Abstract
To develop a maturation function for drug glucuronidation in children, that can be used in population and physiologically based modeling approaches, the physiological and physicochemical basis of a semiphysiological glucuronidation function for children was untangled using Simcyp. The results show that using the currently available in vitro data, in vivo morphine and zidovudine clearances were under predicted by the physiologically based model in Simcyp. The maturation profile was similar to the clinically observed profile except for the first 2 weeks of life, and liver size and UGT2B7 ontogeny are the physiological drivers of the maturation of glucuronidation. Physicochemical drug parameters did not affect this maturation profile, although log P and pKa influenced the absolute value of clearance. The results suggest that the semiphysiological glucuronidation function for young children can be used to predict the developmental clearance profile of other UGT2B7 substrates, though scenarios with nonlinear kinetics and high-extraction ratios require further investigation.
Despite the multifactorial nature of the ontogeny of drug clearance, pediatric population models describe net observed changes in ontogeny with a limited number of covariate relationships. Therefore, these models are only applicable to specific drugs in a specified population, requiring collection and full analysis of the same type of data for every drug in every population. Physiologically based (PB) pharmacokinetic (PK) models use in vitro data on drug kinetics in combination with anatomical measurements and physiological parameters to quantify physiological processes and the interaction of a molecule with certain physicochemical properties with this system. The system-specific parameters in PBPK models are not restricted to specific drugs, making these models more generalizable and useful for selecting first-in-child doses for new compounds in drug development. Only rarely has PBPK modeling, known as “bottom–up approach”, been combined with population PK modeling, known as “top–down approach”, to augment each other in a semiphysiological “middle out approach”.
Previous studies on the UGT2B7-mediated glucuronidation of morphine and zidovudine (see Part I of this article, ref. 1) and the glomerular filtration of antibiotics2 suggest that pediatric population covariate models for clearance contain biological system-specific rather than drug-specific information and that this system-specific information can be extrapolated between drugs that share elimination pathways. In such semiphysiological PK modeling approaches, the ease of analyzing outcome measures with population PK modeling is combined with the mechanistic insight of PBPK modeling.
In this study, the physiological and physicochemical basis of the semiphysiological covariate function for UGT2B7-mediated glucuronidation in children younger than 3 years (see Part I of this article, ref. 1) is investigated. To define preconditions for the use of this semiphysiological function, the influence of system- and drug-specific parameters on the net maturation pattern of in vivo UGT2B7-mediated glucuronidation was investigated. This information is used to identify both patient and drug characteristics that potentially limit the applicability of the semiphysiological glucuronidation function. In addition, the results obtained can be used to establish the information that can be provided by population pediatric covariate models for maturation functions of elimination pathways in PBPK models.
Results
System-specific parameters
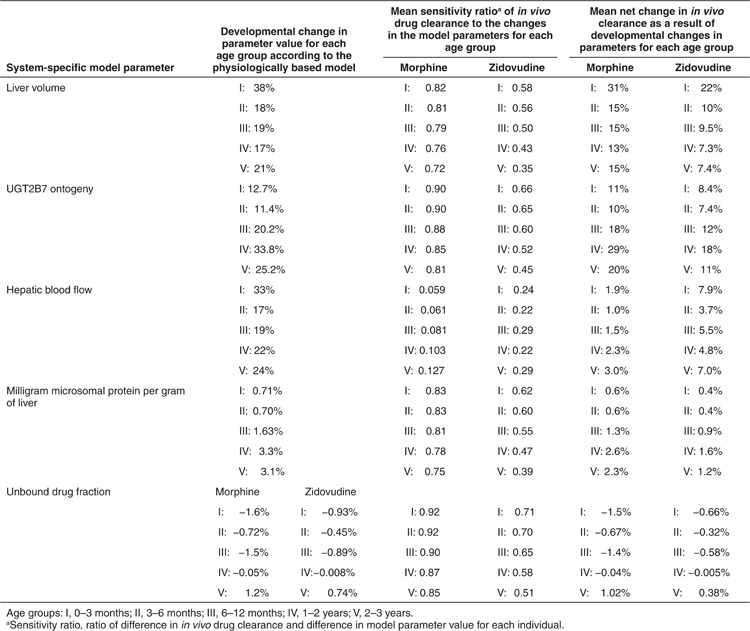
Table 1 presents the changes in parameter values of liver blood flow, liver volume, milligram protein per gram of liver, UGT2B7 ontogeny, and unbound drug fraction in the first 3 years of life according to the pediatric PBPK model in Simcyp in five age-categories. In addition, the sensitivity of in vivo clearance to these changes is quantified as a sensitivity ratio, with a ratio of 0.80 indicating that a 10% increase in the parameter value would increase clearance by 8%. Finally, the percentage change in in vivo clearance as a result of the changes in the underlying system-specific parameters is presented. It is shown that the contribution of each system-specific parameter to the developmental changes in clearance is different for morphine and zidovudine. With respect to the different age groups, the contribution of the parameters to developmental changes in clearance is highly nonlinear and may even be bidirectional. Despite this, liver volume can overall be regarded as the main driver of developmental changes in UGT2B7-mediated glucuronidation, causing an increase in clearance in the different age groups between 13 and 31% for morphine and 7.3 and 22% for zidovudine, with an especially large contribution in the first 3 months of life. The increase in morphine and zidovudine clearance as a result of UGT2B7 ontogeny in the different age groups ranges between 10 and 29% and 7.4 and 18%, respectively. The influence of hepatic blood flow on developmental changes in morphine clearance is <5% in all age groups and can be regarded negligible, whereas for zidovudine, the contribution of changes in hepatic blood flow to increases in clearance ranges between 3.7 and 7.9%. For both drugs, the contribution of changes in milligram protein per gram of liver and unbound drug fraction is negligible in all age groups.
