Abstract
Identifying biomarkers that distinguish Parkinson’s disease (PD) from normal control (NC) individuals has the potential to increase diagnostic sensitivity for the detection of early-stage PD. A previous proteomic study identified potential biomarkers in postmortem ventricular cerebrospinal fluid (V-CSF) from neuropathologically diagnosed PD subjects lacking Alzheimer’s disease (AD) neuropathology. In the present study, we assessed these biomarkers as well as p-tau181, Aβ42, and S100B by ELISA in PD (n = 43) and NC (n = 49) cases. The p-tau181/Aβ42 ratio and ApoA-1 showed statistically significant differences between groups. Multiple regression analysis demonstrated that p-tau181/Aβ42 had a significant odds ratio: OR = 1.42 (95% confidence interval [CI], 1.12–1.84), P = 0.006. Among the molecules investigated, intriguing correlations were observed that require further investigation. Our results suggest coexistent AD CSF biomarkers within the PD group notwithstanding that it was selected to minimize AD neuropathological lesions.
Keywords: Parkinson’s disease, biomarkers, ventricular cerebrospinal fluid, apolipoprotein A-1, p-tau181/Aβ42 ratio
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder clinically characterized by motor and nonmotor abnormalities. Neuropathological analyses reveal loss of dopaminergic neurons and the presence of Lewy bodies, composed of aggregated α-synuclein and other proteins. Although Lewy bodies are a prominent feature of PD, there is debate about whether they play a primary role in the pathogenesis of the disease or are a secondary epiphenomenon.
Discovering biomarkers that distinguish PD from normal control (NC) individuals would greatly enhance the understanding of the etiology of the disease and, more importantly, lead to earlier, more effective treatment strategies. Cerebrospinal fluid (CSF) has been extensively studied for potential biomarkers due to its close proximity to and intercommunication with the brain. Although alterations in total tau, phosphorylated-tau (p-tau) and amyloid-β (Aβ) peptides have become accepted biomarkers for Alzheimer’s disease (AD), there has been a large effort to investigate these molecules in the CSF of PD subjects, as recently reviewed.1 While there is some interlaboratory variability in results, the consensus is total tau or p-tau levels are increased and Aβ42 is decreased in PD patients when compared with normal control (NC) subjects.1 Interestingly, these findings are similar to those observed in Alzheimer’s disease (AD) CSF biomarker studies.2,3
The application of proteomic techniques such as two-dimensional difference gel electrophoresis (2D-DIGE) to neurodegenerative disorders has greatly enhanced the discovery of specific disease biomarkers. In the present study, we attempted to individually validate proteins that were differentially altered in pools of freshly obtained postmortem ventricular CSF (V-CSF) from neuropathologically diagnosed PD and NC individuals.4 Combining powerful, proteomic separations4 with sensitive enzyme-linked immunosorbent assay (ELISA) methods, we compared V-CSF samples from 43 PD cases and 49 NC subjects. All candidate biomarker molecules, fibrinogen (FIB), transthyretin (TTR), clusterin (CLU), apolipoprotein E (ApoE), apolipoprotein A-1 (ApoA-1), and glutathione S-transferase Pi (GST-Pi) have been previously linked to PD.4 In addition, due to the recently reported findings mentioned above, Aβ42 and tau phosphorylated at amino acid 181 (p-tau181) were also quantified. An increased presence of S100B in the CSF is suggestive of astrocytic damage;5–7 therefore, the levels of S100B were also investigated.
Materials and Methods
Patient selection and study cases
Cases
All individuals in this study were enrolled in the Banner Sun Health Research Institute Brain and Body Donation Program, Sun City, Arizona. All individuals or their legal representatives signed the written informed consent approved by the Banner Health Institutional Review Board. Subjects are community volunteers from the metropolitan Phoenix, Arizona, communities as well as patients from clinical private practices (CHA, HS, MNS) and were clinically examined longitudinally8–10 using standardized assessments that included movement11 and cognitive batteries.8 An antemortem PD diagnosis required 2 of 3 cardinal signs (rest tremor, bradykinesia, cogwheel rigidity) and responsiveness to dopaminergic medication.12 Private medical records were also reviewed and abstracted for all subjects.
Neuropathological examination
The Brain and Body Donation Program databases were searched to find cases with an uncomplicated diagnosis of PD free from other concurrent neuropathological conditions, such as AD, dementia with Lewy bodies, frontotemporal lobar degeneration, progressive supranuclear palsy, vascular dementia, or hippocampal sclerosis. The NC group included subjects without evidence of dementia or parkinsonism. Standardized neuropathological examinations were performed on all cases.9 Neuropathological evaluations, at the gross and microscopic levels, were performed by a single observer (TGB) blinded to the clinical information. For the diagnosis of PD, specific clinicopathologic criteria were used13 that included the clinical diagnosis of PD and the presence of Lewy bodies and pigmented neuronal loss in the substantia nigra. At a later stage, the clinical history was appraised in order to adjudicate a definitive clinicopathologic diagnosis. Blocks of tissue were fixed in 4% neutral-buffered formaldehyde and then either dehydrated and embedded in paraffin or cryoprotected and sectioned on a freezing sliding microtome. Paraffin-embedded sections were stained immunohistochemically for phosphorylated α-synuclein. Individual cases were staged following the Unified Staging System for Lewy Body Disorders with a standard set of brain sections as previously published.14 Detailed descriptions of the neuropathological grading for plaque score (maximum 15), neurofibrillary tangle (NFT) score (maximum 15), cerebral amyloid angiopathy (CAA) score (maximum 12) and white matter rarefaction (WMR) score (maximum 12) are given elsewhere.9,15,16 A summary of demographic and relevant neuropathological data is provided in Table 1.
