Abstract
Inflammatory myofibroblastic tumours (IMTs), also known as inflammatory pseudotumours, include a diverse group of lesions characterised by inflammatory cell infiltration and variable fibrotic responses. Their occurrence in the breast is unusual. We present a case of an IMT of the breast in a 46-year-old woman who complained of a breast mass with palpable axillary lymph node. The initial clinical diagnosis was breast cancer, and the patient underwent a conservative excision with apparently negative margins and an axillary lymph node excisional biopsy. A histopathological examination showed the presence of myofibroblastic spindle cells with mixed inflammatory infiltrates, and the pathological diagnosis was IMT. Significantly, the case we present here is unique in showing anaplastic lymphoma kinase 1 (ALK1) overexpression and ALK1 gene amplification in IMT of the breast. Therefore, our case suggests that ALK1 gene amplification in IMT of the breast has important diagnostic and therapeutic implications.
Background
Inflammatory myofibroblastic tumour (IMT) is a rare lesion of unknown aetiology. IMT is classified as soft-tissue tumour by the WHO and defined as ‘a tumour composed of differentiated myofibroblastic spindle cells usually accompanied by numerous plasma cells and/or lymphocytes’.1 IMT has been reported in several anatomical sites. Although the lung is the most common site where IMT occurs, IMT also occurs in extrapulmonary anatomical locations including the mesentery, genitourinary system, retroperitoneum, gastrointestinal tract, liver, upper respiratory tract, mediastinum, central nervous system and pelvis.2 Notably, IMT occurs in the breast with no more than 15 cases reported in the literature so far. Owing to its rare occurrence, IMT in the breast is frequently overlooked by the physician, and is potentially overdiagnosed as a malignancy both clinically and pathologically. In this study, we reported an unusual case of IMT arising from the breast with anaplastic lymphoma kinase 1 (ALK1) overexpression, and described the clinical features, radiological and histopathological findings and treatment outcomes. Finally, we discussed the diagnostic features.
Case presentation
A 46-year-old Chinese woman presented with a tender breast mass only by physical examination for 2 days. She denied any history of trauma and long-term medication. There was nothing particular about her family history and social history. She gave birth to one boy and abortion once. Her medical history was unremarkable except for a pelvic inflammation which was diagnosed by a traditional Chinese physician. She took traditional Chinese drugs for a week but she could not remember the specific names of those drugs.
Differential diagnosis
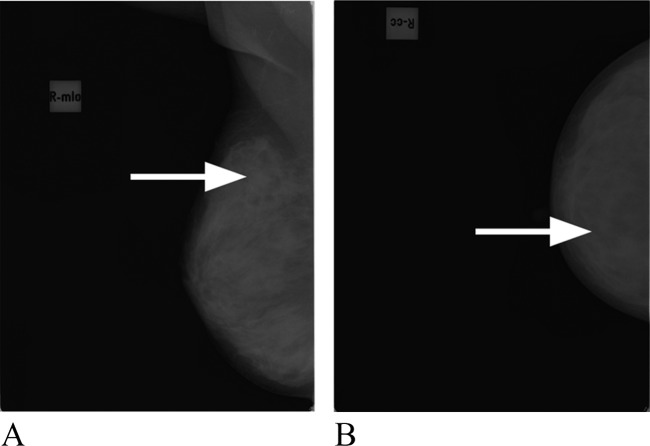
Her physical examination revealed a small, firm, mobile, nontender mass at the upper outer quadrant of the right breast and a palpable lymph node in the right axilla. There was no inflammatory change in this area. The left breast was normal and there was no palpable nodule in the left axilla. Mammography showed the presence of a mass with a diameter of 1 cm, suspicious for malignancy in the upper outer quadrant of the right breast (figure 1). Clinically, the mass was diagnosed as a malignant tumour. Considering that the mass was too small, we decided to perform an excisional biopsy instead of ultrasound-guided core needle biopsy.
Figure 1.
Axial (A) and coronal (B) mammography of the right breast showing a soft tissue mass (arrows) in the external upper quadrant.
Treatment
The circumscribed lesion was excised for frozen section during operation. By pathological examination, the frozen section was diagnosed as carcinoma sarcomatodes. Then, extended excision was performed for a negative margin. The palpable lymph nodes in the right axilla also underwent excisional biopsy and the frozen section was confirmed to have chronic inflammation.
Grossly, the lesion was a well-circumscribed grey-tan nodule measuring 1.1×0.5×0.8 cm within a fibro-fatty breast tissue rim. Necrosis and haemorrhage were not found. The specimen was fixed in 10% buffered formalin, embedded in paraffin wax and sectioned at 5 μm. Then, the sections were analysed by haematoxylin staining and eosin staining and routine immunohistochemistry staining.
