Abstract
Introduction
To assess the efficacy of an abbreviated Stanford V regimen in patients with early-stage Hodgkin lymphoma (HL).
Patients and methods
Patients with untreated nonbulky stage I–IIA supradiaphragmatic HL were eligible for the G4 study. Stanford V chemotherapy was administered for 8 weeks followed by radiation therapy (RT) 30 Gy to involved fields (IF). Freedom from progression (FFP), disease-specific survival (DSS) and overall survival (OS) were estimated.
Results
All 87 enrolled patients completed the abbreviated regimen. At a median follow-up of 10 years, FFP, DSS and OS are 94%, 99% and 94%, respectively. Therapy was well tolerated with no treatment-related deaths.
Conclusions
Mature results of the abbreviated Stanford V regimen in nonbulky early-stage HL are excellent and comparable to the results from other contemporary therapies.
Keywords: abbreviated Stanford V regimen, early-stage Hodgkin lymphoma, involved-field radiotherapy
background
Stage I–II Hodgkin lymphoma (HL) is highly curable [1]. ABVD (doxorubicin, bleomycin, vinblastine and dacarbazine) chemotherapy followed by involved-field radiotherapy (IFRT) is a standard of care with cure rates of >80% [2–5]. The Stanford V regimen is a combined modality approach initially developed for patients with advanced HL with the goals of maintaining high cure rates and reducing acute toxicity as well as late effects of treatment. We have previously reported a 5-year freedom from progression (FFP) of 89% and OS of 96% with minimal impact on fertility for patients with locally extensive or advanced disease [6]. In the present study, we report mature results of the G4 trial for patients with stage I–IIA nonbulky supradiaphragmatic HL in which the duration of Stanford V chemotherapy was reduced from 12 to 8 weeks and radiation dose limited to 30 Gy to the involved field (IF).
patients and methods
This was a multisite study and treatment was delivered at Stanford University Medical Center and at 12 participating centers of Northern California Kaiser Permanente. Patients with previously untreated stage I–IIA supradiaphragmatic classical HL were eligible for the G4 study. Patients with bulky mediastinal adenopathy, defined as a mediastinal mass >one-third of the maximum intrathoracic diameter were excluded. Before enrollment, all patients had their biopsies reviewed and diagnosis confirmed by pathologists in the Department of Pathology at Stanford University Medical Center. Staging studies were carried out that included imaging [chest X-ray, computed tomography (CT) scans of chest, abdomen and pelvis] and routine laboratory tests [complete blood count, metabolic panel and erythrocyte sedimentation rate (ESR)]. Only a minority (n = 8) of patients had a pretreatment positron emission tomography (PET) scan. All patients were presented and discussed at a multidisciplinary conference to confirm stage and protocol eligibility. Patients were included regardless of age, ESR, number of nodal sites or presence of extranodal disease. Patients with a serum bilirubin >2.5 mg/dl, granulocytes ≤2 × 10 d/l, platelets <150 × 10 d/l, serum creatinine ≥2 mg/dl or a positive human immunodeficiency virus test were excluded. The appropriate local institutional review boards approved the study and all participants provided written informed consent before enrollment.
statistical consideration
The main objective of the study was to compare the FFP rate of this reduced therapy to our historical experience (G1 study) that utilized six cycles of vinblastine, bleomycin and methotrexate (VbM) followed by 44 Gy regional radiation therapy (RT) in which the FFP rate was >80% at 4 years [7]. The study was designed so that there was an 80% power to detect a reduction in FFP from 80% to just under 60% using the likelihood ratio test for a one-tailored alpha level of 5%. With regard to superiority, 5-year data would provide ∼80% power to detect an increase in cure rate from 80% to 95%. FFP was calculated from the start of treatment until disease progression or relapse. Overall survival (OS) was calculated from the start of treatment to death from any cause or the last follow-up. Disease-specific survival (DSS) was calculated from the start of treatment to death from disease or the last follow-up. The Kaplan–Meier method was used to estimate FFP and OS curves. [8] Tests of statistical significance in the comparison of survival curves were calculated using the Gehan and the log-rank statistic [9, 10].
