Abstract
Background/Aim:
Inflammatory bowel disease (IBD) is a chronic disease of unknown etiology and considered traditionally as a disease of the western world. Recently, rising trends have been observed in countries previously known to have a low prevalence and incidence. The aim of this study is to collect epidemiological data on IBD outpatients and to add data from the Kingdom of Saudi Arabia (KSA) to the available IBD literature.
Patients and Methods:
The medical records of 693 Saudi patients with IBD over a period of 17 years, between 1993 and 2009, were reviewed. The demographic and clinical data and methods of diagnosis were retrieved.
Results:
The total number of patients in this cohort was 693. It constituted 238 (34.3%) ulcerative colitis (UC) and 455 (65.7%) Crohn's disease (CD) patients. UC was steady throughout the years, whereas only 1.2 CD patients were diagnosed per year in the first 11 years, and 73.7 per year in the last six years. The median age of UC patients was 34 years, ranging from 10 to 80 years with a peak between 21 and 40 years and in CD it was 27 years, ranging from 11 to 73 years with a peak between 11 and 30 years. There was a male preponderance of 1.5:1 and 2:1, respectively. The rest of the data is discussed in this study.
Conclusion:
IBD is no longer a rare disease in KSA. UC is in a steady state, whereas CD is increasing significantly and far outnumbering UC.
Keywords: Crohn's disease, rising trend, Saudi Arabia, ulcerative colitis
Inflammatory bowel disease (IBD) is traditionally considered as a disease of the western world with geographic variations. It is a chronic disease of unknown etiology.[1] Its incidence and prevalence has increased sharply since the early fifties.?[2–6] In other parts of the world including Eastern Europe, South America, and the Pacific region, low prevalence and incidence rates had been reported until a few decades ago.[7–11] However, recent studies have shown rising trends in countries previously known to have a low incidence. In Asia for instance, the incidence and prevalence of IBD are lower than those reported from North America and Europe. However, recent studies have reported an increase in the prevalence of IBD with a predominance of ulcerative colitis (UC) in Asian communities.[12,13] Increasing rates have been reported especially from India, Japan, and the Middle East.[14,15] The rates are higher in Indians in Southeast Asia compared to the Chinese and Malays and the rates of UC are higher than those of Crohn's disease (CD).
This increase is apparently due to the westernization of lifestyles with dietary and environmental changes along with improved sanitation.[16] Similarly, a study from central China has reported an increasing prevalence.[17] The Arab countries are not excluded and several studies mainly from KSA over the last three decades have shown an increasing trend.[18–26]
In an Australian prospective population-based incidence study, an overall incidence rate of IBD of 29.6 per 100,000 has been amongst the highest reported rates and CD has been more frequent than UC.[27]
Also in Africa, a few studies have reported the presence of IBD. Over a period of 12 years, 85 patients have been reported from the National Center for Gastrointestinal and Liver disease in Khartoum, Sudan. The vast majority had UC.[28] In sub-Saharan Africans, the incidence seems to be very low. Only three cases of UC and one case of CD have been reported from a tertiary institution in northern Nigeria over a period of three years.[29]
The aim of this study was to publish our data collected from an outpatient clinic, which will add to the previously reported literature from institutions in the KSA and to emphasize the emergence as well as the rising rates of IBD in KSA from a gastroenterology-oriented polyclinic in Riyadh, KSA.
PATIENTS AND METHODS
We included in this retrospective study all 693 IBD patients diagnosed over a period of 17 years (between January 1993 and December 2009) at Al-Mofarreh Polyclinic, a gastroenterology-oriented private clinic receiving patients from various parts of KSA. Of these, 75 patients were diagnosed before attending the clinic. Only Saudi patients were included in this study. Due to the small number—only five patients—indeterminate colitis (IC) was not included in this analysis. All medical records of the 693 patients with IBD were thoroughly reviewed. Clinical, laboratory, endoscopic, histopathologic, and imaging data were analyzed. Complete blood count (CBC), erythrocyte sedimentation rate (ESR), bilirubin, alanine aminotransferase (ALT), alkaline phosphatase (AP), creatinine, stool, and urine examinations were performed in all patients. Further laboratory tests including serum total iron binding capacity (TIBC), C-reactive protein (CRP), electrolytes, anti-Saccharomyces cerevisiae antibody (ASCA) and perinuclear antineutrophil cytoplasmic antibody (p-ANCA) were performed selectively. Stool culture was performed when infectious causes were suspected and tuberculin skin test or purified protein derivative test (PPD) were performed in the majority of patients. All 693 patients had lower gastrointestinal endoscopy (LGIE) and 114 patients, who simultaneously had upper gastrointestinal symptoms, were also subjected to upper gastrointestinal endoscopy (UGIE). Olympus PCF-230 and XQ-230, Pentax EG-2901 and EC-3801F, as well as Fujinon EG-530WR and EC-430LP5 videoscopes were utilized. Hard copy documentation of all cases and multiple biopsies were obtained from affected and apparently normal sites. Small bowel series (SBS), which was supposed to be performed in all patients with CD, IC, and therapy refractory UC for a proper documentation of the location, extent, and severity of the disease, was declined by some patients due to financial reasons. For the same reasons, computed tomography (CT) scan and magnetic resonance imaging (MRI) were performed selectively. The final diagnosis was achieved by endoscopy and histopathology. At least two of the major endoscopic as well as two of the histologic criteria given by the European Crohn's and Colitis Organization (ECCO) were essential for the diagnosis.[30] However, a combination of clinical and diagnostic parameters should be incorporated into general assessment.
