Abstract
Lipopolysaccharide endotoxin is the only known bacterial product which, when subcutaneously infused into mice in its purified form, can induce obesity and insulin resistance via an inflammation-mediated pathway. Here we show that one endotoxin-producing bacterium isolated from a morbidly obese human's gut induced obesity and insulin resistance in germfree mice. The endotoxin-producing Enterobacter decreased in relative abundance from 35% of the volunteer's gut bacteria to non-detectable, during which time the volunteer lost 51.4 kg of 174.8 kg initial weight and recovered from hyperglycemia and hypertension after 23 weeks on a diet of whole grains, traditional Chinese medicinal foods and prebiotics. A decreased abundance of endotoxin biosynthetic genes in the gut of the volunteer was correlated with a decreased circulating endotoxin load and alleviated inflammation. Mono-association of germfree C57BL/6J mice with strain Enterobacter cloacae B29 isolated from the volunteer's gut induced fully developed obesity and insulin resistance on a high-fat diet but not on normal chow diet, whereas the germfree control mice on a high-fat diet did not exhibit the same disease phenotypes. The Enterobacter-induced obese mice showed increased serum endotoxin load and aggravated inflammatory conditions. The obesity-inducing capacity of this human-derived endotoxin producer in gnotobiotic mice suggests that it may causatively contribute to the development of obesity in its human host.
Keywords: gut microbiota, germfree mice, endotoxin-producing bacterium, obesity, insulin resistance, high-fat diet
The role of the gut microbiota in the pathogenesis of obesity has emerged into an important research area (Backhed et al., 2004). Gram-negative opportunistic pathogens in the gut may be pivotal in obesity (Schumann et al., 1990; Zhang et al., 2010, 2012). Lipopolysaccharide (LPS) endotoxin purified from Escherichia coli induced obese and insulin-resistant phenotypes when subcutaneously infused into mice at a concentration comparable to what can be found in a mouse model of high-fat diet (HFD)-induced obesity (Cani et al., 2007). Endotoxin-induced inflammation seems to be essential for the development of obese and insulin-resistant phenotypes in the mouse model involving LPS infusion, as CD14-knockout mice did not develop these phenotypes after endotoxin infusion (Cani et al., 2007). Epidemiological studies show increased population of endotoxin producers and elevated endotoxin load in various obese cohorts (Lepper et al., 2007; Ruiz et al., 2007; Moreno-Navarrete et al., 2011), but experimental evidence of endotoxin producers having a causative role in human obesity is lacking.
During our clinical studies, we found that Enterobacter, a genus of opportunistic, endotoxin-producing pathogens (Sanders and Sanders, 1997), made up 35% of the gut bacteria in a morbidly obese volunteer (weight 174.8 kg, body mass index 58.8 kg m−2) suffering from diabetes, hypertension and other serious metabolic deteriorations (Table 1). The volunteer lost 30.1 kg after 9 weeks, and 51.4 kg after 23 weeks, on a diet composed of whole grains, traditional Chinese medicinal foods and prebiotics (WTP diet, Supplementary Information; Supplementary Figure 1), with continued amelioration of hyperinsulinemia, hyperglycemia and hypertension until most metabolic parameters improved to normal ranges (Table 1). After 9 weeks on the WTP diet, this Enterobacter population in the volunteer's gut reduced to 1.8%, and became undetectable by the end of the 23-week trial, as shown in the clone library analysis (Table 1; Supplementary Figures 2 and 3). The serum–endotoxin load, measured as LPS-binding protein (Schumann et al., 1990), dropped markedly during weight loss, along with substantial improvement of inflammation, decreased level of interleukin-6 and increased adiponectin (Table 1). Metagenomic sequencing of the volunteer's fecal samples at 0, 9 and 23 weeks on the WTP diet confirmed that during weight loss, the Enterobacteriaceae family was the most significantly reduced population (Supplementary Figure 4). The abundance of 25 KEGG Orthologies involved in the LPS biosynthetic pathway diminished considerably, together indicating a significant reduction of the endotoxin-producing capacity of the volunteer's gut microbiota after the intervention (Supplementary Figures 5–7). In light of previous reports of the pivotal role that endotoxins have in metabolic diseases in mice (Cani et al., 2007), we hypothesized that this endotoxin-producing Enterobacter population may have a causative role in the metabolic deteriorations of its human host. To confirm the causative role it may have in obesity development, we confirm Koch's postulate in an experimental host with an isolated strain of this Enterobacter population (Evans, 1976). We then obtained one clinical isolate (B29) from the volunteer's fecal sample via a ‘sequence-guided isolation' scheme (Rappé et al., 2002; Supplementary Figure 8), and identified it as Enterobacter cloacae through biochemical tests and 16S ribosomal RNA gene sequencing (Supplementary Table 1). We performed whole-genome sequencing on B29, and phylogenetic analysis using CVTree (Qi et al., 2004) and identified its nearest neighbor as E. cloacae subsp. cloacae ATCC 13047 (Supplementary Information). A limulus amebocyte lysate test showed that B29 LPS has strong endotoxin activity (Supplementary Figure 9), and the draft genome sequence revealed LPS biosynthesis genes similar to those in the metagenome from the day 0 fecal sample (Supplementary Figure 10).