Table 1.
Group comparisons for gender distribution, age at death, PMI, brain weight and neuropathological assessments.
| NC | PD | P-value | |
|---|---|---|---|
| Gender (M/F) | 31/18 | 30/13 | 0.51* |
| Mean age at death (yrs) | 83.5 (7.5) | 78.7 (6.6) | 0.02† |
| Mean PMI (h) | 3.3 (3.2) | 3.3 (2.1) | 0.98† |
| Mean brain weight (g) | 1,214 (124) | 1,256 (84) | 0.06† |
| Mean total plaque score | 3.7 (4.12) | 3.1 (3.97) | 0.66†† |
| Mean NFT score | 3.7 (2.23) | 3.4 (1.67) | 0.68†† |
| Mean CAA score | 1.8 (2.90) | 1.0 (1.84) | 0.13†† |
| Mean WMR score | 1.0 (3.16) | 1.3 (2.02) | 0.13†† |
| Median Braak stage | IV | III | 0.72†† |
| Median Lewy body stage | 0 | III | <0.001†† |
Notes:
Chi-square;
two sample t-test;
Kruskall–Wallis test. For mean values, standard deviations are given in parentheses.
Abbreviations: NC, normal control; PD, Parkinson’s disease; M, male; F, female; yrs, years; PMI, postmortem interval; h, hours; NFT, neurofibrillary tangles; CAA, cerebral amyloid angiopathy; WMR, white matter rarefaction.
Ventricular cerebrospinal fluid
Ventricular-CSF was removed in the immediate postmortem from the lateral ventricles by syringe aspiration and centrifuged at 1600 × rpm for 10 minutes. The supernatants were divided into 1 mL samples and stored at −80 °C. The Micro BCA protein assay kit (Pierce, Rockford, IL) was used to quantify the total protein concentration. We initially selected 49 PD and 51 NC cases for study. In the NC group, 2 cases were eliminated from the study: 1 for high total protein levels (>1.5 mg/mL) and 1 for low total protein levels (<0.3 mg/mL). In the PD group, 5 cases were eliminated because of high total protein levels while one case was eliminated for low levels. The final numbers of analyzed specimens compared in this study were 43 PD cases and 49 NC cases.
ELISA analysis
Ten potential biomarkers were measured with the following ELISA kits: S100B (BioVendor R&D, Candler, NC, catalog # RD192090100R); Aβ42 (Innogenetics, Gent, Belgium, catalog # 80177), tau phosphorylated at amino acid 181 (p-tau181, Invitrogen, Carlsbad, CA, catalog # KHO0631), FIB (Immunology Consultants Laboratory, Inc., Newberg, OR, catalog # E-80FIB); TTR (or prealbumin, Immunology Consultants Laboratory, Inc., catalog # E-80PRE); ApoE (MBL Int., Woburn, MA, catalog # 7635); CLU (or apolipoprotein J; BioVendor R&D, Candler, NC, catalog # RD194034200R); ApoA-1 (GenWay, San Diego, CA, catalog # 40-288-20069), and GST-Pi (Alpco, Salem, NH, catalog # K7960). All assays were performed according to the manufacturers’ instructions. The technical parameters of each ELISA kit such as intraassay and interassay CV% and sensitivity are provided by each manufacturer in the data sheets. Samples were run in duplicate. Samples that did not fall within the range of the standard curve or showed greater than 20% variability between duplicates were reassayed.
Statistical analysis
A two-sample t-test was carried out to discern group differences (PD vs. NC) on age at death, postmortem interval (PMI) and brain weight. Chi-square analysis was employed to determine if the proportion of males and females between the groups was significantly different. The Kruskall–Wallis test was used to compare total plaque score, total NFT score, total CAA score, total WMR score, and the median Braak and Lewy body stages for each group. Analysis of covariance (ANCOVA) was performed with the biomarker variables set as dependent variables and group status (PD vs. NC) as the independent variable. Age at death and gender were entered as covariates in order to account for their effect. Similar ANCOVAs were also carried out for the PD and NC groups separately using ApoE ɛ4 carrier status as the independent variable while also adjusting for age at death and gender. Cohen’s d was used to assess the effect size of the group differences for each of the biomarker variables for all analyses.
To assess the predictive value of the biomarkers, a multiple logistic regression model was carried out with group status as the dependent variable and the individual biomarkers as independent variables while adjusting for age at death and gender.
Results
The mean age of death was 78.7 (standard deviation [SD] = 7.5) years for the PD group and 83.5 (SD = 6.6) years for the NC group (P = 0.02) (Table 1). There was no difference in gender distribution, PMI, or brain weight between the groups (Table 1). The ApoE allelic frequency did not differ between groups, NC being ɛ2 = 0.09, ɛ3 = 0.76, and ɛ4 = 0.15; and PD being ɛ2 = 0.07, ɛ3 = 0.83, and ɛ4 = 0.10. The PD and NC groups did not significantly differ in NFT or total plaque scores nor CERAD or Braak stage, the neuropathological hallmarks of AD (Table 1). In addition, there was no difference in WMR or CAA scores between the groups (Table 1). The NC group did not have any subjects with Lewy bodies while the median Unified Lewy body stage of the PD cohort was III (Table 1).
