]. Endometrioid OCs commonly have CTNNB1, ARID1A and PIK3CA mutations, but fewer TP53 mutations [23]. Mucinous OC have a greater prevalence of KRAS mutations [24].
The majority of OCs in women with inherited BRCA1 and BRCA2 mutations are HGS OC [25,26]. Among nonfamilial HGS OCs, somatic mutations or methylation also occur in BRCA1 or BRCA2, and other members of the BRCA1 and BRCA2 homologous recombination pathway, including RAD51, cHK1, cHK2, ATM, ATR and FANCF [27–31]. Homologous recombination defects occur in up to 50% of HGS OCs [19].
BRCA2 mutations are associated with longer survival & better therapy response than BRCA1 mutations
It has been thought that BRCA1- and BRCA2-mutated OCs are part of characteristic ‘BRCA syndrome’, with a constellation of clinicopathological features: younger age at onset [32,33], HGS histologic type (i.e., under-representation of mucinous cancers) [25,26,34], advanced stage at presentation, high probability of durable remission with platinum therapy and better prognosis [35–38]. A recent study reported that BRCA1- and BRCA2-mutated OCs metastasize to viscera more often than BRCA1 and BRCA2 wild-type OCs, suggesting that visceral metastasis is a feature of the BRCA syndrome phenotype [39].
The authors identified 26 studies that assessed the association between BRCA1 and BRCA2 mutations and clinicopathological features (Table 1); most of the studies combined BRCA1 and BRCA2 mutation carriers because of the relative rarity of BRCA1 and BRCA2 mutations [6–10,35–37,40–57]. Among these studies, six analyzed the relationship between chemosensitivity and BRCA1 and BRCA2 mutation, with inconsistent results [35–37,41,50,57]. Although most of the previous studies found that BRCA1- and BRCA2-mutated OCs were associated with better outcomes than were wild-type OCs, many of these studies were limited by small sample sizes, inadequate adjustment for known prognostic factors (such as stage, age at diagnosis, histologic type and extent of surgical debulking), short follow-up and nonuniform therapy in cases and controls. Nine studies analyzed the impact of BRCA1 and BRCA2 mutations separately on OC patient survival, and reported inconsistent conclusions [6–10,36,51,52,54].
Table 1.
Summary of studies reporting chemosensitivity or survival in ovarian cancer cases with mutations in BRCA1/2 compared with nonmutations or sporadic ovarian cancer.
| Study (year) | Mutation-positive population | Carriers | Sporadic/noncarriers | Survival results | p-value | Ref. |
|---|---|---|---|---|---|---|
| Rubin et al. (1996) | Consecutive cases; BRCA1; stages III and IV; matched controls by age, stage, grade and histologic type | 43 | NA | Median survival (months): BRCA1 carriers, 77; controls, 29 | p < 0.001 | [55] |
| Aida et al. (1998) | High-risk families; BRCA1 | 13 | 29 | 5-year survival (%): BRCA1 carriers, 78.6; controls, 30.3 | p < 0.05 | [40] |
| Jóhannsson et al. (1998) | Familial BRCA1 carriers; age- and stage-matched controls | 38 | 97 | Hazard ratio: 1.2; 95% CI: 0.5–2.8 | NS | [48] |
| Pharaoh et al. (1999) | High-risk families; sporadic cases | 151 (BRCA1: 127; BRCA2: 24) | 119 | 5-year survival (%): BRCA1 carriers, 21; BRCA2 carriers, 25; noncarriers, 19 | NS | [52] |
| Boyd et al. (2000) | Consecutive cases; BRCA1/2 tissues; Jewish origin | 88 (BRCA1: 67; BRCA2: 21) | 101 | 5-year survival (%): BRCA1/2 carriers, ~47; noncarriers, ~22 | BRCA1/2 vs noncarriers: p = 0.004 | [43] |
| Zweemer et al. (2001) | Familial cases; BRCA1/2 | 23 (BRCA1: 20; BRCA2: 3) | 17 | 5-year survival (%): BRCA1/2 carriers, ~40; noncarriers, ~ 46 | NS | [56] |
| Ramus et al. (2001) | Consecutive cases; Jewish origin; BRCA1/2 | 27 (BRCA1: 15; BRCA2: 12) | 71 | Median survival (months): BRCA1 carriers, 52; BRCA2 carriers, 49; noncarriers, 35 | NS | [54] |
| Ben David et al. (2002) | Incidence cases; Jewish origin; BRCA1/2 | 229 (BRCA1: 171; BRCA2: 58) | 549 | 3-year survival (%): BRCA1/2 carriers, 65.8; noncarriers, 51.9 | p < 0.001 | [42] |
| Buller et al. (2002) | Incidence cases; BRCA1 carriers; stage-matched noncarriers | 24 | 24 | Median survival (years): BRCA1 carriers, 4.5; noncarriers, 4.6 | NS | [44] |
| Cass et al. (2003) | Consecutive cases; BRCA1/2; Ashkenazi Jewish; stages III and IV | 34 (BRCA1: 22; BRCA2: 12) | 25 | Median survival (months): BRCA1/2 carriers, 91; noncarriers, 54 | p = 0.046 | [45] |
| Kringen et al. (2005) | Familial cases; BRCA1 | 30 | 100 | 5-year survival (%): BRCA1 carriers, 33; noncarriers, 23 | NS | [49] |
| Pal et al. (2007) | Population-based sample; BRCA1/2 | 32 (BRCA1: 20; BRCA2: 12) | 200 | 4-year survival (%): BRCA1 carriers, 37; BRCA2 carriers, 83; noncarriers, 12 |
BRCA1 vs noncarriers: p = 0.17; BRCA2 vs noncarriers: p = 0.013 |
[51] |
| Chetrit et al. (2008) | Case–control; Ashkenazi Jewish; BRCA1/2 | 213 (BRCA1: 159; BRCA2: 54) | 392 | Median survival (months): BRCA1/2, 53.7; noncarriers, 37.9 5-year survival for stage III and IV (%): carriers, 38.1; noncarriers, 24.5 |
Median survival: p = 0.002 5-year survival for stage III and IV: p < 0.001 |
[46] |
| Tan et al. (2008) | Case–control; BRCA1/2 | 22 (BRCA1: 17; BRCA2: 5) | 44 | Median OS (years): BRCA carriers, 8.4; noncarriers, 2.9 Time of first relapse (years): BRCA carriers, 5; noncarriers, 1.6 |
Median OS: p < 0.002 Time of first relapse: p < 00.01 |
[35] |
| Hennessy et al. (2010) | Incidence case; BRCA1/2 | 43 (BRCA1: 31; BRCA2: 13) | 192 | Median PFS (months): BRCA1/2, 20.1; nonmutations, 13.8 | p = 0.032 | [47] |
| Artioli et al. (2010) | Retrospective study; Italian; BRCA1/2 | 48 (BRCA1: 34; BRCA2: 14) | 40 | Median survival (months): BRCA1/2, 145; noncarriers, 280 | NS | [41] |
| Lacour et al. (2011) | Non-Ashkenazi Jewish; BRCA1/2 | 95 (BRCA1: 62; BRCA2: 33) | 183 | OS (months): BRCA mutation carriers, 101.7; sporadic mutation carriers, 54.3 PFS (months): BRCA mutation carriers, 27.9; sporadic mutation carriers, 17.9 |