treatment plan
The Stanford V chemotherapy regimen has been reported previously [6]. In the G4 study, chemotherapy was further abbreviated and administered weekly for 8 weeks as follows: mechlorethamine 6 mg/m2 i.v. on weeks 1 and 5; doxorubicin 25 mg/m2 i.v. weeks 1, 3, 5 and 7; vinblastine 6 mg/m2 i.v. weeks 1, 3, 5, 7; vincristine 1.4 mg/m2 i.v. (dose capped at 2 mg) weeks 2, 4, 6 and 8; bleomycin 5 U/m2 i.v. weeks 2, 4, 6 and 8; etoposide 60 mg/m2 i.v. × 2 days weeks 3 and 7. Prednisone 40 mg/m2 was administered orally every other day for the first 6 weeks and tapered by 10 mg/day over next 2 weeks. Chemotherapy doses (except for vincristine and bleomycin) were reduced to 65% if the absolute neutrophil count (ANC) was < 1000/µl and delayed by 1 week if the ANC was <500/µl. If dose reduction or delay occurred at any time during chemotherapy, granulocyte colony-stimulating factor (G-CSF) (5 µg/kg × 3–5 days) was incorporated into all subsequent treatments on the odd weeks. Serotonin receptor antagonists and decadron were recommended as prechemotherapy antiemetics for weeks 1, 3, 5 and 7. Prophylactic agents administered included ranitidine, 150 mg orally twice a day and cotrimoxazole, double strength, orally twice a day on weekends throughout the treatment period.
One to three weeks following the completion of chemotherapy, patients initiated a course of modified IFRT (30–30.6 Gy in 1.5–1.8 Gy fractions). Radiation fields included all Ann Arbor regions where disease was detected by physical exam or radiographic studies (≥1.5 cm nodes). Modifications of the IF concept included: high neck lymph nodes (above the larynx) were treated only if initially involved; bilateral pulmonary hilar lymph nodes were irradiated if there was any mediastinal disease; bilateral supraclavicular nodes were always treated in conjunction with the mediastinum; the inferior border of the mediastinal field extended no more than 5 cm below the level of the initially involved nodes, and the ipsilateral infraclavicular (subpectoral) nodes were treated whenever the axillary nodes were involved.
Complete blood cell count and chemistry panel were reviewed weekly during the chemotherapy and at the completion of IFRT. Patients were seen for follow-up with relevant laboratory tests and a chest X-ray every 3 months following treatment during years 1 and 2, every 6 months during years 3–5 and annually thereafter. To follow response, CT scans for all abnormal areas at diagnosis were repeated at the conclusion of chemotherapy and at the end of IFRT. CT scans of the chest, abdomen and pelvis were done at the end of years 1, and 2 and later if clinically indicated.
results
patient population
From March 1995 to July 2001, 90 consecutive eligible patients with stage IA–IIA nonbulky HL were evaluated for the study. From this subset, 87 patients enrolled on study and 3 patients declined participation. Patient characteristics are detailed in Table 1. The median age was 30 years (range, 16–59 years). Sixty-five (75%) of patients had mediastinal involvement.
Table 1.
Patient characteristics (N = 87)
| n | % | |
|---|---|---|
| Median age, 30 (16–59) years | ||
| Age > 50 years | 4 | 5 |
| Gender | ||
| Male | 44 | |
| Female | 43 | |
| Subtype of classical HL | ||
| Nodular sclerosis | 73 | 84 |
| Mixed cellularity | 14 | 16 |
| Stage | ||
| IA | 23 | 26 |
| IIA or IIEA | 64 | 74 |
| ESR ≥ 50 mm/h | 12 | 14 |
| Mediastinal involvement | 65 | 75 |
| Ann Arbor nodal sites > 3 | 16 | 18 |
| Ann Arbor nodal sites > 2 | 39 | 45 |
| Extranodal disease | 3 | 3 |
| Unfavorable risk factors | ||
| GHSG criteria | ||
| >2 nodal regionsa, ESR ≥ 50, E sites | 42 | 48 |
| EORTC criteria | ||
| >3 nodal regionsb, ESR ≥ 50, mixed cellularity, age > 50 years | 33 | 38 |
aDefined according to GHSG criteria.
bDefined according to EORTC criteria.