RESULTS
A total of 43,500 patients attended the clinic over a period of 17 years. The vast majority (40,375; 93%) had gastrointestinal symptoms, of whom 3,987 (10%) who had lower gastrointestinal symptoms were subjected to LGIE. A total number of 693 IBD patients constituted 238 (34.3%) UC and 455 (65.7%) cases of CD. The first diagnosis was established in the clinic in 618 (89%) patients with 193 (31.2%) UC and 425 (68.8%) cases of CD. Data from these patients revealed an average of 10.2 patients with UC per year in the first 11 years and 13.3 in the last years, whereas only 13 CD patients (1.2 patients per year) were seen in the first 11 years compared to 412 (68.7 patients per year) in the last six years. The majority of CD patients were seen in the last two years alone (164 patients per year). The ratio of UC to CD has changed drastically. It was 1:0.12 (113:13 patients) in the first 11 years and 1:5.15 (80:412 patients) in the last six years for UC and CD, respectively. During the period of six years, the major ratio shift was in the last two years touching 1:8.1 (36:292 patients). The overall ratio of UC and CD to total number of LGIE examinations was 1:20.7 and 1:1.94, respectively. It was 1:18.8 and 1:163 in the first 11 years and 1:23.3 and 1:4.5 in the last six years (1:21.8 and 1:2.7 alone in the last two years), respectively [Figure 1]. The annual rates of UGIE examinations decreased from 781 in 1993 to 504 per year in 2009 compared to 140 and 427 LGIE examinations, respectively with an almost steady total number of endoscopic examinations through the years [Figure 2].
Figure 1.
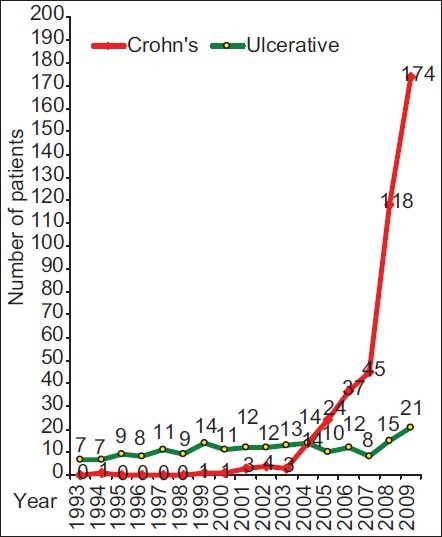
Total number of primarily diagnosed inflammatory bowel disease patients in the clinic (n=618)
Figure 2.
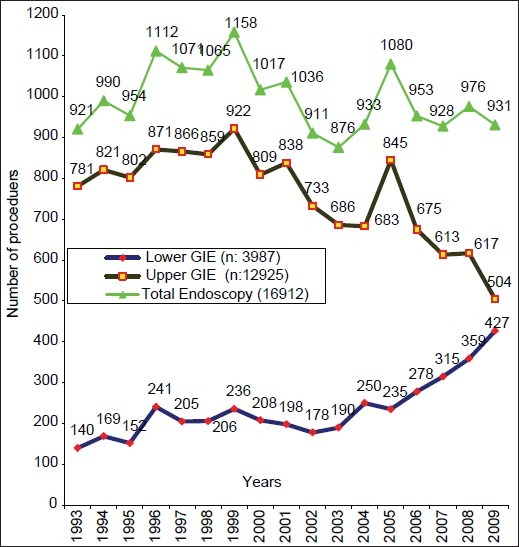
Total number of endoscopic procedures during 1993–2009
The median age was 34 years in UC and 27 years in CD and male to female ratio was 1.5:1 and 2:1, respectively. The age ranged between 10 and 80 years in UC (peak:21–40), and in CD between 11 and 73 (peak:11–30) years. The age distribution is shown in Figure 3. The leading symptoms in UC were abdominal pain (79%), diarrhea (78%), hematochezia (74%), and bloating (48%), whereas abdominal pain was encountered in 91% of the CD patients followed by bloating (71%), diarrhea (66%), hematochezia (45%) and weight loss in 51% [Figure 4]. Extraintestinal manifestations included arthritis/arthralgia, aphthous stomatitis, erythema nodosum, pyoderma gangrenosum, and laryngitis in 64 (14%), 48 (10.5%), 14 (3%), 3 (0.7%), and 2 (0.4%) of the patients, respectively. Primary sclerosing cholangitis, Sjögren's syndrome, psoriasis, and vitiligo were encountered in one patient (0.2%) each.
Figure 3.