Table 1. System-specific parameters investigated in this study. The percentage increase in parameter value in each of the five age groups (I–V) and the mean sensitivity ratios of the clearance of morphine and zidovudine in each group is provided. The calculated percentage change in in vivo morphine and zidovudine clearance as a result of the change in the underlying system-specific parameters are also presented.
Drug-specific parameters
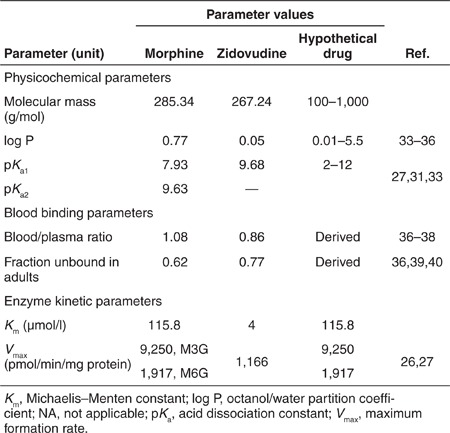
Simulations with hypothetical small-molecular UGT2B7 substrates (Table 2) revealed that the physicochemical drug properties such as molecular weight, octanol/water partition coefficient (log P), and acid dissociation constant (pKa) do not influence the ontogeny profile of in vivo UGT2B7-medidated glucuronidation. Assuming that the changes in mass did not alter the uptake or efflux by hepatocytes or the interaction with the UGT2B7 isoenzyme, molecular mass in the range between 100 and 1,000 g/mol did not influence this clearance at all. Increasing log P between 0.01 and 5.5 yielded a slight decreasing trend in the predicted clearance, whereas increasing the pKa between 2 and 12 yielded an increasing trend in predicted clearance. No strong relationship was observed between the physicochemical properties of the hypothetical drugs and the clearance, nor was there a relationship between the derived blood to plasma ratio and clearance. There was, however, a strong linear correlation (r = 0.978) between the clearance predicted by the PBPK model and the unbound drug fraction of the hypothetical drug in plasma, which was derived from the log P and pKa value using the Simcyp toolbox. According to this relationship, every 0.1 increase in unbound drug fraction resulted in a parallel increase in in vivo clearance of 1.5 l/h across the studied age range.
Table 2. Drug-specific parameters for morphine, zidovudine, and hypothetical drugs used in the physiologically based simulations.
Semiphysiological glucuronidation function
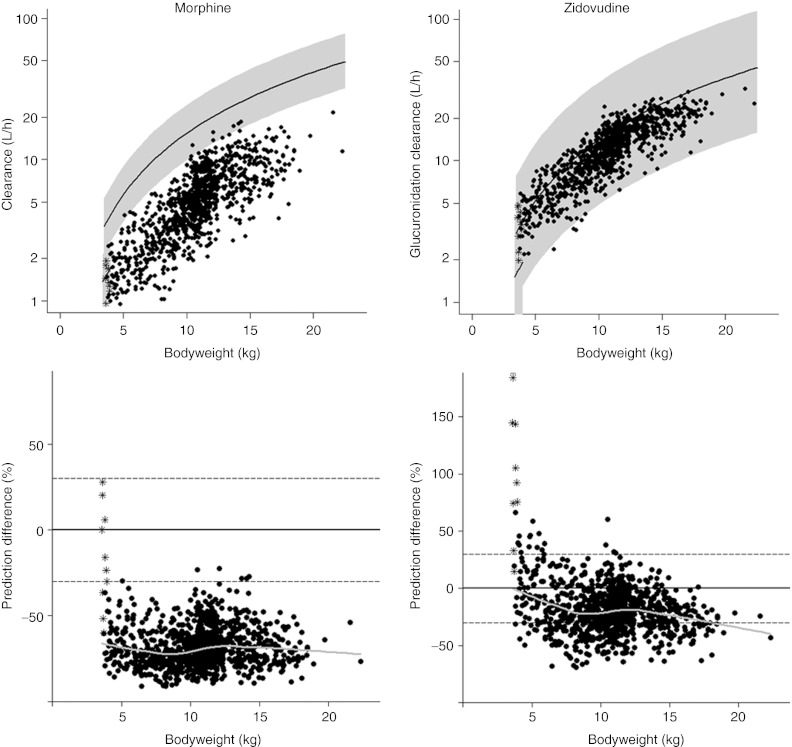
Figure 1 shows the in vivo morphine and zidovudine clearance values in children younger than 3 years obtained in population models using the semiphysiological glucuronidation function (see Part I of this article, ref. 1 and ref. 3) and the PBPK model in Simcyp. The graphs indicate an underprediction of the clearances in children older than 10 days (solid circles) by the PBPK model, yielding a mean percentage difference for this older population of −68.3% for morphine and −19.1% for zidovudine. In neonates younger than 10 days (asterisks), the reduction in glucuronidation capacity as quantified by the semiphysiological glucuronidation function is not observed in the predictions by the PBPK model, yielding a mean percentage difference of −19.4% for morphine and 105% for zidovudine. This illustrates large differences in the prediction of developmental changes in in vivo glucuronidation clearance between the population models with the semiphysiological glucuronidation function and the PBPK model in the first 2 weeks of life. According to the bottom graphs, the prediction difference remains constant throughout the bodyweight range in older children, suggesting that in this subpopulation, the maturation profile predicted by the PBPK model mainly differs from the population models in absolute value while it is similar in shape.