ESR, erythrocyte sedimentation rate; GHSG, German Hodgkin Study Group; EORTC, European Organisation for Research and Treatment of Cancer.
toxicity
All 87 patients completed the planned 8 weeks of abbreviated Standard V chemotherapy and consolidative RT. Six patients (7%) experienced one or more transient grade 3 or 4 nonhematologic toxicities including: constipation, abdominal pain, peripheral neuropathy, allergic reaction to etoposide, weakness, chest pain and mylagias. Thirty-eight patients (44%) required a treatment delay and/or chemotherapy dose reduction for one dose due to grade 3 (n = 16) or grade 4 (n = 23) neutropenia. Two patients had neutropenic fever that required hospitalization. For the majority of patients in this group, subsequent odd weeks of treatment were supported with G-CSF administration for 2–4 days. No grade 3–4 thrombocytopenia was noted. No acute bleomycin toxicity or radiation pneumonitis was identified. There were no treatment-related deaths.
efficacy
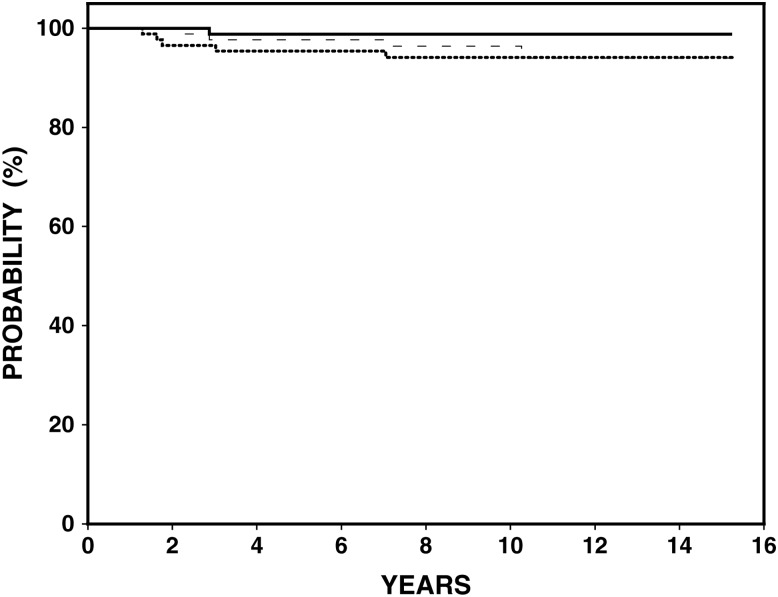
The median follow-up is 10.6 years (range 2–15.5 years). The estimated 10-year FFP is 94% [95% confidence interval (CI) 89.2% to 99.3%], DSS 99% and OS 94% (95% CI 92.5% to 100%) (Figure 1). Five patients relapsed, two within the RT field and three both within RT fields and at distant sites (Table 2). Four of the five relapses occurred at a median of 16 months (range 13–33 months) and in the fifth patient at 81 months. In the latter case, the patient had not completed the recommended follow-up due to two successive full-term pregnancies that precluded imaging studies. Second-line therapy included high-dose therapy with stem cell support (n = 3) and chemotherapy with ABVD alone (n = 2) with disease control achieved in four patients.
Figure 1.
Outcome for 87 patients with early stage Hodgkin lymphoma. At median followup of 10.6 years: estimated freedom from progression (dotted line) 94%, disease specific survival (solid line) 99% and overall survival (dashed line) 94%.