OS: p < 0.0001 PFS: p < 0.0003 |
[50] |
| Ragupathy et al. (2011) | Retrospective analysis; BRCA1/2 | 12 (BRCA1: 4; BRCA2: 8) | 0 | Response to platinum-based chemotherapy (%):100, 50 and 50 for first-, second- and third-line, respectively 3-year survival (%): 92 OS (%): 83 |
– | [53] |
| Vencken et al. (2011) | Retrospective study; BRCA1 and BRCA2 separated | 112 (BRCA1: 99; BRCA2: 13) | 222 | Median OS (years): BRCA1, 5.9; BRCA2, more than 10; sporadic, 2.9 | p < 0.01 | [36] |
| Gallagher et al. (2011) | Incidence case; stage III and IV; BRCA1/2 | 36 (BRCA1: 20; BRCA2: 16) | 74 | Median OS (months): carriers, not reached; noncarriers, 67.8 | p = 0.002 | [37] |
| Yang et al. (2011) | Observational study; BRCA1/2 mutation and BRCA1 methylation | Mutations: 62 (BRCA1: 35; BRCA2: 27; methylation: 33) | 219 | 5-year survival (%): BRCA1, 44; BRCA2, 61; wild-type, 25 | BRCA2 vs wild-type: p = 0.003 | [6] |
| Hyman et al. (2011) | Retrospective study; BRCA1 and BRCA2 separated | 47 (BRCA1: 30; BRCA2: 17) | 143 | 3-year survival (%): BRCA1, 90.7; BRCA2, 100; wild-type, 69.4 | BRCA2 vs wild-type: p = 0.007 | [7] |
| Bolton et al. (2012) | Observational study; BRCA1/2 mutation | 3879 (BRCA1: 909; BRCA2: 304) | 2666 | 5-year survival (%): BRCA1, 44; BRCA2, 52; wild-type, 36 |
BRCA1 vs noncarriers: p < 0.01; BRCA2 vs noncarriers: p < 0.01; BRCA1 vs BRCA2: p = 0.003 |
[8] |
| Liu et al. (2012) | Retrospective study | 197 (BRCA1: 148; BRCA2: 49) | 0 | 5-year survival (%): BRCA1 , 61; BRCA2, 75 | Advanced-stage patients: BRCA1 , p = 0.17; BRCA2, p = 0.08 | [9] |
| Dann et al. (2012) | Retrospective study | 15 (BRCA1: 12; BRCA2: 3) | 38 | Median PFS (months): BRCA-mutant tumor, 21; wild-type, 5 | – | [57] |
| Reitsma et al. (2012) | Retrospective study | 71 (BRCA1: 55; BRCA2: 16) | – | Disease-free survival (months): BRCA1 , 65 months; BRCA2, 95 OS (months): BRCA1 , 71; BRCA2, 116 |
Disease-free survival: p = 0.34 OS: p = 0.56 |
[10] |
NA: Not available; NS: No significance; OS: Overall survival; PFS: Progression-free survival.
In 2003, Cass and colleagues studied the clinical characteristics and treatment responses of Ashkenazi Jewish women with hereditary OC and found that among 18 BRCA1 and 11 BRCA2 mutation patients, BRCA2 carriers had marginally longer disease-free intervals than BRCA1 carriers (57 vs 40 months, respectively; p = 0.2) [45]. In 2007, Pal and colleagues found a statistically significant difference in the 4-year survival rate between six BRCA2 carriers and 115 noncarriers (p = 0.013), but not between 14 BRCA1 carriers and noncarriers (p = 0.17) [51]. In 2008, Chetrit et al. reported on the outcomes of BRCA-associated and sporadic OC as part of the National Israeli Study of Ovarian Cancer [46]. Among 605 patients of Ashkenazi origin, median survival durations of 45.1 and 52.5 months were observed among BRCA1 and BRCA2 mutation carriers, respectively, versus 33.5 months in noncarriers. Vencken et al. compared first-line chemotherapy (including platinum- and nonplatinum-based regimens) response among 99 BRCA1- and 13 BRCA2-mutated and 115 noncarrier epithelial OC patients and found a nonstatistically significant trend towards longer progression-free and overall survival durations for BRCA2 mutation patients than in BRCA1 mutation patients [36].
These studies prompted several larger scale analyses that sought a definitive conclusion regarding the differential clinical associations of BRCA1 and BRCA2 mutations in OC. In a recent report, the authors performed integrated analyses of the multidimensional genomic and clinical data from 316 HGS OC patients in a project for The Cancer Genome Atlas (TCGA) and found that patients with BRCA1 and BRCA2 mutations had differing clinical features. Specifically, patients with BRCA1 mutations were younger at diagnosis (mean age: 55.9 vs 61.8 years for wild-type [p = 0.006] and 60.9 years for BRCA2 [p = 0.03]) [6]. The 5-year survival rate of BRCA2 mutation carriers was significantly higher than that of wild-type cases (61 vs 25%, respectively; p = 0.02). Among BRCA2 mutation carriers, 100% were platinum sensitive (i.e., a complete or partial response to adjuvant chemotherapy) compared with 80% of BRCA1-mutated and 85% of wild-type cases (p = 0.05 and p = 0.02, respectively). Similarly, BRCA2 mutation patients had longer platinum-free survival durations than BRCA1 and wild-type patients. The availability of genomic data (i.e., somatic mutations, DNA copy-number alterations and methylation) in TCGA project for all the analyzed cases allowed the authors to evaluate molecular correlates in a quantitative manner. The result of this analysis led to the determination that BRCA2 cases have a more pronounced ‘mutator phenotype’, as defined by the number of total mutations across the whole exome. BRCA1-mutated cancers exhibited no significant enrichment of mutations.
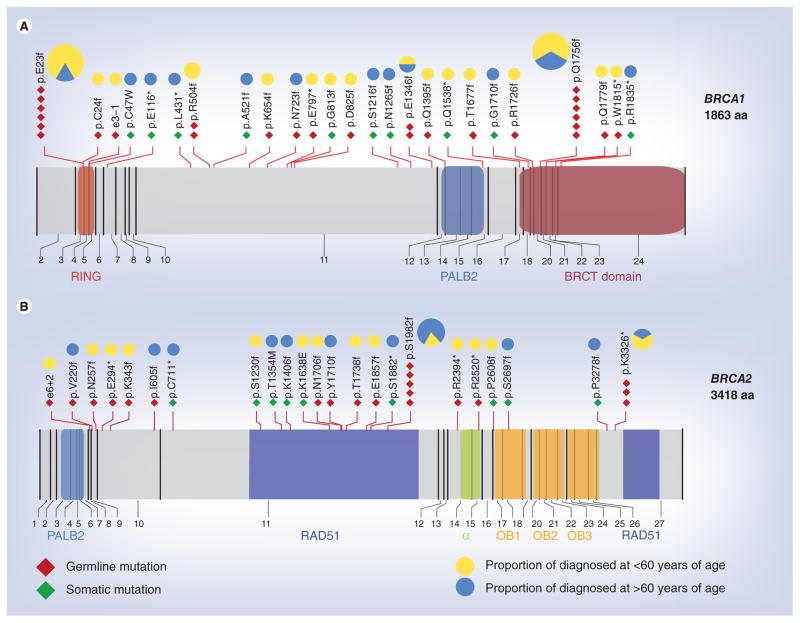
The results of quantitative analysis of the relationships between BRCA1 and BRCA2 mutations and genomic instability in OC suggest BRCA2 plays a more important role in the BRCA dsDNA break-repair pathway. Thus, BRCA2 inactivation would result in extensive gene mutations in cancer cells, making them vulnerable to DNA-damaging chemotherapy drugs. It should be noted that TCGA data set provides a more representative spectrum of BRCA mutations in the general population than previous studies, given that only 7% of patients in TCGA data set are of Askhenazi Jewish origin. The detailed location of the mutations relative to the known functional domains of BRCA1/2 is shown in Figure 1.
Figure 1. Diagrams of BRCA1 and BRCA2 proteins, including functional domains and interacting proteins.