Table 1. Changes of endotoxin load, inflammation indicators, metabolic phenotypes and the gut microbiota during weight loss of a morbidly obese volunteer.
| Measurements | Day 0 | 9 Weeks | 23 Weeks | Reference range |
|---|---|---|---|---|
| Body weight (kg) | 174.8 | 144.8 | 123.5 | — |
| BMI (kg m−2) | 58.78 | 48.66 | 41.50 | 18–23 |
| SBP (mm Hg) | 150 | 120 | 120 | ⩽140 |
| DBP (mm Hg) | 110 | 80 | 75 | ⩽90 |
| Triglycerides (mmol l−1) | 2.68 | 1.72 | 1.18 | 0–1.7 |
| Total cholesterol (mmol l−1) | 5.53 | 4.64 | 4.78 | 3.00–5.17 |
| HDL cholesterol (mmol l−1) | 0.89 | 0.70 | 0.82 | >0.91 |
| LDL cholesterol (mmol l−1) | 3.42 | 3.15 | 3.42 | 0–4.16 |
| Fasting plasma glucose (mmol l−1) | 8.95 | 4.76 | 5.40 | 3.90–6.10 |
| Fasting plasma insulin (μIU ml−1) | 58.7 | 25.8 | 23.0 | 6–27 |
| HbA1c (%) | 7.58 | 5.44 | 4.52 | 3.8–5.8 |
| AST (U l−1) | 122 | 51 | 31 | 10–47 |
| ALT (U l−1) | 97 | 50 | 33 | 0–41 |
| GGT (U l−1) | 168 | 49 | 59 | 0–56 |
| LBP (μg ml−1) | 7.03 | 2.29 | 4.78 | — |
| C-reactive protein (mg l−1) | 14.1 | 9.4 | 9.51 | 0–10 |
| IL-6 (pg ml−1) | 6.71 | 4.46 | 2.76 | — |
| Adiponectin (μg ml−1) | 2.00 | 2.09 | 4.27 | — |
| Enterobactera | 34.98% | 1.77% | 0% | |
| Enterobacteriaceaeb | 13.23% | 0.45% | 0.32% |
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase; BMI, body mass index; DBP, diastolic blood pressure; GGT, gamm-glutamyl transferase; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein cholesterol; IL-6, interleukin-6; LBP, lipopolysaccharide-binding protein; LDL, low-density lipoprotein cholesterol; SBP, systolic blood pressure.
Based on the near full-length 16S ribosomal RNA gene sequence clone libraries of the fecal microbiota.
Based on the metagenomic sequencing analysis of the fecal microbiota.