Figure 1.
In vivo morphine clearance (top left) and zidovudine clearance (top right) vs. bodyweight in children younger than 3 years according to the physiologically based pharmacokinetic model (asterisk for neonates younger than 10 days, and solid dots for children older than 10 days) and the population models using the semiphysiological glucuronidation function (lines are population predictions and the shaded area indicates the 95% prediction interval). The difference in clearance value between the models is depicted vs. bodyweight for both drugs (bottom). The horizontal lines in these graphs show 0% prediction difference (solid line) and ± 30% prediction difference (dotted lines) and the gray line represents the loess curve of the data.
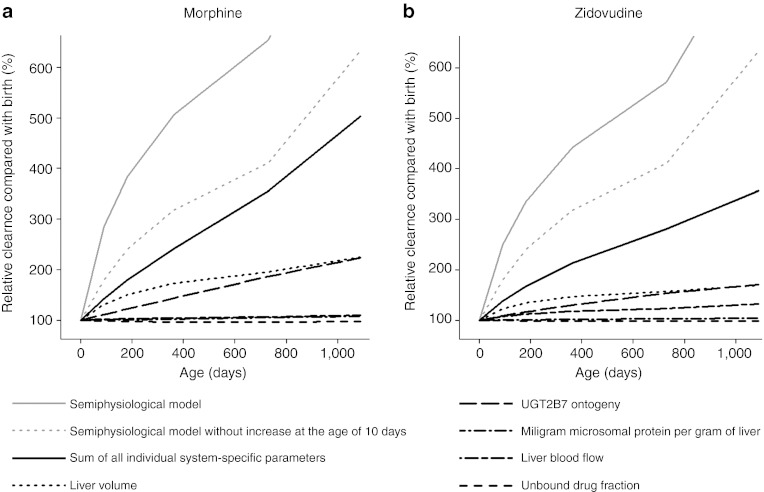
Developmental changes in in vivo morphine and zidovudine clearance relative to birth are depicted in Figure 2 for the population models using the semiphysiological glucuronidation function (gray lines) and the PBPK model (black lines), including the individual contribution of each system-specific parameter in the physiologically based model (nonsolid black lines). It can be seen that for both drugs, the largest contribution to the increase in clearance comes from the increase in liver volume (dotted black line) and UGT2B7 ontogeny (long dashed black line). When not taking into account the rapid increase in drug glucuronidation in the semiphysiological function at 10 days after birth (dashed gray line), the combined influence of the changes in the five system-specific parameters investigated in this study explains 79% of the clinically observed increases in morphine clearance and 41% of the clinically observed increases in zidovudine clearance in the first 3 years of life.
Figure 2.
Developmental changes in in vivo morphine and zidovudine clearance in the first 3 years of life relative to birth. The solid gray line represents the total increase according to the population models using the semiphysiological glucuronidation function and the dotted gray line represents the clearance increase according to these models without taking the rapid increase at the age of 10 days into account. The solid black line represents the sum of the changes by all five system-specific parameters with the non-solid black lines representing the individual contribution of each system-specific parameter.
Discussion
We have previously shown that the clinically observed maturation pattern for morphine glucuronidation in children, who had been quantified in a pediatric population covariate model,3,4 could be directly extrapolated to the glucuronidation of zidovudine in a semiphysiological modeling approach (see Part I of this article, ref. 1). This study investigates the extent to which the semiphysiological glucuronidation function can be extrapolated to other patient populations or other UGT2B7 substrates. Therefore, the physiological and physicochemical basis of this semiphysiological glucuronidation function was investigated using PKPB modeling software (Simcyp, Sheffield, UK). Investigation of the influence of system-specific and drug-specific parameters revealed that increases in liver volume and UGT2B7 ontogeny are the physiological drivers of the developmental changes in in vivo drug glucuronidation (Table 1 and Figure 2). Concerning physicochemical drug characteristics, the log P and pKa of a drug, but not the molecular mass, were found to influence the absolute value of glucuronidation, without influencing the maturation pattern.
Figure 1 shows that the clearance predictions by the PBPK model (symbols), using available in vitro information on UGT2B7 enzyme kinetics of morphine and zidovudine, are generally lower than the clearance values obtained in the population models with the semiphysiological glucuronidation function. In addition, Figure 2 shows that the combined influence of the age-related increases in the system-specific parameters investigated in this study (solid black line) does not fully explain the clinically observed increase in morphine and zidovudine clearance (solid gray line), not even when the rapid increase in glucuronidation described by the semiphysiological glucuronidation function at 10 days after birth is discarded (dashed gray line). These discrepancies are probably the result of a combination of factors:
First, the uridine 5′-diphosphate glucuronisyltransferase enzyme kinetic parameters for morphine and zidovudine were obtained from studies using liver microsomes. Confidence that these values accurately represent in vivo enzyme kinetics is limited by various factors that influence these measured in vitro values causing difficulties in obtaining good predictions on in vivo UGT enzyme kinetics from microsome studies.5,6,7,8,9 In fact, in this study, the reported Km values had to be adjusted to values that yielded accurate clearance predictions by the PBPK model in adults. In a sensitivity analysis, increasing and decreasing Vmax and Km values by 10-fold yielded predicted pediatric glucuronidation clearances that changed by a factor of 2 for M6G formation and a factor of 12 for both zidovudine glucuronidation and M3G formation. It is, however, emphasized that bias in enzyme kinetic parameter values obtained in vitro limits the predictions of absolute glucuronidation clearance by the PBPK model but not the maturation pattern.
