Abstract
New approaches to expedite the development of safe and effective pediatric dosing regimens and first-in-child doses are urgently needed. Model-based approaches require quantitative functions on the maturation of different metabolic pathways. In this study, we directly incorporated a pediatric covariate model for the glucuronidation of morphine into a pediatric population model for zidovudine glucuronidation. This model was compared with a reference model that gave the statistically best description of the data. Both models had adequate goodness-of-fit plots and normalized prediction distribution errors (NPDE), similar population clearance values for each individual, and a Δobjective function value of 13 points (Δ2df). This supports our hypothesis that pediatric pharmacokinetic covariate models contain system-specific information that can be used as semiphysiological functions in pediatric population models. Further research should explore the validity of the semiphysiological function for other UDP-glucuronosyltransferase 2B7 substrates and patient populations and reveal how this function can be used for pediatric physiologically based pharmacokinetic models.
Knowledge on the influence of developmental changes on drug pharmacokinetics (PK) and pharmacodynamics is limited. This may cause the high off-label and unlicensed drug prescription observed in the pediatric population,1 as well as complicate the selection of first-in-child doses during drug development. As it would require a tremendous amount of resources to thoroughly investigate changes in drug PK and pharmacodynamics throughout the entire pediatric age range for each individual drug, new approaches to expedite the development of safe and effective pediatric dosing regimens and first-in-child doses are necessary.
To apply modeling approaches throughout the entire pediatric population, reliable and precise qualitative and quantitative information on developmental changes in various parts of the physiological system that are involved in all the underlying absorption, distribution, metabolism, and elimination processes are required. It is imperative to develop maturation functions that quantify developmental changes in metabolic and elimination pathways, because drug clearance drives drug exposure, and differences in drug exposure are thought to be the major cause of age-dependent differences in pediatric drug dose requirements.2 For metabolic pathways, quantitative information on enzyme activity can be obtained from in vitro studies; however, for instance, for uridine 5′-diphosphate glucuronisyltransferase-mediated glucuronidation, this type of information is limited for the pediatric population.3 We therefore investigated whether maturation functions for metabolic pathways can be obtained from pediatric population PK covariate models that are based on in vivo outcome measures. Population modeling allows for the simultaneous analysis of sparse, dense, and/or unbalanced data, which may even have been collected for clinical purposes (e.g., therapeutic drug monitoring). In fact, by combining historic data from multiple sources, the burden to individual patients can be significantly reduced, while improving the precision of the parameter estimates in a resulting population model. In population models, the net influence of developmental changes in underlying physiological processes on drug clearance is quantified in covariate models.
In this proof-of-concept study, we test the hypothesis that pediatric population PK covariate models contain quantitative information on the influence of maturational changes in the biological system on drug PK and that these covariate models can therefore be extrapolated between drugs that share elimination pathways.4 For this study, an internally and externally validated pediatric covariate relationship for morphine glucuronidation in patients younger than 3 years was directly incorporated into a population model for zidovudine glucuronidation in patients younger than 5 months. As both drugs are primarily eliminated through the UDP-glucuronosyltransferase 2B7-mediated glucuronidation,5,6,7 this model was called the system-specific model. The performance of this model was compared with the performance of a reference model that was based on a comprehensive covariate analysis of the zidovudine data alone.
Results
Patients and data
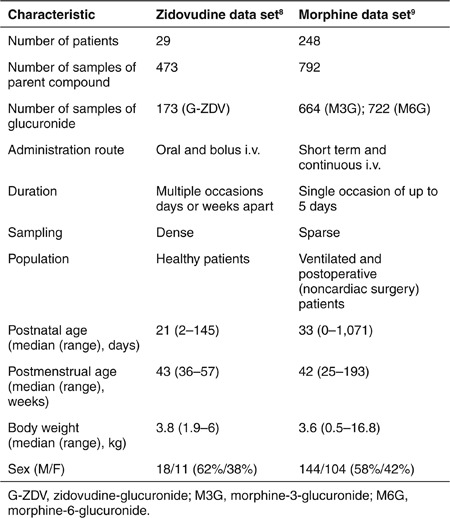
The current analysis is based on 473 zidovudine concentrations and 173 zidovudine-glucuronide concentrations collected on 68 occasions from 29 individuals varying from term neonates to infants up to 5 months of age (PACTG 049).8 For each patient, dense data were available from multiple occasions that were days or weeks apart. Zidovudine was administered both intravenously and orally to each patient. Data were obtained after single-dose administrations on separate occasions and for eight patients, data from administrations that were part of a long-term oral dosing regimen were available as well. This data set was used to develop the two population models in this study. An example of the data records for two patients at the extremes of the age range is provided in the Supplementary Data online.
A data set of morphine and its glucuronides in 248 preterm and term neonates to 3-year-old infants was used to obtain the pediatric covariate model used in the system-specific model.9 In Table 1, study and patient characteristics for the zidovudine data set used for both models in this analysis and the morphine data sets used to obtain the pediatric covariate model are shown for comparison.
Table 1. Patient and study characteristics of the zidovudine data set that was analyzed in this study to build the system-specific model and the reference model, and of the morphine data set that was used to build the pediatric covariate model applied in the system-specific model.