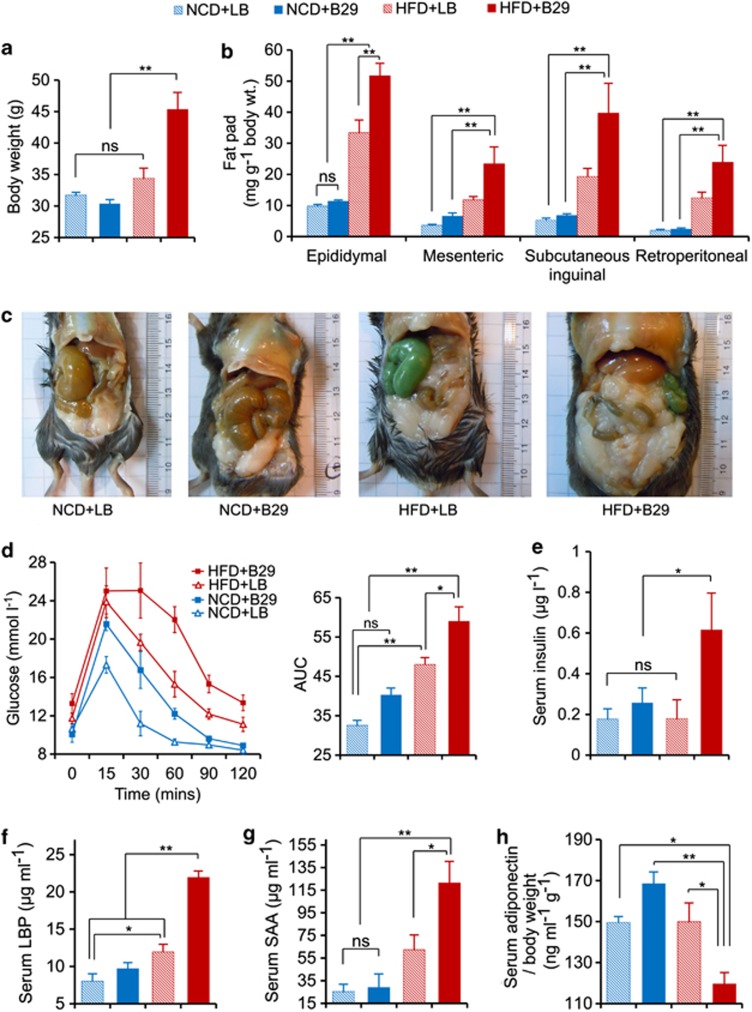
Previous studies show that germfree mice are resistant to HFD-induced obesity (Backhed et al., 2007; Ding et al., 2010; Rabot et al., 2010). To test whether B29 can overcome this resistance to obesity by colonizing the gut of germfree mice (Supplementary Figure 11), we inoculated 1010 cells of B29 every day for the first week into 6- to 10-week-old germfree C57BL/6J mice (n=7 per group) under either normal chow diet (NCD) or HFD. We observed a slight body weight reduction among the mice during the inoculation period (Supplementary Figures 11–14). One mouse in each group died during inoculation because of the translocation of B29 into various organs (Sanders and Sanders, 1997; Supplementary Table 2). After the first week, the HFD-fed gnotobiotic mice inoculated with B29 (HFD+B29) showed a steady weight gain until eventually reaching an obese state comparable to that of the HFD-fed conventional mice (n=8 per group; Figures1a–c; Supplementary Figures 14–17). The excessive fat accumulation in the HFD+B29 gnotobiotic mice was associated with an altered lipometabolism including a leptin-resistant phenotype, reduced expression of fasting-induced adipose factor in the ileum, and increased expression of acetyl-CoA carboxylase 1, fatty acid synthase and peroxisome proliferator-activated receptor-gamma genes in the liver (Supplementary Figures 18–19; Backhed et al., 2004, 2007). The HFD+B29 gnotobiotic mice developed the most significant insulin-resistant phenotype as shown in the oral glucose tolerance test and 2 h post load insulin levels at the end of the trial (Figures 1d and e). This group also had the greatest increases in liver and spleen weights and the greatest decrease in cecum weight (Supplementary Table 3). The NCD-fed mice inoculated with either B29 (NCD+B29) or Luria–Bertani (LB) medium (NCD+LB) both remained lean throughout the trial (Figures 1a–c). The HFD-fed germfree mice inoculated with LB (HFD+LB) experienced significant weight gain over the first 9 weeks but eventually became no different, based on the obesity parameters tested, from the NCD-fed groups by the end of the 16-week trial, except for a moderately increased epididymal fat pad and a low level of insulin resistance (Supplementary Figure 14; Figures 1b and d). Our repeat of the animal test with HFD-fed gnotobiotic mice mono-associated with B29 confirmed that a single endotoxin producer such as B29 can function in the capacity of the whole microbiota for inducing obese and insulin-resistant phenotypes (Supplementary Figure 20). Inoculating 6- to 10-week-old germfree mice (n=4–6 per group) with a strain of Bifidobacterium animals via alternation of NCD and HFD feeding did not induce the same obese phenotype (Supplementary Figure 21), suggesting that obesity cannot be induced by introducing any bacteria in the germfree mice under HFD feeding.
Figure 1.