Additional discrepancies may be the result of the assumption in the PBPK model that morphine and zidovudine were solely eliminated through UGT2B7-mediated glucuronidation, whereas some studies have suggested that morphine is, to a small degree, also eliminated through sulfation or unchanged elimination in the very young.10,11 Moreover, active drug uptake or efflux by hepatocytes and biliary clearance of morphine and zidovudine were assumed to be zero. Whereas animal studies with morphine have shown energy-dependent carrier-mediated uptake of morphine in hepatocytes,12,13 other studies found active hepatic uptake to not limit hepatic morphine metabolism.14 Finally, biliary clearance of morphine was reported in rat livers.14
Furthermore, concerning the system-specific parameters in the PBPK model, while the maturational changes in liver volume are based on a large number of observations,15 the pediatric information on other system-specific parameters is more limited, decreasing the level of confidence in the ontogeny profiles of these parameters. In particular, the UGT2B7 expression and function in the PBPK model increases linearly with age from 8.9% from adult values at birth to adult values at the age of 20 years. Literature data, however, suggest the expression and function of UGT isoenzymes to increase rapidly in the first few weeks of life.16 Given that morphine and zidovudine clearances were found to be sensitive to changes in the UGT2B7 ontogeny, a suboptimal representation of this ontogeny profile may explain the discrepancy in the morphine and zidovudine clearance values (Figure 1) and the discrepancy between the increases in morphine and zidovudine clearances in (Figure 2). As liver volume and UGT2B7 ontogeny are the main drivers of UGT2B7-mediated glucuronidation and as the maturation profile of liver volume is well established,15 an improved function for UGT2B7 ontogeny in the PBPK model can be obtained by subtracting the maturation function for liver volume from the semiphysiological glucuronidation function in children. As such, pediatric population PK modeling, which is based on in vivo outcome measures, can indeed provide maturation functions for pediatric PBPK models.
Concerning drug characteristics, the molecular weight of hypothetical drugs was not found to influence drug glucuronidation, whereas the log P and pKa of the hypothetical drug molecule influenced only the absolute value of glucuronidation, which is reflected in the population clearance value. These results thereby support the hypothesis that this value is drug specific. Since in a semiphysiological modeling approach for new UGT2B7 substrates the value of this constant is estimated based on the population analysis of outcome measures, these results suggest that the semiphysiological glucuronidation function can predict developmental changes in glucuronidation clearance of all small-molecular substrates for UGT2B7.
A relevant question in this context is whether the semiphysiological glucuronidation function can be applied to drugs with saturable kinetics. In this study, a linear relationship was found between unbound drug concentration in plasma and the absolute value of glucuronidation; however, the Michaelis–Menten parameters were kept constant in each simulation. Michaelis–Menten parameters influence drug metabolism significantly, but due to the nonlinear correlation between substrate concentration and intrinsic drug clearance, interpretation of the results from simulations with varying Michaelis–Menten constants is complex. We, therefore, conclude that further simulations with varying Michaelis–Menten constants are necessary to investigate the applicability of the semiphysiological glucuronidation function to drugs with saturable kinetics.
In addition, the application of the semiphysiological glucuronidation function in scenarios of drugs with varying extraction ratios needs further investigation. The ontogeny of UGT2B7 isoenzymes may have a one to one effect on low-extraction substrates as in this case, in vivo clearance closely reflects intrinsic clearance. By contrast, high-extraction substrates may be affected less by an increased level in UGT activity as their clearance is limited by hepatic blood flow. With similar extraction ratios of morphine and zidovudine of 0.5 and 0.65, respectively,17,18,19 the influence of changes in the underlying physiological system is expected to be rather similar for both drugs, resulting in the same ranking of system-specific parameters in Table 1 and the possibility to extrapolate the semiphysiological glucuronidation function between these drugs (see Part I of this article, ref. 1). The small difference in extraction ratio that does exist between these two drugs may explain why the influence of hepatic blood flow on in vivo clearance is more pronounced for zidovudine than for morphine.
In conclusion, direct extrapolation of the pediatric covariate model in a semiphysiological modeling approach as described in Part I of this article1 is possible between UGT2B7 substrates that have linear kinetics and similar extraction ratios. The relatively large impact of changes in liver volume on in vivo drug glucuronidation suggest that the applicability of the semiphysiological glucuronidation function in patients with a decreased liver size or liver function may be reduced. The currently available maturation profile for UGT2B7 ontogeny in the PBPK model can be improved based on information obtained in the covariate model of a pediatric population model.