System-specific and reference model for zidovudine
Figure 1 shows the structural model for zidovudine, which is the same for both the system-specific model and the reference model, and the covariate equations, which are different between the two models.
Figure 1.
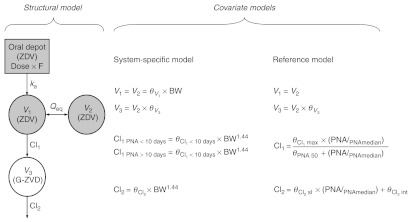
Schematic representation of the structural model for the zidovudine models (left) and equations of the covariate relationships in the reference model (middle) and the system-specific model (right). BW, body weight; Cl, clearance of designated route; F, bioavailability; G-ZDV, zidovudine-glucuronide; ka, absorption rate constant; PNA, postnatal age with subscript “median” indicating the median value of the individuals at the different occasions; Qeq intercompartmental clearance; V, distribution volume of designated compartment; ZDV, zidovudine; θV, distribution volume of designated compartment as fraction of 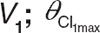 , maximum value of the zidovudine glucuronidation clearance; θPNA 50, postnatal age at which half the maximum value of zidovudine glucuronidation clearance is reached;
, maximum value of the zidovudine glucuronidation clearance; θPNA 50, postnatal age at which half the maximum value of zidovudine glucuronidation clearance is reached; 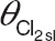 , slope of the line describing age-related changes in zidovudine-glucuronide elimination clearance;
, slope of the line describing age-related changes in zidovudine-glucuronide elimination clearance;  , y-intercept of the line describing age-related changes in zidovudine-glucuronide elimination clearance;
, y-intercept of the line describing age-related changes in zidovudine-glucuronide elimination clearance; 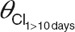 , population value of zidovudine glucuronidation clearance value in children within 10 days of birth;
, population value of zidovudine glucuronidation clearance value in children within 10 days of birth; 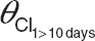 , population value of zidovudine glucuronidation clearance value in children older than 10 days;
, population value of zidovudine glucuronidation clearance value in children older than 10 days; 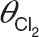 , population value of zidovudine-glucuronide elimination clearance value.
, population value of zidovudine-glucuronide elimination clearance value.
For the structural model, a two-compartment model (V1 and V2) was found to describe the bimodal decline in zidovudine concentrations in time. Both distribution volumes were set to be equal. A one-compartment model was used to describe the time course of the zidovudine-glucuronide (V3) with the distribution volume estimated as a fraction of the central compartment 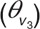 . Zidovudine absorption was described by first-order absorption (ka) and the oral bioavailability (F) was estimated.
. Zidovudine absorption was described by first-order absorption (ka) and the oral bioavailability (F) was estimated.
For the error model, significant interindividual variability could be identified in both models for the absorption rate constant (ka), the formation (Cl1) and elimination (Cl2) clearance of zidovudine-glucuronide, the distribution volume of the central compartment (V1), and the bioavailability (F). The interindividual variability and residual error for both models were best described by a proportional error model.
The covariate model was different for the system-specific model and the reference model (Figure 1).
System-specific model. A previously published covariate model for glucuronidation of morphine in patients varying from preterm neonates to children of 3 years9,10 was directly incorporated in the model for zidovudine. This covariate model consisted of a body weight-based exponential equation with an exponent of 1.44 for the formation and elimination of zidovudine-glucuronide, with a reduced formation clearance of zidovudine-glucuronide in neonates within 10 days of birth, and linear relationships between body weight and distribution volumes.9
Reference model. A comprehensive covariate analysis identified age (either postnatal or postmenstrual age) and body weight as predictive and statistically significant covariates for the formation (Cl1) and elimination (Cl2) clearance of zidovudine-glucuronide. Due to the relatively small range in body weight and age of the patients in the current zidovudine data set (Table 1), only small differences in objective function and diagnostics between models using either of the three covariates or between models using these covariates in different equations (i.e., linear, exponential, or sigmoidal) were obtained. On the basis of objective function, postnatal age was found to be a slightly superior covariate for both Cl1 and Cl2. The inclusion of this covariate was most optimal in a sigmoidal relationship on Cl1 and in a linear relationship with estimated y-intercept on Cl2. No other statistically significant covariates were identified.
In Table 2, the model parameter estimates obtained for the system-specific and reference models are provided, showing similar values for the structural parameters and the parameters of the error model. The table also shows that for both models the coefficients of variation of the fixed effects remain well below 50%, indicating that the parameters can be estimated with acceptable precision. The coefficients of variation of some of the variance estimates of the interindividual variability did exceed 50%, indicating that the information in the data set was uninformative for precise estimation of these parameter values. The NONMEM code for two final models is provided in the Supplementary Data online.
Table 2. Final parameter estimates of the system-specific model and the reference model for zidovudine glucuronidation.
