Abstract
Background. Garlic (Allium sativum) has been shown to have important benefits in individuals at high cardiovascular risk. The aim of the present study was to evaluate the effects of the administration of aged garlic extract (AGE) on the risk factors that constitute the cluster of metabolic syndrome (MS). Methods and Design. Double-blind, crossover, randomized, placebo-controlled clinical trial to assess the effect of 1.2 g/day of AGE (Kyolic), for 24 weeks of treatment (12 weeks of AGE and 12 weeks of placebo), on subjects with MS. Results. The administration of AGE increased the plasma levels of adiponectin (P = 0.027). No serious side effects associated with the intervention were reported. Conclusion. The present results have shown for the first time that the administration of AGE for 12 weeks increased plasma adiponectin levels in patients with MS. This suggests that AGE might be a useful, novel, nonpharmacological therapeutic intervention to increase adiponectin and to prevent cardiovascular (CV) complications in individuals with MS.
1. Introduction
Metabolic syndrome (MS) is characterized by the presence of insulin resistance, low-degree inflammation, dysglycemia, low plasma high-density lipoprotein cholesterol (HDL-C), increased triglycerides (TG), elevated blood pressure, and abdominal obesity [1]. MS has been associated with an increased risk of type 2 diabetes mellitus (DM2) and cardiovascular diseases (CVDs) [1, 2]. The prevalence of MS varies between 15% and 40%, being greater in the population of Hispanic origin [3].
Abdominal obesity is considered a key characteristic of MS, which is related to decreased insulin-mediated glucose uptake [4]. Adipose tissue is known to express and secrete a variety of adipokines, including leptin, adiponectin, resistin, and visfatin, as well as cytokines and chemokines such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and monocyte chemoattractant protein-1 (MCP-1) [5–8]. The release of adipokines by either adipocytes or adipose tissue-infiltrated macrophages plays a key role in the development of insulin resistance and DM2, as well as the increased risk of cardiovascular disease associated with obesity. Renin-angiotensin system components are also activated in adipose tissue, leading to hypertension and insulin resistance [4]. Adiponectin is considered to be a protective protein with antidiabetic, anti-inflammatory, and antiatherogenic effects [9]. Reduced plasma adiponectin levels have been reported in obese individuals, particularly in those with visceral obesity, and have been negatively correlated with insulin resistance. Furthermore, decreased adiponectin levels were found to be associated with a higher incidence of DM2 [4]. Leptin was shown to promote the development of atherosclerosis by inducing oxidative stress in endothelial cells, increasing platelet aggregation, and hypertrophy and proliferation of vascular smooth muscle cells [4]. Additionally, it was shown that a high leptin level predicts subsequent development of DM2 [6]. Thus, leptin/adiponectin imbalance has a key role in the metabolic alterations associated with obesity [5–10].
Multiple therapeutic approaches such as renin-angiotensin system blockers and inhibitors, statins as well as nutrient and dietary interventions [11–14], have been proposed to reduce metabolic and cardiovascular risk in patients with MS. Garlic (Allium sativum L.) has been used as a nutrient with beneficial cardiovascular effects [15]. However, the beneficial effects of garlic are offset by the fact that fresh garlic causes indigestion and that its pungent odor lingers on breath and skin [16]. An alternative source of garlic that is odorless and rich in antioxidants is aged garlic extract (AGE) [17]. AGE has shown beneficial effects in several alterations related to the development of cardiovascular diseases, such as antioxidant and antithrombotic properties [18–20]. Thus, in the present study we aimed to investigate the effects of AGE on adipokines, inflammatory substances, endothelial function, and metabolic risk factors that constitute the cluster of metabolic syndrome in an urban Colombian population.
2. Materials and Methods
2.1. Study Design
Double-blind, crossover, randomized, placebo-controlled clinical trial to assess the effect of AGE (Kyolic) on the cardiovascular risk factors of subjects with MS.