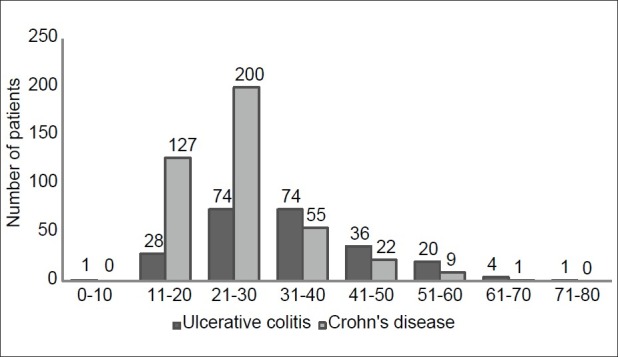
Age distribution of inflammatory bowel disease patients
Figure 4.
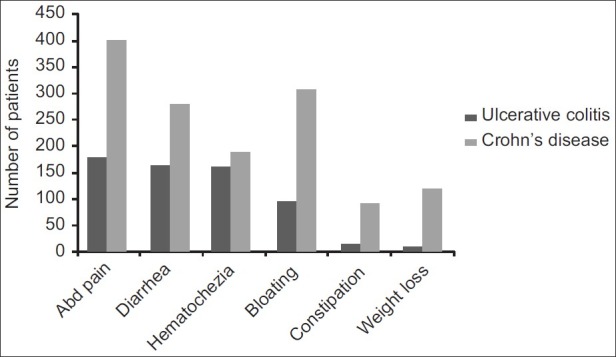
Features of inflammatory bowel disease
Abnormal laboratory findings were mainly raised ESR and CRP, low hemoglobin, thrombocytosis, and raised ALT levels in 44, 37, 28, 24, and 11% in UC patients and 25, 18, 25, 15, and 7% in CD patients, respectively.
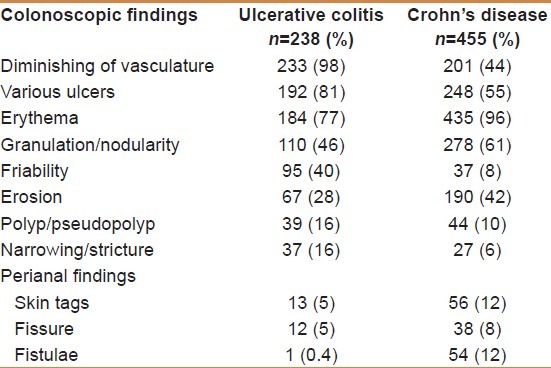
As shown in Table 1, the most encountered endoscopic findings were diminishing of vasculature (98%), ulcers (81%), and erythema (77%) in UC compared to erythema (96%), granulation/nodularity/cobble stone appearance (61%), and apthous and minute ulcers (55%) in CD. Pseudopolyps and narrowing were each encountered in 16% of UC compared to 10 and 6% in CD patients, respectively. Backwash ileitis was encountered in 4%. Perianal involvement in UC were skin tags (5%), anal fissure (5%), and perianal fistula (0%), whereas they were seen in 12, 8, and 12% in CD, respectively. The UGI tract was involved in 11% of the CD patients.
Table 1.
Colonoscopic findings in patients with inflammatory bowel disease (n=693)
The frequent findings of SBS in 251 CD patients were wall thickening, narrowing/strictures, ulcerations, cobble stoning/nodularity, bowel separation, and fistulae (entero-enteric, entero-colic) in 64, 41, 39, 27, 20, and 14%, respectively. Pseudosacculation was found in 6% and marked segmentation in 3%. Thirty-one percent of the radiological findings were nonspecific or normal. The rectum and sigmoid and descending colon were by far the most affected sites in UC patients (95, 87, and 73%) respectively, whereas the ileum (47%) and ileum and colon (33%) were the most affected sites in CD. SBS and CT scan performed in 69 and 20% of cases showed radiological features of CD in 69 and 47% of the examined patients, respectively. In addition to fistulae detected radiologically in 34 patients with CD, 54 patients were diagnosed endoscopically mainly with perianal fistulae.
Histopathological findings were mixed inflammatory cells, mucosal ulcerations, lamina propria edema, lymphoid hyperplasia, focal granulation, lymphoid aggregates, granulomas, dilated lympatics, and crypt abscess in 90, 56, 55, 52 48, 28, 21, 10, and 8%, respectively.
The final diagnosis in both UC and CD was established by endoscopy and positive histopathology in all patients. The duration from the onset of symptoms to definitive diagnosis was less than six months in 18 and 21% and less than one year in 35 and 38% of UC and CD patients, respectively. However, the lapse between presentation at the clinic and establishment of diagnosis was shorter. The majority of the cases of UC (92%) and CD (88%) were diagnosed within six months, most of them (77% of UC and 53% of CD) within one month of presentation.