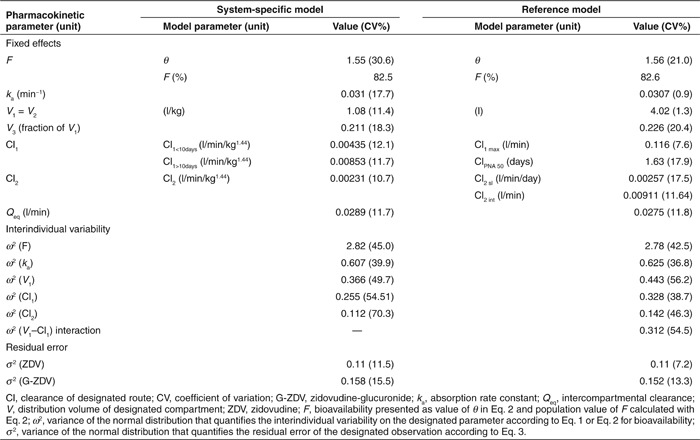
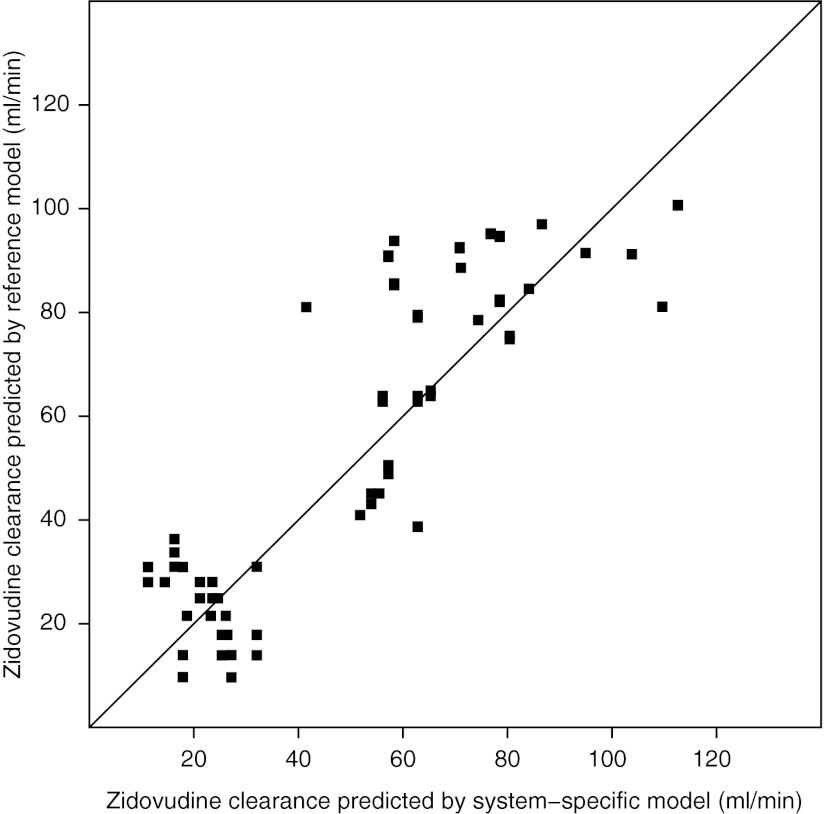
Figure 2 shows population-predicted zidovudine clearances (Cl1) for the reference model vs. the system-specific model. As data were available on different occasions that were days or weeks apart, the covariates (body weight and postnatal age) differed for each child on the different occasions, yielding different clearance predictions per child per occasion. The figure shows that both models estimate similar population clearance values for each individual at each occasion, despite the differences in covariate model as depicted in Figure 1.
Figure 2.
Population-predicted zidovudine clearances (Cl1) for the reference model vs. the system-specific model for each individual at each separate study occasion.
Model evaluation
The reference model was statistically superior over the system-specific model in describing the zidovudine data, as demonstrated by a difference in objective function value of 13 points at a 2-point difference in degrees of freedom. Figure 3 shows the goodness-of-fit graphs that are stratified by age into one group that is older than and another group that is younger than 38 days (the median age of the individuals at the different occasions). Visual inspection of these graphs shows that both models can describe the observed zidovudine concentrations in children older and younger than the median age without bias and that the difference between the plots of the two models is negligible.
Figure 3.
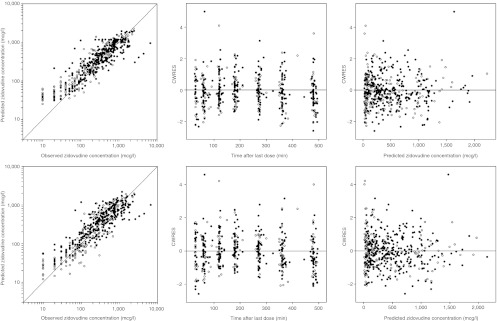
Basic goodness-of-fit plots for zidovudine of the system-specific model (top) and the reference model (bottom). On the left are population-predicted concentrations vs. observed concentrations, in the middle are conditional weighted residuals (CWRES) vs. time after last dose, and on the right are CWRES vs. population-predicted concentrations. The plots are stratified by age, with solid circles indicating observations in children on occasions that they were younger than 38 days, the median age of the individuals at the different occasions, and open circles indicating observations in children on occasions that they were older than 38 days.