2.2. Population
Men and women over 18 years old with diagnosis of MS, attending primary health care clinics from the metropolitan area of Bucaramanga, Colombia. The MS diagnosis was based on the presence of central obesity (waist circumference ≥90 cm (male), ≥80 cm (female)) and two of the following criteria: TG ≥150 mg/dL, HDL-C <40 mg/dL (male), <50 mg/dL (female), blood pressure ≥130/85 mmHg, and fasting plasma glucose ≥100 mg/dL. The exclusion criteria were (1) allergies to garlic; (2) current treatment with lipid-lowering drugs, antihypertensive drugs, and/or hypoglycemic medications; (3) psychiatric disorders that prevent proper decision making; (4) patients with infections or inflammatory assets; (5) presence of coronary artery disease, with a current or past ischemic event; (6) presence of severe chronic or terminal illnesses; and (7) presence of diseases that compromise the immune system.
2.3. Procedures
This study was registered in ClinicalTrials.gov with the identifier code NCT01168700. The study was approved by the ethical committee of the Cardiovascular Foundation in Bucaramanga, Colombia. All subjects provided written informed consent before entering the study. Patients were randomly assigned by blocks to receive either 1.2 g/day of AGE (Kyolic) or placebo, and after 12 weeks of supplementation, the treatment was invested for another 12 weeks (Figure 1). Each treatment was provided in an identical capsule that was taken twice daily with breakfast and dinner (2 capsules of each). All subjects received routine recommendations of lifestyle changes (having a diet lower in fat and sugar and increasing physical activity with 30 minutes/day of moderate walking). Participants were followed up every four weeks with clinical evaluations and registration of potential undesirable effects and use of any other medication. During the baseline and at the end of each phase of treatment (week 12 and week 24), the following were determined.
Figure 1.
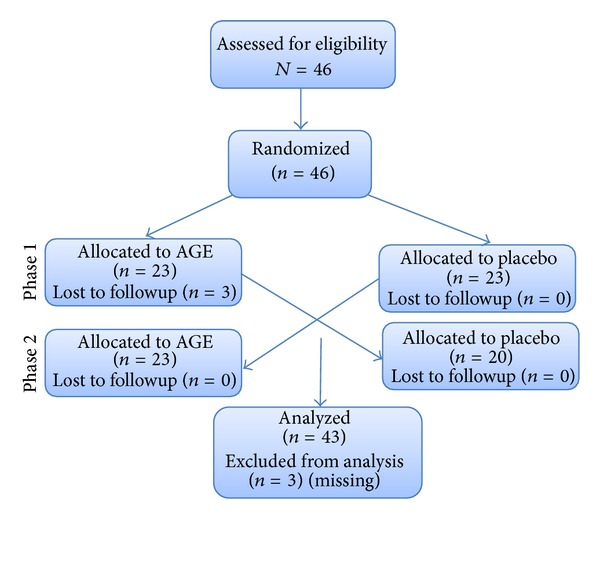
CONSORT diagram showing the flow of participants through each phase of the randomized crossover trial.
2.3.1. Anthropometrical Measurements
Weight, height, body mass index (BMI), waist (WC) and hip circumferences (HC), and blood pressure.
2.3.2. Biochemical Determinations
Routine clinical tests were processed in the Clinical Research Laboratory from the Ophthalmological Foundation of Santander-FOSCAL, Floridablanca, Colombia. Measurements of adipokines and inflammatory factors were performed at the Department of Physiology, Faculty of Medicine, Complutense University of Madrid, Spain. Glycemia and lipid profile were quantified by using a routine colorimetric method (Biosystem BTS-303 Photometric, Barcelona, Spain). Interleukin-6, adiponectin, and C-reactive protein were measured using an immunoassay (R&D Systems, MN, USA).
2.3.3. Endothelial Function
Endothelial function was evaluated by flow mediated vasodilatation (FMV). The FMV was performed using a high-resolution Doppler ultrasound, measuring the changes in diameter of the brachial artery in response to increased blood flow (reactive hyperemia). This method was previously standardized in our population [21, 22].
