Abstract
Background
Epidemiological studies have reported inconsistent associations between cruciferous vegetable (CV) intake and colorectal cancer (CRC) risk. To our knowledge, a comprehensive and quantitative assessment of the association between CV intake and CRC has not been reported.
Methods
Relevant articles were identified by searching MEDLINE. We pooled the relative risks (RR) from individual studies using a random-effect model and carried out heterogeneity and publication bias analyses.
Results
Twenty-four case–control and 11 prospective studies were included in our analysis. When all studies were pooled, we yielded a significantly inverse association between CV (RR: 0.82; 95% confidence interval 0.75–0.90) intake and CRC risk. Specific analysis for cabbage and broccoli yielded similar result. When separately analyzed, case–control studies of CV intake yield similar results, and the results from the prospective studies showed borderline statistical significance. Moreover, significant inverse associations were also observed in colon cancer and its distal subsite both among prospective and case–control studies.
Conclusions
Findings from this meta-analysis provide evidence that high intake of CV was inversely associated with the risk of CRC and colon cancer in humans. Further analysis on other specific CV, food preparation methods, stratified results by anatomic cancer site, and subsite of colon cancer should be extended in future study.
Keywords: colorectal cancer, cruciferous vegetables, dietary, epidemiology, meta-analysis
introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer worldwide in males and the second in females, with over 1.2 million new cases diagnosed in 2008 accounting for 9.7% of all incident cancers [1]. Epidemiological and experimental studies have provided strong evidence that smoking, physical inactivity, overweight and obesity can cause CRC [2]. Despite the role of diet as a contributing factor in CRC development, convincing evidence only demonstrated that intakes of alcohol (among males) and red and processed meat are considered to be dietary causes of CRC [3].
Cruciferous vegetables (CV) contain a variety of bioactive components such as folate, vitamin C, tocopherols, and carotenoids [4]. However, the most frequently attributable anticancer constituent of CV is glucosinolates (GLS), the precursors of isothiocyanates (ITCs) as well as indole-3-carbinol (I3C), which may contribute to reduce risk of CRC. Evidence from animal studies has indicated that the joint induction of phase I (i.e. cytochrome P450s) and phase II enzymes (e.g. glutathione S-transferases, GST) by a variety of CV results in a favorable metabolic profile for the elimination of certain chemical carcinogens [5, 6]. On the other hand, CV as a good source of dietary fiber which can prevent CRC by several plausible mechanisms, including increased fecal bulk and dilution of carcinogens in the colonic lumen, reduced transit time, and bacterial fermentation of fiber to short-chain fatty acids [7, 8]. In an updated report from the World Cancer Research Fund and the American Institute for Cancer Research (WCRF/AICR), dietary fiber intake has been upgraded as a convincing protective dietary factor for CRC [9].
A recent meta-analysis which included 19 prospective studies reported that there is a weak but statistically significant inverse association between vegetable intake and CRC [summary relative risk (RR): 0.91; 95% confidence interval (CI) 0.86–0.96] [10]. Nevertheless, the epidemiological results of whether intake of CV may protect against CRC are still controversial. Some epidemiological reviews published in the mid-1990s [11, 12] indicated an inverse association between CV intake and CRC, but some cohort studies have cast doubt on the strength of the inverse association between CV intake and CRC risk [13–16]. Therefore, we carried out a meta-analysis on all prospective and case–control studies published up to April 2012 to evaluate the relationship between CV intake and CRC.
methods
literature search
We carried out a comprehensive literature search up to April 2012 using the MEDLINE (PubMed), with restrictions to the studies of humans and articles published in English using the following search key words and medical subject heading terms: (Brassicaceae OR Brassica OR cruciferous vegetables OR broccoli OR cabbage OR cauliflower OR Brussels sprouts OR mustard plants OR sauerkraut OR cole slaw OR collards OR bok choy OR turnip greens OR vegetables) AND (colorectal OR colorectum OR colon OR rectal OR rectum) AND (cancer OR neoplasm OR carcinoma OR tumor). Furthermore, we also searched the reference lists of all included studies. We followed standard criteria for conducting and reporting meta-analyses [17].
study selection criteria
Published studies were included if they (i) used a case–control or prospective study design; (ii) evaluated the association between CV intake and CRC risk; (iii) presented odds ratio (OR), RR, or hazard ratio (HR) estimates with 95% CI, standard errors (SE), or data necessary to calculate these. When multiple publications from the same study were available, we used the publication with the largest number of cases and most applicable information.
data abstraction and quality assessment
For each eligible study, two investigators (Q-JW and YY) independently carried out the eligibility evaluation, data abstraction, and quality assessment; disagreements were resolved by consensus. Data abstracted from each study included are as follows: the first author's last name, year of publication, study region and design, study sample size (number of cases and controls or cohort size), age range or the mean age of studies, duration years of follow-up for cohort studies, measures and types of CV and intake categories, study-specific adjusted ORs or RRs with their 95% CIs for the highest versus lowest category of CV intake (if multiple estimates were available, we abstracted the estimate that adjusted for the most covariates), and factors matched by or adjusted for in the design or data analysis.
To assess the study quality, a 10-star system on the basis of the Newcastle–Ottawa Scale [18–21] was used in this meta-analysis. The full score was 10 and the high-quality study was defined as a study with quality scores ≥7.
statistical analysis
The study-specific adjusted RRs were used as the common measure of association across studies. Because the absolute risk of CRC is low in human, the ORs in case–control studies should approximate the RRs or HRs; therefore, we reported all results as RRs for simplicity. Some studies presented individual risk estimates according to the different types of CV and did not report the effect of total CV intake. In this situation, the study-specific effect size in overall analysis was calculated by pooling the risk estimates of the various CV types, using the inverse-variance method [22]. For studies that reported results separately for males and females or proximal and distal colon or colon and rectal cancer, but not combined, we pooled the results using a fixed-effect model to obtain an overall combined estimate before combining with the rest of the studies [8, 10].