Gnotobiotic mice mono-associated with E. cloacae B29 become obese and insulin resistant with increased endotoxin load and provoked systemic inflammation under HFD feeding (data collected at the end of 16 weeks after inoculation). (a) Body weight; (b) mass of epididymal, mesenteric, subcutaneous inguinal and retroperitoneal fat pad; (c) abdominal photographs; (d) oral glucose tolerance test (OGTT) and the areas under the curve (AUC) for the plasma glucose; (e) serum 2 h post load insulin; (f) enzyme-linked immunosorbent assay (ELISA) analysis of serum LPS-binding protein (LBP); (g) serum amyloid A (SAA); and (h) adiponectin corrected for bodyweight. The two-way analysis of variance (ANOVA) revealed a significant effect of the diet (P<0.01), a significant effect of B29 (P<0.01) and a significant diet × B29 interaction effect (P<0.01) on body weight, mass of epididymal, mesenteric, subcutaneous inguinal and retroperitoneal fat pad, serum LBP; a significant effect of the diet (P<0.01) and a significant effect of B29 (P<0.01) on OGTT; a significant effect of B29 (P<0.05) on serum 2 h post load insulin. Data are shown as means±s.e.m. (n=6). NS, no significant difference; *P<0.05; **P<0.01. Color code for animal groups: NCD+LB, blue slash; NCD+B29, blue; HFD+LB, red slash; HFD+B29, red. LB, Luria–Bertani medium.
A slightly increased endotoxin load can induce a low-grade, chronic inflammation as a driving force for insulin resistance and altered lipometabolism in mice (Hotamisligil et al., 1996; Cani et al., 2007). The serum LPS-binding protein was significantly higher in the HFD+B29 gnotobiotic mice than in the NCD+B29 gnotobiotic mice (Figure 1f), despite the fact that B29 reached a significantly greater population size in the gut of the NCD-fed gnotobiotic mice (Supplementary Figure 13). As B29 was the only LPS producer in the gnotobiotic-mouse gut (Supplementary Figure 22), the increased serum–endotoxin load in the HFD+B29 gnotobiotic mice could only come from B29. As the gene expression levels of the two tight junction proteins occludin and ZO-1 (Cani et al., 2008) in the ileum were not significantly different among the groups (Supplementary Figure 23), the high amount of endotoxin translocation from the gut to the serum in the HFD+B29 gnotobiotic mice may be facilitated by chylomicrons induced by long-chain fatty acids in the HFD (Cani et al., 2007; Ghoshal et al., 2009), rather than by impaired gut barrier function (Cani et al., 2007; Zhang et al., 2010, 2012). In accordance with the increased endotoxin load, the HFD+B29 gnotobiotic mice had the greatest increase in serum amyloid A protein levels and the greatest decrease in adiponectin secretion, suggesting that these mice had the greatest increase in systemic inflammation (Figures 1g and h). The expression of the tumor necrosis factor-alpha, interleukin-1β, interleukin-6, I kappa B kinase epsilon and Toll-like receptor 4 pro-inflammatory genes increased significantly in the liver and epididymal fat pad but not in the ileum of the HFD+B29 gnotobiotic mice (Supplementary Figure 24), indicating local inflammation induced in the former two tissues but not in the gut, in contrast to a previous report (Ding et al., 2010). The HFD+LB germfree mice had moderately higher levels of serum serum amyloid A and liver tumor necrosis factor-alpha expression than the NCD-fed groups, suggesting that the HFD induced some host inflammation (Tripathy et al., 2003), which is, however, much lower than that induced by B29. Taken together, our results suggest that endotoxin-induced inflammation may have a pivotal role in obesity induced by E. cloacae B29, supporting the existence of a putative chain of causation from endotoxin producers in the gut to the obesity end points.
Germfree mice have been extensively used for obesity studies. For example, Gordon et al. showed that co-inoculation of germfree mice with the plant polysaccharide-fermenting Bacteroides thetaiotaomicron and the methane-producing Methanobrevibacter smithii significantly increased the epididymal fat pad but not the total bodyweight (Samuel and Gordon, 2006). As a step forward, our study has followed a procedure modified from Koch's Postulates (Evans, 1976) and, for the first time, established a gnotobiotic-mouse obesity model combining HFD with a human-originated endotoxin producer. This work suggests that the overgrowth of an endotoxin-producing gut bacterium is a contributing factor to, rather than a consequence of, the metabolic deteriorations in its human host. In fact, this strain B29 is probably not the only contributor to human obesity in vivo, and its relative contribution needs to be assessed. Nevertheless, by following the protocol established in this study, we hope to identify more such obesity-inducing bacteria from various human populations, gain a better understanding of the molecular mechanisms of their interactions with other members of the gut microbiota, diet and host for obesity, and develop new strategies for reducing the devastating epidemic of metabolic diseases.
Acknowledgments
We appreciate Professor R. Losick, L Neuhauser, M Obin and M Pop for critical reading of the manuscript and kind suggestions. We are also grateful to the following individuals for their kind assistance during the study: S Xiao, J Shen, X Pang, M Zhang, XJ Zhang, Y Zhao, L Wang, J Wang, Y Zhang, G Wu, G Wang, H Ou, J Qi, JJ Wang, X Zhang, R Wang, M Song, J Xu, H Tang, T Liu, Q Zhang, N Zhao, C Zhang, Y Fan, S Liu, YZ Fan, T Wang, Z Hu, R Xi, XY Zhang, C Liu, H Wu, X Guo, X Li, G Ning, S Yang and G Zhao.