2.3.4. Statistical Analysis
The averages and proportions obtained in a descriptive analysis for all clinically relevant variables measured during the baseline evaluation were compared. Then, the treatment effect of the crossover design was evaluated through the difference in change between baseline versus posttreatment according to the intervention phase. Based on the frequencies distribution of the outcome variables, the Student's t-test or Wilcoxon signed-ranks test was used. In outcome variables where significant differences were observed, further analysis of changes was performed using analysis of covariance (ANCOVA), adjusting by phase, treatment, and their interaction (treatment × phase) to determine if changes were due to a carryover effect. All analyses were conducted using Stata statistical software, release 11.0 (Stata Corporation, College Station, TX, USA). A P < 0.05 was considered statistically significant.
3. Results
The 46 patients included in the study were distributed in two sequences of treatment: AGE-placebo and placebo-AGE. Three subjects (all of them of the first group) voluntarily discontinued the treatment during the phase 1. Demographic, anthropometric, and biochemical characteristics obtained in the 43 participants who completed the study are shown in Table 1. A significant difference in age was found between the AGE-placebo and the placebo-AGE groups at the baseline.
Table 1.
Baseline demographic, anthropometrical, endothelial function and biochemical characteristics of the general population.
| Variable | Global | AGE-placebo Group (n = 20) |
Placebo-AGE group (n = 23) |
P value |
|---|---|---|---|---|
| Age (years) | 40.79 (10.71) | 44.75 (10.5) | 37.34 (9.8) | 0.02∗,Ψ |
| SBP (mm Hg) | 123.73 (15.63) | 127 (15) | 120 (15) | 0.15* |
| DBP (mm Hg) | 81.59 (10.86) | 82 (12) | 80 (9) | 0.56* |
| BMI (kg/m2) | 33.07 (5.01) | 33.7 (5.4) | 32.5 (4.5) | 0.54* |
| WC (cm) | 100.44 (10.32) | 102.4 (11.6) | 98.7 (8.8) | 0.23* |
| HC (cm) | 109.42 (8.89) | 108.8 (8.4) | 109.8 (9.4) | 0.46* |
| Glucose (mg/dL) | 87.12 (14.99) | 91.85 (17.53) | 83 (11.19) | 0.10* |
| Cholesterol (mg/dL) | 198.61 (30.82) | 201.9 (36.61) | 195.73 (25.24) | 0.51* |
| LDL (mg/dL) | 116.96 (28.16) | 119.25 (35.10) | 114.95 (20.98) | 0.61* |
| HDL (mg/dL) | 37.47 (5.68) | 37.51 (6.55) | 37.42 (4.94) | 0.87* |
| TAG (mg/dL) | 220.91 (71.96) | 225.65 (80.07) | 216.78 (65.65) | 0.88# |
| CRP (mg/L) | 5.79 (4.73) | 7.04 (5.73) | 4.70 (3.39) | 0.21* |
| IL-6 (units/mL) | 1.97 (0.88) | 1.93 (0.87) | 2.00 (0.90) | 0.68* |
| Adiponectin (ng/mL) | 5936.56 (1813.74) | 5516.7 (1665.24) | 6301.65 (1893.59) | 0.15* |
| Leptin (ng/mL) | 26.19 (19.28) | 27.29 (18.64) | 25.22 (20.17) | 0.60# |
| Insulin (μU/mL) | 22.26 (25.87) | 28.81 (36.07) | 16.58 (9.03) | 0.10# |
| HOMA index | 5.04 (6.51) | 6.82 (9.03) | 3.49 (2.22) | 0.05# |
| FMV (%) | 11.06 (5.98) | 10.68 (5.39) | 11.38 (6.37) | 0.70* |
SBP: systolic blood pressure; DBP: diastolic blood pressure; BMI: body mass index; WC: waist circumference; HC: hip circumference, LDL-C: low density lipoprotein cholesterol; HDL-C: high density lipoprotein cholesterol; TG: triglycerides; CRP: C-reactive protein; IL-6: interleukin-6; FMV: flow-mediated vasodilatation. Values are expressed as mean ± SD. *AGE group versus placebo group with independent Student's t-test. #AGE group versus placebo group with Wilcoxon signed-ranks test. Ψ P < 0.05.