Microscopically, the proliferating spindle cells comprised most of the tumour and formed sarciniform patterns (figure 2A). In some margins, the spindle cells infiltrated into fat tissue. The stroma was mixed with diffuse plasma cells and small lymphocytes infiltrated. Spindle cells were cytologically bland; mitotic rate was 0–1 mitotic figures/10 high-power fields with no atypical forms. Surrounding breast tissue was unremarkable.
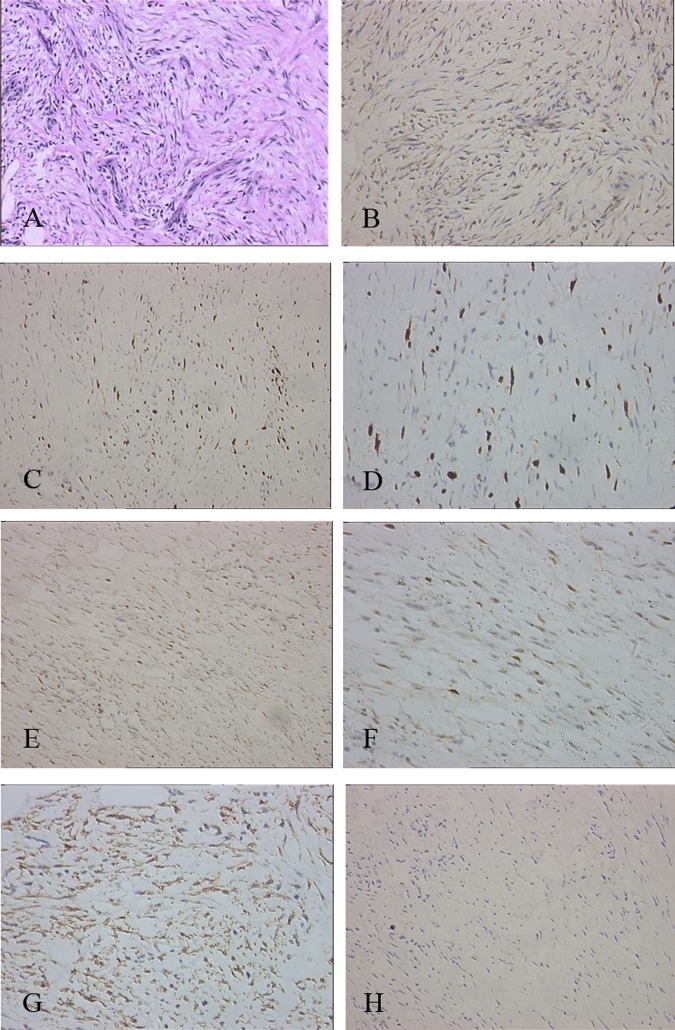
Figure 2.
(A) Proliferation of spindle cells was accompanied by numerous plasma cells and small lymphocytes (×200 haematoxylin-eosin). (B) Immunohistochemical staining showing the tumour cells were positive for smooth muscle actin (×200 ). (C) The tumour cells were positive for vimentin (×200 ). (D) The tumour cells were negative for S-100 protein ( ×200 . (E) The tumour cells were negative for cytokeratins (200×). (F) The tumour cells were negative for CD34 (200×). (G) The tumour cells were positive for Ki-67 (200×). (H) The tumour cells were positive for p53 (200×).
Immunohistochemical staining showed that the spindle cells were positive for smooth muscle actin and vimentin, but negative for S-100 protein, cytokeratins and CD34 (figure 2). In order to exclude the possibility of an inflammatory pseudotumour-like reticulum cell sarcoma, the immunohistochemical staining of CD21 and CD35 were both negative. These features were consistent with an IMT. To further identify the lesion, we performed immuohistochemical staining using a panel of antibodies against proteins involved in the malignant tumour phenotype. We found that the spindle cells were immunoreactive for ALK1 (figure 3A), Ki67Ki-67 and P53 p53 (figure 2). Based on the strong staining of ALK1 which indicated ALK1 overexpression, we further detected the ALK1 gene amplification by fluorescence in situ hybridisation (FISH) and indeed found ALK1 amplification in this sample by FISH (figure 3B).
Figure 3.