Methods
PBPK simulations. Simcyp version 11 (Simcyp, Sheffield, UK) was used to investigate the influence of system-specific and drug-specific parameters on the ontogeny of in vivo UGT2B7-mediated drug glucuronidation in the first 3 years of life (see Part I of this article, ref. 1). The pediatric database was selected for the simulations and parameters were set to include patients with a maximum age of 3 years. The accuracy of pediatric clearance predictions for 11 drugs by the Simcyp software package was illustrated previously.20 A total of 1,000 individuals were simulated and the same random number generation seed was used for repeated simulations, yielding the same set of individuals for each simulation, allowing for a direct comparison of clearance predictions between individuals in simulations with varying parameter values. A uniform age distribution was used and the male/female ratio was set to 1. For each of the 1,000 simulated individuals, age- and sex-appropriate bodyweight and height were determined based on UK reference growth charts taking interindividual variability into account.21 The parallel-tube model was used to derive whole blood hepatic drug clearances from intrinsic hepatic clearance, hepatic blood flow, and the unbound drug fraction in blood.
Morphine and zidovudine were used as model compounds as both drugs are specific substrates for the UGT2B7 isoenzyme22,23,24 and used in the proof-of-concept studies for the development of the semiphysiological glucuronidation function (see Part I of this article, ref. 1). In the simulations, clinically relevant intravenous bolus doses of 0.1 mg/kg morphine or 3 mg/kg zidovudine were administered.
System-specific parameters. The pediatric database in Simcyp is based on system-specific parameter values and variability measures in these parameters that represent a “healthy” population. Simulations in this population form the basis for exploring the influence of changes in the system-specific parameters that can be the result of not only maturation but also disease.
Hepatic blood flow, liver volume, milligram protein per gram of liver, UGT2B7 ontogeny, and unbound drug fraction were the system-specific parameters that were investigated. “UGT2B7 ontogeny” denotes the fractional expression and function of the UGT2B7 isoenzyme in children as compared with adults. The percentage change of each of the parameters was obtained by calculating the mean parameter value of all individuals with an age in the first 2 weeks and the last 2 weeks of each interval and by determining the percentage increase in these values per group for the patients in the following groups: I, the first 3 months of life (0–3 months); II, the second 3 months (3–6 months); III, the second half year (6–12 months); IV, the second year (1–2 years); and V, the third year (2–3 years).
Subsequently, one at a time, the values of each system-specific parameter were increased with a physiologically relevant value based on the mean increase in each age group. For hepatic blood flow and liver volume, the value was 23%, for milligram protein per gram of liver it was 2%, for the UGT2B7 ontogeny profile, 21%, and for the unbound drug fraction, the values were 0.51% for morphine and 0.33% for zidovudine. As the user cannot alter the UGT2B7 ontogeny profile in the Simcyp software, an alternative scenario was simulated to represent an increase of 21% in the UGT2B7 ontogeny profile. The age-appropriate ontogeny factor for each individual, which is derived from the ontogeny profile, is a scalar for the intrinsic glucuronidation clearance that takes place in both the liver and the kidneys. Similarly, liver density is a scalar for intrinsic UGT2B7-mediated glucuronidation in the liver, whereas milligram microsomal protein per gram of kidney is a scalar of intrinsic UGT2B7-mediated glucuronidation clearance in the kidney. Therefore, simulation of a scenario in which both liver density and milligram microsomal protein per gram of kidney are increased by 21% were used to represent a situation with a 21% increased UGT2B7 ontogeny profile.
To quantify the influence of these physiologically relevant changes in the system-specific parameters on the predicted in vivo clearance, a sensitivity ratio was calculated for each individual. For this sensitivity ratio, the difference in in vivo clearance predictions was divided by the percentage difference in the system-specific parameter. Mean sensitivity ratios were calculated for each system-specific parameter in each of the age groups. By multiplying the percentage change in a system-specific parameter in each age group by the mean sensitivity ratio for that age group, the percentage change in in vivo glucuronidation clearance as a result of the changes in the underlying parameter value in that age range was determined.
Drug-specific parameters. Morphine and zidovudine were added to the compound database in Simcyp by obtaining their drug-specific parameters from the literature. In all simulations, the assumption was made that there is no elimination through pathways other than UGT2B7-mediated glucuronidation, that there is no active drug transport into or out of the hepatocytes, and that there is no biliary clearance. Inclusion of these processes would increase the predicted drug clearance.
The obtained drug-specific parameters for each drug are presented in Table 2. With respect to the Michaelis–Menten parameters, values for the formation of the two morphine glucuronides were obtained from the liver microsomes from five separate adults25 and values for the glucuronidation of zidovudine were obtained from the liver microsomes from four adults.26 To verify the obtained drug-specific parameters, predictions of morphine and zidovudine clearances for 1,000 healthy adult volunteers were compared with reported clearance values in adults of 93 l/h for morphine27,28,29 and 91.2 l/h for zidovudine.30,31,32 This yielded a 75% mean underprediction for morphine and a 71% mean underprediction for zidovudine. On the basis of the literature reports that show that for UGTs albumin and fatty acids in in vitro assays influence Km values but not Vmax values,5,6 the clearance predictions for morphine and zidovudine in adults were optimized by adjusting the Km values. For morphine, the optimized Km value was 115.8 µmol/l for the formation of both M3G and M6G, and for zidovudine this was a value of 4 µmol/l. These values were subsequently used in the pediatric simulations.