At the end of the study the crossover analysis was conducted, and a significant difference in the adiponectin delta was found comparing AGE versus Placebo, Δ: 313.79 (95%IC: −48.34~675.92) versus Δ: −271.88 (95%IC: −649.64~105.87), respectively (Table 2). The ANCOVA confirmed that the significant difference in adiponectin was due to the treatment, not the phase of the study (no carryover effect) as no significant changes were observed in the interaction treatment phase (Table 3). No significant changes were observed in any of the other anthropometrical measurements, endothelial function, and biochemical variables (Table 2). No serious side effects were associated with AGE administration.
Table 2.
Change differences in the anthropometrical measurements, endothelial function, and biochemical characteristics in the crossover analysis.
| Parameters | AGE | Placebo | P value |
|---|---|---|---|
| Change differences (n = 43) |
Change differences (n = 43) |
||
| SBP (mm Hg) | −2.59 ± 1.91 | −1.72 ± 1.60 | 0.727* |
| DBP (mm Hg) | −1.07 ± 1.32 | −0.31 ± 1.17 | 0.670* |
| BMI (kg/m2) | 0.01 ± 0.21 | −0.11 ± 0.17 | 0.952# |
| WC (cm) | −0.99 ± 0.47 | 0.32 ± 0.50 | 0.062* |
| HC (cm) | −0.91 ± 0.39 | −0.39 ± 0.58 | 0.462# |
| WHR | −0.001 ± 0.004 | −0.005 ± 0.005 | 0.358* |
| Glucose (mg/dL) | 2.04 ± 1.68 | 3.46 ± 1.87 | 0.766# |
| Cholesterol (mg/dL) | −4.41 ± 4.47 | 6.0 ± 4.27 | 0.172# |
| LDL-C (mg/dL) | 3.94 ± 5.32 | 6.51 ± 4.46 | 0.869# |
| HDL-C (mg/dL) | −1.64 ± 0.86 | −1.16 ± 0.69 | 0.911# |
| TG (mg/dL) | −18.76 ± 12.42 | −3.98 ± 13.84 | 0.453# |
| CRP (mg/L) | 0.21 ± 0.85 | 0.07 ± 0.77 | 0.976# |
| IL-6 (units/mL) | 0.08 ± 0.16 | 0.01 ± 0.18 | 0.682 # |
| Adiponectin (ng/mL) | 313.79 ± 179.44 | −271.88 ± 187.18 | 0.027∗Ψ |
| Leptin (ng/mL) | −1.67 ± 1.68 | −0.79 ± 1.26 | 0.993# |
| Insulin (μU/mL) | −2.94 ± 2.60 | 2.26 ± 1.38 | 0.269# |
| HOMA index | −0.67 ± 0.70 | 0.67 ± 0.34 | 0.142# |
| FMV (%) | −0.81 ± 5.09 | −1.34 ± 9.78 | 0.836* |
SBP: systolic blood pressure; DBP: diastolic blood pressure; BMI: body mass index; WC: waist circumference; HC: hip circumference; WHR: waist-hip ratio; LDL-C: low density lipoprotein cholesterol; HDL-C: high density lipoprotein cholesterol; TG: triglycerides; CRP: C-reactive protein; IL-6: interleukin 6; FMV: flow-mediated vasodilatation. Values are expressed as mean ± SEM. *AGE group versus placebo group with Student's t-test. #AGE group versus placebo group with Wilcoxon signed-ranks test. Ψ P < 0.05.
Table 3.