The mutations identified in the The Cancer Genome Atlas project are indicated. Germline and somatic mutations in (A) BRCA1 and (B) BRCA2. Mutations are mapped to the corresponding exons of BRCA1 and BRCA2. The pie chart on top of each mutated location indicates the distribution of age at diagnosis of patients with the mutation. The size of each pie chart is based on the number of mutations in that location.
aa: Amino acids; BRCT: BRCA1 C-terminal; RING: RING-type zinc finger.
Subsequent to our report, two independent studies also provided supporting evidence that BRCA2 mutations are associated with a better prognosis of OC [7,8]. Hyman and colleagues demonstrated that among 143 BRCA-nonmutated, 30 BRCA1-mutated and 17 BRCA2-mutated stage III or IV serous ovarian, fallopian tube or primary peritoneal cancer patients, the 3-year survival rates were 69.4, 90.7 and 100%, respectively [7]. A multivariate analysis revealed that BRCA2 mutations (p = 0.007), but not BRCA1 mutations (p = 0.31), were associated with improved overall survival compared with wild-type.
Recently, a pooled observational study interrogated a total of 3739 epithelial OC cases (909 BRCA1 mutation carriers, 304 BRCA2 mutation carriers and 2666 noncarriers) from the USA, Europe, Israel, Hong Kong, Canada, Australia and the UK, and concluded that BRCA2 mutation carriers had the best prognosis [8]. Specifically, the 5-year overall survival was 36% for noncarriers, 44% for BRCA1 carriers and 52% for BRCA2 carriers. In a Cox proportional-hazards regression model, BRCA2 mutation carriers had more survival advantages than BRCA1 mutation carriers (hazard ratio [HR]: 0.73 for BRCA1; 95% CI: 0.64–0.84, HR: 0.49 for BRCA2; 95% CI: 0.39–0.61). The result of these studies suggested that BRCA2 inactivation by mutation resulted in better survival than BRCA1 inactivation and wild-type. Consistent with these findings, the result of a recent study demonstrated that BRCA2 overexpression is associated with shorter progression-free survival in OC [58].
Next, Liu et al. identified that among 197 BRCA-related OC patients, 5-year overall survival was 75% in 49 BRCA2-mutated patients versus 61% in 148 BRCA1-mutated patients, and there was a trend toward improved outcomes in BRCA2-associated OCs in advanced-stage patients (p = 0.08) [9]. Similarly, Reitsma et al. evaluated clinico-pathological features and survival of 55 BRCA1- and 16 BRCA2-related adnexal cancer, and also found a nonsignificant trend of more favorable outcomes in BRCA2-mutated patients with fewer relapse (38 vs 58%) and a longer time to first time relapse (42 vs 25 months) than BRCA1 carriers [10].
The results of recent studies suggest that BRCA2 mutations have a stronger impact on synthetic lethality-associated sensitivity to chemotherapy compared with BRCA1 mutations, which may underlie their increased survival advantage; however, the role of BRCA1 is nevertheless important. First, in most reported studies, BRCA1-mutated OCs are diagnosed approximately 5–10 years earlier than BRCA2-mutated and non-BRCA-mutated cancers (median age, 50–55 years vs 60, respectively) [8,36,43,45,52,54]. Therefore, inactivation of BRCA1 likely plays a more important role in OC initiation than platinum sensitivity. This observation should probably be attributed to heterogeneity of the functional outcomes of different BRCA1 mutations. BRCA1 plays a versatile role in tumor suppression through its ability to participate in the DNA-damage response, checkpoint control, mitotic spindle assembly, sister chromatid decatenation and centrosome duplication [59–63]. The failure of one of these functions could predispose BRCA1-mutated cells to tumorigenesis, but not necessarily render the developed cancer cell sensitive to DNA-damaging agents such as cisplatin. Indeed, Bolton et al. observed that survival association of BRCA1 mutation carriers depended on the mutation location, with N-terminal mutations of the BRCA1 protein associated with worse survival and C-terminal mutations found to be associated with better survival [8]. Consistent with this finding, a recent study demonstrated that a mutation in the RING-type zinc finger (RING) domain (N-terminal) of BRCA1 showed poor response to cisplatin and PARP inhibitors [64]. The authors’ analysis of TCGA data and the study of Hyman et al. both revealed a nonstatistically significant trend toward better survival in BRCA1 mutation carriers compared with noncarrier patients. The HR for BRCA1 carriers reported in the TCGA analysis (0.76) [6] and in an analysis by the Memorial Sloan-Kettering Cancer Center (0.70) [7] was very similar to those from Bolton et al. (0.73). However, with a larger sample size, the Bolton et al. study observed a statistically significant survival advantage for the BRCA1-mutated cases, although it is clear that BRCA2-mutated cases had the best survival advantage.
Other differing associations of BRCA1 & BRCA2 mutations
Unlike BRCA1 mutations, which are almost exclusively associated with female breast cancer and OC, BRCA2 mutations also confer a higher risk for pancreatic, prostate, male breast cancers and melanoma [65–68]. Women with BRCA1-associated breast cancer are more likely to have estrogen receptor-negative disease and significantly poorer overall prognoses than noncarrier sand BRCA2-associated breast cancer [69–71]. Evidence indicates that considerable differences in OC risk exist between women with BRCA1 mutations and those with BRCA2 mutations. Specifically, the lifetime risk for OC in patients with BRCA1 mutations is 39–46% compared with 10–27% in patients with BRCA2 mutations [2,72–74]. Furthermore, only 2–3% of women with BRCA2 mutations will develop OC by 50 years of age versus 10–21% of women with BRCA1 mutations.
Different roles of BRCA1 & BRCA2 in DNA-repair pathway
The observed differences in clinical outcome basis of BRCA1 versus BRCA2 mutations may be rooted in different functions of these proteins in dsDNA-repair pathway. BRCA1 was first localized to chromosome 17 via a genetic linkage analysis [75] and was cloned in 1994 [76]. The C-terminus of BRCA1 contains a BRCA1 C-terminal (BRCT) domain, which facilitates phosphoprotein binding. The N-terminus has a RING domain, which has E3 ubiquitin ligase activity. BRCA1 interacts with PALB2 through a domain toward the C-terminal region. Because PALB2 also interacts with BRCA2, BRCA1 may affect BRCA2 function through PALB2 [77]. BRCA2 was found to be related to hereditary breast cancer in 1995 [78]. BRCA2 contains a DNA-binding domain, which binds both ssDNA and dsDNA. BRCA2 has eight BRC repeats that bind RAD51. The C-terminus of BRCA2 also binds RAD51 in a phosphorylation-regulated manner (Figure 1) [79–81].
Both BRCA1 and BRCA2 have been reported to play key roles in DNA-damage repair; however, they appear to have distinct but complementary functions. BRCA1 plays more diverse roles including sensing DNA damage and replication stress, mediating signaling responses, signaling cell cycle checkpoints and mediating other transcriptional responses to DNA damage [82]. In the homologous recombination pathway, BRCA1 is mainly a scaffold protein, enabling interactions between different components of the homologous recombination machinery, and is required for the initial steps of the dsDNA break-repair response and signal amplification. By contrast, BRCA2 is directly involved in loading RAD51 to damage sites or stalled replication forks [83,84]. BRCA2 acts by navigating RAD51 to ssDNA, enabling RAD51 to displace replication protein-A from ssDNA and stabilizing RAD51–ssDNA filaments by blocking ATP hydrolysis, which is a key regulatory step in DNA pairing [85,86]. RAD51 plays a central role in recombination, assembling onto ssDNA as a nucleoprotein filament and catalyzing the exchange of homologous DNA sequences [87].