To identify how physicochemical drug properties influence the maturation pattern of UGT2B7-mediated glucuronidation, in vivo clearance values of hypothetical UGT2B7-specific substrates with physicochemical properties that are common for small-molecular drugs were simulated with Simcyp. In these simulations, the implicit assumption was made that the changes in physicochemical properties influenced neither the active transport of the hypothetical drug into or out of the hepatocytes nor the interaction of the hypothetical drug with the UGT2B7 isoenzyme. Hypothetical drugs with molecular weights of 100, 200, 500, 800, and 1,000 g/mol were simulated in combination with log P values of 0.01, 1, and 5.5. Neutral compounds were simulated as well as monoprotic acids and bases with pKa values of 2 or 5 and 8.5 or 12, respectively, and diprotic acids and bases with pKa values of 2 and 5 and 8.5 and 10, respectively. An ampholyte was used with a pKa of 5 and 9. These values are summarized in Table 2. The Simcyp toolbox was used to calculate the blood/plasma ratio and unbound drug fraction for each hypothetical drug based on log P and pKa values. The Michaelis–Menten parameters obtained for morphine were used, and in the simulations an intravenous drug dose of 0.1 mg/kg was administered.
The influence of changes in physicochemical drug parameters on the predicted in vivo clearance was assessed by changing one parameter value at a time and calculating the percentage difference between the clearance predictions between two simulations. When the highest individual prediction difference was <5%, the parameter was classified as not significantly influencing drug glucuronidation. When the highest individual prediction difference was >5% and the difference between mean prediction difference of individuals in the first month of life and individuals in the 35th month of life was <5%, a parameter was classified as influencing the absolute value of drug glucuronidation. When the difference between mean prediction difference of individuals in the first month of life and individuals in the 35th month of life was >5%, the parameter was classified as influencing the ontogeny profile in addition to influencing the absolute value of drug glucuronidation clearance.
Semiphysiological glucuronidation function. The semiphysiological glucuronidation function is obtained from a pediatric population covariate model quantifying the net observed maturational changes in drug glucuronidation clearance in children younger than the age of 3 years including preterm and term neonates. This model was obtained and validated in a population analysis of pediatric morphine data3,4 and could also be directly extrapolated to zidovudine (see Part I of this article, ref. 1). In this model, the overall developmental changes in drug glucuronidation in children younger than 3 years are quantified according to Eq. 1:
in which CLind represents the individual glucuronidation clearance, CLpop represents the population clearance, fneonate<10 represents a reduced glucuronidation fraction in neonates younger than 10 days, and BW represents the bodyweight of an individual pediatric patient in kilograms. The population clearance for each drug was estimated from clinical outcome measures in a population analysis. The reduction in glucuronidation clearance in neonates with a postnatal age younger than 10 days (fneonate<10) is 50% and is independent from gestational age. The final element of Eq. 1 quantifies the overall ontogeny of in vivo drug glucuronidation in this young population using bodyweight as a surrogate descriptor in an exponential equation with an exponent of 1.44.
Using available in vitro data as input parameters, the morphine and zidovudine clearance predictions by the physiologically based PK model in Simcyp were compared with the clearance values according to the population models using the semiphysiological glucuronidation function. This was done by plotting clearance values from these models vs. bodyweight, which is the primary covariate in the semiphysiological glucuronidation function, and by plotting the prediction difference between these clearance values for each drug for each of the 1,000 simulated individuals vs. bodyweight.
In addition, for each simulated individual in this study, the morphine and zidovudine clearances according to the population models using the semiphysiological function were determined as well. The percentage increase in morphine and zidovudine clearance values in each of the five age groups according to these models were calculated as described above and compared with the increase in morphine and zidovudine clearances predicted by the PBPK model for each age group.
Author Contributions
A.R.H., M.D., and C.A.J.K. designed the research. C.A.J.K. analyzed the research. A.R.H., M.D., D.T., and C.A.J.K. wrote the manuscript.
Conflict of Interest
A.R.H. was a joint founder of, and is currently part-time secondee to Simcyp Limited (a Certara company). T.N.J. is a full-time employee of Simcyp Limited (a Certara company).
Study Highlights

Acknowledgments
This study was performed within the framework of Dutch Top Institute Pharma project number D2-10.
References
- Krekels E.H.J.et al. From pediatric covariate model to semiphysiological function for maturation: part I—extrapolation of a covariate model from morphine to zidovudine CPT Pharmacometrics Syst. Pharmacol 1eXX.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cock R.F.W.et al. Maturation of GFR in preterm and term neonates reflected by clearance of different antibiotics PAGE 20 2011. Abstr 2096.