Analysis of covariance (ANCOVA), adjusting by phase, treatment, and their interaction (treatment × phase).
| Parameter | AGE | Placebo | P value | ANCOVA | |
|---|---|---|---|---|---|
| Change differences (n = 43) | Change differences (n = 43) | P value treatment | P value phase | ||
| Adiponectin (ng/mL) | 313.79 ± 179.44 | −271.88 ± 187.18 | 0.027∗Ψ | 0.031& | 0.428 |
ΨP < 0.05. & P < 0.05 ANCOVA model. P value treatment and phase: analysis of covariance.
4. Discussion
The present study demonstrates for the first time that the administration of AGE to subjects with MS for 12 weeks increased adiponectin plasma concentrations. The ANCOVA indicated that this outcome was not due to a carryover effect.
Our group previously reported that in dyslipidemic subjects, the presence of coronary artery disease is associated with an elevation of certain inflammatory markers but not with further endothelial dysfunction [23]. In the present study, after the AGE intervention there were no significant changes either in endothelial function or in inflammation, which may relate both to the short period of intervention and the participation of subjects with low cardiovascular risk. However, there was a significant increase in adiponectin, an anti-inflammatory adipokine with cardioprotective properties [24].
Low adiponectin levels are observed in obese subjects with and without severe coronary atherosclerosis and in subjects with abdominal obesity [10, 25], and decreased adiponectin levels (<4 μg/mL) are associated with a twofold increase in the prevalence of coronary heart disease, independent of other cardiovascular risk factors [26]. Moreover, hypoadiponectinemia is associated with insulin resistance and DM2 [27, 28], as well as atherosclerosis and hypertension [29].
Adiponectin exerts an anti-inflammatory effect through activation of its three receptors (AdipoR1, AdipoR2, and T-cadherin) [9]. The activation of AdipoR1 and R2 results in increased hepatic and skeletal muscle fatty acid oxidation, increased skeletal muscle lactate production, reduced hepatic gluconeogenesis, increased cellular glucose uptake, and inhibition of inflammation and oxidative stress [30]. Activation of T-cadherin is protective in vascular endothelial cells against oxidative stress-induced apoptosis [31]. Several mechanisms have been suggested to explain the anti-inflammatory effects of adiponectin, including direct actions on inflammatory cells, actions on NF-κB, and interaction with TNF-α [9]. It has been demonstrated that adiponectin inhibits the expression of adhesion molecules in endothelial cells and inhibits smooth muscle cell proliferation, the differentiation of monocytes into macrophages, as well as the formation of foam cells and the secretion of TNF-α by macrophages [32–34]. Also, increased adiponectin levels are related to improvement in the differentiation of preadipocytes into adipocytes, which is usually impaired in obese subjects [35]. In fact, 1,2-vinyldithiin (1,2-DT), a garlic-derived organosulfur compound, has been shown to affect the differentiation of human preadipocytes into adipocytes [36]. Interestingly, a significant reduction of the expression of the two major adipogenic transcription factors, PPARγ2 and CCAAT/enhancer binding protein (C/EBPα), was observed in 1,2-DT-treated preadipocytes. The 1,2-DT-mediated decrease in PPARγ2 expression is associated with reduced PPARγ activity, suggesting that the negative effect of 1,2-DT on preadipocytes differentiation could be mainly due to an inhibitory effect on PPARγ2, the master regulator of adipogenesis. The role of these mechanisms of action of 1,2-DT in the beneficial effects of AGE increasing the levels of adiponectin remains to be elucidated. Additionally, our results showing that a short period of AGE administration increases the adiponectin level suggest that the effect of AGE improving the insulin resistance could be another new interesting mechanism to explain the well-known beneficial cardiometabolic effect of garlic.