Table 2.
Characteristics of patients who relapsed
| Patient | Stage | Unfavorable risk factor(s)a | Time to relapse | Sites of relapse |
|
|---|---|---|---|---|---|
| (months) | Within RT field | Distant | |||
| 1 | 2 | >3 nodal sites and age > 50 years | 16 | X | X |
| 2 | 2 | ESR > 50 | 13 | X | X |
| 3 | 2 | ESR > 50 | 16 | X | |
| 4 | 2 | >3 nodal sites | 81 | X | X |
| 5 | 2 | >3 nodal sites, ESR > 50, mixed cellularity histology | 33 | X | |
aUnfavorable factors according to GHSG or EORTC
ESR, erythrocyte sedimentation rate.
Five patients developed secondary cancers: metastatic colon cancer (n = 1), melanoma in unirradiated sites (n = 2) and breast cancer (n = 2). Both patients with breast cancer were older than 30 years at the outset of treatment. One patient had received RT to her involved left axilla at age 38 and 6 years later developed a left breast invasive ductal carcinoma. Of note, this patient had a significant family history of breast cancer in both her maternal grandmother and maternal aunt. The second patient treated at age 33, developed mucinous breast cancer in a nonirradiated site of the left breast 5½ years after completion of therapy. No patient developed therapy related secondary leukemia or myelodysplastic syndrome. Overall, four patients have died: transplant related complications (n = 2), metastatic colon cancer (n = 1) and swine influenza (n = 1).
discussion
Over the past two decades, changes in the management of early-stage HL have emphasized the reduction of late effects by reducing radiation field size and/or dose, eliminating radiation entirely and/or modifying chemotherapy (i.e. intensity or number of cycles). The abbreviated Stanford V for 8 weeks followed by IFRT regimen results in excellent outcomes that demonstrate no detriment in FFP compared with our prior study with six cycles of VbM chemotherapy and 44 Gy regional RT for patients with nonbulky stage I-IIA HL. With 10-year FFP, DSS and OS of 94%, 99% and 94%, respectively, these results are comparable to other results reported recently in the literature [3, 5, 11]. The regimen was well tolerated with no primary treatment-related deaths. Although the median age of the patients included in our study (30 years) is younger than those included in the National Cancer Institute of Canada Clinical Trials Group (NCIC CTG) HD.6 trial (36 years) and the German Hodgkin Study Group (GHSG) HD10 trial (39 years), it is similar to the median age of other trials for stage I–II disease, such as European Organisation for Research and Treatment of Cancer (EORTC) H8F (30 years), EORTC H8U (32 years), GHSG HD11 (33 years) and GHSG HD14 (32 years) [2, 3, 5, 11, 12].