- Knibbe C.A.et al. Morphine glucuronidation in preterm neonates, infants and children younger than 3 years Clin. Pharmacokinet 48371–385.2009 [DOI] [PubMed] [Google Scholar]
- Krekels E.H.et al. Predictive performance of a recently developed population pharmacokinetic model for morphine and its metabolites in new datasets of (preterm) neonates, infants and children Clin. Pharmacokinet 5051–63.2011 [DOI] [PubMed] [Google Scholar]
- Manevski N., Moreolo P.S., Yli-Kauhaluoma J., &, Finel M. Bovine serum albumin decreases Km values of human UDP-glucuronosyltransferases 1A9 and 2B7 and increases Vmax values of UGT1A9. Drug Metab. Dispos. 2011;39:2117–2129. doi: 10.1124/dmd.111.041418. [DOI] [PubMed] [Google Scholar]
- Rowland A., Gaganis P., Elliot D.J., Mackenzie P.I., Knights K.M., &, Miners J.O. Binding of inhibitory fatty acids is responsible for the enhancement of UDP-glucuronosyltransferase 2B7 activity by albumin: implications for in vitro-in vivo extrapolation. J. Pharmacol. Exp. Ther. 2007;321:137–147. doi: 10.1124/jpet.106.118216. [DOI] [PubMed] [Google Scholar]
- Hewitt N.J.et al. Primary hepatocytes: current understanding of the regulation of metabolic enzymes and transporter proteins, and pharmaceutical practice for the use of hepatocytes in metabolism, enzyme induction, transporter, clearance, and hepatotoxicity studies Drug Metab. Rev 39159–234.2007 [DOI] [PubMed] [Google Scholar]
- Miners J.O., Knights K.M., Houston J.B., &, Mackenzie P.I. In vitro-in vivo correlation for drugs and other compounds eliminated by glucuronidation in humans: pitfalls and promises. Biochem. Pharmacol. 2006;71:1531–1539. doi: 10.1016/j.bcp.2005.12.019. [DOI] [PubMed] [Google Scholar]
- Engtrakul J.J., Foti R.S., Strelevitz T.J., &, Fisher M.B. Altered AZT (3′-azido-3′-deoxythymidine) glucuronidation kinetics in liver microsomes as an explanation for underprediction of in vivo clearance: comparison to hepatocytes and effect of incubation environment. Drug Metab. Dispos. 2005;33:1621–1627. doi: 10.1124/dmd.105.005058. [DOI] [PubMed] [Google Scholar]
- McRorie T.I., Lynn A.M., Nespeca M.K., Opheim K.E., &, Slattery J.T. The maturation of morphine clearance and metabolism. Am. J. Dis. Child. 1992;146:972–976. doi: 10.1001/archpedi.1992.02160200094036. [DOI] [PubMed] [Google Scholar]
- Choonara I., Ekbom Y., Lindström B., &, Rane A. Morphine sulphation in children. Br. J. Clin. Pharmacol. 1990;30:897–900. doi: 10.1111/j.1365-2125.1990.tb05458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto K., Eaton D.L., &, Klaassen C.D. Uptake of morphine and nalorphine by isolated rat hepatocytes. J. Pharmacol. Exp. Ther. 1978;206:181–189. [PubMed] [Google Scholar]
- Déchelotte P., Sabouraud A., Sandouk P., Hackbarth I., &, Schwenk M. Uptake, 3-, and 6-glucuronidation of morphine in isolated cells from stomach, intestine, colon, and liver of the guinea pig. Drug Metab. Dispos. 1993;21:13–17. [PubMed] [Google Scholar]
- Doherty M.M., Poon K., Tsang C., &, Pang K.S. Transport is not rate-limiting in morphine glucuronidation in the single-pass perfused rat liver preparation. J. Pharmacol. Exp. Ther. 2006;317:890–900. doi: 10.1124/jpet.105.100446. [DOI] [PubMed] [Google Scholar]
- Johnson T.N., Tucker G.T., Tanner M.S., &, Rostami-Hodjegan A. Changes in liver volume from birth to adulthood: a meta-analysis. Liver Transpl. 2005;11:1481–1493. doi: 10.1002/lt.20519. [DOI] [PubMed] [Google Scholar]
- Krekels E.H.J., Danhof M., Tibboel D., &, Knibbe C.A.J. Ontogeny of Hepatic Glucuronidation; Methods and Results. Curr.Drug Metab. 2012;13:728–43. doi: 10.2174/138920012800840455. [DOI] [PubMed] [Google Scholar]
- Stanski D.R., Greenblatt D.J., &, Lowenstein E. Kinetics of intravenous and intramuscular morphine. Clin. Pharmacol. Ther. 1978;24:52–59. doi: 10.1002/cpt197824152. [DOI] [PubMed] [Google Scholar]
- Crotty B.et al. Hepatic extraction of morphine is impaired in cirrhosis Eur. J. Clin. Pharmacol 36501–506.1989 [DOI] [PubMed] [Google Scholar]
- Naritomi Y., Terashita S., Kagayama A., &, Sugiyama Y. Utility of hepatocytes in predicting drug metabolism: comparison of hepatic intrinsic clearance in rats and humans in vivo and in vitro. Drug Metab. Dispos. 2003;31:580–588. doi: 10.1124/dmd.31.5.580. [DOI] [PubMed] [Google Scholar]
- Johnson T.N., Rostami-Hodjegan A., &, Tucker G.T. Prediction of the clearance of eleven drugs and associated variability in neonates, infants and children. Clin. Pharmacokinet. 2006;45:931–956. doi: 10.2165/00003088-200645090-00005. [DOI] [PubMed] [Google Scholar]
- Cole T.J., Freeman J.V., &, Preece M.A. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat. Med. 1998;17:407–429. [PubMed] [Google Scholar]