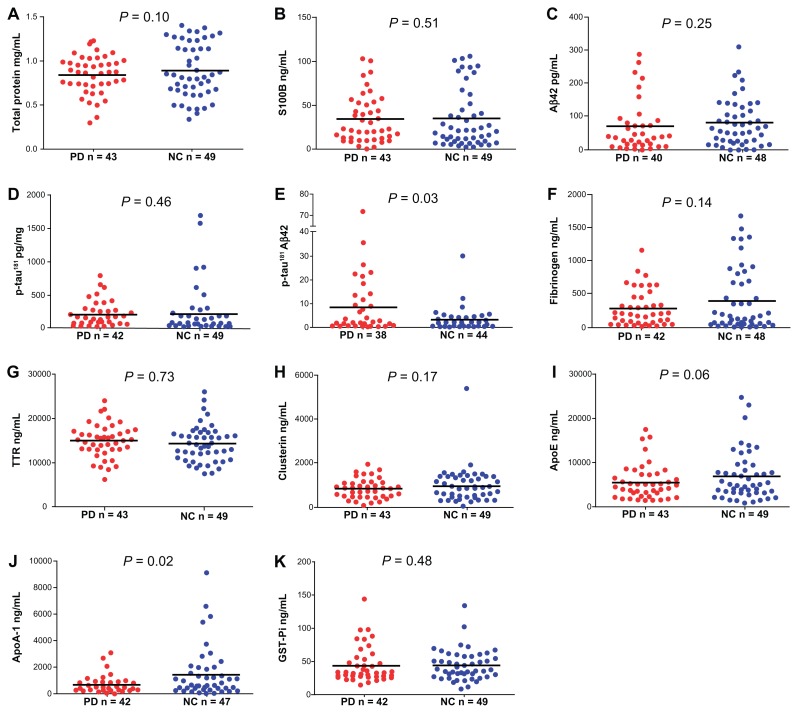
Figure 1 displays the group differences (PD vs. NC) for the 10 biomarker measures as well as total protein levels, and Table 2 shows the results of the statistical analyses. The p-tau181/Aβ42 ratio and ApoA-1 were the only biomarkers that differed statistically between the groups (Table 2). The PD group demonstrated higher p-tau181/Aβ42 ratios (Fig. 1E, P = 0.03) and lower ApoA-1 (Fig. 1J, P = 0.02) levels relative to the NC group. The effect sizes for p-tau181/Aβ42 ratio and ApoA-1 were medium, indicating these differences are of moderate importance (Table 2).
Figure 1.
Scatter plots of total protein concentration and ELISA measurements in CSF. (A) Total protein concentration in mg/mL. (B) S100B concentration in ng/mL. (C) Aβ42 concentration in pg/mL. (D) p-tau181 concentration in pg/mL. (E) p-tau181/Aβ42 ratio. (F) Fibrinogen concentration in ng/mL. (G) TTR concentration in ng/mL. (H) Clusterin concentration in ng/mL. (I) ApoE concentration in ng/mL. (J) ApoA-1 concentration in ng/mL. (K) GST-Pi concentration in ng/mL.
Notes: Mean values are indicated by the horizontal line. Results of the statistical analyses are given in Table 2.
Abbreviations: PD, Parkinson’s disease; NC, normal control; Aβ42, amyloid-beta 42; p-tau181, tau phosphorylated at amino acid 181; TTR, transthyretin; ApoE, apolipoprotein E; ApoA-1, apolipoprotein A-1; GST-Pi, glutathione S-transferase Pi.
Table 2.
Group differences for biomarker measures.
| NC | PD | F-value | P-value | Effect size | |
|---|---|---|---|---|---|
| Total protein (mg/mL) | 0.89 (0.31) | 0.84 (0.21) | 2.84 | 0.10 | 0.19 |
| S100B (ng/mL) | 35 (32) | 35 (27) | 0.43 | 0.51 | 0.00 |
| Aβ42 (pg/mL) | 81 (70) | 68 (72) | 1.35 | 0.25 | 0.19 |
| p-tau181 (pg/mL) | 205 (359) | 194 (190) | 0.55 | 0.46 | 0.04 |
| p-tau181/Aβ42 | 2.87 (4.55) | 8.15 (12.98) | 5.24 | 0.03 | 0.54 |
| FIB (ng/mL) | 417 (469) | 302 (267) | 2.24 | 0.14 | 0.30 |
| TTR (ng/mL) | 14,342 (4,099) | 14,981 (3,774) | 0.12 | 0.73 | 0.16 |
| CLU (ng/mL) | 986 (784) | 848 (434) | 1.88 | 0.17 | 0.22 |
| ApoE (ng/mL) | 6,920 (5,507) | 5,665 (3,946) | 3.51 | 0.06 | 0.26 |
| ApoA-1 (ng/mL) | 1,454 (1,845) | 718 (630) | 6.04 | 0.02 | 0.53 |
| GST-Pi (ng/mL) | 44 (23) | 44 (27) | 0.50 | 0.48 | 0.00 |
Notes: Mean (SD); df = (1, 88); effect size—Cohen’s d.
Abbreviations: NC, normal control; PD, Parkinson’s disease; Aβ42, amyloid-beta 42; p-tau181, tau phosphorylated at amino acid 181; FIB, fibrinogen; TTR, transthyretin; CLU, clusterin; ApoE, apolipoprotein E; ApoA-1, apolipoprotein A-1; GST-Pi, glutathione S-transferase Pi.
Multiple logistic regression analysis (Table 3) was used to assess the predictive value of the individual biomarkers, and only the p-tau181/Aβ42 ratio yielded a significant effect (odds ratio [OR] = 1.42 (95% confidence interval [CI], 1.12, 1.84), P = 0.006].
Table 3.