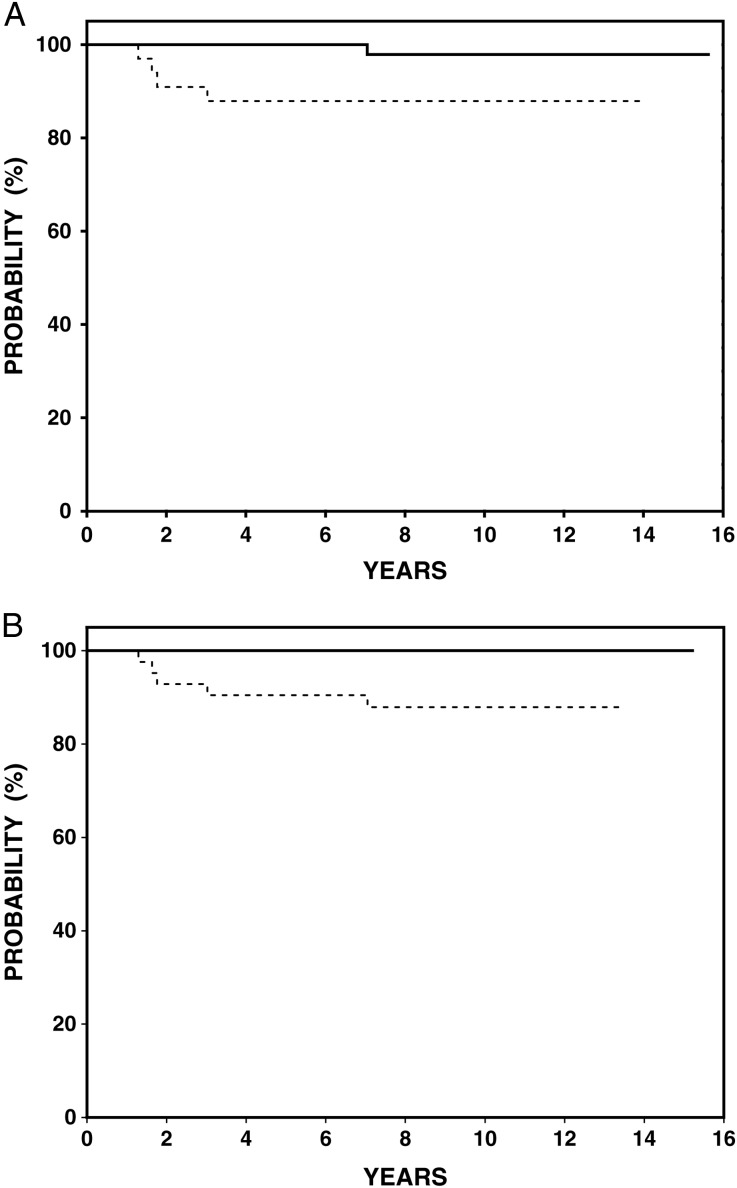
Clinical trials groups generally consider bulky mediastinal disease (variably defined) or the presence of B symptoms to be unfavorable prognostic factors in stage I–II HL, and therefore worthy of more intensive therapy. However, European groups have also included additional prognostic factors to stratify patients into more aggressive treatment protocols [2–4]. For example, patients with elevated ESR (>50) or >3 regions of involvement and stage I–II disease were treated on trials for ‘unfavorable’ stage I–II (GHSG HD11 or EORTC H9U) with ABVD × 4–6 + IFRT or bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine and prednisone baseline × 4 + IFRT [3, 4, 13]. Our results are comparable to the results of the ‘favorable’ trials of those groups (GHSG HD10 and EORTC H9F) [5, 13], despite our inclusion of 38% of patients with unfavorable risk factors receiving this attenuated therapy (Figure 2A and B). As risk factors such as elevated ESR, mixed cellularity histology and number of sites of disease were identified in the era when RT alone was used in the management of HL, it is possible that these no longer remain important in the era of combined modality therapy. For patients with favorable disease, the GHSG HD10 trial has reported excellent 8 year FFTF of 86% with as few as two cycles of ABVD + 20 Gy IFRT [5]. Our study results are comparable in patients considered ‘favorable’ by both GHSG and EORTC criteria with a FFP of 98%. While cumulative doses of doxorubicin are similar, the bleomycin dose is 50% lower in abbreviated Stanford V (20 U/m2) compared with 2 cycles of ABVD (40 U/m2). Therefore, in situations where it is desired to limit exposure to bleomycin our combined modality treatment regimen offers an acceptable alternative. It is possible that the subset of ‘favorable’ patients in our study would do well with further reduction of IFRT to 20 Gy, and this is currently being evaluated in our G5 trial [14].
Figure 2.
Freedom from progression (FFP) according to European prognostic criteria. (A) German Hodgkin Study Group (GHSG): FFP 100% for favorable patients (solid line, n = 45) and 88% for unfavorable (dotted line, n = 42) (95% confidence interval 78.5% to 94.8%), P = 0.018. (B) European Organisation for Research and Treatment of Cancer (EORTC): FFP 97.8% for favorable patients (solid line, n = 54) and 88% (95% confidence interval 94.1% to 100%), for unfavorable (dotted line, n = 33) (95% confidence interval 77.4% to 99.8%), P = 0.04.