The possible heterogeneity in results across studies was examined by using the Cochran Q and I2 statistics [23]. For the Q statistic, a P-value <0.1 was considered to be representative of statistically significant heterogeneity. I2 represents the proportion of total variation contributed by between-study variation [23]. When substantial heterogeneity was detected, the summary estimate based on the random-effect model (DerSimonian–Laird method) [24] was reported, which assumes that the studies included in the meta-analysis had varying effect sizes. Otherwise, the summary estimate based on the fixed-effect model (the inverse variance method) [25] was reported, which assumes that the studies included in the meta-analysis had the same effect size. Summary estimates were calculated for CV, cabbage, and broccoli intakes. Heterogeneity between subgroups was evaluated by meta-regression in our analysis. Subgroup analyses were carried out based on study quality, study design (prospective versus case–control studies), type of control subjects for case–control (population-based versus hospital-based controls), geographic location (Europe, America, and Asia), gender (males versus females), anatomic site of CRC (colon versus rectum cancer), and cancer subsite of colon (proximal versus distal colon cancer). Moreover, we carried out sensitivity analyses excluding one study at a time to explore whether the results were strongly influenced by a specific study.
Publication bias was evaluated via Egger's linear regression [26], Begg's rank correlation methods [27] and funnel plots. A P-value <0.05 for Egger's or Begg's tests was considered representative of significant statistical publication bias. Statistical analyses were carried out with Stata (version 11.0; StataCorp, College Station, TX), and all statistical tests were two-sided.
results
literature search
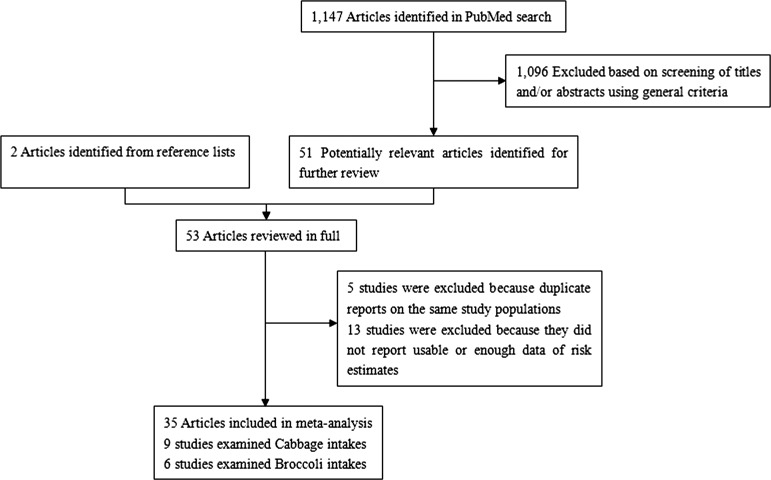
We identified 1147 potentially relevant articles from our search of the PubMed databases. Of these, 1096 articles were excluded after the first screening based on abstracts or titles, leaving 51 articles for full-text review. Handsearching of the bibliographic references of these articles identified two additional articles [28, 29], of a total of 53 articles for full-text review. Figure 1 shows a flow diagram, identifying the relevant studies. After exclusion, the remaining 35 articles [13–16, 28–58] were included in the systematic review and meta-analysis.
Figure 1.
Selection of studies for inclusion in meta-analysis
study characteristics and quality assessment
Characteristics of the 35 included articles are shown in supplementary Table S1, available at Annals of Oncology online. All included articles, which included 24 275 cases and 1 295 063 subjects, were published between 1978 and 2012, consisting of 11 prospective studies (10 cohort studies [13–16, 29, 31–35], one nested case–control study [30]) and 24 case–control studies [28, 36–58] in which 2 articles [38, 39] using the same control population reported the results of colon and rectum cancer separately. Hence we pooled the results of these two studies. Of the 11 prospective studies, eight were conducted in the United States [13, 14, 16, 29, 31–34], one each in China [30], Finland [15], and The Netherlands [35]. Sample sizes ranged from 17 633 [29] to 488 043 [13], and the number of CRC cases varied from 145 [29] to 2972 [13].
Of the 24 case–control studies, 6 were conducted in the United States [40, 47, 52, 55, 56, 58], 3 in Australia [36, 49, 57], 2 each in Canada [38, 39], Singapore [44, 54], Italy [28, 51], Japan [42, 48], and the UK [41, 46], one each in China [43], Spain [53], India [37], Russia [50], and Uruguay [45]. The number of patients enrolled in these studies ranged from 43 [52] to 3575 [28], and the number of control subjects varied from 41 [52] to 31 782 [48]. Control subjects were drawn from the general population in 12 studies [36, 38–41, 43, 44, 46, 49, 55–57], hospitals in 8 studies [28, 37, 42, 45, 47, 48, 51, 54], or both in 3 studies [50, 53, 58].
Study-specific quality scores are summarized in supplementary Tables S2 and S3, available at Annals of Oncology online. The range of quality scores was from 4 to 9; the median score was 7. The median scores of prospective and case–control studies were 7 and 6, separately. High-quality studies (i.e. those studies that had 7 awarded stars) included 10 prospective [13–15, 29–35] and 11 case–control studies [28, 36, 38, 39, 42–46, 49, 57].
cruciferous vegetables
In a random-effect pooled analysis of these studies, high-CV intake (comparing the highest with the lowest category) was associated with a reduced CRC risk (RR: 0.82; 95% CI 0.75–0.90) (Table 1). Statistically significant heterogeneity was observed in the study results (Q = 98.57, P < 0.001, I2 = 66.5%). There was no indication of publication bias with Egger's test (P = 0.08) or with Begg's test (P = 0.218).
Table 1.