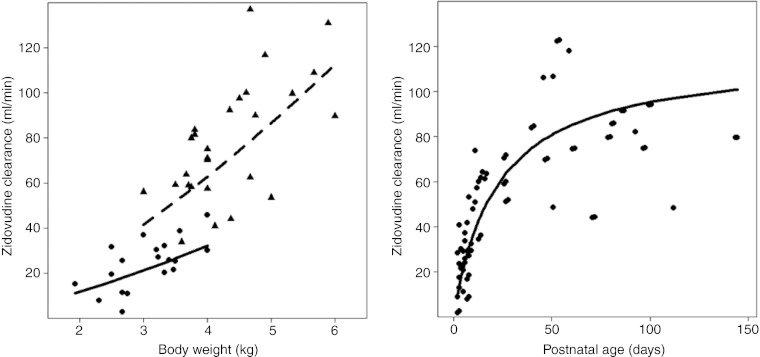
The plots in Figure 4 show that the covariate relationships for zidovudine clearance (Cl1) of both the system-specific and reference model describe individual glucuronidation clearances without bias, despite the use of different covariates (i.e., body weight for the system-specific model and postnatal age for the reference model). Accuracy of the individual zidovudine clearance values compared with the population values described by the covariate relationships was numerically quantified as mean percentage error and was 20.5% for the reference model and 11.3% for the system-specific model. The precision, numerically quantified as root mean square error, was 19.2% for both models.
Figure 4.
Individual post hoc parameter values of the glucuronidation clearance to zidovudine-glucuronide (Cl1) for each individual at each separate study occasion vs. the most predictive covariate, which is body weight for the system-specific model (left) and postnatal age for the reference model (right). The covariate relationships describing the population clearance values are indicated with lines. For the plot of the system-specific model (left), individual post hoc parameter estimates and population estimates of children younger than 10 days are indicated with circles and a solid line, respectively, for children older than 10 days, triangles and a dotted line are used, respectively.
In terms of predictive performance, the two models perform similar as well, as expressed by the results of the normalized prediction distribution error (NPDE) analysis shown in Figure 5. This figure shows that the system-specific model and the reference model can accurately predict the median zidovudine concentrations, but they slightly overestimate the variability in the observations. In addition, there is no bias in NPDEs in time or across the concentration range for any of the models.
Figure 5.
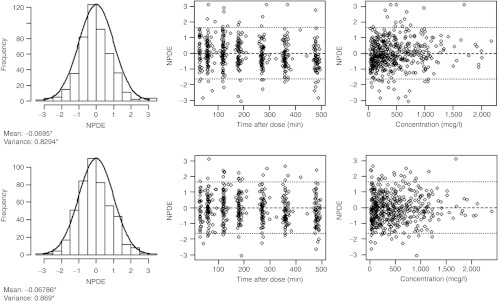
Results of the normalized prediction distribution error (NPDE) analysis for zidovudine from the system-specific model (top) and the reference model (bottom). In the histograms, the distributions of the NPDEs in the overall data set are shown with the solid line depicting a normal distribution and the values below specifying the mean and variance of the NPDE distribution in the histogram. A significant (P < 0.05) deviation of the distribution from a mean of 0 and a variance of 1 is indicated with an asterisk (*). The NPDE distribution against time after last dose (middle) and against the observed concentrations (right) are also shown.
Discussion
Our group previously described and defined a distinction between drug-specific and system-specific parameters in population models.11 This investigation is a proof-of-concept study to examine whether the context of system-specific properties can be extended to include not only static descriptors of the physiological system but also temporal changes in the physiological system as a result of developmental changes in the pediatric population. It was shown that a covariate model for the glucuronidation of morphine in preterm and term neonates to children up to the age of 3 years9,10 also accurately reflects developmental changes in zidovudine glucuronidation clearance in term neonates and infants. Similar results have been obtained by our group for the glomerular filtration of antibiotics in neonates.12 These results support the hypothesis that pediatric covariate models for drug clearance may serve as semiphysiological functions in population PK models, although further research should reveal the generalizability of this approach in scenarios with different drug and patient characteristics.
For the maturation of metabolic pathways, functions obtained with population modeling from in vivo sources reflect the net effect of maturational changes in multiple underlying physiological systems. This may include changes in expression and function of drug metabolizing enzymes and active transporters, changes in cardiac output and organ perfusion, changes in acid–base balance, and changes in the amount and composition of drug-binding plasma proteins and other blood components that may influence plasma protein binding.13 Unfortunately, quantitative knowledge on all these underlying processes is incomplete especially for the pediatric population, impeding the use of pediatric physiologically based PK (PBPK) models. In addition, data on enzyme activity obtained from in vitro data may not accurately reflect the in vivo situation, especially for uridine 5′-diphosphate glucuronisyltransferase enzymes.3,14,15 Combining information from population PK models with PBPK approaches may therefore be necessary to obtain functions to describe the maturational changes in metabolic and/or elimination pathways. These modeling approaches are crucial for determining evidence-based pediatric dosing algorithms and first-in-child doses.