Multiple logistic regression analysis for biomarkers.
| Biomarker | Odds ratio | 95% CI | P-value |
|---|---|---|---|
| Total protein | 0.38 | 0.03, 5.12 | 0.47 |
| S100B | 0.99 | 0.96, 1.02 | 0.53 |
| Aβ42 | 1.01 | 1.00, 1.02 | 0.17 |
| p-tau181 | 1.00 | 0.99, 1.00 | 0.26 |
| p-tau181/Aβ42 | 1.42 | 1.12, 1.84 | 0.006 |
| FIB | 1.00 | 1.00, 1.00 | 0.56 |
| TTR | 1.00 | 1.00, 1.00 | 0.41 |
| CLU | 1.00 | 1.00, 1.00 | 0.10 |
| ApoE | 1.00 | 1.00, 1.00 | 0.27 |
| ApoA-1 | 1.00 | 1.00, 1.00 | 0.24 |
| GST-Pi | 1.00 | 0.98, 1.02 | 0.95 |
Abbreviations: Aβ42, amyloid-beta 42; p-tau181, tau phosphorylated at amino acid 181; FIB, fibrinogen; TTR, transthyretin; CLU, clusterin; ApoE, apolipoprotein E; ApoA-1, apolipoprotein A-1; GST-Pi, glutathione S-transferase Pi.
Within-group analyses of the NC and PD cohorts were conducted to determine the effect of ApoE ɛ4 carrier status on the individual biomarkers. For the NC subjects, ApoE ɛ4 carriers had significantly higher ApoE levels than ɛ4 noncarriers (P = 0.003). Within the NC group, ɛ4 carriers also had significantly higher TTR levels (P = 0.04). For the PD group, there were no significant differences between ɛ4 carriers and noncarriers on any of the biomarkers.
Correlations among the biomarkers are displayed in Table 4. The p-tau181/Aβ42 ratio correlated moderately with S100B (r = 0.42). However, this correlation is probably due to the effect of p-tau181 (r = 0.65) because the correlation of Aβ42 and S100B is −0.01. On the other hand, p-tau181 and Aβ42 did not show significant effects as individual variables. ApoA-1 demonstrated a strong correlation with FIB (r = 0.62) and moderate correlations with CLU (r = 0.57) and ApoE (r = 0.45). ApoE correlated strongly with CLU (r = 0.74) and moderately with Aβ42 (r = 0.45). In addition, FIB showed a moderate correlation with S100B (r = 0.59) (Table 4).
Table 4.
Correlation analysis of biomarkers.
| S100B | Aβ42 | p-tau181 | p-tau181/Aβ42 | FIB | TTR | CLU | ApoE | ApoA-1 | GST-Pi | |
|---|---|---|---|---|---|---|---|---|---|---|
| S100B | – | −0.01 | 0.65* | 0.42* | 0.59* | 0.38* | 0.36* | 0.19 | 0.38* | 0.34* |
| Aβ42 | −0.01 | – | 0.07 | −0.30 | 0.10 | 0.08 | 0.38* | 0.45* | 0.19 | 0.06 |
| p-tau181 | 0.65* | 0.07 | – | 0.51 | 0.54 | 0.19 | 0.31 | 0.25* | 0.38 | 0.27 |
| p-tau181/Aβ42 | 0.42* | −0.30 | 0.51 | – | 0.34* | 0.14 | 0.04 | −0.05 | 0.05 | 0.02 |
| FIB | 0.59* | 0.10 | 0.54 | 0.34* | – | 0.15 | 0.40* | 0.26 | 0.62* | 0.11 |
| TTR | 0.38* | 0.08 | 0.19 | 0.14 | 0.15 | – | 0.30 | 0.09 | 0.13 | 0.19 |
| CLU | 0.36* | 0.38* | 0.31 | 0.04 | 0.40* | 0.30 | – | 0.74* | 0.57* | 0.20 |
| ApoE | 0.19 | 0.45* | 0.25* | −0.05 | 0.26 | 0.09 | 0.74* | – | 0.45* | 0.14 |
| ApoA-1 | 0.38* | 0.19 | 0.38 | 0.05 | 0.62* | 0.13 | 0.57* | 0.45* | – | 0.14 |
| GST-Pi | 0.34* | 0.06 | 0.27 | 0.02 | 0.11 | 0.19 | 0.20 | 0.14 | 0.14 | – |
Note:
P < 0.05.
Abbreviations: Aβ42, amyloid-beta 42; p-tau181, tau phosphorylated at amino acid 181; FIB, fibrinogen; TTR, transthyretin; CLU, clusterin; ApoE, apolipoprotein E; ApoA-1, apolipoprotein A-1; GST-Pi, glutathione S-transferase Pi.
In our recent proteomic analysis of the same PD and NC subjects, FIB, ApoA-1, and GST-Pi had lower density values in the PD pool compared to the NC pool, while TTR, ApoE, and CLU values were higher in the PD group relative to the NC.4 In the current ELISA study, the average quantities of FIB, TTR, and ApoA-1 followed the same trends as in the proteomic study. Both ApoE and ApoA-1 had lower average ELISA values in the PD group, the opposite of the 2D-DIGE results. GST-pi had the same average quantities in the ELISA study. Due to the wide individual variability in the ELISA quantifications (Fig. 1), the average fold change in the proteins ranged from 1.04 to 1.4 with the exception of ApoA-1, which was 2X lower in the PD subjects (Table 2). The 2D-DIGE analysis revealed stronger changes between PD and ND pools (1.6–4.3X).4
Discussion
Proteomic analysis combining 2D separation of V-CSF pools of neuropathologically diagnosed PD and NC, revealed the presence of 6 biomarker molecules that differed between these groups.4 These 6 proteins were individually measured on the same group of PD and NC individuals by ELISA. Due to the large body of information regarding alterations in the levels of Aβ42, p-tau181 and, to a lesser degree, S100B in lumbar-derived CSF in PD, we also quantified these molecules in our study. The p-tau181/Aβ42 ratio was significantly higher in the PD group, while ApoA-1 level was significantly lower in the PD group relative to the NC group. The effect size for these differences was medium, indicating they are of moderate importance.