DISCUSSION
The recent years has witnessed a rapidly increasing rate of IBD and in particular CD in the KSA.[25,26] In the current study, the annual rate of UC remained steady throughout the study period, whereas CD increased sharply, particularly over the last years of the study and CD patients are by now far exceeding those of UC. The markedly increasing CD trend in this private clinic might be at least partially explained by the increasing awareness of patients and physicians as well as the easy access to the clinic. These findings are similar to those reported recently from the United States and the KSA.[3,25,26] The characteristics of IBD in Asia have diverged from those in the western communities.[31,32] In contrast, our results with predominant CD differed in some aspects from data from other Asian countries and Finland, which reported a predominance of UC.[12–14,33] The predominance of CD is in keeping with data from Australia and the United States.[27,34]
There was a male preponderance of both UC and CD in concordance with other studies from KSA, Turkey, and China[25,35,36] The median age of UC and CD was comparable to other data from KSA, China, Lebanon, the United Kingdom, and Greece.[24,25,36–39]
It is generally accepted that the diagnosis of IBD is based on history, physical examinations, laboratory investigations, endoscopy, histopathology, and imaging. Endoscopy, in addition to histopathology, is essential to confirm the diagnosis of IBD, evaluate the extent of the disease, and assess the disease activity and response to treatment and complications such as strictures, dysplasia, or neoplasia. The endoscopic findings of UC are reported frequently as Baron Endoscopic Score or Mayo Score, a combination of the clinical Truelove and Witts Severity Index and Baron score.[40,41] The endoscopic indices for CD used frequently in clinical practice are the Crohn's Disease Endoscopic Index of Severity (CDEIS) and Simple Endoscopic Score for CD (SES-CD).[42,43] The first is reliable and reproducible but time consuming and has poor correlation with clinical activity, whereas the second is easier, faster, and more reliable with clinical activity.
In the present study, the final diagnosis was confirmed by endoscopy and histopathology. They were positive in 96 and 94% of the patients. The remaining cases were confirmed by UGIE and histopathology (20%). Similar to other studies, the majority of UC patients in our series were between the second and fourth decade of life, whereas only a minor proportion (2%) was above the age of 65 compared to 12% in other reports, and only one (0.2%) CD patient was above the age of 65 compared to 9% in other series.[44,45] The main age peak of our CD patients was between 14 and 28 years with a second smaller peak between 30 and 35 years. Majority of the patients (80%) were below the age of 28 years, and 18% in the pediatric age group.[44,45]
Abdominal pain, diarrhea, and hematochezia were the most common presenting features in UC patients, whereas abdominal pain, diarrhea, and weight loss were the most common in CD patients. Although weight loss was observed in only 5% of the UC patients, it was encountered in 51% of the CD patients, which concurs with most local studies of CD.[24,25,46–50] These data may help to differentiate UC from CD clinically in the early stages of presentation.
Similar to other data,[24,25] the frequently observed abnormal laboratory investigations in UC and CD patients were elevated ESR and CRP, anemia, and thrombocytosis. Some other laboratory tests may prove useful in differentiating CD from UC. CD-specific ASCA and UC-specific p-ANCA could enhance the diagnostic accuracy and minimize the need for invasive investigations particularly in pediatric patients.[51] Many other serological markers such as outer membrane protein C (OmpC), antilaminaribioside carbohydrate antibiodies (ALCA), antichitobioside carbohydrate antibodies (ACCA), and antimannobioside carbohydrate antibodies (AMCA) have been reported to be promising tools for the prediction of the course of the disease. Most of them are specifically associated with CD, except p-ANCA which is UC specific.[51–57]
The frequently encountered leading colonoscopic findings in the UC patients were diminishing of vasculature, ulcers, erythema, granulation/nodularities, and friability, whereas the leading findings in CD patients were erythema, aphthous ulcers, nodularities/cobble stoning, erosions, and diminished vasculature, similar to other studies from the KSA.[24–26] According to the Vienna classification of CD patients,[58,59] the terminal ileum alone (L1) was affected in our series in 47% compared to 16–29% by other reports, whereas colonic involvement alone (L2) occurred in 9% of patients which is less than what has been reported worldwide. Thirty-three percent of our patients had simultaneous involvement of terminal ileum and colon (L3), which is comparable with other studies.[60–62] Upper gastrointestinal involvement (L4) was encountered in 11% of the patients, which is higher than other local reports varying between 0 and 5%.[24,25,48,49] Similar to other reports, the rectum and sigmoid and descending colon were the major sites of UC.[21,22,24,25] Transverse and ascending colon were involved in only 11 and 9% of cases, respectively, which might be, underdiagnosed as completion of colonoscopy might be difficult in some nonsedated patients or even contraindicated in severe colitis. Four percent of the patients with intubated terminal ileum had backwash ileitis. Backwash ileitis has been reported to be associated with colonic carcinoma in some patients with UC, which was not the case in our series.[63] Anal fissures, skin tags, and perianal fistulae were seen in 5, 5, and 0% of the UC patients and 8, 12, and 12% of the CD patients, respectively, whereas piles was seen in 34 and 27% of the UC and CD patients, respectively, which concurs with some local reports[24,25] and differs from others.[47–49]
The leading radiological findings of SBE in CD patients were wall thickening, narrowing/strictures, ulcerations, and cobble stoning/nodularity. According to Louis et al.,[64] at the time of diagnosis, 90% of the CD patients have inflammatory and 10% have complicated (fibrostenotic or penetrating) disease. In our series, 54 (12%) of our CD patients had various external fistulae and 34 (7.5%) had internal fistulae; 14 patients out of them had combined internal and external fistulae resulting in 16% (74 patients) cases of penetrating disease in the form of internal and external fistulae at the time of diagnosis.