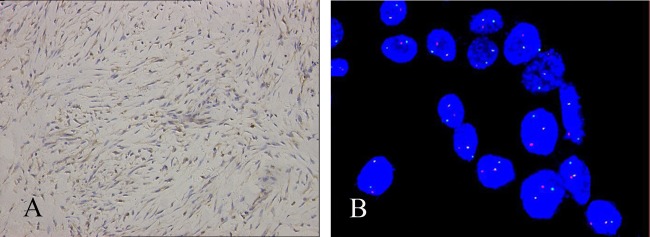
(A) Immunohistochemical staining showing the tumour cells was positive for anaplastic lymphoma kinase 1 (ALK1) (×200 ). (B) Fluorescence in situ hybridisation analysis showing the amplification of ALK1 gene (× 100 ).
Outcome and follow-up
The patient is still alive without recurrence.
Discussion
IMT was considered as a non-neoplastic reactive inflammatory condition with an exaggerated response to tissue injury. However, its potential for local recurrence has challenged the concept of a reactive postinflammatory lesion. Presently, IMT is recognised as a neoplasm of intermediate biological potential. This distinctive neoplasm is composed of myofibroblastic mesenchymal spindle cells accompanied by an inflammatory infiltrate of plasma cells, lymphocytes and eosinophils. Although IMT occurs primarily in the lung, recent reports have shown that it also occurs in soft tissue and viscera of children and young adults. The IMT of breast has been reported in 19 cases in 16 published papers, and 18 cases reported in the literature have risen spontaneously without any history of prior external or intraparenchymal trauma. Only one case reported a post-traumatic IMT of the breast.3 Our case here is the first case with anaplastic lymphoma kinase (ALK) overexpression both with immunohistochemistry and FISH exam.
We describe here a case of IMT arising in the breast of a 46-year-old woman. IMT rarely occurs in patients over 40 years old. Three histological types of IMT have been identified as follows: (1) inflammatory type: a myxoid, vascular and inflammatory proliferation resembling granulation tissue or nodular fasciitis; (2) cellular type: compact spindle cells with inflammation resembling a fibrous histiocytoma or a fibromatosis and (3) hypocellular type: dense plate-like collagen which resembles a desmoid tumour or a scar.4 Most of the spindle cells have the characteristics of a myofibroblast, and are immunohistochemically positive for vimentin, muscle-specific actin and smooth muscle actin in the major cases. In the present case, remarkable histological feature and other morphological findings were consistent with the cellular type of IMT. In addition, immunohistochemical staining showed a positive reaction for smooth muscle actin and vimentin, and a negative reaction for S-100 protein, cytokeratins, CD21, CD35 and CD34, which is similar to IMT reported at other sites.
The diagnosis of IMT is often confused with other spindle cell tumours, such as low-grade fibromatosis-like spindle cell carcinoma, myofibroblastoma, leiomyosarcomas and malignant fibrous histiocytomas. To distinguish IMT from these tumours, it is important to note that low-grade fibromatosis-like spindle cell carcinoma has no inflammatory infiltration and is positive for CD117, whereas myofibroblastomas are well-demarcated lesions formed by clusters of spindle cells separated by bands of hyalinised collagen. In addition, the mitotic rate and atypical forms of leiomyosarcomas are higher than those of IMT, and malignant fibrous histiocytomas are negative for immunoreactivity of smooth muscle actin.
Recent studies have suggested that ALK, a receptor tyrosine kinase encoded by a gene located on the short arm of chromosome 2 at 2p23, is implicated in the pathogenesis of IMT.5 The aberrant fusion of ALK gene with TPM3, TPM4 and some unknown genes would result in the overexpression of ALK protein, and ALK gene rearrangements occur in about 50–75% of IMT.6 Dysregulation of ALK has been shown to play an important role in tumorigenesis by promoting abnormal phosphorylation of cellular substrates, but the prognostic consequences of ALK expression in IMT are unclear. A review of extrapulmonary IMTs documented a recurrence rate of 31% among ALK-negative tumours and 69% among ALK-positive tumours.7 Although ALK1 gene amplification of IMT is usually presented, it is not clear whether IMT in breast is very different from IMT in other organs. The present case documents, for the first time, an ALK-positive breast IMT with gene amplification and protein overexpression. Future studies should explore the contribution of ALK dysregulation to the pathogenesis of breast IMT.