- Court M.H.et al. Evaluation of 3′-azido-3′-deoxythymidine, morphine, and codeine as probe substrates for UDP-glucuronosyltransferase 2B7 (UGT2B7) in human liver microsomes: specificity and influence of the UGT2B7*2 polymorphism Drug Metab. Dispos 311125–1133.2003 [DOI] [PubMed] [Google Scholar]
- Coffman B.L., Rios G.R., King C.D., &, Tephly T.R. Human UGT2B7 catalyzes morphine glucuronidation. Drug Metab. Dispos. 1997;25:1–4. [PubMed] [Google Scholar]
- Barbier O.et al. 3′-azido-3′-deoxythimidine (AZT) is glucuronidated by human UDP-glucuronosyltransferase 2B7 (UGT2B7) Drug Metab. Dispos 28497–502.2000 [PubMed] [Google Scholar]
- Morrish G.A., Foster D.J., &, Somogyi A.A. Differential in vitro inhibition of M3G and M6G formation from morphine by ®- and (S)-methadone and structurally related opioids. Br. J. Clin. Pharmacol. 2006;61:326–335. doi: 10.1111/j.1365-2125.2005.02573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchaipichat V., Winner L.K., Mackenzie P.I., Elliot D.J., Williams J.A., &, Miners J.O. Quantitative prediction of in vivo inhibitory interactions involving glucuronidated drugs from in vitro data: the effect of fluconazole on zidovudine glucuronidation. Br. J. Clin. Pharmacol. 2006;61:427–439. doi: 10.1111/j.1365-2125.2006.02588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart-Harris R., Joel S.P., McDonald P., Currow D., &, Slevin M.L. The pharmacokinetics of morphine and morphine glucuronide metabolites after subcutaneous bolus injection and subcutaneous infusion of morphine. Br. J. Clin. Pharmacol. 2000;49:207–214. doi: 10.1046/j.1365-2125.2000.00141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbsguth U., Rentsch K.M., Eich-Höchli D., Diterich I., &, Fattinger K. Oral diacetylmorphine (heroin) yields greater morphine bioavailability than oral morphine: bioavailability related to dosage and prior opioid exposure. Br. J. Clin. Pharmacol. 2008;66:781–791. doi: 10.1111/j.1365-2125.2008.03286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarton E.et al. Sex differences in morphine analgesia: an experimental study in healthy volunteers Anesthesiology 931245–54.discussion 6A (2000 [DOI] [PubMed] [Google Scholar]
- Acosta E.P., Page L.M., &, Fletcher C.V. Clinical pharmacokinetics of zidovudine. An update. Clin. Pharmacokinet. 1996;30:251–262. doi: 10.2165/00003088-199630040-00001. [DOI] [PubMed] [Google Scholar]
- Klecker R.W., Jret al. Plasma and cerebrospinal fluid pharmacokinetics of 3′-azido-3′-deoxythymidine: a novel pyrimidine analog with potential application for the treatment of patients with AIDS and related diseases Clin. Pharmacol. Ther 41407–412.1987 [DOI] [PubMed] [Google Scholar]
- Gitterman S.R., Drusano G.L., Egorin M.J., &, Standiford H.C. Population pharmacokinetics of zidovudine. The Veterans Administration Cooperative Studies Group. Clin. Pharmacol. Ther. 1990;48:161–167. doi: 10.1038/clpt.1990.131. [DOI] [PubMed] [Google Scholar]
- Kaufman J.J., Semo N.M., &, Koski W.S. Microelectrometric titration measurement of the pKa's and partition and drug distribution coefficients of narcotics and narcotic antagonists and their pH and temperature dependence. J. Med. Chem. 1975;18:647–655. doi: 10.1021/jm00241a001. [DOI] [PubMed] [Google Scholar]
- Kaufman J.J., Koski W.S., &, Bennon D.W. Temperature and pH sensitivity of the partition coefficient as related to the blood-brain barrier to drugs. Exp. Eye Res. 1977;25 (suppl.):201–203. doi: 10.1016/s0014-4835(77)80018-3. [DOI] [PubMed] [Google Scholar]
- Luzier A., &, Morse G.D. Intravascular distribution of zidovudine: role of plasma proteins and whole blood components. Antiviral Res. 1993;21:267–280. doi: 10.1016/0166-3542(93)90032-e. [DOI] [PubMed] [Google Scholar]
- Teijeiro S.A., Moroni G., Motura M., &, Brinon M.C. Lipophilic character of pyrimidinic nucleoside derivatives: correlation between shake flask, chromatographic (RP-TLC and RP-HPLC) and theoretical methods. J.Liq.Chrom.& Rel.Technol. 2000;23:855–872. [Google Scholar]
- Tunblad K., Jonsson E.N., &, Hammarlund-Udenaes M. Morphine blood-brain barrier transport is influenced by probenecid co-administration. Pharm. Res. 2003;20:618–623. doi: 10.1023/a:1023250900462. [DOI] [PubMed] [Google Scholar]
- Skopp G., Pötsch L., Ganssmann B., Aderjan R., &, Mattern R. A preliminary study on the distribution of morphine and its glucuronides in the subcompartments of blood. J. Anal. Toxicol. 1998;22:261–264. doi: 10.1093/jat/22.4.261. [DOI] [PubMed] [Google Scholar]
- Olsen G.D. Morphine binding to human plasma proteins. Clin. Pharmacol. Ther. 1975;17:31–35. doi: 10.1002/cpt197517131. [DOI] [PubMed] [Google Scholar]
- Quevedo M.A., Ribone S.R., Moroni G.N., &, Briñón M.C. Binding to human serum albumin of zidovudine (AZT) and novel AZT derivatives. Experimental and theoretical analyses. Bioorg. Med. Chem. 2008;16:2779–2790. doi: 10.1016/j.bmc.2008.01.007. [DOI] [PubMed] [Google Scholar]