The proposed semiphysiological modeling approach can be performed using data routinely obtained in pediatric pharmacokinetic trials. In fact, this approach allows for the use of denser information or information from a wider age range than may be available for the analysis of the unstudied drug. This is especially important in the pediatric population where often only limited data are available for ethical and practical reasons. The system-specific model of this analysis was, for instance, based on data from 248 patients ranging from preterm neonates to infants of 3 years, whereas in the current zidovudine analysis, data from only 29 patients ranging from term neonates to infants of 5 months were available (Table 1). The small range in age and body weight of the patients in the zidovudine data set made it difficult to discriminate model performance between submodels. The covariate relationship identified for morphine glucuronidation was probably not identified as most significant in the comprehensive covariate analysis of the current zidovudine data due to the indistinctive curvature of this relationship in the body weight range of the zidovudine data set. Nonetheless, in the system-specific model, direct incorporation of the pediatric covariate model obtained with morphine did provide a good description of the population and individual zidovudine clearance parameters as shown in Figure 4. In this approach, it is, however, a prerequisite that the covariate models are validated both internally and externally and that the population to which the covariate model applies is well defined in terms of other potentially important covariates, like, for instance, genetics or disease status. In case covariates other than size and/or age are identified in a pediatric covariate model, we envision that the complete covariate model describes the biological system and that therefore this complete covariate model should be extrapolated between drugs.
In contrast to PBPK models, a drawback of population PK modeling is that the analysis has to be repeated for each individual drug for each pediatric age group. This study suggests that the between-drug extrapolation of semiphysiological pediatric covariate functions can be used to develop pediatric population PK models of hitherto unstudied drugs in a more time-efficient manner. However, the use of the semiphysiological glucuronidation function in the population analysis of zidovudine still relied on the availability of at least a limited amount of pediatric data to determine the population value of the clearance, which is mainly determined by the drug-specific parameters Km and Vmax. This does not pose a problem for marketed drugs that are already being used off-label in a population, but when in drug development a drug has never been used in a pediatric age range before, a methodology that does not rely on in vivo pediatric data of the drug under investigation is required. To date, there is no suitable methodology based on population PK modeling available to extrapolate pediatric PK parameters from older to younger age ranges in the drug development process.16 If system-specific profiles on developmental changes in specific metabolic pathways were, however, available over the entire pediatric age range, these profiles could be used in a semiphysiological modeling approach to design successive studies in children of decreasing ages for unstudied drugs. These studies could then be of a confirmative rather than an explorative nature.
Concerning the net observed maturational changes in drug clearance in this study, it is emphasized that the weight that each change in the physiological system has may be different for drugs with different physicochemical and PK properties. Morphine and zidovudine are quite similar with respect to these properties. Their molecular masses are 285 and 267 g/mol, respectively. The acid dissociation constant (pKa) values for morphine are 7.9 and 9.617 and for zidovudine this value is around 9.718,19 and the octanol/water partition coefficient (log P) values for these compounds were reported to be around 0.7517 and 0.05,20,21 respectively. Plasma protein binding in adults ranges between 23 and 38% for both drugs,20,22,23 and their hepatic extraction ratios in adults range between 0.5 and 0.65.24,25,26 We cannot exclude that these similarities in drug characteristics have contributed to the good extrapolation potential of the pediatric covariate model for glucuronidation observed in this study, therefore, further studies on the applicability of the semiphysiological function for glucuronidation in children are required. For this, in silico studies using a PBPK modeling approach could be performed to reveal whether, how, and to what extent differences in physicochemical drug properties influence the between-drug extrapolation potential of the semiphysiological glucuronidation function. This can be done by simulating in vivo pediatric drug clearance of hypothetical drugs with various physicochemical drug properties that are all eliminated through the same pathway (see Part II of this article, ref. 27). Investigation of maturation patterns in individual physiological parameters and PBPK simulations of scenarios in which values of these parameters are altered may reveal whether the changes in drug clearance quantified by a pediatric semiphysiological function mainly result from changes in a single or multiple parameters (see Part II of this article, ref. 27). This can be used to determine whether a population covariate relationship can be directly incorporated in PBPK models or whether further deconvolution of the covariate relationship is necessary. The advantage of PBPK model in drug development would be that it may aid in determining the absolute value of drug clearance without prior pediatric in vivo data.
In conclusion, this proof-of-concept study supports our hypothesis that pediatric population covariate models that describe the developmental changes in drug elimination pathways constitute system-specific rather than drug-specific information. This system-specific information can be used in a semiphysiological manner in the development of pediatric population PK models of drugs that share clearance pathways. Further analysis of the physicochemical and physiological basis of the pediatric semiphysiological glucuronidation function should reveal whether this function can be directly incorporated in PBPK models for all substrates or whether it is necessary to separate the covariate relationship further into components to describe the influence of the various physiological changes.
Methods
Study design. Two population PK models were developed for a single data set of zidovudine and its glucuronide.
System-specific model: In this model, a covariate model for morphine glucuronidation in patients younger than 3 years that was validated both internally and externally using various tools9,10 was directly incorporated.
Reference model: For this model, a comprehensive covariate analysis was performed yielding a PK model with a set of covariate relationships that best described the current zidovudine data according to predefined statistical criteria.
The performance of both models was evaluated according to a previously published framework for the validation of pediatric population models,28 and the results of this evaluation for the system-specific model (A) were compared with the results obtained for the reference model (B).