BRCA2’s more direct involvement in homologous recombination is consistent with the association of BRCA2 mutations and hypermutator phenotype that the authors found in OC. Most BRCA2 mutations found in TCGA study were in the RAD51-binding domain or caused truncations that would delete the RAD51-binding region (Figure 1); therefore, these mutations are expected to attenuate or abolish the interaction with RAD51, resulting in failure to load RAD51 to DNA-damage sites. BRCA1 is also implicated in RAD51 recruitment to the sites of DNA damage through its interactions with PALB2 and BRCA2. This interaction appears to be dependent on CHK2-mediated phosphorylation of S988 on BRCA1 [82]. Interestingly, most BRCA1 mutations do not result in deletion of S988 or the PALB2 interaction region that regulates BRCA2 (Figure 1); thus, BRCA1 mutation may have less impact on RAD51-mediated homologous recombination. Consistent with this notion, mutations on the C-terminal region of BRCA1, including the PALB2-binding region, were found to be correlated with better survival advantage by Bolton et al. [8]. Thus, this group of BRCA1-mutated cases may impact survival in a manner similar to that BRCA2-mutated bases because of the effect of these mutants on BRCA2.
Implications for BRCA-related OC treatment
BRCA2 mutation status may be a genetic marker for predicting prognosis and chemotherapy response. Because BRCA2 mutations are associated with longer platinum-free survival durations than BRCA1 mutations and BRCA wild-type, a patient’s BRCA status may influence the choice of chemotherapy agents for recurrent disease. Whether BRCA1- and BRCA2-mutated OC patients would experience different responses to chemotherapy drugs other than cisplatin remains unclear. It should be noted that BRCA2-deficient tumor cells manifest a strong response to melphalan in vivo and in vitro [88,89].
The differences in survival and chemotherapy responses among BRCA1 and BRCA2 mutation and noncarriers have important implications for the future of OC clinical trial designs. Because BRCA1 and BRCA2 mutations, especially BRCA2 mutations, have a clear impact on survival, any clinical trial that evaluates effectiveness of new therapeutic agents should consider BRCA1 and BRCA2 mutations status and perform multivariant analyses with this mutation status as a variable; for BRCA2, it is relatively simple. For BRCA1, the situation is more complicated because mutations at the N-terminal and C-terminal region of the proteins have opposite association with survival. Further analysis will be needed to stratify the BRCA1 mutations. Without a balanced BRCA mutation distribution, any survival difference observed in different arms of the clinical trials can be at least partially attributed to difference in distributions.
Recent findings demonstrate that PARP inhibitors have cytotoxic effects on BRCA1- or BRCA2-deficient cells [90,91]. The prevailing explanation for these findings is a phenomenon called synthetic lethality [92]. Promising results from clinical trials in BRCA-associated carcinomas (including OC) have been reported (Table 2) [93–98]. An important consideration is whether BRCA1- and BRCA2-mutated OCs’ differing response to platinum-based chemotherapy, as observed in recent studies, may also result in differing sensitivity to agents that target the resultant homologous recombination defects. Some preclinical data suggest that BRCA2−/− cells respond better to PARP inhibitors than BRCA1−/− cells [90,99]. Although current trials of PARP inhibitors have not been large enough to detect differences in efficacy among the BRCA gene mutations, trends can be observed. In Gelmon et al.’s study, which included 11 BRCA1- and five BRCA2-mutated OC patients treated by PAR P inhibitors, a 60% (three out of five) response rate for BRCA2 versus 24% (11 out of 60) for BRCA-wild-type and 36% (four out of 11) for BRCA1 was observed [96]. A similar trend was seen in the cohort that received 400 mg of olaparib twice daily in Audeh et al.’s study [94]. These promising yet early results indicate that further stratification is needed to evaluate the differing effects of PARP inhibitors treatment in individuals with BRCA2 and BRCA1 mutations. In addition, upcoming trials of PARP inhibitors that specifically enrich for BRCA1 and BRCA2 carriers may be at particular risk for bias if differences between these two biologically distinct groups are not considered. Knowing the differing role of BRCA1 and BRCA2 in DNA-damage repair, the authors anticipate that BRCA2-mutated cases will also render OC more sensitive to PARP inhibitors.
Table 2.
Clinical trials of PARP inhibitors including ovarian cancer patients.
| Study (year) | Study population | Phase | Treatment | Patients (n) | RR (%)† | CBR (%) | PFS or RD | Toxicity | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Fong et al. (2009) | Refractory solid tumors (OC, breast, colorectal, melanoma, sarcoma and others) | I | Olaparib: dose escalation and expansion at 600 mg twice daily | 60 (OC: 21) | 53 | 60 | – | Grade 1 or 2 | [93] |
| Audeh et al. (2010) | BRCA-associated recurrent advanced OC | II | Olaparib: 400 mg twice daily and 100 mg twice daily | 57 | 400 mg dose: RECIST, 33; RECIST/GCIG, 61; 100 mg dose: RECIST, 13; RECIST/GCIG, 17 | 400 mg dose: 66; 100 mg dose: 42 | PFS (months): olaparib 400 mg dose, 5.8; 100 mg dose, 1.9 | Mainly grade ≤2 | [94] |
| Fong et al. (2010) | BRCA-associated OC that progressed after platinum agent | II | Olaparib: dose escalation and expansion at 200 mg twice daily | 50 | RECIST/GCIG, 40 | Overall: 46; platinum sensitive: 69; platinum resistant: 46; platinum refractory: 23 (p < 0.05) | RD of all responding patients (weeks): 28 | Mainly grade ≤2 | [95] |
| Gelmon et al. (2011) | Advanced high-grade serous undifferentiated OC; triple-negative breast cancer | II | Olaparib: 400 mg twice daily | 91 (OC: 65) | Total: 29; BRCA1/2 mutations: 41; non-BRCA: 24 | Total: 67; BRCA1/2 mutations: 76; non-BRCA: 63 | PFS (days): total, 219; BRCA1/2 mutations, 221; non-BRCA, 192 | 36% grade ≥3 | [96] |
| Kaye et al. (2012) | Recurred within 12 months of prior platinum treatment; BRCA1/2-mutated OC | II | Olaparib: 400 mg or 200 mg twice daily, or PLD 50 mg/m2 every 28 days | 97 | Olaparib 400 mg: RECIST, 31; RECIST/GCIG, 59 200 mg: RECIST, 25; RECIST/GCIG, 38 PLD: RECIST, 18; RECIST/GCIG, 39 |
Olaparib 400 mg: 59 200 mg: 47 PLD: 52 |
PFS (months): olaparib 400 mg, 8.8; 200 mg, 6.5; PLD 50 mg/m2, 7.1 | Generally grade ≤2 | [97] |
| Ledermann et al. (2012) | Platinum-sensitive, relapsed, high-grade serous OC | II | Olaparib 400 mg twice daily or placebo | 265 | Olaparib 12; Placebo 4 | Olaparib: 53; placebo: 25 | PFS (months): olaparib, 8.4; placebo, 4.8 | Most grade 1 or 2 | [98] |
RR according to RECIST criteria unless stated otherwise.
CBR: Clinical benefit rate (response rate + stable disease); GCIG: Gynecologic Cancer Intergroup; OC: Ovarian cancer; PFS: Progression-free survival; PLD: Pegylated liposomal doxorubicin; RD: Response duration; RECIST: Response Evaluation Criteria In Solid Tumors; RR: Response rate (complete + partial responses).
BRCA2 mutation carriers experience better response to chemotherapy; however, it is challenging to identify therapeutic strategies for non-BRCA2 carriers and carriers with secondary resistance to DNA damaging drugs or PARP inhibitors. Understanding the homologous recombination pathway and the impact of mutations in genes in the pathway should provide important insight into areas for future explorations and drug development that mimic BRCA2 mutation in tumor cells. Recently, Issaeva et al. found that cisplatin-resistant breast cancer cells with BRCA2 secondary mutations are still sensitive to 6-thioguanine [100]. In addition, proteasome inhibitors [101], cyclin-dependent kinase inhibitors [102] and HSP90 inhibitors [103] have been reported to inhibit RAD51 foci formation. These drugs may re-sensitize drug-resistant cancers with secondary BRCA2 mutations by inhibiting homologous recombination.