The p-tau181/Aβ42 ratio yielded a small, but statistically significant OR. This finding was seen despite specifically excluding pathologically confirmed PD subjects with concomitant AD. Other studies, using lumbar-CSF, have reported significantly higher p-tau levels in PD with dementia (PDD) than in PD and NC17 and a trend or significantly lower values for Aβ42 in PDD and/or PD compared with NC.17–21 However, a review of 7 independent studies in which Aβ42 levels were appraised in lumbar-CSF found no change in levels of this peptide between PD and NC in 6 studies, and only one group observed lower levels of Aβ42 in PD, but the results were not statistically significant.22 Additionally, total tau and p-tau have also been reported to have no significant changes between PD without dementia and/or PDD compared with NC cases.19,20 In a longitudinal study of CSF, lower baseline Aβ42 levels predicted cognitive decline in PD patients but without significant alterations in total tau and p-tau.23 It is known that α-synuclein interacts with tau, inducing the aggregation of both of these molecules,24 and that the binding of α-synuclein to tau also causes tau phosphorylation.25 Similarly, overexpression of APP/Aβ has been shown to induce the accumulation of neuronal α-synuclein.26 The diversity of observations reveals the challenge posed by attempting to compare results obtained with living patients with evolving and uncertain pathology and postmortem studies of end-stage disease. Postmortem studies have the advantage of clearly delineated pathology. Many subjects with PD also have a relatively high level of AD histopathology, and many, in fact, meet neuropathological criteria for AD.14 Although the PD subjects in this study were selected for relatively low levels of AD histopathology, it is possible that the biochemical changes of AD may still be more advanced in these PD subjects as compared with NC individuals. It is also possible that some proteins such as Aβ42 and p-tau may simply be generally associated with neurodegeneration and cellular failure, as they have been linked to other neurological disorders including AD,27,28 progressive supranuclear palsy and corticobasal degeneration,29 Creutzfeldt- Jakob disease,30–33 and cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL).34 Therefore, despite its statistical significance, the predictive value of the p-tau181/Aβ42 ratio in PD may be somewhat limited.
While some investigators have reported a decrease in Aβ42 levels in ApoE ɛ4 carriers compared with ApoE ɛ4 noncarriers with PD,23,35 we did not find any correlations of this type, nor did we observe correlations between ApoE ɛ4 carriers and the remainder of the CSF biomarkers. However, a moderately significant and positive correlation was apparent between Aβ42 and ApoE carrier status when the PD and NC cohorts were combined. Intriguingly, within the NC group only, ApoE ɛ4-positive subjects had significantly higher ApoE levels and TTR levels.
S100B is a multifunctional Ca2+ binding protein that can act as an intracellular regulator or extracellular signal molecule that interacts with a wide variety of proteins including the receptor for advanced glycation-end products (RAGE).36 S100B can be neuroprotective at low/physiological levels or toxic at higher concentrations.7,36,37 Although not connected to a PD diagnosis, increased levels of S100B in the serum of PD patients positively correlated with the progression of disease.38 Although controversial, this suggests S100B may also serve as a general marker of brain injury or neurodegeneration due to its abundant presence in oligodendrocytes and astrocytes.7,37 In this context, it has been reported that increased levels of S100B result in glycogen synthase kinase 3β (GSK3β) and hyperphosphorylation of tau.39 Our analyses revealed that S100B was significantly and positively correlated with p-tau181.
CLU and ApoE as well as FIB and ApoA-1 were strongly correlated. However, the significance of these statistical associations is not clear. It was recently observed that the CLU genetic locus is apparently associated with PD and that this association is independent of ApoE ɛ4 status.40 The amounts of CLU in the CSF in PD patients are variable according to the duration of the disease, being more elevated during the first 2 years.41 Increased levels of plasma FIB have been linked to an elevated risk for PD.42 There seems to be a positive association between elevated high density lipoprotein cholesterol and, hence, ApoA-1, with enhanced fibrin clot permeability and lysis43 that may have some protective effects. ApoA-1 has also been found to be decreased in individuals with other neurodegenerative diseases.44,45 Oxidation of this molecule can lead to elevations of tumor necrosis factor-α that can contribute to neuronal death.46 As decreased levels of blood lipoproteins, including ApoA-1, are known to occur during fasting, and as PD and other chronic diseases are associated with weight loss, it is possible that the decreased ApoA-1 concentrations in PD CSF are secondary to fasting, undernourishment, and weight loss. We had previously reported decreased ApoA-1 in AD CSF,44 another chronic disease that is also associated with weight loss.
Our initial strategy was to investigate, by 2D-DIGE/mass spectrometry, if there were specific molecular differences in pooled CSF specimens originating from neuropathologically diagnosed PD, without other neurodegenerative comorbidities, when compared with an age-matched pool of NC CSF. Those molecules that were substantially increased or decreased in the pooled pair-wise comparisons were subsequently investigated at the individual level by ELISA. However, it should be recognized that ELISA and 2D-DIGE technically differ. ELISAs identify the total level of a given protein based on specific antibody-antigen binding, while 2D-DIGE analyses detect all molecular forms of a given molecule including those that differ by size and charge. Therefore, initial screening by 2D-DIGE may identify a specific isoform of a protein as a potential biomarker that may not be detected by the more broad specificity antibody-antigen binding of ELISAs.