Patients and data. Data were obtained from a multicenter study to evaluate the safety, tolerability, and PK of zidovudine as a prophylaxis to prevent mother-to-child HIV transmission in healthy neonates and infants born to HIV-infected women. The study protocol was approved by institutional review boards of the participating institutions and written informed consent was obtained from the parents or legal guardians of each patient.
Model development. NONMEM VI (ICON, Ellicott City, MD) was used to perform the data analysis, with PLT Tools version 3.0.0.29 in combination with R version 2.10.0 for the visualization of the data. All parameter estimates were obtained with the first-order conditional estimation method with interaction. Model development for the reference model and the system-specific model was performed in three steps: choice of structural model; choice of error model; and choice of the covariate model.
For both models, the first two steps in the model development process (i.e., the choice of the structural and error model) were the same. One- and two-compartment models were tested for the structural model. For the error model, interindividual variability on the model parameters was tested assuming a log-normal distribution described by an exponential distribution model depicted in Eq. 1. For bioavailability (F), interindividual variability was described using Eq. 2 to avoid individual bioavailability estimates of more than 100%
 |
In these equations, Pi is the individual parameter estimate for the ith individual, θ represents the population parameter estimate for parameter P, and ηi is a random variable for the ith individual from a normal distribution with a mean of zero and estimated variance of ω2. For the intraindividual variability and residual error in the observed zidovudine and zidovudine-glucuronide concentrations, proportional, additive, and combination error models were tested.
The likelihood ratio (assumed to be χ2 distributed) was used to assess whether the difference between (sub)models was statistically significant. A decrease in the objective function corresponding to P < 0.01 was considered to be significant. In addition, the following basic goodness-of-fit plots were used for diagnostic purposes (i) observed vs. individually predicted concentrations, (ii) observed vs. population-predicted concentrations, (iii) conditional weighted residuals vs. time, and (iv) conditional weighted residuals vs. population-predicted concentrations. Furthermore, the 95% confidence intervals of the model parameters and the correlation matrix were assessed.
The third and final step of the model development process (i.e., choice of the covariate model) was different for the reference model and the system-specific model:
System-specific model: The previously obtained and validated covariate model for morphine glucuronidation in children within 3 years9,10 was directly incorporated into the model for zidovudine. Specifically, a body weight-based exponential equation with an exponent of 1.44 for the formation and elimination of zidovudine-glucuronide with a reduced formation clearance of zidovudine-glucuronide in neonates within 10 days of birth was included, as was a linear correlation for distribution volume of the parent compound and metabolite. Although this pediatric covariate model describes the rate of developmental changes in clearance and distribution volume, the population values of these parameters for zidovudine were estimated by NONMEM.
Reference model: A comprehensive covariate analysis with forward inclusion and backward deletion of covariates was performed to obtain a covariate model with the best description of the current zidovudine data according to statistical criteria. The following covariates were tested for significance: postnatal age, postmenstrual age, gestational age at birth, body weight, sex, and creatinine clearance. The continuous covariates were tested in linear equations, exponential equations with estimated exponents, or sigmoidal equations. A decrease in the objective function corresponding to P < 0.01 for the forward inclusion of covariates was considered to be significant. In addition, the aforementioned diagnostic criteria were used. When more than one significant covariate was identified, the most significant covariate was included in the model and the resulting model served as the basis for the subsequent exploration of additional covariate effects. For the backward deletion of covariates, an increase in objective function corresponding to P < 0.001 was considered to be significant.
Model evaluation. Although the system-specific and reference models are not nested, they are based on the same patients and data, and therefore, the −2 log likelihood, by means of the NONMEM objective function, was used to statistically compare the description of the zidovudine data by the system-specific model with the description of the zidovudine data by the reference model. To directly compare clearance predictions between the two models, population clearance predictions from the reference model were plotted vs. population clearance predictions from the system-specific model. For each patient, data were obtained at multiple occasions, as age and bodyweight change rapidly in this young population one population prediction was obtained per patient per occasion.
According to the framework for the validation of pediatric population models,28 the basic goodness-of-fit plots of the models were inspected. These plots were stratified by age into a group that was younger and a group that was older than 38 days (the median age of the individuals at the different occasions) to ascertain that the entire age range was described equally well. In addition, the covariate relationships describing the population-predicted zidovudine clearance (Cl1) and the individual post hoc clearance estimates of each individual at each separate study occasion were plotted in one graph for each model to visually assess the description of the individual zidovudine glucuronidation clearances (Cl1) by the covariate relationships. Finally, bias and precision of the individual zidovudine glucuronidation clearance values compared with the population-predicted clearances described by the covariate relationships were quantified by calculating the percentage mean prediction error (%MPE, Eq. 3) and the root mean square error (RMSE, Eq. 4), respectively.