It has been estimated that more than 50% of HGS epithelial OC could show dysfunction of BRCA1 or BRCA2 through genetic or epi-genetic events. There are reports that epithelial OC cases with somatic BRCA1/2 mutations or deficient expression show a survival advantage over noncarriers [15,47,57]; however, data from TCGA and others suggest that silencing of BRCA1 through promoter methylation does not result in an improved overall survival [19,104]. Larger studies that include comprehensive genomic screening of BRCA1 and BRCA2 in primary epithelial OCs will be needed to determine if alterations at the somatic and epigenetic level have similar clinical effects to germline mutations.
Conclusion & future perspective
Over the past two decades, significant progress has been made in elucidating the functions of BRCA1 and BRCA2 and their mutations on cancer risk and prognosis. Given the growing awareness of the prognostic importance of BRCA2 mutations and how their sensitivity differs from that of BRCA1 mutations and wild-type, as well as the potential of PARP inhibitors to change how OC is treated, it is increasingly likely that knowing patients’ BRCA mutation status will be important making informed treatment recommendations and clinical trial designs. BRCA mutation detection by sequencing is readily available to OC and breast cancer patients in the USA and Europe. The recent findings on BRCA2 mutations should encourage those in other countries to adopt the use of this molecular marker in OC treatment [105]. Other BRCA2 mutations cancer types may manifest similar clinical features to those of OC. Finally, for cancers that are not associated with BRCA2 mutations, efforts should be made to develop agents that mimic BRCA2 mutation as a new class of targeted therapeutics that can be specifically delivered to cancer cells.
Executive summary.
BRCA1 and BRCA2 mutations are associated with high-grade serous ovarian cancer (OC).
The clinical effects of BRCA1 and BRCA2 mutations have commonly been analyzed together; however, it is becoming increasingly apparent that these mutations do not have the same effects in OC. Recent reports demonstrated that BRCA2 mutations are associated with better survival and therapeutic response than BRCA1-mutated and wild-type serous OC patients.
BRCA1 and BRCA2 have different roles in homologous recombination repair.
It is critical to understand the difference of these two genes mutation in OC and the potentially important implications for future clinical management of patients with OC.
Acknowledgments
The authors would like to thank A Sutton in the Department of Scientific Publications (The University of Texas MD Anderson Cancer Center, TX, USA) for editing this manuscript.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
This study was supported by a grant from the NIH (U24CA143835) to I Shmulevich and W Zhang, a grant from the Blanton-Davis Ovarian Cancer Research Program to W Zhang, an Ovarian Cancer SPORE grant (P50 CA083639) to AK Sood and a grant supported by National Natural Science Foundation of China (grant no. 81101673) to G Liu. D Yang is an Odyssey Fellow at MD Anderson Cancer Center, and supported by The Diane Denson Tobola Fellowship in Ovarian Cancer Research fellowship and The Harold C and Mary L Daily Endowment Fund. Y Sun is supported by The Linda K Manning Fellowship in Ovarian Cancer and The A Lavoy Moore Endowment Fund. G Liu is a New Century outstanding fellow at Tianjin Medical University General Hospital. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Pennington KP, Swisher EM. Hereditary ovarian cancer: beyond the usual suspects. Gynecol Oncol. 2012;124(2):347–353. doi: 10.1016/j.ygyno.2011.12.415. [DOI] [PubMed] [Google Scholar]
- 2.King MC, Marks JH, Mandell JB. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302(5645):643–646. doi: 10.1126/science.1088759. [DOI] [PubMed] [Google Scholar]
- 3.Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25(11):1329–1333. doi: 10.1200/JCO.2006.09.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakai W, Swisher EM, Karlan BY, et al. Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature. 2008;451(7182):1116–1120. doi: 10.1038/nature06633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swisher EM, Sakai W, Karlan BY, Wurz K, Urban N, Taniguchi T. Secondary BRCA1 mutations in BRCA1-mutated ovarian carcinomas with platinum resistance. Cancer Res. 2008;68(8):2581–2586. doi: 10.1158/0008-5472.CAN-08-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6▪.Yang D, Khan S, Sun Y, et al. Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. JAMA. 2011;306(14):1557–1565. doi: 10.1001/jama.2011.1456. A comprehensive analysis of The Cancer Genome Atlas data on different effect of BRCA1 and BRCA2 mutations on drug response and hypermutator phenotype in ovarian cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7▪.Hyman DM, Zhou Q, Iasonos A, et al. Improved survival for BRCA2-associated serous ovarian cancer compared with both BRCA-negative and BRCA1-associated serous ovarian cancer. Cancer. 2011;118(15):3703–3709. doi: 10.1002/cncr.26655. Another important report of improved survival of BRCA2-mutated ovarian cancer patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8▪▪.Bolton Kl, Chenevix-Trench G, Goh C, et al. Association between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian cancer. JAMA. 2012;307(4):382–390. doi: 10.1001/jama.2012.20. Large multicenter study on differing effect of BRCA1 and BRCA2 mutations on ovarian cancer survival. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J, Cristea MC, Frankel P, et al. Clinical characteristics and outcomes of BRCA-associated ovarian cancer: genotype and survival. Cancer Genet. 2012;205(1–2):34–41. doi: 10.1016/j.cancergen.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reitsma W, de Bock GH, Oosterwijk JC, Ten Hoor KA, Hollema H, Mourits MJ. Clinicopathologic characteristics and survival in BRCA1- and BRCA2-related adnexal cancer: are they different? Int J Gynecol Cancer. 2012;22(4):579–585. doi: 10.1097/IGC.0b013e31823d1b5c. [DOI] [PubMed] [Google Scholar]
- 11.Whittemore AS, Gong G, Itnyre J. Prevalence and contribution of BRCA1 mutations in breast cancer and ovarian cancer: results from three U.S. population-based case–control studies of ovarian cancer. Am J Hum Genet. 1997;60(3):496–504. [PMC free article] [PubMed] [Google Scholar]
- 12.Risch HA, McLaughlin JR, Cole DE, et al. Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am J Hum Genet. 2001;68(3):700–710. doi: 10.1086/318787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubin SC, Blackwood MA, Bandera C, et al. BRCA1, BRCA2, and hereditary nonpolyposis colorectal cancer gene mutations in an unselected ovarian cancer population: relationship to family history and implications for genetic testing. Am J Obstet Gynecol. 1998;178(4):670–677. doi: 10.1016/s0002-9378(98)70476-4. [DOI] [PubMed] [Google Scholar]