There is a paucity of literature about the duration of symptoms before the diagnosis. Mekhijian et al. (1979) reported in the National Cooperative Crohn's Disease Study that half of all CD patients have symptoms for six months or less before diagnosis.[65] In our series, 88% of the CD patients were symptomatic and were diagnosed within six months or less. Similarly, 92% of the UC patients were diagnosed in our series within six months of symptoms. Furthermore, 77% of the UC patients and 53% of the CD patients were diagnosed in our series within one month or less of presentation, indicating a high index of physician's and patient's awareness. Finally, due to the expenses of the visits and endoscopy, the examined population might not represent the community, which, in addition to the retrospective nature of the study, will possibly lead to some limitations.
CONCLUSION
In conclusion, IBD is no longer a rare disease in the KSA. The current data show a steady state of UC with a strikingly increasing prevalence of CD, by far outnumbering UC. CD is a disease of the youth. Concerns with the etiology and the role of an increasingly westernized lifestyle with increase in the consumption of fast food and smoking warrant further studies. Improvement in hygienic standards in genetically predisposed individuals may also contribute to the rising trend. Increasing the awareness of physicians and patients will lead to an early diagnosis and contribute to a satisfactory outcome.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Fiocchi C. Inflammatory bowel disease: Etiology and pathogenesis. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- 2.Loftus EV, Sandborn WJ. Epidemiology of inflammatory bowel disease. Gastroenterol Clin North Am. 2002;1:1–20. doi: 10.1016/s0889-8553(01)00002-4. [DOI] [PubMed] [Google Scholar]
- 3.Gerland CF, Lilienfield AM, Mendeloff Al, Markowitz JA, Terrel KB, Gerland FC. Incidence rates of ulcerative colitis and Crohn's disease in 15 areas of the United States. Gastroenterology. 1981;81:115–24. [PubMed] [Google Scholar]
- 4.Lashner BA. Epidemiology of inflammatory bowel disease. Gastroenterol Clin North Am. 1995;24:467–74. [PubMed] [Google Scholar]
- 5.Loftus EV, Jr, Silverstein MD, Sandborn WJ, Termaine WJ, Harmsen WS, Zinsmeister AR. Crohn's disease in Olmsted Country, Minnesota 1940-1993: Incidence, prevalence and survival. Gastroenterology. 1998;114:1161–8. doi: 10.1016/s0016-5085(98)70421-4. [DOI] [PubMed] [Google Scholar]
- 6.Russel MG, Dorant E, Volovics A, Brummer RJ, Pop P, Muris JW, et al. High incidence of inflammatory bowel disease in The Netherlands: Results of a prospective study. The South Limburg IBD Study Group. Dis Colon Rectum. 1998;41:33–40. doi: 10.1007/BF02236893. [DOI] [PubMed] [Google Scholar]
- 7.Powell JJ, Harvey RS, Ashwood P, Woistencroft R, Gershwin ME, Thompson RP. Immune potentiation of ultrafine dietary particles in normal subjects and patients with inflammatory bowel disease. J Autoimmun. 2000;14:99–105. doi: 10.1006/jaut.1999.0342. [DOI] [PubMed] [Google Scholar]
- 8.Shivananda S, Lennard-Jones J, Logan R, Fear N, Price A, Carpenter L, et al. Incidence of inflammatory bowel disease across Europe: Is there a difference between north and south? Results of the European Collaborative Study on Inflammatory Bowel Disease (EC-IBD) Gut. 1996;39:690–7. doi: 10.1136/gut.39.5.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan CC, Kang JY, Guan R, Yap I, Tay HH. Inflammatory bowel disease: An uncommon problem in Singapore. J Gastroenterol Hepatol. 1992;7:360–2. doi: 10.1111/j.1440-1746.1992.tb00997.x. [DOI] [PubMed] [Google Scholar]
- 10.Sung JJ, Hsu RK, Chan FK, Liew CT, Lau JW, Li AK. Crohn's disease in the Chinese population.An experience from Hong Kong. Dis Colon Rectum. 1994;37:1307–9. doi: 10.1007/BF02257802. [DOI] [PubMed] [Google Scholar]
- 11.Jacobsen C, Wewer AV, Urne F, Andersen J, Faerk J, Kramer I, et al. Incidence of ulcerative colitis and Crohn's disease in danish children: Still rising or leveling out? J Crohns Colitis. 2008;2:152–7. doi: 10.1016/j.crohns.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Sood A, Midha V. Epidemiology of inflammatory bowel disease in Asia. Indian J Gastroenterol. 2007;26:285–9. [PubMed] [Google Scholar]