Another mechanism that could be associated with the adiponectin increase is the nitric oxide (NO) pathway. There appears to be a reciprocal relationship between adiponectin and NO [37]. Adiponectin increases the stability of eNOS mRNA and half-life, enhances the association of eNOS with Hsp90, and stimulates the phosphorylation of eNOS, which together lead to increased NO production [38, 39]. Moreover, NO appears to positively regulate adiponectin levels [40]. It has been suggested that AGE could increase NO bioavailability [40, 41] by (a) increasing cellular antioxidant capacity by providing cellular thiol antioxidants like cysteine and reduced glutathione, (b) maintaining functionally relevant levels of tetrahydrobiopterin and preventing oxidative inactivation of tetrahydrobiopterin, which prevents NO synthase uncoupling and superoxide anion generation, and (c) maintaining NO bioavailability in endothelial cells even under conditions of increased vascular oxidant stress [41]. AGE is rich in water-soluble organosulfur bioactive compounds such as S-allylcysteine and S-allylmercaptocysteine which are cellular donors of thiol containing reducing equivalents [41] and as such might explain the cardiovascular benefits of AGE.
In summary, we showed for the first time that AGE administration for 12 weeks increases adiponectin levels. The importance of this observation in the prevention of CVD remains to be determined, and further and larger studies are needed.
Conflict of Interests
This project has been funded in part by Wakunaga of America, providing the AGE supplements and the placebo. This company did not participate in the study design, data collection, and data analysis or in the decision to publish it. None of the authors have conflict of interests.
References
- 1.Alberti KGMM, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 2.Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. Journal of the American College of Cardiology. 2010;56(14):1113–1132. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 3.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third national health and nutrition examination survey. The Journal of the American Medical Association. 2002;287(3):356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 4.Langenberg C, Bergstrom J, Scheidt-Nave C, Pfeilschifter J, Barrett-Connor E. Cardiovascular death and the metabolic syndrome: role of adiposity-signaling hormones and inflammatory markers. Diabetes Care. 2006;29(6):1363–1369. doi: 10.2337/dc05-2385. [DOI] [PubMed] [Google Scholar]
- 5.Yudkin JS. Inflammation, obesity, and the metabolic syndrome. Hormone and Metabolic Research. 2007;39(10):707–709. doi: 10.1055/s-2007-985898. [DOI] [PubMed] [Google Scholar]
- 6.Guerro-Millo M. Adipose tissue and adipokines: for better or worse. Diabetes and Metabolism. 2004;30(1):13–19. doi: 10.1016/s1262-3636(07)70084-8. [DOI] [PubMed] [Google Scholar]
- 7.Antuna-Puente B, Feve B, Fellahi S, Bastard JP. Adipokines: the missing link between insulin resistance and obesity. Diabetes and Metabolism. 2008;34(1):2–11. doi: 10.1016/j.diabet.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Rabe K, Lehrke M, Parhofer KG, Broedl UC. Adipokines and insulin resistance. Molecular Medicine. 2008;14(11-12):741–751. doi: 10.2119/2008-00058.Rabe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson K, Prins J, Venkatesh B. Clinical review: adiponectin biology ant its role in inflammation and critical illness. Critical Care. 2011;15(article 221) doi: 10.1186/cc10021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weyer C, Funahashi T, Tanaka S, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hiperinsulinemia. Journal of Clinical Endocrinology and Metabolism. 2001;86(5):1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 11.de las Heras N, Martín-Fernández B, Miana M, et al. The protective effect of irbesartan in rats fed a high-fat diet is associated with modification of leptin-adiponectin imbalance. Journal of Hypertension, Supplement. 2009;27(supplement 6):37–41. doi: 10.1097/01.hjh.0000358836.64052.43. [DOI] [PubMed] [Google Scholar]