- 14.Pal T, Permuth-Wey J, Betts JA, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005;104(12):2807–2816. doi: 10.1002/cncr.21536. [DOI] [PubMed] [Google Scholar]
- 15.Press JZ, De Luca A, Boyd N, et al. Ovarian carcinomas with genetic and epigenetic BRCA1 loss have distinct molecular abnormalities. BMC Cancer. 2008;8:17. doi: 10.1186/1471-2407-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levine DA, Argenta PA, Yee CJ, et al. Fallopian tube and primary peritoneal carcinomas associated with BRCA mutations. J Clin Oncol. 2003;21(22):4222–4227. doi: 10.1200/JCO.2003.04.131. [DOI] [PubMed] [Google Scholar]
- 17.Crum CP, Drapkin R, Miron A, et al. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Curr Opin Obstet Gynecol. 2007;19(1):3–9. doi: 10.1097/GCO.0b013e328011a21f. [DOI] [PubMed] [Google Scholar]
- 18.Norquist BM, Garcia RL, Allison KH, et al. The molecular pathogenesis of hereditary ovarian carcinoma: alterations in the tubal epithelium of women with BRCA1 and BRCA2 mutations. Cancer. 2010;116(22):5261–5271. doi: 10.1002/cncr.25439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bowtell DD. The genesis and evolution of high-grade serous ovarian cancer. Nat Rev Cancer. 2010;10(11):803–808. doi: 10.1038/nrc2946. [DOI] [PubMed] [Google Scholar]
- 21.Kuo KT, Mao TL, Jones S, et al. Frequent activating mutations of PIK3CA in ovarian clear cell carcinoma. Am J Pathol. 2009;174(5):1597–1601. doi: 10.2353/ajpath.2009.081000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones S, Wang TL, Shih Ie M, et al. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science. 2010;330(6001):228–231. doi: 10.1126/science.1196333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiegand KC, Shah SP, Al-Agha OM, et al. ARID1A mutations in endometriosis- associated ovarian carcinomas. N Engl J Med. 2010;363(16):1532–1543. doi: 10.1056/NEJMoa1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cuatrecasas M, Villanueva A, Matias-Guiu X, Prat J. K-RAS mutations in mucinous ovarian tumors: a clinicopathologic and molecular study of 95 cases. Cancer. 1997;79(8):1581–1586. doi: 10.1002/(sici)1097-0142(19970415)79:8<1581::aid-cncr21>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 25.Gilks CB, Prat J. Ovarian carcinoma pathology and genetics: recent advances. Hum Pathol. 2009;40(9):1213–1223. doi: 10.1016/j.humpath.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 26.Piek JM, Torrenga B, Hermsen B, et al. Histopathological characteristics of BRCA1- and BRCA2-associated intraperitoneal cancer: a clinic-based study. Fam Cancer. 2003;2(2):73–78. doi: 10.1023/a:1025700807451. [DOI] [PubMed] [Google Scholar]
- 27.Geisler JP, Hatterman-Zogg MA, Rathe JA, Buller RE. Frequency of BRCA1 dysfunction in ovarian cancer. J Natl Cancer Inst. 2002;94(1):61–67. doi: 10.1093/jnci/94.1.61. [DOI] [PubMed] [Google Scholar]
- 28.Hilton JL, Geisler JP, Rathe JA, Hattermann-Zogg MA, Deyoung B, Buller RE. Inactivation of BRCA1 and BRCA2 in ovarian cancer. J Natl Cancer Inst. 2002;94(18):1396–1406. doi: 10.1093/jnci/94.18.1396. [DOI] [PubMed] [Google Scholar]
- 29.Lim Sl, Smith P, Syed N, et al. Promoter hypermethylation of FANCF and outcome in advanced ovarian cancer. Br J Cancer. 2008;98(8):1452–1456. doi: 10.1038/sj.bjc.6604325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Z, Li M, Lu S, Zhang Y, Wang H. Promoter hypermethylation of FANCF plays an important role in the occurrence of ovarian cancer through disrupting Fanconi anemia–BRCA pathway. Cancer Biol Ther. 2006;5(3):256–260. doi: 10.4161/cbt.5.3.2380. [DOI] [PubMed] [Google Scholar]
- 31.D’Andrea AD. The Fanconi anemia/BRCA signaling pathway: disruption in cisplatin-sensitive ovarian cancers. Cell Cycle. 2003;2(4):290–292. [PubMed] [Google Scholar]
- 32.Soegaard M, Kjaer SK, Cox M, et al. BRCA1 and BRCA2 mutation prevalence and clinical characteristics of a population-based series of ovarian cancer cases from Denmark. Clin Cancer Res. 2008;14(12):3761–3767. doi: 10.1158/1078-0432.CCR-07-4806. [DOI] [PubMed] [Google Scholar]
- 33.Risch HA, McLaughlin JR, Cole De, et al. Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: a kin-cohort study in Ontario, Canada. J Natl Cancer Inst. 2006;98(23):1694–1706. doi: 10.1093/jnci/djj465. [DOI] [PubMed] [Google Scholar]
- 34.Shaw PA, McLaughlin JR, Zweemer RP, et al. Histopathologic features of genetically determined ovarian cancer. Int J Gynecol Pathol. 2002;21(4):407–411. doi: 10.1097/00004347-200210000-00011. [DOI] [PubMed] [Google Scholar]
- 35.Tan DS, Rothermundt C, Thomas K, et al. ‘BRCAness’ syndrome in ovarian cancer: a case–control study describing the clinical features and outcome of patients with epithelial ovarian cancer associated with BRCA1 and BRCA2 mutations. J Clin Oncol. 2008;26(34):5530–5536. doi: 10.1200/JCO.2008.16.1703. [DOI] [PubMed] [Google Scholar]
- 36.Vencken PM, Kriege M, Hoogwerf D, et al. Chemosensitivity and outcome of BRCA1- and BRCA2-associated ovarian cancer patients after first-line chemotherapy compared with sporadic ovarian cancer patients. Ann Oncol. 2011;22(6):1346–1352. doi: 10.1093/annonc/mdq628. [DOI] [PubMed] [Google Scholar]
- 37.Gallagher DJ, Konner JA, Bell-McGuinn KM, et al. Survival in epithelial ovarian cancer: a multivariate analysis incorporating BRCA mutation status and platinum sensitivity. Ann Oncol. 2011;22(5):1127–1132. doi: 10.1093/annonc/mdq577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turner N, Tutt A, Ashworth A. Hallmarks of ‘BRCAness’ in sporadic cancers. Nat Rev Cancer. 2004;4(10):814–819. doi: 10.1038/nrc1457. [DOI] [PubMed] [Google Scholar]
- 39.Gourley C, Michie Co, Roxburgh P, et al. Increased incidence of visceral metastases in Scottish patients with BRCA1/2-defective ovarian cancer: an extension of the ovarian BRCAness phenotype. J Clin Oncol. 2010;28(15):2505–2511. doi: 10.1200/JCO.2009.25.1082. [DOI] [PubMed] [Google Scholar]
- 40.Aida H, Takakuwa K, Nagata H, et al. Clinical features of ovarian cancer in Japanese women with germ-line mutations of BRCA1. Clin Cancer Res. 1998;4(1):235–240. [PubMed] [Google Scholar]
- 41.Artioli G, Borgato L, Cappetta A, et al. Overall survival in BRCA-associated ovarian cancer: case–control study of an Italian series. Eur J Gynaecol Oncol. 2010;31(6):658–661. [PubMed] [Google Scholar]
- 42.Ben David Y, Chetrit A, Hirsh-Yechezkel G, et al. Effect of BRCA mutations on the length of survival in epithelial ovarian tumors. J Clin Oncol. 2002;20(2):463–466. doi: 10.1200/JCO.2002.20.2.463. [DOI] [PubMed] [Google Scholar]
- 43.Boyd J, Sonoda Y, Federici MG, et al. Clinicopathologic features of BRCA-linked and sporadic ovarian cancer. JAMA. 2000;283(17):2260–2265. doi: 10.1001/jama.283.17.2260. [DOI] [PubMed] [Google Scholar]
- 44.Buller RE, Shahin MS, Geisler JP, Zogg M, De Young BR, Davis CS. Failure of BRCA1 dysfunction to alter ovarian cancer survival. Clin Cancer Res. 2002;8(5):1196–1202. [PubMed] [Google Scholar]
