Abstract
Background/Aim:
To evaluate the immunohistochemical expression of matrix metalloproteinase-7 (MMP-7) in colorectal adenomas, and to correlate this expression with different clinicopathological parameters.
Patients and Methods:
The study was retrospectively designed. Thirty three paraffin blocks from patients with colorectal adenoma and 20 samples of non-tumerous colonic tissue taken as control group were included in the study. MMP-7 expression was assessed by immunohistochemistry method. The scoring of immunohistochemical staining was conducted utilizing a specified automated cellular image analysis system (Digimizer).
Results:
The frequency of positive immunohistochemical expression of MMP-7 was significantly higher in adenoma than control group (45.45% versus 10%) (P value < 0.001). Strong MMP-7 staining was mainly seen in adenoma cases (30.30%) in comparison with control (0%) the difference is significant (P < 0.001). The three digital parameters of MMP-7 immunohistochemical expression (Area (A), Number of objects (N), and intensity (I)) were significantly higher in adenoma than control. Mean (A and I) of MMP-7 showed a significant correlation with large sized adenoma (≥ 1cm) (P < 0.05), also a significant positive correlation of the three digital parameters (A, N, and I) of MMP-7 expression with villous configuration and severe dysplasia in colorectal adenoma had been identified (P < 0.05).
Conclusion:
MMP-7 plays an important role in the growth and malignant conversion of colorectal adenomas as it is more likely to be expressed in advanced colorectal adenomatous polyps with large size, severe dysplasia and villous histology. The use of automated cellular image analysis system (Digmizer) to quantify immunohistochemical staining yields more consistent assay results, converts semi-quantitative assay to a truly quantitative assay, and improves assay objectivity and reproducibility.
Keywords: Colorectal adenoma, digimizer, matrix metalloproteinase-7
Colorectal cancer (CRC) is a major public health problem and there is renewed interest in understanding the basic principles of its molecular biology.[1] Surrogate endpoint markers provide opportunities to understand cancer development and also to evaluate the efficacy of intervention. Based on epidemiologic, therapeutic, pathophysiological, clinical and cost benefit, adenomas are considered to be surrogate endpoints in CRC because removal of adenomatous polyps (adenomas) has been shown to reduce the risk of development of CRC.[2] There is currently a multitiered approach to reducing the burden of colorectal cancer through both the earlier diagnosis of cancer and the detection and removal of benign polyp precursors. These clinical strategies have been informed by our understanding of the molecular pathology underpinning colorectal carcinogenesis.[3] Multiple factors including alterations in matrix metalloproteinases (MMPs) seem to be associated with polyp development. MMPs are a family of zinc-dependent and calcium-dependent endopeptidases that degrade matrix glycoproteins, play an important role in tumor invasion and metastases, and are inhibited by tissue inhibitors of metalloproteinases (TIMPs). MMP-7, matrilysin, appears to be expressed by neoplastic cells and may function in the early phase of neoplastic growth.[4] Up-regulation of MMP-7 occurs very early in colonic epithelial cells as a result of mutation of adenomatous polyposis coli (APC) gene. Mutation of APC is the putative “gatekeeping” event in colorectal carcinogenesis, leading to translocation of β-catenin to the nucleus. Over expression of MMP-7 has been found in 85% of colorectal adenocarcinomas and associated with a poor prognosis.[5]
The aim of the present study is to evaluate the immunohistochemical expression of MMP-7 in colorectal adenoma using specified automated cellular image analysis system and to correlate this expression with different clinicopathological parameters “Age and gender of the patients, number, size, site, histopathological type and degree of dysplasia”.
PATIENTS AND METHODS
The study was retrospectively designed. A total of 53 tissue samples (paraffin blocks and tissue biopsies) were included in the study.
Out of the total number of tissue samples, 33 paraffin blocks from patients with colorectal adenomas were collected from Gastroenterology and Hepatology Center, Gastroenterology unit at Al-Khadhmiya Teaching Hospital and private laboratories in Baghdad, Iraq, for the period 2006-2010. The control group included 20 samples of non-tumerous colonic tissue taken from autopsy cases from the Institute of Forensic Medicine. These specimens were processed and paraffin embedded in the same center. The clinicopathological parameters were obtained from patients’ admission case sheets and pathology reports. An absolute confidentiality of the patients’ vital information was maintained for ethical purposes and an ethical approval was obtained from institutions in which the study was carried out.
From each block, 2 sections of 5μm thickness were taken, one section was stained with Hematoxylin and Eosin (H and E) and slides were revised for the histopathological type and grade of dysplasia. The other section was stained immunohistochemically using three steps- indirect streptavidin method for Monoclonal Mouse Anti-Human Matrix Metalloproteinase-7 (MMP-7, Matrilysin), clone 3F264, manufactured by US Biological. Brown cytoplasmic and membranous staining is considered positive reaction. Positive control is breast carcinoma tissue. Technical negative control was obtained by omission of primary antibody.