- 12.Koh KK, Sakuma I, Quon MJ. Differential metabolic effects of distinct statins. Atherosclerosis. 2011;215(1):1–8. doi: 10.1016/j.atherosclerosis.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 13.Nagamia S, Pandian A, Cheema F, et al. The role of quinapril in the presence of a weight loss regimen: endothelial function and markers of obesity in patients with the metabolic syndrome. Preventive cardiology. 2007;10(4):204–209. doi: 10.1111/j.1520-037x.2007.06556.x. [DOI] [PubMed] [Google Scholar]
- 14.de las Heras N, Valero M, Martín-Fernández B, et al. Effect of rosuvastatin on metabolic and endocrine alterations induced by high fat-diet in rats. Proceedings of the 4th International Congress on Prediabetes and Metabolic Syndrome; 2011; Madrid, Spain. p. p. 46. [Google Scholar]
- 15.Rivlin RS. Historical perspective on the use of garlic. Journal of Nutrition. 2001;131(3):951S–954S. doi: 10.1093/jn/131.3.951S. [DOI] [PubMed] [Google Scholar]
- 16.Amagase H, Petesch BL, Matsuura H, Kasuga S, Itakura Y. Intake of garlic and its bioactive compounds. Journal of Nutrition. 2001;131(3s):955–962. doi: 10.1093/jn/131.3.955S. [DOI] [PubMed] [Google Scholar]
- 17.Borek C. Antioxidant health effects of aged garlic extract. Journal of Nutrition. 2001;131(3):1010S–1015S. doi: 10.1093/jn/131.3.1010S. [DOI] [PubMed] [Google Scholar]
- 18.Ide N, Lau BHS. Aged garlic extract attenuates intracellular oxidative stress. Phytomedicine. 1999;6(2):125–131. doi: 10.1016/S0944-7113(99)80047-6. [DOI] [PubMed] [Google Scholar]
- 19.Dillon SA, Lowe GM, Billington D, Rahman K. Dietary supplementation with aged garlic extract reduces plasma and urine concentrations of 8-iso-prostaglandin F2α in smoking and nonsmoking men and women. Journal of Nutrition. 2002;132(2):168–171. doi: 10.1093/jn/132.2.168. [DOI] [PubMed] [Google Scholar]
- 20.Morihara N, Sumioka I, Moriguchi T, Uda N, Kyo E. Aged garlic extract enhances production of nitric oxide. Life Sciences. 2002;71(5):509–517. doi: 10.1016/s0024-3205(02)01706-x. [DOI] [PubMed] [Google Scholar]
- 21.Silva SY, Villamizar C, Villamizar N, et al. Colombian study to assess the use of noninvasive determination of the endothelium-mediated vasodilation (CANDEV) II. Does location of the occlusion device affects the accuracy of the diagnosis? Endothelium: Journal of Endothelial Cell Research. 2005;12(3):107–111. doi: 10.1080/10623320500189798. [DOI] [PubMed] [Google Scholar]
- 22.Accini JL, Sotomayor A, Trujillo F, Barrera JG, Bautista L, López-Jaramillo P. Colombian study to assess the use of noninvasive determination of endothelium-mediated vasodilatation (CANDEV). Normal values and factors associated. Endothelium. 2001;8(2):157–166. doi: 10.3109/10623320109165324. [DOI] [PubMed] [Google Scholar]
- 23.Lahera V, Rueda-Clausen CF, López-Jaramillo P, Luengas C, Oubia MDP, Cachofeiro V. Inflammation but not endothelial dysfunction is associated with the severity of coronary artery disease in dyslipidemic subjects. Mediators of Inflammation. 2009;2009:8 pages. doi: 10.1155/2009/469169.469169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang WS, Lee WJ, Funahashi T, et al. Weight reduction increases plasma levels of an adipose-derived anti-inflammatory protein, adiponectin. Journal of Clinical Endocrinology and Metabolism. 2001;86(8):3815–3819. doi: 10.1210/jcem.86.8.7741. [DOI] [PubMed] [Google Scholar]