- 13.Niriella MA, De Silva AP, Dayaratne AH, Ariyasinghe MH, Navarathne MM, Peiris RS, et al. Prevalence of inflammatory bowel disease in two districts of Sri Lanka: A hospital based survey. BMC Gastroenterol. 2010;10:32–32. doi: 10.1186/1471-230X-10-32. PP not available in Pubmed and not mentioned in PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goh K, Xiao SD. Inflammatory bowel disease: A survey of the epidemiology in Asia. J Dig Dis. 2009;10:1–6. doi: 10.1111/j.1751-2980.2008.00355.x. [DOI] [PubMed] [Google Scholar]
- 15.Ahuja V, Tandon RK. Inflammatory bowel disease in the Asia-Pacific area: A comparison with developed countries and regional differences. J Dig Dis. 2010;11:134–47. doi: 10.1111/j.1751-2980.2010.00429.x. [DOI] [PubMed] [Google Scholar]
- 16.Thia KT, Loftus EV, Jr, Sandborn WJ, Yang SK. An update on the epidemiology of inflammatory bowel disease in Asia. Am J Gastroenterol. 2008;103:3167–82. doi: 10.1111/j.1572-0241.2008.02158.x. [DOI] [PubMed] [Google Scholar]
- 17.Jiang L, Xia B, Li J, Ye M, Yan W, Deng C, et al. Retrospective survey of 452 patients with inflammatory bowel disease in Wuhan city, central China. Inflamm Bowel Dis. 2006;12:212–7. doi: 10.1097/01.MIB.0000201098.26450.ae. [DOI] [PubMed] [Google Scholar]
- 18.Mokhtar A, Khan MA. Crohn's disease in Saudi Arabia. Saudi Med J. 1982;3:270–4. [Google Scholar]
- 19.Al-Nakib B, Radhakrishan S, Jacob GS, Al-Ruwaih A. Inflammatory bowel disease in Kuwait. Am J Gastroenterol. 1984;79:191–4. [PubMed] [Google Scholar]
- 20.Mohamed AE, Al-Karawi MA, Hanid MA, Yasawy I. Lower gastrointestinal tract pathology in Saudis: Results of endoscopic biopsy findings in 1600 patients. Ann Saudi Med. 1987;7:306–11. [Google Scholar]
- 21.Hossain J, Al-Mofleh IA, Laajam MA, Al-Rashed RS, Al-Faleh FZ. Crohn's disease in Arab. Ann Saudi Med. 1991;11:40–6. doi: 10.5144/0256-4947.1991.40. [DOI] [PubMed] [Google Scholar]
- 22.Satti MB, Al-Quorain A, Al-Gindan Y, Al-Hamdan A. Chronic idiopathic ulcerative colitis in Saudi Arabia: A clinicopathological study of 76 cases. Ann Saudi Med. 1996;16:637–40. doi: 10.5144/0256-4947.1996.637. [DOI] [PubMed] [Google Scholar]
- 23.Isbister WH, Hubler M. Inflammatory bowel disease in Saudi Arabia.Presentation at initial management. J Gastroenterol Hepatol. 1998;11:1119–24. doi: 10.1111/j.1440-1746.1998.tb00587.x. [DOI] [PubMed] [Google Scholar]
- 24.Al-Gindan Y, Satti MB, Al-Quorain A, Al-Hamdan A. Crohn's disease in Saudi Arabia: A clinicopathological study of 12 cases. Saudi J Gastroenterol. 1996;6:150–5. [PubMed] [Google Scholar]
- 25.Al-Ghamdi AS, Al-Mofleh IA, Al-Rashed RS, Al-Amri SM, Al-Jebreen AM, Isnani AC. Epidimiology and outcome of Crohn's disease in a teaching hospital in Riyadh. World J Gastroenterol. 2004;10:1341–4. doi: 10.3748/wjg.v10.i9.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Mofarreh MA, Al-Mofleh IA, Al-Teimi IN, Al-Jebreen AM. Crohn's disease in Saudi outpatient population: Is it still rare? Saudi J Gastroenterol. 2009;15:111–6. doi: 10.4103/1319-3767.45357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson J, Hair C, Knight R, Catto-Smith A, Bell S, Kamm M, et al. High incidence of inflammatory bowel disease in Australia: A prospective population-based Australian incidence study. Inflamm Bowel Dis. 2010;16:1550–6. doi: 10.1002/ibd.21209. [DOI] [PubMed] [Google Scholar]
- 28.Khalifa SE, Mudawi HM, Fedail SS. Presentation and management outcome of inflammatory bowel disease in Sudan. Trop Gastroenterol. 2005;26:194–6. [PubMed] [Google Scholar]
- 29.Ukwenya AY, Ahmed A, Odigie VI, Mohammed A. Inflammatory bowel disease in Nigerians: Still a rare diagnosis? Ann Afr Med. 2011;10:175–9. doi: 10.4103/1596-3519.82067. [DOI] [PubMed] [Google Scholar]
- 30.Van Assche G, Dignass A, Panes J, Beaugerie L, Karagiannis J, Allez M, et al. European Crohn's and colitis organisation (ECCO) The second European evidence-based consensus on the diagnosis and management of Crohn's disease: Definitions and diagnosis. J Crohns Colitis. 2010;4:7–27. doi: 10.1016/j.crohns.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Kim ES, Kim WH. Inflammatory bowel disease in Korea: Epidemiological, genomic, clinical, and therapeutic characteristics. Gut Liver. 2010;4:1–14. doi: 10.5009/gnl.2010.4.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walker DG, Williams HR, Kane SP, Mawdsley JE, Arnold J, McNeil I, et al. Differences in inflammatory bowel disease phenotype between South Asians and Northern Europeans living in North West London, UK. Am J Gastroenterol. 2011;106:1281–9. doi: 10.1038/ajg.2011.85. [DOI] [PubMed] [Google Scholar]