Immunostaining of IMT has revealed the overexpression of various cell cycle and apoptosis regulatory proteins with potential diagnostic and prognostic utility. Occasional cases of IMT have manifested disruption of the autoregulatory feedback loop involving p53, and may be associated with the aggressiveness of IMT.8 Our case exhibited considerable nuclear immunostaining with both p53 and Ki-67. Positivity for the nuclear protein Ki-67, an index of proliferative activity, is often associated with malignancy and elevated in some IMTs, which range from 0 to 10%, although the prognostic significance with regard to IMT is equivocal. One study found that 19% (3/16) of patients who died of inflammatory fibrosarcoma were positive for Ki-67 protein ranging from 1% to 5%.9 Another study reported that 38% (3/8) of IMTs with Ki-67 nuclear staining (up to 10%) were associated with multifocal origin, aggressive tumour behaviour and rapid growth.8 Immunopositivity for proteins with oncogenic potential such as ALK and Ki-67 may help predict the outcome in our case.
Complete surgical resection remains the choice of treatment for extrapulmonary IMT, but after the excision, no definitive clinical, histopathological, cytogenetical or molecular genetic features are available to predict the risk of recurrence or metastasis. It is reported that the dominance of a myofibroblastic or fibroblastic phenotype in this lesion may indicate that this lesion will recur and be locally aggressive. For extra-lung IMT, the recurrence rate is about 25%, and the metastasis rate is less than 5%. The clinical behaviour of most cases of IMT in the breast is benign, except a recurrence case in the literature.2 The prognosis of IMT in the breast seems to be good, and this case is alive without any signs of recurrence 5 years after surgery.
In conclusion, IMT of the breast is a rare lesion that may mimic malignant breast tumours clinically and pathologically. Caution should be exercised when evaluating spindle cell lesions. An accurate diagnosis is crucial to avoid unnecessary radical surgery. Significantly, the case we presented here is unique in showing ALK1 overexpression and ALK1 gene amplification in IMT of the breast. The role of ALK1 gene amplification in IMT of the breast is still unclear and needs to be further studied, which has important diagnostic and therapeutic implications.
Learning points.
Inflammatory myofibroblastic tumour (IMT) of the breast can be misdiagnosed as breast carcinoma in preoperative clinical examination and even frozen section is hard to make the right decision.
Immunostaining may help the differential diagnosis of IMT to other spindle cell tumours.
The role of anaplastic lymphoma kinase 1 gene amplification in IMT of the breast is also important as other organs and needs to be studied further.
After extended excision with a negative margin, the clinical behaviour of breast IMT is benign.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Fletcher CDM, Unni KK, Mertens F. World Health Organization classification of tumors. Pathology and genetics of tumors of soft tissue and bone. Lyon: IARC Press, 2002:48–106 [Google Scholar]
- 2.Khanafshar E, Phillipson J, Schammel DP, et al. Inflammatory myofibroblastic tumor of the breast. Ann Diagn Pathol 2005;9:123–9 [DOI] [PubMed] [Google Scholar]
- 3.Vecchio GM, Amico P, Grasso G, et al. Post-traumatic inflammatory pseudotumor of the breast with atypical morphological features a potential diagnostic pitfall. Report of a case and a critical review of the literature. Pathol Res Pract 2011;207:322–6 [DOI] [PubMed] [Google Scholar]
- 4.Zen Y, Kasahara Y, Horita K, et al. Inflammatory pseudotumor of the breast in a patient with a high serum IgG4 level histologic similarity to sclerosing pancreatitis. Am J Surg Pathol 2005;29:275–8 [DOI] [PubMed] [Google Scholar]
- 5.Armstrong F, Lamant L, Hieblot C, et al. TPM3-ALK expression induces changes in cytoskeleton organisation and confers higher metastatic capacities than other ALK fusion proteins. Eur J Cancer 2007;43:640–6 [DOI] [PubMed] [Google Scholar]
- 6.Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol 2007;31:509–20 [DOI] [PubMed] [Google Scholar]
- 7.Cook JR, Dehner LP, Collins MH, et al. Anaplastic lymphoma kinase (ALK) expression in the inflammatory myofibroblastic tumor a comparative immunohistochemical study. Am J Surg Pathol 2001;25:1364–71 [DOI] [PubMed] [Google Scholar]
- 8.Brooks JK, Nikitakis NG, Frankel BF, et al. Oral inflammatory myofibroblastic tumor demonstrating ALK, p53, MDM2, CDK4, pRb, and Ki-67 immunoreactivity in an elderly patient. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005;99:716–26 [DOI] [PubMed] [Google Scholar]
- 9.Meis-Kindblom JM, Kjellström C, Kindblom LG. Inflammatory fibrosarcoma update, reappraisal, and perspective on its place in the spectrum of inflammatory myofibroblastic tumors. Semin Diagn Pathol 1998;15:133–43 [PubMed] [Google Scholar]