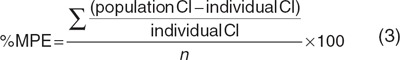 |
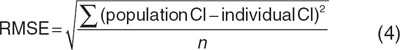 |
To compare the predictive properties of both models, an NPDE analysis,30 which is a simulation-based diagnostic, was used. The entire data set was simulated 1,000 times in NONMEM and subsequently each observed concentration was compared with the reference distribution of the simulated data points using the NPDE add-on package in R.31
Author Contributions
E.H.J.K., M.N., D.T., E.C., M.M., and C.A.J.K. designed the research. M.N., E.C., and M.M. performed the research. E.H.J.K., E.P., M.D., and C.A.J.K. analyzed the research. E.H.J.K., M.N., E.P., D.T., E.C., M.D., M.M., and C.A.J.K. wrote the manuscript.
Conflict of Interest
All authors declared no conflict of interest.
Study Highlights

Acknowledgments
This study was performed within the framework of Top Institute Pharma project number D2-104. The work of C.A.J.K. is supported by the Innovational Research Incentives Scheme (Veni grant, July 2006) of the Dutch Organization for Scientific Research (NWO). Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) (U01 AI068632), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institute of Mental Health (AI068632). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was supported by the Statistical and Data Analysis Center at Harvard School of Public Health, under the NIAID cooperative agreement no. 5 U01 AI41110 with the Pediatric AIDS Clinical Trials Group (PACTG) and no. 1 U01 AI068616 with the IMPAACT Group. Support of the sites was provided by the NIAID and the NICHD International and Domestic Pediatric and Maternal HIV Clinical Trials Network funded by NICHD (contract number N01-DK-9-001/HHSN267200800001C). We thank Gregory Sivolapenko from the Laboratory of Pharmacokinetics of the University of Patras for his support.
Supplementary Material
References
- Cuzzolin L., Atzei A., &, Fanos V. Off-label and unlicensed prescribing for newborns and children in different settings: a review of the literature and a consideration about drug safety. Expert Opin. Drug Saf. 2006;5:703–718. doi: 10.1517/14740338.5.5.703. [DOI] [PubMed] [Google Scholar]
- Alcorn J., &, McNamara P.J. Ontogeny of hepatic and renal systemic clearance pathways in infants: part I. Clin. Pharmacokinet. 2002;41:959–998. doi: 10.2165/00003088-200241120-00003. [DOI] [PubMed] [Google Scholar]
- Krekels E.H.J., Danhof M., Tibboel D., &, Knibbe C.A.J. Ontogeny of hepatic glucuronidation; methods and results. Curr Drug Metab. 2012;13:728–43. doi: 10.2174/138920012800840455. [DOI] [PubMed] [Google Scholar]
- Knibbe C.A., Krekels E.H., &, Danhof M. Advances in paediatric pharmacokinetics. Expert Opin. Drug Metab. Toxicol. 2011;7:1–8. doi: 10.1517/17425255.2011.539201. [DOI] [PubMed] [Google Scholar]
- Court M.H.et al. Evaluation of 3′-azido-3′-deoxythymidine, morphine, and codeine as probe substrates for UDP-glucuronosyltransferase 2B7 (UGT2B7) in human liver microsomes: specificity and influence of the UGT2B7*2 polymorphism Drug Metab. Dispos 311125–1133.2003 [DOI] [PubMed] [Google Scholar]
- Coffman B.L., Rios G.R., King C.D., &, Tephly T.R. Human UGT2B7 catalyzes morphine glucuronidation. Drug Metab. Dispos. 1997;25:1–4. [PubMed] [Google Scholar]
- Barbier O.et al. 3′-azido-3′-deoxythimidine (AZT) is glucuronidated by human UDP-glucuronosyltransferase 2B7 (UGT2B7) Drug Metab. Dispos 28497–502.2000 [PubMed] [Google Scholar]
- Boucher F.D.et al. Phase I evaluation of zidovudine administered to infants exposed at birth to the human immunodeficiency virus J. Pediatr 122137–144.1993 [DOI] [PubMed] [Google Scholar]
- Knibbe C.A.et al. Morphine glucuronidation in preterm neonates, infants and children younger than 3 years Clin. Pharmacokinet 48371–385.2009 [DOI] [PubMed] [Google Scholar]
- Krekels E.H.et al. Predictive performance of a recently developed population pharmacokinetic model for morphine and its metabolites in new datasets of (preterm) neonates, infants and children Clin. Pharmacokinet 5051–63.2011 [DOI] [PubMed] [Google Scholar]
- Danhof M., de Jongh J., De Lange E.C., Della Pasqua O., Ploeger B.A., &, Voskuyl R.A. Mechanism-based pharmacokinetic-pharmacodynamic modeling: biophase distribution, receptor theory, and dynamical systems analysis. Annu. Rev. Pharmacol. Toxicol. 2007;47:357–400. doi: 10.1146/annurev.pharmtox.47.120505.105154. [DOI] [PubMed] [Google Scholar]
- De Cock R.F.W.et al. Maturation of GFR in preterm and term neonates reflected by clearance of different antibiotics PAGE Abstr 209620.2011 [Google Scholar]