In summary, the search for adequate biomarkers for complex neurodegenerative disorders such as AD and PD has been fraught with frustrations. This reflects the tremendous amount of human variability as well as the heterogeneous presentations of disease illustrated by the different pathological expression and clinical course of a particular neurodegenerative disorder that, not infrequently, has a parallel course with other concurrent morbidities. In addition, there is a large amount of genetic diversity among humans, which is compounded by complicated genetic pleiotropic interactions, epigenetic alterations, and transcriptomic interactions as well as posttranslational modifications and specific protein/ligand events that, at the phenotypic level, dictate the final qualitative and quantitative molecular expression in a given subject. Moreover, the different disease stages at which the fluids are collected and the diverse methodologies utilized in their processing and storage further complicate comparison and validation of biomarker appraisals. The wide range of human variability is reflected in a proteomics evaluation of V-CSF that revealed that out of 249 proteins identified in 10 elderly subjects, 38% were unique to 1 case and not found in the other 9.47 Even more surprising was that only 6% of the identified proteins were present in all 10 subjects.47
The actual utility of biomarkers identified at the end of a long duration, chronic disease process to serve as an early PD indicator is uncertain and must be assessed in additional studies since postmortem studies in neuropathologically confirmed PD may vary substantially from values obtained in living subjects. Our decision to use postmortem CSF was due to fact that physiological specimens obtained from living patients are at diverse stages of disease while postmortem subjects are all at the end of the disease course. Furthermore, studies of living patients do not have the definite diagnosis of PD that comes with postmortem studies. The use of samples from living subjects is additionally complicated because some PD patients have other concurrent neurodegenerative conditions that will generate confounding factors that are difficult to interpret. However, setting these situations in the context of previous investigations does reveal the extraordinary complexity of PD and its evolution. Efforts to define PD biomarkers in both postmortem and living subjects have demonstrated an exceptional diversity of pathological sequences and reactions that unfold as the disease appears and progresses. These findings suggest that molecular diagnosis may require a constellation of markers and assessments.
Footnotes
Author Contributions
Conceived and designed the experiments: CLM, TGB, CHA, MM-A, TAK, AER. Analyzed the data: CLM, MM-A, BND, DGW, AER. Wrote the first draft of the manuscript: CLM, TAK, AER. Contributed to the writing of the manuscript: CHA, TGB, BND, HAS, SAJ, MNS. Agree with manuscript results and conclusions: CLM, TGB, CHA, MM-A, TAK, BND, DGW, HAS, SAJ, MNS, AER. Jointly developed the structure and arguments for the paper: CHA, TGB, MNS, AER. Made critical revisions and approved final version: CLM, TGB, CHA, MM-A, TAK, BND, DGW, HAS, SAJ, MNS, AER. All authors reviewed and approved of the final manuscript.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
Funding
This study is supported by the Arizona Parkinson’s Disease Consortium (contract number 1001). The National Institute on Aging grant R01 AG019795. The Brain and Body Donation Program at Banner Sun Health Research Institute is supported by the National Institute of Neurological Disorders and Stroke (U24 NS072026), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901 and 1001 to the Arizona Parkinson’s Disease Consortium) and the Michael J. Fox Foundation for Parkinson’s Research. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Competing Interests
CLM, MM-A, TAK, BND, DGW, SAJ and AER have no conflicts of interest to declare. TGB receives funding from AVID-Radiopharmaceuticals, Bayer Healthcare and GE Healthcare. CHA has received consulting payments from Eli Lilly, Merck Serono, Informa Healthcare, Impax, Ipsen, Merz, and Teva. CHA has grants/grants pending from the Michael J. Fox Foundation and has received royalties from Springer. HAS is a consultant for Ipsen and Merz pharmaceuticals, as well as Medtronic. HAS receives research support from Chelsea Therapeutics, Teva Neuroscience, Schering-Plough, Kalaco Scientific and Avid Radiopharmaceuticals. MNS receives grant/contract support from Avid, Bayer, Baxter, BMS, Lilly, GE, Janssen, Celgene, Ceregene and Pfizer. MNS is a consultant/advisor for EASAI, Bayer, Lilly, Avid, Takeda, Amerisciences and BMS. MNS receives royalties from Amerisciences and Wiley.