Summary risk estimates of the association between cruciferous vegetable consumption and colorectal cancer risk
| Number of studies | Summary RR (95% CI) | Q-statistic | I2 | Ph* | Ph** | |
|---|---|---|---|---|---|---|
| Value (%) | ||||||
| Overall studies | ||||||
| CV | 35 | 0.82 (0.75–0.90) | 98.57 | 66.5 | <0.001 | – |
| Cabbage | 9 | 0.76 (0.60–0.97) | 19.20 | 58.3 | 0.014 | – |
| Broccoli | 6 | 0.82 (0.65–1.02) | 11.07 | 54.8 | 0.050 | – |
| Subgroup analyses for CV | ||||||
| High-quality studies (scores ≥7) | 20 | 0.88 (0.80–0.97) | 48.57 | 60.9 | <0.001 | 0.046 |
| Study design | ||||||
| Prospective studies | 11 | 0.93 (0.87–1.00) | 15.40 | 35.1 | 0.118 | 0.025 |
| Case–control studies | 23 | 0.74 (0.65–0.84) | 74.37 | 70.4 | <0.001 | |
| Type of control subjects | ||||||
| Population based | 11 | 0.79 (0.66–0.93) | 35.18 | 71.6 | <0.001 | 0.487 |
| Hospital based | 9 | 0.71 (0.56–0.89) | 30.76 | 74.0 | <0.001 | |
| Geographic location | ||||||
| Europe | 10 | 0.89 (0.76–1.04) | 24.23 | 62.8 | 0.004 | 0.822 |
| America | 14 | 0.81 (0.72–0.92) | 34.51 | 62.3 | 0.001 | |
| Asia | 7 | 0.82 (0.64–1.05) | 16.86 | 64.4 | 0.010 | |
| Gender | ||||||
| Male | 16 | 0.80 (0.69–0.93) | 54.75 | 72.6 | <0.001 | 0.546 |
| Female | 16 | 0.87 (0.77–0.98) | 29.26 | 48.7 | 0.015 | |
| Anatomic cancer site | ||||||
| Colon | 16 | 0.78 (0.69–0.89) | 37.06 | 59.5 | 0.001 | 0.225 |
| Rectum | 9 | 0.91 (0.74–1.13) | 21.21 | 62.3 | 0.007 | |
| Cancer subsite of colon | ||||||
| Proximal | 6 | 0.80 (0.67–0.95) | 8.66 | 42.3 | 0.123 | 0.709 |
| Distal | 6 | 0.74 (0.56–0.98) | 13.84 | 63.9 | 0.017 | |
| Adjustment for confounders | ||||||
| Body mass index | ||||||
| Yes | 14 | 0.90 (0.85–0.95) | 16.58 | 21.6 | 0.219 | 0.020 |
| No | 19 | 0.70 (0.59–0.83) | 72.10 | 75.0 | <0.001 | |
| Alcohol | ||||||
| Yes | 15 | 0.89 (0.84–0.94) | 21.07 | 33.5 | 0.100 | 0.061 |
| No | 19 | 0.73 (0.61–0.86) | 73.77 | 75.6 | <0.001 | |
| Physical activity | ||||||
| Yes | 13 | 0.90 (0.85–0.96) | 15.62 | 23.2 | 0.209 | 0.060 |
| No | 21 | 0.72 (0.59–0.88) | 63.05 | 76.2 | <0.001 | |
| Total energy intake | ||||||
| Yes | 16 | 0.90 (0.85–0.95) | 16.14 | 7.0 | 0.373 | 0.014 |
| No | 18 | 0.71 (0.59–0.85) | 75.87 | 77.6 | <0.001 | |
| Meat intake | ||||||
| Yes | 9 | 0.92 (0.85–0.99) | 7.63 | 0 | 0.470 | 0.290 |
| No | 25 | 0.78 (0.69–0.89) | 86.33 | 72.2 | <0.001 | |
| Family history of CRC/adenomatous polyposis | ||||||
| Yes | 13 | 0.93 (0.87–0.99) | 15.76 | 23.9 | 0.202 | 0.020 |
| No | 21 | 0.73 (0.64–0.84) | 73.43 | 72.8 | <0.001 | |
| Smoking status | ||||||
| Yes | 14 | 0.91 (0.86–0.97) | 18.58 | 30.0 | 0.137 | 0.019 |
| No | 20 | 0.72 (0.62–0.84) | 72.68 | 73.9 | <0.001 | |
| Subgroup analyses for cabbage | ||||||
| High-quality studies (scores ≥7) | 2 | 0.88 (0.55–1.39) | 0 | 0 | 0.935 | 0.633 |
| Study design | ||||||
| Prospective studies | 1 | 0.96 (0.54–1.72) | N/A | N/A | N/A | 0.557 |
| Case–control studies | 8 | 0.74 (0.57–0.97) | 18.98 | 63.1 | 0.008 | |
| Subgroup analyses for broccoli | ||||||
| High-quality studies (scores ≥7) | 5 | 0.86 (0.76–0.97) | 10.55 | 43.1 | 0.103 | 0.576 |
| Study design | ||||||
| Prospective studies | 3 | 0.91 (0.80–1.03) | 1.41 | 0 | 0.494 | 0.359 |
| Case–control studies | 3 | 0.60 (0.32–1.13) | 7.32 | 72.7 | 0.026 | |
CI, confidence interval; CV, cruciferous vegetables; N/A, not available; RR, relative risk.
*P-value for heterogeneity within each subgroup.