This work was supported by Project 30730005 of the National Nature Science Foundation of China (NSFC), 863 Projects 2008AA02Z315 and 2009AA02Z310, Key Projects 2007DFC30450 and 075407001 of International Cooperation Program Grants and Project in the National Science and Technology Pillar Program 2006BAI11B08.
Footnotes
Supplementary Information accompanies the paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci USA. 2007;104:979–984. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- Ding S, Chi MM, Scull BP, Rigby R, Schwerbrock NM, Magness S, et al. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS One. 2010;5:e12191. doi: 10.1371/journal.pone.0012191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AS. Causation and disease: the Henle-Koch postulates revisited. Yale J Biol Med. 1976;49:175–195. [PMC free article] [PubMed] [Google Scholar]
- Ghoshal SJ, Witta J, Zhong J, de Villiers W, Eckhardt E. Chylomicrons promote intestinal absorption of lipopolysaccharides. J Lipid Res. 2009;50:90–97. doi: 10.1194/jlr.M800156-JLR200. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM, et al. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- Lepper PM, Schumann C, Triantafilou K, Rasche FM, Schuster T, Frank H, et al. Association of lipopolysaccharide-binding protein and coronary artery disease in men. J Am Coll Cardiol. 2007;50:25–31. doi: 10.1016/j.jacc.2007.02.070. [DOI] [PubMed] [Google Scholar]
- Moreno-Navarrete JM, Ortega F, Serino M, Luche E, Waget A, Pardo G, et al. Circulating lipopolysaccharide-binding protein (LBP) as a marker of obesity-related insulin resistance. Int J Obes (Lond) 2011;36:1442–1449. doi: 10.1038/ijo.2011.256. [DOI] [PubMed] [Google Scholar]
- Qi J, Luo H, Hao B. CVTree: a phylogenetic tree reconstruction tool based on whole genomes. Nucleic Acids Res. 2004;32:45–47. doi: 10.1093/nar/gkh362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabot S, Membrez M, Bruneau A, Gerard P, Harach T, Moser M, et al. Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J. 2010;24:4948–4959. doi: 10.1096/fj.10-164921. [DOI] [PubMed] [Google Scholar]
- Rappé MS, Connon SA, Vergin KL, Giovannoni SJ. Cultivation of the ubiquitous SAR 11 marine bacterioplankton clade. Nature. 2002;418:630–633. doi: 10.1038/nature00917. [DOI] [PubMed] [Google Scholar]
- Ruiz AG, Casafont F, Crespo J, Cayon A, Mayorga M, Estebanez A, et al. Lipopolysaccharide-binding protein plasma levels and liver TNF-alpha gene expression in obese patients: evidence for the potential role of endotoxin in the pathogenesis of non-alcoholic steatohepatitis. Obes Surg. 2007;17:1374–1380. doi: 10.1007/s11695-007-9243-7. [DOI] [PubMed] [Google Scholar]
- Samuel BS, Gordon JI. A humanized gnotobiotic mouse model of host-archaeal-bacterial mutualism. Proc Natl Acad Sci USA. 2006;103:10011–10016. doi: 10.1073/pnas.0602187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders J. WE, Sanders CC. Enterobacter spp.: pathogens poised to flourish at the turn of the century. Clin Microbiol Rev. 1997;10:220–241. doi: 10.1128/cmr.10.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann RR, Leong SR, Flaggs GW, Gray PW, Wright SD, Mathison JC, et al. Structure and function of lipopolysaccharide binding protein. Science. 1990;249:1429–1431. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- Tripathy D, Mohanty P, Dhindsa S, Syed T, Ghanim H, Aljada A, et al. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes. 2003;52:2882–2887. doi: 10.2337/diabetes.52.12.2882. [DOI] [PubMed] [Google Scholar]
- Zhang C, Zhao Y, Zhang M, Pang X, Wang L, Zhao L. Structural resilience of the gut microbiota in adult mice under high-fat dietary perturbations. ISME J. 2012;6:1848–1857. doi: 10.1038/ismej.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Zhang M, Wang S, Han R, Cao Y, Hua W, et al. Interactions between gut microbiota, host genetics and diet relevant to development of metabolic syndromes in mice. ISME J. 2010;4:232–241. doi: 10.1038/ismej.2009.112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.