References
- 1.Buongiorno M, Compta Y, Marti MJ. Amyloid-beta and tau biomarkers in Parkinson’s disease-dementia. J Neurol Sci. 2011;310(1–2):25–30. doi: 10.1016/j.jns.2011.06.046. [DOI] [PubMed] [Google Scholar]
- 2.Fagan AM, Holtzman DM. Cerebrospinal fluid biomarkers of Alzheimer’s disease. Biomark Med. 2010;4(1):51–63. doi: 10.2217/BMM.09.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Humpel C. Identifying and validating biomarkers for Alzheimer’s disease. Trends Biotechnol. 2011;29(1):26–32. doi: 10.1016/j.tibtech.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maarouf CL, Beach TG, Adler CH, et al. Cerebrospinal fluid biomarkers of neuropathologically diagnosed Parkinson’s disease subjects. Neurol Res. 2012;34(7):669–76. doi: 10.1179/1743132812Y.0000000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayakata T, Shiozaki T, Tasaki O, et al. Changes in CSF S100B and cytokine concentrations in early-phase severe traumatic brain injury. Shock. 2004;22(2):102–7. doi: 10.1097/01.shk.0000131193.80038.f1. [DOI] [PubMed] [Google Scholar]
- 6.Petzold A, Keir G, Lim D, Smith M, Thompson EJ. Cerebrospinal fluid (CSF) and serum S100B: release and wash-out pattern. Brain Res Bull. 2003;61(3):281–5. doi: 10.1016/s0361-9230(03)00091-1. [DOI] [PubMed] [Google Scholar]
- 7.Rothermundt M, Peters M, Prehn JH, Arolt V. S100B in brain damage and neurodegeneration. Microsc Res Tech. 2003;60(6):614–32. doi: 10.1002/jemt.10303. [DOI] [PubMed] [Google Scholar]
- 8.Caviness JN, Driver-Dunckley E, Connor DJ, et al. Defining mild cognitive impairment in Parkinson’s disease. Mov Disord. 2007;22(9):1272–7. doi: 10.1002/mds.21453. [DOI] [PubMed] [Google Scholar]
- 9.Beach TG, Sue LI, Walker DG, et al. The Sun Health Research Institute Brain Donation Program: description and experience, 1987–2007. Cell Tissue Bank. 2008;9(3):229–45. doi: 10.1007/s10561-008-9067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adler CH, Caviness JN, Sabbagh MN, et al. Motor impairment in normal aging, possible Parkinson’s disease, and definite Parkinson’s disease: longitudinal evaluation of a cohort of prospective brain donors. Mov Disord. 2004;19(9):1118. doi: 10.1016/s1353-8020(02)00012-3. [DOI] [PubMed] [Google Scholar]
- 11.Adler CH, Caviness JN, Sabbagh MN, et al. Heterogeneous neuropathological findings in Parkinson’s disease with mild cognitive impairment. Acta Neuropathol. 2010;120(6):827–8. doi: 10.1007/s00401-010-0744-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–4. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56(1):33–9. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- 14.Beach TG, Adler CH, Lue L, et al. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol. 2009;117(6):613–34. doi: 10.1007/s00401-009-0538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sabbagh MN, Cooper K, DeLange J, et al. Functional, global and cognitive decline correlates to accumulation of Alzheimer’s pathology in MCI and AD. Curr Alzheimer Res. 2010;7(4):280–6. doi: 10.2174/156720510791162340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roher AE, Esh C, Kokjohn TA, et al. Circle of Willis atherosclerosis is a risk factor for sporadic Alzheimer’s disease. Arterioscler Thromb Vasc Biol. 2003;23(11):2055–62. doi: 10.1161/01.ATV.0000095973.42032.44. [DOI] [PubMed] [Google Scholar]
- 17.Compta Y, Marti MJ, Ibarretxe-Bilbao N, et al. Cerebrospinal tau, phosphotau, and beta-amyloid and neuropsychological functions in Parkinson’s disease. Mov Disord. 2009;24(15):2203–10. doi: 10.1002/mds.22594. [DOI] [PubMed] [Google Scholar]
- 18.Montine TJ, Shi M, Quinn JF, et al. CSF Abeta(42) and tau in Parkinson’s disease with cognitive impairment. Mov Disord. 2010;25(15):2682–5. doi: 10.1002/mds.23287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parnetti L, Tiraboschi P, Lanari A, et al. Cerebrospinal fluid biomarkers in Parkinson’s disease with dementia and dementia with Lewy bodies. Biol Psychiatry. 2008;64(10):850–5. doi: 10.1016/j.biopsych.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 20.Alves G, Bronnick K, Aarsland D, et al. CSF amyloid-beta and tau proteins, and cognitive performance, in early and untreated Parkinson’s disease: the Norwegian ParkWest study. J Neurol Neurosurg Psychiatry. 2010;81(10):1080–6. doi: 10.1136/jnnp.2009.199950. [DOI] [PubMed] [Google Scholar]
- 21.Mollenhauer B, Trenkwalder C, von Ahsen N, et al. Beta-amlyoid 1–42 and tau-protein in cerebrospinal fluid of patients with Parkinson’s disease dementia. Dement Geriatr Cogn Disord. 2006;22(3):200–8. doi: 10.1159/000094871. [DOI] [PubMed] [Google Scholar]
- 22.Constantinescu R, Zetterberg H, Holmberg B, Rosengren L. Levels of brain related proteins in cerebrospinal fluid: an aid in the differential diagnosis of parkinsonian disorders. Parkinsonism Relat Disord. 2009;15(3):205–12. doi: 10.1016/j.parkreldis.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Siderowf A, Xie SX, Hurtig H, et al. CSF amyloid {beta} 1–42 predicts cognitive decline in Parkinson disease. Neurology. 2010;75(12):1055–61. doi: 10.1212/WNL.0b013e3181f39a78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giasson BI, Forman MS, Higuchi M, et al. Initiation and synergistic fibrillization of tau and alpha-synuclein. Science. 2003;300(5619):636–40. doi: 10.1126/science.1082324. [DOI] [PubMed] [Google Scholar]
- 25.Jensen PH, Hager H, Nielsen MS, Hojrup P, Gliemann J, Jakes R. Alpha-synuclein binds to Tau and stimulates the protein kinase A- catalyzed tau phosphorylation of serine residues 262 and 356. J Biol Chem. 1999;274(36):25481–9. doi: 10.1074/jbc.274.36.25481. [DOI] [PubMed] [Google Scholar]