- 33.Jussila A, Virta LJ, Kautiainen H, Rekiaro M, Nieminen U, Färkkilä MA. Increasing incidence of inflammatory bowel diseases between 2000 and 2007: A nationwide register study in Finland. Inflamm Bowel Dis. 2011;18:555–61. doi: 10.1002/ibd.21695. doi: 10.1002/ibd.21695. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 34.Loftus CG, Loftus EV, Jr, Harmsen WS, Zinsmeister AR, Tremaine WJ, Melton LJ, 3rd, et al. Update on the incidence and prevalence of Crohn's disease and ulcerative colitis in Olmsted County, Minnesota, 1940-2000. Inflamm Bowel Dis. 2007;13:254–61. doi: 10.1002/ibd.20029. [DOI] [PubMed] [Google Scholar]
- 35.Tozun N, Atug O, Imeryuz N, Hamzaoglu HO, Tiftikci A, Parlak E, et al. Members of the Turkish IBD Study Group.Clinical characteristics of inflammatory bowel disease in Turkey: A multicenter epidemiologic survey. J Clin Gastroenterol. 2009;43:51–7. doi: 10.1097/MCG.0b013e3181574636. [DOI] [PubMed] [Google Scholar]
- 36.Cao Q, Si JM, Gao M, Zhou G, Hu WL, Li JH. Clinical presentation of inflammatory bowel disease: A hospital based retrospective study of 379 patients in eastern China. Chin Med J (Engl) 2005;118:747–52. [PubMed] [Google Scholar]
- 37.Abdul-Baki H, ElHajj I, El-Zahabi LM, Azar C, Aoun E, Zantout H, et al. Clinical epidemiology of inflammatory bowel disease in Lebanon. Inflamm Bowel Dis. 2007;13:475–80. doi: 10.1002/ibd.20022. [DOI] [PubMed] [Google Scholar]
- 38.Bardhan KD, Simmonds N, Royston C, Dhar A, Edwards CM Rotherham IBD Database Users Group. A United Kingdom inflammatory bowel disease database: Making the effort worthwhile. J Crohns Colitis. 2010;4:405–12. doi: 10.1016/j.crohns.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 39.Katsanos KH, Stamou P, Tatsioni A, Zoumbas S, Kavvadia S, Giga A, et al. Northwest Greece IBD Study Group.Prevalence of inflammatory bowel disease related dysplasia and cancer in 1500 colonoscopies from a referral center in northwestern Greece. J Crohns Colitis. 2011;5:19–23. doi: 10.1016/j.crohns.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Baron JH, Connell AM, Lennard-Jones JE. Variation between observers in describing mucosal appearances in proctocolitis. Br Med J. 1964;1:89–92. doi: 10.1136/bmj.1.5375.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cooney RM, Warren BF, Altman DG, Abreu MT, Travis SP. Outcome measurement in clinical trials for ulcerative colitis. Towards Standardization. Trials. 2007;8:17–17. doi: 10.1186/1745-6215-8-17. doi 10: 1186/1745-6215-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mary JY, Modigliani R. Development and validation of an endoscopic index of the severity for Crohn's disease: A prospective multicentre study. Groupe d’Etudes Thérapeutiques des Affections Inflammatoires du Tube Digestif (GETAID) Gut. 1989;30:983–9. doi: 10.1136/gut.30.7.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Daperno M, D’Haens G, Van Assche G, Baert F, Bulois P, Maunoury V, et al. Development and validation of a new, simplified endoscopic activity score for Crohn's disease: The SES-CD. Gastrointest Endosc. 2004;60:505–12. doi: 10.1016/s0016-5107(04)01878-4. [DOI] [PubMed] [Google Scholar]
- 44.Jess T, Loftus EV, Jr, Harmsen WS, Zinsmeister AR, Tremaine WJ, Melton LJ, 3rd, et al. Survival and cause specific mortality in patients with inflammatory bowel disease: A long term outcome study in Olmsted County. Minnesota, 1940-2004. Gut. 2006;55:1248–54. doi: 10.1136/gut.2005.079350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ananthakrishnan AN, McGinley EL, Binion DG. Inflammatory bowel disease in the elderly is associated with worse outcomes: A national study of hospitalizations. Inflamm Bowel Dis. 2009;15:182–9. doi: 10.1002/ibd.20628. [DOI] [PubMed] [Google Scholar]
- 46.El-Mouzan MI, Abdullah AM, Al Habbal MT. Epidemiology of Juvenile-onset Inflammatory Bowel disease in Central Saudi Arabia. J Trop Pediatr. 2006;52:69–71. doi: 10.1093/tropej/fmi039. [DOI] [PubMed] [Google Scholar]
- 47.Al-Salamah SM. Surgery for small bowel Crohn's disease: Experience of a tertiary referral center. Saudi J Gastroenterol. 2005;11:85–92. doi: 10.4103/1319-3767.33324. [DOI] [PubMed] [Google Scholar]