Concerns regarding the potential long-term risks of any RT have led to programs testing the use of chemotherapy alone. Most notable is the NCIC CTG HD.6 trial. This trial compared a standard RT containing regimen (subtotal lymphoid irradiation (STLI) alone for favorable disease and combined modality therapy with two cycles of ABVD and STLI for unfavorable disease) to an experimental arm consisting of chemotherapy alone (either four or six cycles of ABVD, depending on the response by CT imaging after two cycles of therapy for both favorable and unfavorable patients) [11]. At a median follow-up of 11.3 years, the freedom from disease progression favored the radiation-containing regimens (92% vs. 87%, P = 0.05), however, OS was superior among patients treated with chemotherapy alone (94% versus 87%, P = 0.04) [11]. The differences in OS were largely related to deaths due to secondary cancers and other causes, some of which were not clearly radiation related. The data related to the use of RT in this study are difficult to compare with contemporary studies because such extensive radiation treatment (sub–total nodal irradiation, STNI) has been abandoned and is no longer used in combined modality programs. In fact, the use of reduced radiation fields (IFRT) has been associated with a significant decrease in the risk for second cancers [15]. In the G4 trial, we observed five second cancers, only one of which (a breast cancer) arose in an irradiated site.
The long-term disease control of 87% and an OS of 94% reported for the NCIC CTG HD.6 trial with ABVD chemotherapy alone are excellent and set a benchmark to which current and future trials with combined modality therapy will need to be compared. [11] The concept of risk adaptation, utilized in the NCIC study, is now being incorporated into other clinical trials, although escalation or de-escalation of therapy is based on interim PET imaging, rather than response on conventional CT imaging [16–18].
In summary, mature overall results from the G4 study conducted at Stanford and Kaiser community practices of the abbreviated Stanford V regimen and low-dose IFRT are excellent. Continued efforts to improve risk assessment in early-stage HL are critical to tailor treatment intensity and allow for an individualized risk adapted therapy approach that minimizes late effects without compromising high cure rates.
funding
This work was supported by the National Institutes of Health (R01 CA56060). The remaining authors have declared no conflicts of interest.
disclosure
Sandra J. Horning is currently employed by Genentech and has stock ownership in Roche.
references
- 1.Herbst C, Rehan FA, Brillant C, et al. Combined modality treatment improves tumor control and overall survival in patients with early stage Hodgkin's lymphoma: a systematic review. Haematologica. 2009;95:494–500. doi: 10.3324/haematol.2009.015644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferme C, Eghbali H, Meerwaldt JH, et al. Chemotherapy plus involved-field radiation in early-stage Hodgkin's disease. N Engl J Med. 2007;357:1916–1927. doi: 10.1056/NEJMoa064601. [DOI] [PubMed] [Google Scholar]
- 3.Eich HT, Diehl V, Gorgen H, et al. Intensified chemotherapy and dose-reduced involved-field radiotherapy in patients with early unfavorable Hodgkin's lymphoma: final analysis of the German Hodgkin Study Group HD11 trial. J Clin Oncol. 2010;28:4199–4206. doi: 10.1200/JCO.2010.29.8018. [DOI] [PubMed] [Google Scholar]
- 4.Ferme C, Divine M, Vranovsky A, et al. Four ABVD and Involved-Field Radiotherapy in Unfavorable Supradiaphragmatic Clinical Stages (CS) I-II Hodgkin's Lymphoma (HL): Preliminary Results of the EORTC-GELA H9-U Trial. Blood. 2005;106:813. ASH Annual Meeting Abstracts. [Google Scholar]