- 45.Cass I, Baldwin RL, Varkey T, Moslehi R, Narod SA, Karlan BY. Improved survival in women with BRCA-associated ovarian carcinoma. Cancer. 2003;97(9):2187–2195. doi: 10.1002/cncr.11310. [DOI] [PubMed] [Google Scholar]
- 46.Chetrit A, Hirsh-Yechezkel G, Ben-David Y, Lubin F, Friedman E, Sadetzki S. Effect of BRCA1/2 mutations on long-term survival of patients with invasive ovarian cancer: the national Israeli study of ovarian cancer. J Clin Oncol. 2008;26(1):20–25. doi: 10.1200/JCO.2007.11.6905. [DOI] [PubMed] [Google Scholar]
- 47.Hennessy BT, Timms KM, Carey MS, et al. Somatic mutations in BRCA1 and BRCA2 could expand the number of patients that benefit from poly (ADP ribose) polymerase inhibitors in ovarian cancer. J Clin Oncol. 2010;28(22):3570–3576. doi: 10.1200/JCO.2009.27.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jóhannsson OT, Ranstam J, Borg A, Olsson H. Survival of BRCA1 breast and ovarian cancer patients: a population-based study from southern Sweden. J Clin Oncol. 1998;16(2):397–404. doi: 10.1200/JCO.1998.16.2.397. [DOI] [PubMed] [Google Scholar]
- 49.Kringen P, Wang Y, Dumeaux V, et al. TP53 mutations in ovarian carcinomas from sporadic cases and carriers of two distinct BRCA1 founder mutations; relation to age at diagnosis and survival. BMC Cancer. 2005;5:134. doi: 10.1186/1471-2407-5-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lacour RA, Westin SN, Meyer LA, et al. Improved survival in non-Ashkenazi Jewish ovarian cancer patients with BRCA1 and BRCA2 gene mutations. Gynecol Oncol. 2011;121(2):358–363. doi: 10.1016/j.ygyno.2010.12.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pal T, Permuth-Wey J, Kapoor R, Cantor A, Sutphen R. Improved survival in BRCA2 carriers with ovarian cancer. Fam Cancer. 2007;6(1):113–119. doi: 10.1007/s10689-006-9112-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pharoah PD, Easton DF, Stockton DL, Gayther S, Ponder BA. Survival in familial, BRCA1-associated, and BRCA2-associated epithelial ovarian cancer. United Kingdom Coordinating Committee for Cancer Research (UKCCCR) Familial Ovarian Cancer Study Group. Cancer Res. 1999;59(4):868–871. [PubMed] [Google Scholar]
- 53.Ragupathy K, Ferguson M. Pattern and chemosensitivity of ovarian cancer in patients with BRCA1/2 mutations. J Obstet Gynaecol. 2011;31(2):178–179. doi: 10.3109/01443615.2010.539719. [DOI] [PubMed] [Google Scholar]
- 54.Ramus SJ, Fishman A, Pharoah PD, Yarkoni S, Altaras M, Ponder BA. Ovarian cancer survival in Ashkenazi Jewish patients with BRCA1 and BRCA2 mutations. Eur J Surg Oncol. 2001;27(3):278–281. doi: 10.1053/ejso.2000.1097. [DOI] [PubMed] [Google Scholar]
- 55.Rubin SC, Benjamin I, Behbakht K, et al. Clinical and pathological features of ovarian cancer in women with germ-line mutations of BRCA1. N Engl J Med. 1996;335(19):1413–1416. doi: 10.1056/NEJM199611073351901. [DOI] [PubMed] [Google Scholar]
- 56.Zweemer RP, Verheijen RH, Coebergh JW, et al. Survival analysis in familial ovarian cancer, a case control study. Eur J Obstet Gynecol Reprod Biol. 2001;98(2):219–223. doi: 10.1016/s0301-2115(01)00318-9. [DOI] [PubMed] [Google Scholar]
- 57.Dann RB, Deloia JA, Timms KM, et al. BRCA1/2 mutations and expression: response to platinum chemotherapy in patients with advanced stage epithelial ovarian cancer. Gynecol Oncol. 2012;125(3):677–682. doi: 10.1016/j.ygyno.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 58.Mankoo PK, Shen R, Schultz N, Levine DA, Sander C. Time to recurrence and survival in serous ovarian tumors predicted from integrated genomic profiles. PLoS ONE. 2011;6(11):e24709. doi: 10.1371/journal.pone.0024709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yarden RI, Pardo-Reoyo S, Sgagias M, Cowan KH, Brody LC. BRCA1 regulates the G2/M checkpoint by activating Chk1 kinase upon DNA damage. Nat Genet. 2002;30(3):285–289. doi: 10.1038/ng837. [DOI] [PubMed] [Google Scholar]
- 60.Joukov V, Groen AC, Prokhorova T, et al. The BRCA1/BARD1 heterodimer modulates ran-dependent mitotic spindle assembly. Cell. 2006;127(3):539–552. doi: 10.1016/j.cell.2006.08.053. [DOI] [PubMed] [Google Scholar]
- 61.Lou Z, Minter-Dykhouse K, Chen J. BRCA1 participates in DNA decatenation. Nat Struct Mol Biol. 2005;12(7):589–593. doi: 10.1038/nsmb953. [DOI] [PubMed] [Google Scholar]
- 62.Sankaran S, Crone DE, Palazzo RE, Parvin JD. BRCA1 regulates gamma-tubulin binding to centrosomes. Cancer Biol Ther. 2007;6(12):1853–1857. doi: 10.4161/cbt.6.12.5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Starita LM, Machida Y, Sankaran S, et al. BRCA1-dependent ubiquitination of gamma-tubulin regulates centrosome number. Mol Cell Biol. 2004;24(19):8457–8466. doi: 10.1128/MCB.24.19.8457-8466.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64▪.Drost R, Bouwman P, Rottenberg S, et al. BRCA1 RING function is essential for tumor suppression but dispensable for therapy resistance. Cancer Cell. 2011;20(6):797–809. doi: 10.1016/j.ccr.2011.11.014. Important study on different domain mutations of BRCA1 inducing different effects on tumor suppression and therapy resistance. [DOI] [PubMed] [Google Scholar]
- 65.Narod SA, Neuhausen S, Vichodez G, et al. Rapid progression of prostate cancer in men with a BRCA2 mutation. Br J Cancer. 2008;99(2):371–374. doi: 10.1038/sj.bjc.6604453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ferrone CR, Levine DA, Tang LH, et al. BRCA germline mutations in Jewish patients with pancreatic adenocarcinoma. J Clin Oncol. 2009;27(3):433–438. doi: 10.1200/JCO.2008.18.5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liede A, Karlan BY, Narod SA. Cancer risks for male carriers of germline mutations in BRCA1 or BRCA2: a review of the literature. J Clin Oncol. 2004;22(4):735–742. doi: 10.1200/JCO.2004.05.055. [DOI] [PubMed] [Google Scholar]
- 68.Hahn SA, Greenhalf B, Ellis I, et al. BRCA2 germline mutations in familial pancreatic carcinoma. J Natl Cancer Inst. 2003;95(3):214–221. doi: 10.1093/jnci/95.3.214. [DOI] [PubMed] [Google Scholar]
- 69.Moller P, Evans DG, Reis MM, et al. Surveillance for familial breast cancer: Differences in outcome according to BRCA mutation status. Int J Cancer. 2007;121(5):1017–1020. doi: 10.1002/ijc.22789. [DOI] [PubMed] [Google Scholar]
- 70.Lee EH, Park SK, Park B, et al. Effect of BRCA1/2 mutation on short-term and long-term breast cancer survival: a systematic review and meta-analysis. Breast Cancer Res Treat. 2010;122(1):11–25. doi: 10.1007/s10549-010-0859-2. [DOI] [PubMed] [Google Scholar]
- 71.Foulkes WD, Metcalfe K, Sun P, et al. Estrogen receptor status in BRCA1- and BRCA2-related breast cancer: the influence of age, grade, and histological type. Clin Cancer Res. 2004;10(6):2029–2034. doi: 10.1158/1078-0432.ccr-03-1061. [DOI] [PubMed] [Google Scholar]
- 72.Satagopan JM, Boyd J, Kauff ND, et al. Ovarian cancer risk in Ashkenazi Jewish carriers of BRCA1 and BRCA2 mutations. Clin Cancer Res. 2002;8(12):3776–3781. [PubMed] [Google Scholar]