- 26.Masliah E, Rockenstein E, Veinbergs I, et al. Beta-amyloid peptides enhance alpha-synuclein accumulation and neuronal deficits in a transgenic mouse model linking Alzheimer’s disease and Parkinson’s disease. Proc Natl Acad Sci U S A. 2001;98(21):12245–50. doi: 10.1073/pnas.211412398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prvulovic D, Hampel H. Amyloid beta (Abeta) and phospho-tau (p-tau) as diagnostic biomarkers in Alzheimer’s disease. Clin Chem Lab Med. 2011;49(3):367–74. doi: 10.1515/CCLM.2011.087. [DOI] [PubMed] [Google Scholar]
- 28.Trojanowski JQ, Vandeerstichele H, Korecka M, et al. Update on the biomarker core of the Alzheimer’s Disease Neuroimaging Initiative subjects. Alzheimers Dement. 2010;6(3):230–8. doi: 10.1016/j.jalz.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noguchi M, Yoshita M, Matsumoto Y, Ono K, Iwasa K, Yamada M. Decreased beta-amyloid peptide42 in cerebrospinal fluid of patients with progressive supranuclear palsy and corticobasal degeneration. J Neurol Sci. 2005;237(1–2):61–5. doi: 10.1016/j.jns.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 30.Skinningsrud A, Stenset V, Gundersen AS, Fladby T. Cerebrospinal fluid markers in Creutzfeldt-Jakob disease. Cerebrospinal Fluid Res. 2008;5:14. doi: 10.1186/1743-8454-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang GR, Gao C, Shi Q, et al. Elevated levels of tau protein in cerebrospinal fluid of patients with probable Creutzfeldt-Jakob disease. Am J Med Sci. 2010;340(4):291–5. doi: 10.1097/MAJ.0b013e3181e92a1f. [DOI] [PubMed] [Google Scholar]
- 32.Otto M, Wiltfang J, Cepek L, et al. Tau protein and 14-3-3 protein in the differential diagnosis of Creutzfeldt-Jakob disease. Neurology. 2002;58(2):192–7. doi: 10.1212/wnl.58.2.192. [DOI] [PubMed] [Google Scholar]
- 33.Otto M, Esselmann H, Schulz-Shaeffer W, et al. Decreased beta- amyloid 1–42 in cerebrospinal fluid of patients with Creutzfeldt-Jakob disease. Neurology. 2000;54(5):1099–102. doi: 10.1212/wnl.54.5.1099. [DOI] [PubMed] [Google Scholar]
- 34.Formichi P, Parnetti L, Radi E, Cevenini G, Dotti MT, Federico A. CSF biomarkers profile in CADASIL-A model of pure vascular dementia: usefulness in differential diagnosis in the dementia disorder. Int J Alzheimers Dis. 2010;2010 doi: 10.4061/2010/959257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prince JA, Zetterberg H, Andreasen N, Marcusson J, Blennow K. APOE epsilon4 allele is associated with reduced cerebrospinal fluid levels of Abeta42. Neurology. 2004;62(11):2116–8. doi: 10.1212/01.wnl.0000128088.08695.05. [DOI] [PubMed] [Google Scholar]
- 36.Donato R, Sorci G, Riuzzi F, et al. S100B’s double life: intracellular regulator and extracellular signal. Biochim Biophys Acta. 2009;1793(6):1008–22. doi: 10.1016/j.bbamcr.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 37.Steiner J, Bogerts B, Schroeter ML, Bernstein HG. S100B protein in neurodegenerative disorders. Clin Chem Lab Med. 2011;49(3):409–24. doi: 10.1515/CCLM.2011.083. [DOI] [PubMed] [Google Scholar]
- 38.Schaf DV, Tort AB, Fricke D, et al. S100B and NSE serum levels in patients with Parkinson’s disease. Parkinsonism Relat Disord. 2005;11(1):39–43. doi: 10.1016/j.parkreldis.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Esposito G, Scuderi C, Lu J, et al. S100B induces tau protein hyperphosphorylation via Dickopff-1 up-regulation and disrupts the Wnt pathway in human neural stem cells. J Cell Mol Med. 2008;12(3):914–27. doi: 10.1111/j.1582-4934.2008.00159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao J, Huang X, Park Y, Hollenbeck A, Chen H. An exploratory study on CLU, CR1 and PICALM and Parkinson disease. PLoS One. 2011;6(8):e24211. doi: 10.1371/journal.pone.0024211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prikrylova Vranova H, Mares J, Nevrly M, et al. CSF markers of neurodegeneration in Parkinson’s disease. J Neural Transm. 2010;117(10):1177–81. doi: 10.1007/s00702-010-0462-z. [DOI] [PubMed] [Google Scholar]
- 42.Wong KT, Grove JS, Grandinetti A, et al. Association of fibrinogen with Parkinson disease in elderly Japanese-American men: a prospective study. Neuroepidemiology. 2010;34(1):50–4. doi: 10.1159/000260070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zabczyk M, Hondo L, Krzek M, Undas A. High-density cholesterol and apolipoprotein AI as modifiers of plasma fibrin clot properties in apparently healthy individuals. Blood Coagul Fibrinolysis. 2013;24(1):50–4. doi: 10.1097/MBC.0b013e32835a083c. [DOI] [PubMed] [Google Scholar]
- 44.Roher AE, Maarouf CL, Sue LI, Hu Y, Wilson J, Beach TG. Proteomics-derived cerebrospinal fluid markers of autopsy-confirmed Alzheimer’s disease. Biomarkers. 2009;14(7):493–501. doi: 10.3109/13547500903108423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Puchades M, Hansson SF, Nilsson CL, Andreasen N, Blennow K, Davidsson P. Proteomic studies of potential cerebrospinal fluid protein markers for Alzheimer’s disease. Brain Res Mol Brain Res. 2003;118(1–2):140–6. doi: 10.1016/j.molbrainres.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 46.Keeney JT, Swomley AM, Forster S, Harris JL, Sultana R, Butterfield DA, Apolipoprotein A-I. Insights from redox proteomics for its role in neurodegeneration. Proteomics Clin Appl. 2012;7(1-2):109–22. doi: 10.1002/prca.201200087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wenner BR, Lovell MA, Lynn BC. Proteomic analysis of human ventricular cerebrospinal fluid from neurologically normal, elderly subjects using two-dimensional LC-MS/MS. J Proteome Res. 2004;3(1):97–103. doi: 10.1021/pr034070r. [DOI] [PubMed] [Google Scholar]