- Kearns G.L., Abdel-Rahman S.M., Alander S.W., Blowey D.L., Leeder J.S., &, Kauffman R.E. Developmental pharmacology–drug disposition, action, and therapy in infants and children. N. Engl. J. Med. 2003;349:1157–1167. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- Manevski N., Moreolo P.S., Yli-Kauhaluoma J., &, Finel M. Bovine serum albumin decreases Km values of human UDP-glucuronosyltransferases 1A9 and 2B7 and increases Vmax values of UGT1A9. Drug Metab. Dispos. 2011;39:2117–2129. doi: 10.1124/dmd.111.041418. [DOI] [PubMed] [Google Scholar]
- Rowland A., Gaganis P., Elliot D.J., Mackenzie P.I., Knights K.M., &, Miners J.O. Binding of inhibitory fatty acids is responsible for the enhancement of UDP-glucuronosyltransferase 2B7 activity by albumin: implications for in vitro–in vivo extrapolation. J. Pharmacol. Exp. Ther. 2007;321:137–147. doi: 10.1124/jpet.106.118216. [DOI] [PubMed] [Google Scholar]
- Cella M., Zhao W., Jacqz-Aigrain E., Burger D., Danhof M., &, Della Pasqua O. Paediatric drug development: are population models predictive of pharmacokinetics across paediatric populations. Br. J. Clin. Pharmacol. 2011;72:454–464. doi: 10.1111/j.1365-2125.2011.03992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J.J., Semo N.M., &, Koski W.S. Microelectrometric titration measurement of the pKa's and partition and drug distribution coefficients of narcotics and narcotic antagonists and their pH and temperature dependence. J. Med. Chem. 1975;18:647–655. doi: 10.1021/jm00241a001. [DOI] [PubMed] [Google Scholar]
- Acosta E.P., Page L.M., &, Fletcher C.V. Clinical pharmacokinetics of zidovudine. An update. Clin. Pharmacokinet. 1996;30:251–262. doi: 10.2165/00003088-199630040-00001. [DOI] [PubMed] [Google Scholar]
- Uchaipichat V., Winner L.K., Mackenzie P.I., Elliot D.J., Williams J.A., &, Miners J.O. Quantitative prediction of in vivo inhibitory interactions involving glucuronidated drugs from in vitro data: the effect of fluconazole on zidovudine glucuronidation. Br. J. Clin. Pharmacol. 2006;61:427–439. doi: 10.1111/j.1365-2125.2006.02588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzier A., &, Morse G.D. Intravascular distribution of zidovudine: role of plasma proteins and whole blood components. Antiviral Res. 1993;21:267–280. doi: 10.1016/0166-3542(93)90032-e. [DOI] [PubMed] [Google Scholar]
- Teijeiro S.A., Moroni G., Motura M., &, Brinon M.C. Lipophilic character of pyrimidinic nucleoside derivatives: correlation between shake flask, chromatographic (RP-TLC and RP-HPLC) and theoretical methods. J. Liq. Chrom. & Rel. Technol. 2000;23:855–872. [Google Scholar]
- Olsen G.D. Morphine binding to human plasma proteins. Clin. Pharmacol. Ther. 1975;17:31–35. doi: 10.1002/cpt197517131. [DOI] [PubMed] [Google Scholar]
- Quevedo M.A., Ribone S.R., Moroni G.N., &, Briñón M.C. Binding to human serum albumin of zidovudine (AZT) and novel AZT derivatives. Experimental and theoretical analyses. Bioorg. Med. Chem. 2008;16:2779–2790. doi: 10.1016/j.bmc.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Stanski D.R., Greenblatt D.J., &, Lowenstein E. Kinetics of intravenous and intramuscular morphine. Clin. Pharmacol. Ther. 1978;24:52–59. doi: 10.1002/cpt197824152. [DOI] [PubMed] [Google Scholar]
- Crotty B.et al. Hepatic extraction of morphine is impaired in cirrhosis Eur. J. Clin. Pharmacol 36501–506.1989 [DOI] [PubMed] [Google Scholar]
- Naritomi Y., Terashita S., Kagayama A., &, Sugiyama Y. Utility of hepatocytes in predicting drug metabolism: comparison of hepatic intrinsic clearance in rats and humans in vivo and in vitro. Drug Metab. Dispos. 2003;31:580–588. doi: 10.1124/dmd.31.5.580. [DOI] [PubMed] [Google Scholar]
- Krekels E.H.J.et al. From pediatric covariate model to semiphysiological function for maturation: part II—sensitivity to physiological and physicochemical properties CPT Pharmacomet Syst. Pharmacol 1eXX.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krekels E.H., van Hasselt J.G., Tibboel D., Danhof M., &, Knibbe C.A. Systematic evaluation of the descriptive and predictive performance of paediatric morphine population models. Pharm. Res. 2011;28:797–811. doi: 10.1007/s11095-010-0333-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D.M.PLT Tools . < http://www.pltsoft.com > ( 2011
- Brendel K., Comets E., Laffont C., Laveille C., &, Mentré F. Metrics for external model evaluation with an application to the population pharmacokinetics of gliclazide. Pharm. Res. 2006;23:2036–2049. doi: 10.1007/s11095-006-9067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comets E., Brendel K., &, Mentré F. Computing normalised prediction distribution errors to evaluate nonlinear mixed-effect models: the NPDE add-on package for R. Comput. Methods Programs Biomed. 2008;90:154–166. doi: 10.1016/j.cmpb.2007.12.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.