**P-value for heterogeneity between subgroups with meta-regression analysis.
cabbage
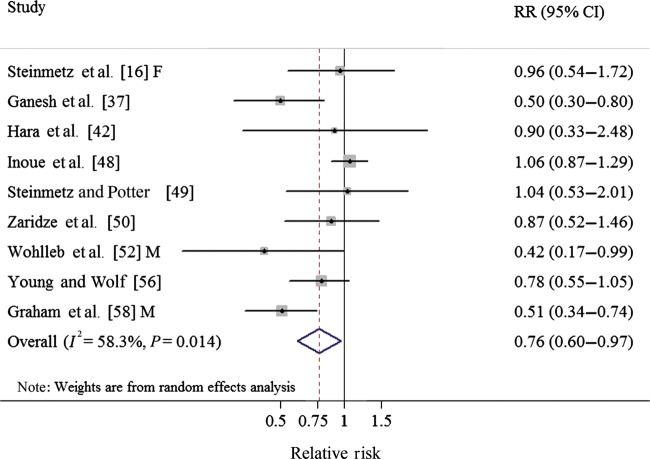
One cohort [16] and eight case–control studies [37, 42, 48–50, 52, 56, 58] investigated the association between cabbage intake and CRC risk. In a fixed-effect pooled analysis of these studies, high cabbage intake (comparing the highest with the lowest category) was associated with a reduced risk of CRC (RR: 0.76; 95% CI 0.60–0.97) (Table 1, Figure 2). There was moderate heterogeneity among the nine studies (Q = 19.20, P = 0.234, I2 = 58.3%), and no publication bias was found by the Egger's test (P = 0.196) or with Begg's test (P = 0.917).
Figure 2.
Forest plot (random-effect model) of cabbage consumption and colorectal cancer risk. Squares indicate study-specific relative risks (size of the square reflects the study-specific statistical weight); horizontal lines indicate 95% CIs; the diamond indicates the summary relative risk estimate with its 95% CI. CI, confidence interval; F, females; M, males; RR, relative risk.
broccoli
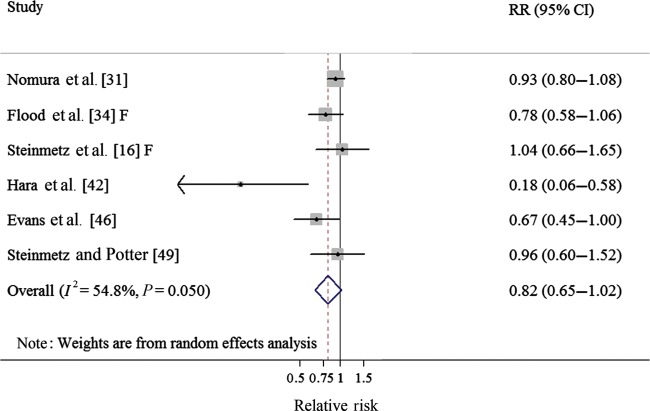
Three cohort studies [16, 31, 34] and three case–control studies [42, 46, 49] investigated the association between broccoli intake and CRC risk. The summary RR was 0.82 (95% CI 0.65–1.02), borderline significant, for high broccoli intake (comparing the highest with the lowest category) (Table 1, Figure 3), with moderate heterogeneity (Q = 11.07, P = 0.05, I2 = 54.8%), and no publication bias was found by the Egger's test (P = 0.164) or with Begg's test (P = 0.260).
Figure 3.
Forest plot (random-effect model) of broccoli consumption and colorectal cancer risk. Squares indicate study-specific relative risks (size of the square reflects the study-specific statistical weight); horizontal lines indicate 95% CIs; the diamond indicates the summary relative risk estimate with its 95% CI. CI, confidence interval; F, females; RR, relative risk.
subgroup and sensitivity analyses
In subgroup analyses of CV intake and CRC, all strata showed inverse associations but some associations were not statistically significant (Table 1). Similar results were observed for the intake of cabbage. Although the summary results of broccoli intake were not significant, there were inverse associations in all the strata and some showed statistically significance (Table 1). However, considering study design is the source of heterogeneity of CV intake and CRC (P-value for meta-regression = 0.025), we separated the studies by study design and carried out the subgroup analysis (supplementary Table S4, available at Annals of Oncology online).
In a sensitivity analysis of CV intake and CRC risk, we sequentially removed one study at a time and re-analyzed the data. The 36 study-specific RRs ranged from a low of 0.81 (95% CI 0.74–0.88) after omitting the study by Pietinen et al. [15] to a high of 0.84 (95% CI 0.78–0.92) after omitting the study by Kune et al. [57], but were in general similar. Meanwhile, we removed six studies [40, 51, 53, 55, 57, 58] in which RRs and 95% CI were not reported but calculated from raw data. The result from this analysis (RR: 0.88; 95% CI 0.81–0.95) was similar. Like CV intake, similar sensitivity analyses for cabbage and broccoli did not significantly change the results (data not shown). Furthermore, we chose not using the inverse-variance method to pool the risk estimates of the various CV types—the result also was robust (RR: 0.85; 95% CI 0.77–0.93).