- 73.Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72(5):1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Prevalence and penetrance of BRCA1 and BRCA2 mutations in a population-based series of breast cancer cases. Anglian Breast Cancer Study Group. Br J Cancer. 2000;83(10):1301–1308. doi: 10.1054/bjoc.2000.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hall JM, Lee Mk, Newman B, et al. Linkage of early-onset familial breast cancer to chromosome 17q21. Science. 1990;250(4988):1684–1689. doi: 10.1126/science.2270482. [DOI] [PubMed] [Google Scholar]
- 76.Miki Y, Swensen J, Shattuck-Eidens D, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266(5182):66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 77.Sy SM, Huen MS, Chen J. PALB2 is an integral component of the BRCA complex required for homologous recombination repair. Proc Natl Acad Sci USA. 2009;106(17):7155–7160. doi: 10.1073/pnas.0811159106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wooster R, Bignell G, Lancaster J, et al. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995;378(6559):789–792. doi: 10.1038/378789a0. [DOI] [PubMed] [Google Scholar]
- 79.Esashi F, Christ N, Gannon J, et al. CDK-dependent phosphorylation of BRCA2 as a regulatory mechanism for recombinational repair. Nature. 2005;434(7033):598–604. doi: 10.1038/nature03404. [DOI] [PubMed] [Google Scholar]
- 80.Ayoub N, Rajendra E, Su X, Jeyasekharan AD, Mahen R, Venkitaraman AR. The carboxyl terminus of BRCA2 links the disassembly of Rad51 complexes to mitotic entry. Curr Biol. 2009;19(13):1075–1085. doi: 10.1016/j.cub.2009.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Davies OR, Pellegrini L. Interaction with the BRCA2 C terminus protects RAD51-DNA filaments from disassembly by BRC repeats. Nat Struct Mol Biol. 2007;14(6):475–483. doi: 10.1038/nsmb1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82▪▪.Roy R, Chun J, Powell SN. BRCA1 and BRCA2: different roles in a common pathway of genome protection. Nat Rev Cancer. 2011;12(1):68–78. doi: 10.1038/nrc3181. Comprehensive and well-written review highlighting the different roles of BRCA1 and BRCA2 in homologous recombination. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lou Z, Chini CC, Minter-Dykhouse K, Chen J. Mediator of DNA damage checkpoint protein 1 regulates BRCA1 localization and phosphorylation in DNA damage checkpoint control. J Biol Chem. 2003;278(16):13599–13602. doi: 10.1074/jbc.C300060200. [DOI] [PubMed] [Google Scholar]
- 84.Hashizume R, Fukuda M, Maeda I, et al. The RING heterodimer BRCA1–BARD1 is a ubiquitin ligase inactivated by a breast cancer-derived mutation. J Biol Chem. 2001;276(18):14537–14540. doi: 10.1074/jbc.C000881200. [DOI] [PubMed] [Google Scholar]
- 85.Jensen RB, Carreira A, Kowalczykowski SC. Purified human BRCA2 stimulates RAD51-mediated recombination. Nature. 2010;467(7316):678–683. doi: 10.1038/nature09399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Holloman WK. Unraveling the mechanism of BRCA2 in homologous recombination. Nat Struct Mol Biol. 2011;18(7):748–754. doi: 10.1038/nsmb.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baumann P, Benson FE, West SC. Human Rad51 protein promotes ATP-dependent homologous pairing and strand transfer reactions in vitro. Cell. 1996;87(4):757–766. doi: 10.1016/s0092-8674(00)81394-x. [DOI] [PubMed] [Google Scholar]
- 88.Osher DJ, Kushner YB, Arseneau J, Foulkes WD. Melphalan as a treatment for BRCA-related ovarian carcinoma: can you teach an old drug new tricks? J Clin Pathol. 2011;64(10):924–926. doi: 10.1136/jcp.2010.086405. [DOI] [PubMed] [Google Scholar]
- 89.Evers B, Schut E, van der Burg E, et al. A high-throughput pharmaceutical screen identifies compounds with specific toxicity against BRCA2-deficient tumors. Clin Cancer Res. 2009;16(1):99–108. doi: 10.1158/1078-0432.CCR-09-2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 91.Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434(7035):913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 92.Chen A. PARP inhibitors: its role in treatment of cancer. Chin J Cancer. 2011;30(7):463–471. doi: 10.5732/cjc.011.10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361(2):123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 94.Audeh MW, Carmichael J, Penson RT, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010;376(9737):245–251. doi: 10.1016/S0140-6736(10)60893-8. [DOI] [PubMed] [Google Scholar]
- 95.Fong PC, Yap TA, Boss DS, et al. Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J Clin Oncol. 2010;28(15):2512–2519. doi: 10.1200/JCO.2009.26.9589. [DOI] [PubMed] [Google Scholar]
- 96.Gelmon KA, Tischkowitz M, Mackay H, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a Phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 2011;12(9):852–861. doi: 10.1016/S1470-2045(11)70214-5. [DOI] [PubMed] [Google Scholar]
- 97.Kaye SB, Lubinski J, Matulonis U, et al. Phase II, open-label, randomized, multicenter study comparing the efficacy and safety of olaparib, a poly (ADP-ribose) polymerase inhibitor, and pegylated liposomal doxorubicin in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer. J Clin Oncol. 2012;30(4):372–379. doi: 10.1200/JCO.2011.36.9215. [DOI] [PubMed] [Google Scholar]
- 98.Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366(15):1382–1392. doi: 10.1056/NEJMoa1105535. [DOI] [PubMed] [Google Scholar]
- 99.Turner NC, Lord CJ, Iorns E, et al. A synthetic lethal siRNA screen identifying genes mediating sensitivity to a PARP inhibitor. EMBO J. 2008;27(9):1368–1377. doi: 10.1038/emboj.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Issaeva N, Thomas HD, Djureinovic T, et al. 6-thioguanine selectively kills BRCA2-defective tumors and overcomes PARP inhibitor resistance. Cancer Res. 2010;70(15):6268–6276. doi: 10.1158/0008-5472.CAN-09-3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jacquemont C, Taniguchi T. Proteasome function is required for DNA damage response and fanconi anemia pathway activation. Cancer Res. 2007;67(15):7395–7405. doi: 10.1158/0008-5472.CAN-07-1015. [DOI] [PubMed] [Google Scholar]
- 102.Deans AJ, Khanna KK, Mcnees CJ, Mercurio C, Heierhorst J, Mcarthur GA. Cyclin-dependent kinase 2 functions in normal DNA repair and is a therapeutic target in BRCA1-deficient cancers. Cancer Res. 2006;66(16):8219–8226. doi: 10.1158/0008-5472.CAN-05-3945. [DOI] [PubMed] [Google Scholar]
- 103.Dungey FA, Caldecott KW, Chalmers AJ. Enhanced radiosensitization of human glioma cells by combining inhibition of poly(ADP-ribose) polymerase with inhibition of heat shock protein 90. Mol Cancer Ther. 2009;8(8):2243–2254. doi: 10.1158/1535-7163.MCT-09-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chiang JW, Karlan BY, Cass L, Baldwin RL. BRCA1 promoter methylation predicts adverse ovarian cancer prognosis. Gynecol Oncol. 2006;101(3):403–410. doi: 10.1016/j.ygyno.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 105.Liu GY, Zhang W. Will Chinese ovarian cancer patients benefit from knowing the BRCA2 mutation status? Chin J Cancer. 2012;31(1):1–4. doi: 10.5732/cjc.011.10432. [DOI] [PMC free article] [PubMed] [Google Scholar]