- 5.Engert A, Plutschow A, Eich HT, et al. Reduced treatment intensity in patients with early-stage Hodgkin's lymphoma. N Engl J Med. 2010;363:640–652. doi: 10.1056/NEJMoa1000067. [DOI] [PubMed] [Google Scholar]
- 6.Horning SJ, Hoppe RT, Breslin S, et al. Stanford V and radiotherapy for locally extensive and advanced Hodgkin's disease: mature results of a prospective clinical trial. J Clin Oncol. 2002;20:630–637. doi: 10.1200/JCO.2002.20.3.630. [DOI] [PubMed] [Google Scholar]
- 7.Horning SJ, Hoppe RT, Mason J, et al. Stanford-Kaiser Permanente G1 study for clinical stage I to IIA Hodgkin's disease: subtotal lymphoid irradiation versus vinblastine, methotrexate, and bleomycin chemotherapy and regional irradiation. J Clin Oncol. 1997;15:1736–1744. doi: 10.1200/JCO.1997.15.5.1736. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan EL, Meier P. Non-parametric estimation of incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 9.Gehan E. A generalized Wilcoxon test for comparing arbitrarily singly-censored samples. Biometrika. 1965;52:203–223. [PubMed] [Google Scholar]
- 10.Peto R, Peto J. Asymptotically efficient rank invariant test procedures. J R Stat Soc A. 1972;135:185–198. [Google Scholar]
- 11.Meyer RM, Gospodarowicz MK, Connors JM, et al. ABVD Alone versus Radiation-Based Therapy in Limited-Stage Hodgkin's Lymphoma. N Engl J Med. 2012;366:399–408. doi: 10.1056/NEJMoa1111961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Tresckow B, Plutschow A, Fuchs M, et al. Dose-intensification in early unfavorable Hodgkin's lymphoma: final analysis of the German Hodgkin study group HD14 trial. J Clin Oncol. 2012;30:907–13. doi: 10.1200/JCO.2011.38.5807. [DOI] [PubMed] [Google Scholar]
- 13.Noordijk EM, Thomas J, Ferme C, et al. First results of the EORTC-GELA H9 randomized trials: the H9-F trial (comparing 3 radiation dose levels) and H9-U trial (comparing 3 chemotherapy schemes) in patients with favorable or unfavorable early stage Hodgkin's lymphoma (HL) ASCO Meeting Abstracts. 2005;23:6505. [Google Scholar]
- 14.Advani R, Horning SJ, Jonathan E, et al. Abbreviated 8-week chemotherapy (CT) plus involved node radiotherapy (INRT) for nonbulky stage I-II Hodgkin lymphoma: preliminary results of the Stanford G5 Study. ASCO Annual Meeting Abstracts. 2011;29:8064. [Google Scholar]
- 15.De Bruin ML, Sparidans J, van't Veer MB, et al. Breast cancer risk in female survivors of Hodgkin's lymphoma: lower risk after smaller radiation volumes. J Clin Oncol. 2009;27:4239–4246. doi: 10.1200/JCO.2008.19.9174. [DOI] [PubMed] [Google Scholar]
- 16.Gallamini A, Patti C, Viviani S, et al. Early chemotherapy intensification with BEACOPP in advanced-stage Hodgkin lymphoma patients with a interim-PET positive after two ABVD courses. Br J Haematol. 2011;152:551–560. doi: 10.1111/j.1365-2141.2010.08485.x. [DOI] [PubMed] [Google Scholar]
- 17.Radford J, O'Doherty M, Barrington S, et al. Results of the 2nd planned interim analysis of the RAPID trial (involved field radiotherapy versus no further treatment) in patients with clinical stages 1A and 2A Hodgkin lymphoma with a ‘negative’ FDG-PET scan after 3 cycles ABVD. Blood (ASH Annual Meeting Abstract) 2008;112 369. [Google Scholar]
- 18.Eastern Cooperative Oncology Group. Chemotherapy based on PET scan in treating patients with stage I or stage II Hodgkin lymphoma. ClinicalTrials.gov. 2012 Bethesda (MD), National Library of Medicine (US), http://clinicaltrials.gov/ct2/show/NCT01390584 . [Google Scholar]