discussion
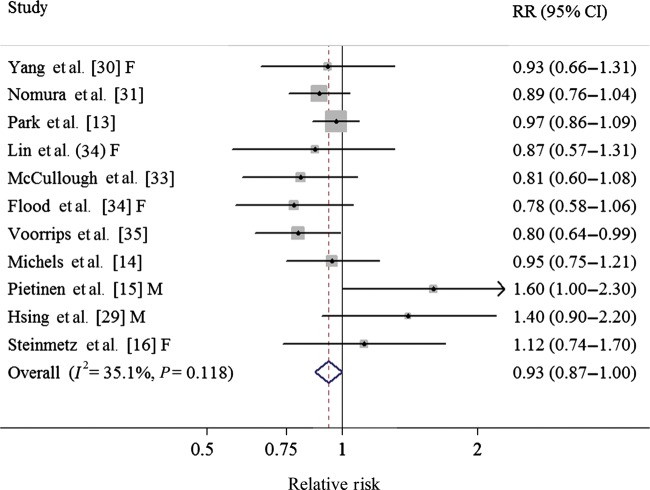
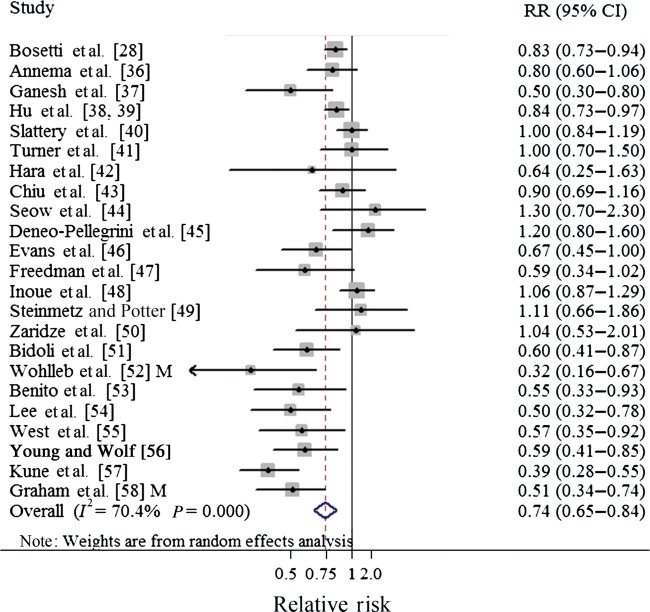
To our knowledge, this is the first meta-analysis evaluating an association between CV intake and CRC. In general, the results of this meta-analysis suggested that intake of CV may reduce the risk of CRC in humans (comparing the highest with the lowest category). Specific analysis for cabbage yielded similar inverse association but the result of broccoli showed borderline significance. Our findings from the case–control studies suggested a reduction between CV intake and CRC risk, but the results from the prospective studies showed borderline statistical significance (Table 1, Figures 4 and 5). Additionally, we also observed significant inverse associations in colon cancer and its distal cancer subsite among both prospective and case–control studies (supplementary Table S4, available at Annals of Oncology online).
Figure 4.
Forest plot (fixed-effect model) of cruciferous vegetables consumption and colorectal cancer risk in prospective studies. Squares indicate study-specific relative risks (size of the square reflects the study-specific statistical weight); horizontal lines indicate 95% CIs; the diamond indicates the summary relative risk estimate with its 95% CI. CI, confidence interval; F, females; M, males; RR, relative risk.
Figure 5.
Forest plot (random-effect model) of cruciferous vegetables consumption and colorectal cancer risk in case–control studies. Squares indicate study-specific relative risks (size of the square reflects the study-specific statistical weight); horizontal lines indicate 95% CIs; the diamond indicates the summary relative risk estimate with its 95% CI. CI, confidence interval; M, males; RR, relative risk.
The inverse association between CV intake and risk of CRC is biologically plausible. CV is a good source of GLS which can be hydrolyzed by the plant enzyme myrosinase into biologically active compounds (ITCs and I3C). Nonetheless, the anti-carcinogenic actions of GLS are commonly attributed to ITCs. The CRC cancer-protective effects of CV and their GLS breakdown products likely involve complex interactions of several mechanisms: the modulation of xenobiotic-metabolizing enzymes to protect against chemically induced tumors [59], antioxidant effects, and the induction of apoptosis and cell-cycle arrest [60, 61]. Furthermore, evidence from animal models of colon cancer also showed that CV has generally been shown to inhibit chemical carcinogenesis [60, 62]. On the other hand, CV are good sources of fiber which can prevent CRC by several plausible mechanisms, including increased fecal bulk and dilution of carcinogens in the colonic lumen, reduced transit time, and bacterial fermentation of fiber to short-chain fatty acids [7, 8].
Compared with retrospective studies, prospective studies are less susceptible to bias (e.g. recall bias, selection bias) due to their nature. Furthermore, case–control studies had a lower median quality score than prospective studies (6 versus 7). In the subgroup analyses of CV intake and CRC risk by study quality, we observed that the magnitude of risk reduction reported in high-quality studies was weaker than that reported in the overall analysis (18% versus 12%), which indicated that the association may have been changed by poor study methodologies. Likewise, in the subgroup analyses by type of control subjects, the protective effect in hospital-based control subjects was stronger than that in population-based ones (Table 1), which might mean hospital-based case–control studies more inclined to selection bias [19].
For the subgroup analysis of CV intake and CRC risk by anatomic CRC site and subsite of colon cancer, we observed a statistically significant inverse association between CV intake and colon cancer and its proximal and distal subsites. These results are consistent with a recent meta-analysis of the relation between fruit and vegetable and CRC [10] and a large cohort study in The Netherlands [35]. However, the result of a study in 2007 that has pooled 14 prospective cohorts showed that associations with colon cancer risk are not significant [pooled multivariate RR: 0.99 (95% CI 0.93–1.06) for the highest tertile versus lowest] for CV [63]. Compared with the number of studies on colon cancer, fewer studies conducted analyses of rectal cancer (16 versus 9), which may diminish our statistical power to detect an association. Thus, more prospective and well-designed studies should focus on this.
As with all meta-analyses, several limitations must be addressed. First, as the observational nature of the data, it is possible that the observed significant inverse association between CV intakes and CRC risk could be due to unmeasured or residual confounding. Higher intake of CV may be associated with other health behaviors (e.g. higher levels of PA, lower intakes of alcohol, and red meat). However, many of the studies adjusted for known confounding factors (e.g. BMI, alcohol consumption, PA). In addition, the results generally showed inverse association in the subgroup analyses when we stratified the results according to adjustment for confounding factors or other study characteristics. Second, because most studies used FFQ to assess CV intake, our results may have been influenced by misclassification. On the other hand, exposure levels of ITCs can be further affected by different food preparation methods [64]. Boiling CV results in a 30%–60% loss of intact GLS due to thermal degradation and leaching [65]. However, none of the studies separates the CV intake by cooking methods. Therefore, future epidemiological studies should consider whether the inverse association of CV will be affected by the food preparation methods. Third, significant heterogeneity and possible publication bias must be considered. There was significant heterogeneity for all studies combined (Q = 98.57, P < 0.001, I2 = 66.5%) in the pooled analysis of CV intake. This could be explained by many factors, mainly the design of this study (Table 1). Publication bias can be a problem in meta-analyses of published studies but we found no statistical evidence of publication bias in this meta-analysis. Last but not least, due to different methods used to report CV intake among studies and because we did not attempt to contact the authors for additional information, we failed to carry out a dose–response analysis between CV intake and CRC.