- 25.Rueda-Clausen CF, Lahera V, Calderón J, et al. The presence of abdominal obesity is associated with changes in vascular function independently of other cardiovascular risk factors. International Journal of Cardiology. 2010;139(1):32–41. doi: 10.1016/j.ijcard.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Kumada M, Kihara S, Sumitsuji S, et al. Association of hypoadiponectinemia with coronary artery disease in men. Arteriosclerosis, Thrombosis, and Vascular Biology. 2003;23:85–89. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 27.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. The Journal of Biological Chemistry. 1996;271(18):10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 28.Maeda N, Shimomura I, Kishida K, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nature Medicine. 2002;8(7):731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 29.Han SH, Sakuma I, Shin EK, Koh KK. Antiatherosclerotic and anti-insulin resistance effects of adiponectin: basic and clinical studies. Progress in Cardiovascular Diseases. 2009;52(2):126–140. doi: 10.1016/j.pcad.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nature Medicine. 2002;8(11):1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 31.Joshi MB, Philippova M, Ivanov D, Allenspach R, Erne P, Resink TJ. T-cadherin protects endothelial cells from oxidative stress-induced apoptosis. The FASEB Journal. 2005;19(12):1737–1739. doi: 10.1096/fj.05-3834fje. [DOI] [PubMed] [Google Scholar]
- 32.Arita Y, Kihara S, Ouchi N, et al. Adipocyte-derived plasma protein adiponectin acts as a platelet-derived growth factor-BB-binding protein and regulates growth factor-induced common postreceptor signal in vascular smooth muscle cell. Circulation. 2002;105(24):2893–2898. doi: 10.1161/01.cir.0000018622.84402.ff. [DOI] [PubMed] [Google Scholar]
- 33.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. The British Journal of Nutrition. 2004;92(3):347–355. doi: 10.1079/bjn20041213. [DOI] [PubMed] [Google Scholar]
- 34.Lenz A, Diamond FB., Jr. Obesity: the hormonal milieu. Current Opinion in Endocrinology, Diabetes and Obesity. 2008;15(1):9–20. doi: 10.1097/MED.0b013e3282f43a5b. [DOI] [PubMed] [Google Scholar]
- 35.Tian FS, Luo R, Zhao ZQ, Wu Y, Ban DJ. Blockade of the RAS increases plasma adiponectin in subjects with metabolic syndrome and enhances differentiation and adiponectin expression of human preadipocytes. Experimental and Clinical Endocrinology and Diabetes. 2010;118(4):258–265. doi: 10.1055/s-0029-1237706. [DOI] [PubMed] [Google Scholar]
- 36.Keophiphath M, Priem F, Jacquemond-Collet I, Clément K, Lacasa D. 1,2-Vinyldithiin from garlic inhibits differentiation and inflammation of human preadipocytes. Journal of Nutrition. 2009;139(11):2055–2060. doi: 10.3945/jn.109.105452. [DOI] [PubMed] [Google Scholar]
- 37.Ouchi N, Ohishi M, Kihara S, et al. Association of hypoadiponectinemia with impaired vasoreactivity. Hypertension. 2003;42(3):231–234. doi: 10.1161/01.HYP.0000083488.67550.B8. [DOI] [PubMed] [Google Scholar]
- 38.Hattori Y, Suzuki M, Hattori S, Kasai K. Globular adiponectin upregulates nitric oxide production in vascular endothelial cells. Diabetologia. 2003;46(11):1543–1549. doi: 10.1007/s00125-003-1224-3. [DOI] [PubMed] [Google Scholar]
- 39.Xi W, Satoh H, Kase H, Suzuki K, Hattori Y. Stimulated HSP90 binding to eNOS and activation of the PI3-Akt pathway contribute to globular adiponectin-induced NO production: vasorelaxation in response to globular adiponectin. Biochemical and Biophysical Research Communications. 2005;332(1):200–205. doi: 10.1016/j.bbrc.2005.04.111. [DOI] [PubMed] [Google Scholar]
- 40.Razny U, Kiec-Wilk B, Wator L, et al. Increased nitric oxide availability attenuates high fat diet metabolic alterations and gene expression associated with insulin resistance. Cardiovascular Diabetology. 2011;10(article 68) doi: 10.1186/1475-2840-10-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weiss N, Papatheodorou L, Morihara N, Hilge R, Ide N. Aged garlic extract restores nitric oxide bioavailability in cultured human endothelial cells even under conditions of homocysteine elevation. Journal of Ethnopharmacology. 2013;145(1):162–167. doi: 10.1016/j.jep.2012.10.045. [DOI] [PubMed] [Google Scholar]