- 48.Contractor QQ, Contractor TQ, Ul Haque I, El Mahdi EE. Crohn's disease among Saudis in Al Gassim Region. Saudi J Gastroenterol. 2005;11:157–63. doi: 10.4103/1319-3767.33319. [DOI] [PubMed] [Google Scholar]
- 49.Azzam NA, Al-Jebreen AM, Abdo AA, Al-Sawat KA, A-Mofleh IA, Al-Rashed RS. Emerge in Crohn's disease incidence in Saudi Arabia: Tertiary care centre experience. The 9th GI and Liver Disease Conference; 7-10 May 2007; Abha: KSA. p. A48. [Google Scholar]
- 50.Toonisi TS. Crohn's disease in Saudi Arabia. Indian Pediatr. 1993;30:1101–4. [PubMed] [Google Scholar]
- 51.Dubinsky MC, Lin YC, Dutridge D, Picornell Y, Landers CJ, Farrior S, et al. Serum immune responses predict rapid disease progression among children with Crohn's disease: Immune responses predict disease progression. Am J Gastroenterol. 2006;101:360–7. doi: 10.1111/j.1572-0241.2006.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sandborn WJ. Serologic markers in inflammatory bowel disease: State of the art. Rev Gastroenterol Disord. 2004;4:167–74. [PubMed] [Google Scholar]
- 53.Mow WS, Vasiliauskas EA, Lin YC, Fleshner PR, Papadakis KA, Taylor KD, et al. Association of antibody responses to microbial antigens and complications of small bowel Crohn's disease. Gastroenterology. 2004;126:414–24. doi: 10.1053/j.gastro.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 54.Fleshner PF, Vasiliauskas EA, Kam LY, Karp LC, Gaiennie J, Yang H, et al. High level perinuclear antineutrophil cytoplasmic antibody (P.ANCA) in ulcerative colitis patients before colectomy predicts the development of chronic pouchitis after ileal pouch anal anastomosis. Gut. 2001;49:671–7. doi: 10.1136/gut.49.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vasiliauskas EA, Kam LY, Karp LC, Gaiennie J, Yang H, Targan SR. Marker antibody expression stratifies Crohn.s disease into immunologically hemogenous subgroups with distinct clinical characteristics. Gut. 2000;47:487–96. doi: 10.1136/gut.47.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferrante M, Henckaerts L, Joossens M, Pierik M, Joossens S, Dotan N, et al. New serological markers in inflammatory bowel disease are associated with complicated disease behaviour. Gut. 2007;56:1394–403. doi: 10.1136/gut.2006.108043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dotan I, Fishman S, Dgani Y, Schwartz M, Karban A, Lerner A, et al. Antibodies against laminaribioside and chitobioside are novel serologic markers in Crohn's disease. Gastroenterology. 2006;131:366–78. doi: 10.1053/j.gastro.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 58.Gasche C, Scholmerich J, Brynskov J, D’Haens G, Hanauer SB, Irvine EJ, et al. A simple classification of Crohn's disease: Report of the working party for the world congress of gastroenterology, Vienna 1998. Inflamm Bowel Dis. 2000;6:8–15. doi: 10.1097/00054725-200002000-00002. [DOI] [PubMed] [Google Scholar]
- 59.Leiss D, Boerner N. Vienna classification of Crohn's disease: Helpful or dispensable? A critical view. Z Gastroenterol. 2007;45:265–72. doi: 10.1055/s-2006-927283. [DOI] [PubMed] [Google Scholar]
- 60.Rubesin SE, Scotiniotis I, Birnbaum BA, Ginsberg GG. Radiologic and endoscopic diagnosis of Crohn's disease. Surg Clin North Am. 2001;81:39–70. doi: 10.1016/s0039-6109(05)70273-5. viii. Review. [DOI] [PubMed] [Google Scholar]
- 61.Thomson-Fawcett MW, Mortensen NJ. Crohn's disease. In: Philips RK, editor. Colorectal surgery. London: WB Saunders; 1998. pp. 179–215. [Google Scholar]
- 62.Nakahara T, Yao T, Sakurai T, Okada M, Iida M, Fuchigami T, et al. Long-term prognosis of Crohn's diasease. Nihon Shokakibyo Gakkai Zasshi. 1991;88:1305–12. [PubMed] [Google Scholar]
- 63.Heuschen UA, Hinz U, Allemeyer EH, Stern J, Lucas M, Autschbach F, et al. Backwash ileitis is strongly associated with colorectal carcinoma in ulcerative colitis. Gastroenterology. 2001;120:841–7. doi: 10.1053/gast.2001.22434. [DOI] [PubMed] [Google Scholar]
- 64.Louis E, Collard A, Oger AF, Degroote E, Aboul Nasr El Yafi FA, Belaiche J. Behavior of Crohn's disease according to the vienna classification: Changing pattern over the course of the disease. Gut. 2001;49:777–82. doi: 10.1136/gut.49.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mekhijian HS, Switz DN, Melnyk C, Rankin GB, Brooks RK. Clinical features and natural history of Crohn's disease. Gastroenterology. 1979;77:898–906. [PubMed] [Google Scholar]