The strengths of this meta-analysis include a large sample size with 24 275 cases and 1 295 063 subjects which provided sufficient power to detect the putative association between CV intake and CRC, although compared with CV, fewer studies and cases were included in the subgroup analyses of cabbage and broccoli. The relatively large number of included studies also allowed us to conduct subgroup analyses according to a number of study characteristics to identify potential sources of heterogeneity.
In summary, this meta-analysis suggested that high intake of CV can decrease risk of CRC and colon cancer. More in-depth studies are warranted to report more detailed results, including other specific vegetables within the CV family, stratified results by anatomic cancer site, subsite of colon cancer, food preparation methods, or adjustment for potential confounders.
funding
This work was supported by the fund of the State Key Project Specialized for Infectious Diseases of China (2008ZX10002-015 and 2012ZX10002008-002). EV was also supported by the Fogarty International Clinical Research Scholars and Fellows Support Center at the Vanderbilt Institute for Global Health, funded by the Fogarty International Center, NIH, through an R24 Training Grant (grant number R24TW007988), and the Cancer Prevention and Control Training Program at the University of Alabama at Birmingham funded through the National Institutes of Health (5R25 CA047888).
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
Q-JW and Y-BX designed research; Q-JW, YY and Y-BX conducted research; Q-JW, YY analyzed data; Q-JW wrote the draft; all authors read, reviewed and approved the final manuscript. Y-BX had primary responsibility for final content.
references
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Giovannucci E, Kana W. Cancer of the colon and rectum. In: Schottenfeld D, Fraumeni JJ, editors. Cancer Epidemiology and Prevention. 3rd edition. New York: Oxford University Press; 2006. pp. 809–829. [Google Scholar]
- 3.World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity and the Prevention of Cancer: A Global Perspective. Washington, DC: American Institute for Cancer Research; 2007. [Google Scholar]
- 4.Kurilich AC, Tsau GJ, Brown A, et al. Carotene, tocopherol, and ascorbate contents in subspecies of Brassica oleracea. J Agric Food Chem. 1999;47:1576–1581. doi: 10.1021/jf9810158. [DOI] [PubMed] [Google Scholar]
- 5.Conaway CC, Yang YM, Chung FL. Isothiocyanates as cancer chemopreventive agents: their biological activities and metabolism in rodents and humans. Curr Drug Metab. 2002;3:233–255. doi: 10.2174/1389200023337496. [DOI] [PubMed] [Google Scholar]
- 6.Hecht SS. Inhibition of carcinogenesis by isothiocyanates. Drug Metab Rev. 2000;32:395–411. doi: 10.1081/dmr-100102342. [DOI] [PubMed] [Google Scholar]
- 7.Lipkin M, Reddy B, Newmark H, et al. Dietary factors in human colorectal cancer. Annu Rev Nutr. 1999;19:545–586. doi: 10.1146/annurev.nutr.19.1.545. [DOI] [PubMed] [Google Scholar]
- 8.Aune D, Chan DS, Lau R, et al. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ. 2011;343:d6617. doi: 10.1136/bmj.d6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Cancer Research Fund/American Institute for Cancer Research. http://www.dietandcancerreport.org/cup/current_progress/colorectal_cancer.php. 1 July 2012, date last accessed.
- 10.Aune D, Lau R, Chan DS, et al. Nonlinear reduction in risk for colorectal cancer by fruit and vegetable intake based on meta-analysis of prospective studies. Gastroenterology. 2011;141:106–118. doi: 10.1053/j.gastro.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 11.Steinmetz KA, Potter JD. Vegetables, fruit, and cancer prevention: a review. J Am Diet Assoc. 1996;96:1027–1039. doi: 10.1016/S0002-8223(96)00273-8. [DOI] [PubMed] [Google Scholar]
- 12.Verhoeven DT, Goldbohm RA, van Poppel G, et al. Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiol Biomarkers Prev. 1996;5:733–748. [PubMed] [Google Scholar]
- 13.Park Y, Subar AF, Kipnis V, et al. Fruit and vegetable intakes and risk of colorectal cancer in the NIH-AARP diet and health study. Am J Epidemiol. 2007;166:170–180. doi: 10.1093/aje/kwm067. [DOI] [PubMed] [Google Scholar]
- 14.Michels KB, Edward G, Joshipura KJ, et al. Prospective study of fruit and vegetable consumption and incidence of colon and rectal cancers. J Natl Cancer Inst. 2000;92:1740–1752. doi: 10.1093/jnci/92.21.1740. [DOI] [PubMed] [Google Scholar]
- 15.Pietinen P, Malila N, Virtanen M, et al. Diet and risk of colorectal cancer in a cohort of Finnish men. Cancer Causes Control. 1999;10:387–396. doi: 10.1023/a:1008962219408. [DOI] [PubMed] [Google Scholar]
- 16.Steinmetz KA, Kushi LH, Bostick RM, et al. Vegetables, fruit, and colon cancer in the Iowa Women's Health Study. Am J Epidemiol. 1994;139:1–15. doi: 10.1093/oxfordjournals.aje.a116921. [DOI] [PubMed] [Google Scholar]
- 17.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 18.Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses Available from http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp .
- 19.Yang WS, Va P, Wong MY, et al. Soy intake is associated with lower lung cancer risk: results from a meta-analysis of epidemiologic studies. Am J Clin Nutr. 2011;94:1575–1583. doi: 10.3945/ajcn.111.020966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larsson SC, Orsini N, Wolk A. Vitamin B6 and risk of colorectal cancer: a meta-analysis of prospective studies. JAMA. 2010;303:1077–1083. doi: 10.1001/jama.2010.263. [DOI] [PubMed] [Google Scholar]
- 21.Oh SW, Myung SK, Park JY, et al. Aspirin use and risk for lung cancer: a meta-analysis. Ann Oncol. 2011;22:2456–2465. doi: 10.1093/annonc/mdq779. [DOI] [PubMed] [Google Scholar]
- 22.Zhou Y, Zhuang W, Hu W, et al. Consumption of large amounts of Allium vegetables reduces risk for gastric cancer in a meta-analysis. Gastroenterology. 2011;141:80–89. doi: 10.1053/j.gastro.2011.03.057. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 24.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 25.Woolf B. On estimating the relation between blood group and disease. Ann Hum Genet. 1955;19:251–253. doi: 10.1111/j.1469-1809.1955.tb01348.x. [DOI] [PubMed] [Google Scholar]
- 26.Egger M, Davey SG, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 28.Bosetti C, Filomeno M, Riso P, et al. Cruciferous vegetables and cancer risk in a network of case-control studies. Ann Oncol. 2012;23:2198–2203. doi: 10.1093/annonc/mdr604. [DOI] [PubMed] [Google Scholar]
- 29.Hsing AW, McLaughlin JK, Chow WH, et al. Risk factors for colorectal cancer in a prospective study among U.S. white men. Int J Cancer. 1998;77:549–553. doi: 10.1002/(sici)1097-0215(19980812)77:4<549::aid-ijc13>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 30.Yang G, Gao YT, Shu XO, et al. Isothiocyanate exposure, glutathione S-transferase polymorphisms, and colorectal cancer risk. Am J Clin Nutr. 2010;91:704–711. doi: 10.3945/ajcn.2009.28683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nomura AM, Wilkens LR, Murphy SP, et al. Association of vegetable, fruit, and grain intakes with colorectal cancer: the Multiethnic Cohort Study. Am J Clin Nutr. 2008;88:730–737. doi: 10.1093/ajcn/88.3.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin J, Zhang SM, Cook NR, et al. Dietary intakes of fruit, vegetables, and fiber, and risk of colorectal cancer in a prospective cohort of women (United States) Cancer Causes Control. 2005;16:225–233. doi: 10.1007/s10552-004-4025-1. [DOI] [PubMed] [Google Scholar]
- 33.McCullough ML, Robertson AS, Chao A, et al. A prospective study of whole grains, fruits, vegetables and colon cancer risk. Cancer Causes Control. 2003;14:959–970. doi: 10.1023/b:caco.0000007983.16045.a1. [DOI] [PubMed] [Google Scholar]
- 34.Flood A, Velie EM, Chaterjee N, et al. Fruit and vegetable intakes and the risk of colorectal cancer in the Breast Cancer Detection Demonstration Project follow-up cohort. Am J Clin Nutr. 2002;75:936–943. doi: 10.1093/ajcn/75.5.936. [DOI] [PubMed] [Google Scholar]
- 35.Voorrips LE, Goldbohm RA, van Poppel G, et al. Vegetable and fruit consumption and risks of colon and rectal cancer in a prospective cohort study: The Netherlands Cohort Study on Diet and Cancer. Am J Epidemiol. 2000;152:1081–1092. doi: 10.1093/aje/152.11.1081. [DOI] [PubMed] [Google Scholar]
- 36.Annema N, Heyworth JS, McNaughton SA, et al. Fruit and vegetable consumption and the risk of proximal colon, distal colon, and rectal cancers in a case-control study in Western Australia. J Am Diet Assoc. 2011;111:1479–1490. doi: 10.1016/j.jada.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 37.Ganesh B, Talole SD, Dikshit R. A case-control study on diet and colorectal cancer from Mumbai, India. Cancer Epidemiol. 2009;33:189–193. doi: 10.1016/j.canep.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 38.Hu J, Mery L, Desmeules M, et al. Diet and vitamin or mineral supplementation and risk of rectal cancer in Canada. Acta Oncol. 2007;46:342–354. doi: 10.1080/02841860600746982. [DOI] [PubMed] [Google Scholar]
- 39.Hu J, Morrison H, Mery L, et al. Diet and vitamin or mineral supplementation and risk of colon cancer by subsite in Canada. Eur J Cancer Prev. 2007;16:275–291. doi: 10.1097/01.cej.0000228411.21719.25. [DOI] [PubMed] [Google Scholar]
- 40.Slattery ML, Curtin K, Sweeney C, et al. Diet and lifestyle factor associations with CpG island methylator phenotype and BRAF mutations in colon cancer. Int J Cancer. 2007;120:656–663. doi: 10.1002/ijc.22342. [DOI] [PubMed] [Google Scholar]
- 41.Turner F, Smith G, Sachse C, et al. Vegetable, fruit and meat consumption and potential risk modifying genes in relation to colorectal cancer. Int J Cancer. 2004;112:259–264. doi: 10.1002/ijc.20404. [DOI] [PubMed] [Google Scholar]
- 42.Hara M, Hanaoka T, Kobayashi M, et al. Cruciferous vegetables, mushrooms, and gastrointestinal cancer risks in a multicenter, hospital-based case-control study in Japan. Nutr Cancer. 2003;46:138–147. doi: 10.1207/S15327914NC4602_06. [DOI] [PubMed] [Google Scholar]
- 43.Chiu BC, Ji BT, Dai Q, et al. Dietary factors and risk of colon cancer in Shanghai, China. Cancer Epidemiol Biomarkers Prev. 2003;12:201–208. [PubMed] [Google Scholar]
- 44.Seow A, Quah SR, Nyam D, et al. Food groups and the risk of colorectal carcinoma in an Asian population. Cancer. 2002;95:2390–2396. doi: 10.1002/cncr.10971. [DOI] [PubMed] [Google Scholar]
- 45.Deneo-Pellegrini H, Boffetta P, De Stefani E, et al. Plant foods and differences between colon and rectal cancers. Eur J Cancer Prev. 2002;11:369–375. doi: 10.1097/00008469-200208000-00009. [DOI] [PubMed] [Google Scholar]
- 46.Evans RC, Fear S, Ashby D, et al. Diet and colorectal cancer: an investigation of the lectin/galactose hypothesis. Gastroenterology. 2002;122:1784–1792. doi: 10.1053/gast.2002.33659. [DOI] [PubMed] [Google Scholar]
- 47.Freedman AN, Michalek AM, Marshall JR, et al. Familial and nutritional risk factors for p53 overexpression in colorectal cancer. Cancer Epidemiol Biomarkers Prev. 1996;5:285–291. [PubMed] [Google Scholar]
- 48.Inoue M, Tajima K, Hirose K, et al. Subsite-specific risk factors for colorectal cancer: a hospital-based case-control study in Japan. Cancer Causes Control. 1995;6:14–22. doi: 10.1007/BF00051676. [DOI] [PubMed] [Google Scholar]
- 49.Steinmetz KA, Potter JD. Food-group consumption and colon cancer in the Adelaide Case-Control Study. I. Vegetables and fruit. Int J Cancer. 1993;53:711–719. doi: 10.1002/ijc.2910530502. [DOI] [PubMed] [Google Scholar]
- 50.Zaridze D, Filipchenko V, Kustov V, et al. Diet and colorectal cancer: results of two case-control studies in Russia. Eur J Cancer. 1992;29A:112–115. doi: 10.1016/0959-8049(93)90586-5. [DOI] [PubMed] [Google Scholar]
- 51.Bidoli E, Franceschi S, Talamini R, et al. Food consumption and cancer of the colon and rectum in north-eastern Italy. Int J Cancer. 1992;50:223–229. doi: 10.1002/ijc.2910500211. [DOI] [PubMed] [Google Scholar]
- 52.Wohlleb JC, Hunter CF, Blass B, et al. Aromatic amine acetyltransferase as a marker for colorectal cancer: environmental and demographic associations. Int J Cancer. 1990;46:22–30. doi: 10.1002/ijc.2910460107. [DOI] [PubMed] [Google Scholar]
- 53.Benito E, Obrador A, Stiggelbout A, et al. A population-based case-control study of colorectal cancer in Majorca. I. Dietary factors. Int J Cancer. 1990;45:69–76. doi: 10.1002/ijc.2910450114. [DOI] [PubMed] [Google Scholar]
- 54.Lee HP, Gourley L, Duffy SW, et al. Colorectal cancer and diet in an Asian population—a case-control study among Singapore Chinese. Int J Cancer. 1989;43:1007–1016. doi: 10.1002/ijc.2910430609. [DOI] [PubMed] [Google Scholar]
- 55.West DW, Slattery ML, Robison LM, et al. Dietary intake and colon cancer: sex- and anatomic site-specific associations. Am J Epidemiol. 1989;130:883–894. doi: 10.1093/oxfordjournals.aje.a115421. [DOI] [PubMed] [Google Scholar]
- 56.Young TB, Wolf DA. Case-control study of proximal and distal colon cancer and diet in Wisconsin. Int J Cancer. 1988;42:167–175. doi: 10.1002/ijc.2910420205. [DOI] [PubMed] [Google Scholar]
- 57.Kune S, Kune GA, Watson LF. Case-control study of dietary etiological factors: the Melbourne Colorectal Cancer Study. Nutr Cancer. 1987;9:21–42. doi: 10.1080/01635588709513908. [DOI] [PubMed] [Google Scholar]
- 58.Graham S, Dayal H, Swanson M, et al. Diet in the epidemiology of cancer of the colon and rectum. J Natl Cancer Inst. 1978;61:709–714. [PubMed] [Google Scholar]
- 59.Steinkellner H, Rabot S, Freywald C, et al. Effects of cruciferous vegetables and their constituents on drug metabolizing enzymes involved in the bioactivation of DNA-reactive dietary carcinogens. Mutat Res. 2001;480–481:285–297. doi: 10.1016/s0027-5107(01)00188-9. [DOI] [PubMed] [Google Scholar]
- 60.Smith TK, Mithen R, Johnson IT. Effects of Brassica vegetable juice on the induction of apoptosis and aberrant crypt foci in rat colonic mucosal crypts in vivo. Carcinogenesis. 2003;24:491–495. doi: 10.1093/carcin/24.3.491. [DOI] [PubMed] [Google Scholar]
- 61.Smith TK, Lund EK, Clarke RG, et al. Effects of Brussels sprout juice on the cell cycle and adhesion of human colorectal carcinoma cells (HT29) in vitro. J Agric Food Chem. 2005;53:3895–3901. doi: 10.1021/jf048025v. [DOI] [PubMed] [Google Scholar]
- 62.Uhl M, Kassie F, Rabot S, et al. Effect of common Brassica vegetables (Brussels sprouts and red cabbage) on the development of preneoplastic lesions induced by 2-amino-3-methylimidazo[4,5-f]quinoline (IQ) in liver and colon of Fischer 344 rats. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;802:225–230. doi: 10.1016/j.jchromb.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 63.Koushik A, Hunter DJ, Spiegelman D, et al. Fruits, vegetables, and colon cancer risk in a pooled analysis of 14 cohort studies. J Natl Cancer Inst. 2007;99:1471–1483. doi: 10.1093/jnci/djm155. [DOI] [PubMed] [Google Scholar]
- 64.Higdon JV, Delage B, Williams DE, et al. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol Res. 2007;55:224–236. doi: 10.1016/j.phrs.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang Y. Cancer-preventive isothiocyanates: measurement of human exposure and mechanism of action. Mutat Res. 2004;555:173–190. doi: 10.1016/j.mrfmmm.2004.04.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.