Scoring of immunohistochemical staining
Scoring of immunohistochemical staining was performed using specialized automated cellular image analysis system, Digimizer software, version 3.7.0.[6]
Each immunohistochemically stained slide was scanned by alight microscope (Proway, China) for the positive brown immunostaining and three fields that reflect the best of the overall immunostaining were chosen and captured using a Sony digital camera (digital still camera DSH-H55).
Determination of staining intensity cut-off value
To obtain a cut-off value for intensity of immunostaining, photomicrographs presented at the website of NordiCQ showing different grades of brown color intensity (strong (+3) for dark brown, moderate (+2) for brown, weak (+1) for light brown to yellow) were analyzed by digimizer software, and the digital value of intensity was classed into three categories: weak, moderate and strong, taking into consideration that the digital value of intensity of staining is inversely proportional to digital number in the digimizer color scale [Table 1].[6]
Table 1.
Determination of staining intensity cut-off value[6]

Statistical analysis
Data was analyzed using SPSS program (Statistical Package for Social Sciences) version 16 and Microsoft Office Excel 2007. Numeric data were expressed as mean + SEM, frequency was used to express discrete data. ANOVA was used to analyze numeric data while Chi-square was used to analyze discrete data, and benferroni test was used for multiple comparisons. Scattered graph was used to show relation between various markers. P Value of less than 0.05 was considered significant. For Digimizer software, the integrated statistics window displays statistics (n, mean of area, mean of average intensity, Standard deviation (SD), minimum and maximum) of the measurements in the measurements list; these measurements were saved as Excel 2007 spreadsheet file.
RESULTS
Clinicopathological parameters
The results concerning clinicopathological parameters assessed in patients studied are shown in Table 2.
Table 2.
Clinicopathological parameters of patients studied
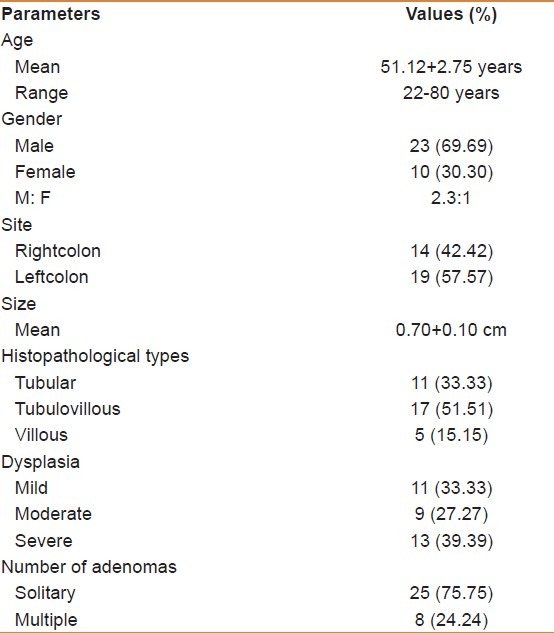
Comparison of MMP-7 immunohistochemical expressions in colorectal adenoma and control group.
The frequency of MMP-7 – positive cases was significantly higher in adenoma than control group (45.45% versus 10%) (P value < 0.001). The classification of the MMP-7 positive cases of adenoma and control groups into different grades of intensity (negative, weak, moderate, and strong) according to the tabulated values of NordiCQ laboratories showed that strong MMP-7 staining was mainly seen in adenoma cases 10 (30.30%) in comparison with control 0 (0%), the difference is significant (P < 0.001) [Figures 1–3] [Table 3].
Figure 1.
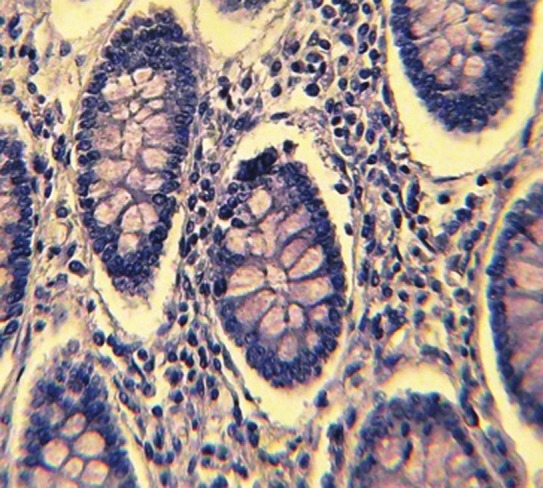
Normal colonic tissue with negative MMP-7 immunohistochemical expression (×40)
Figure 3.
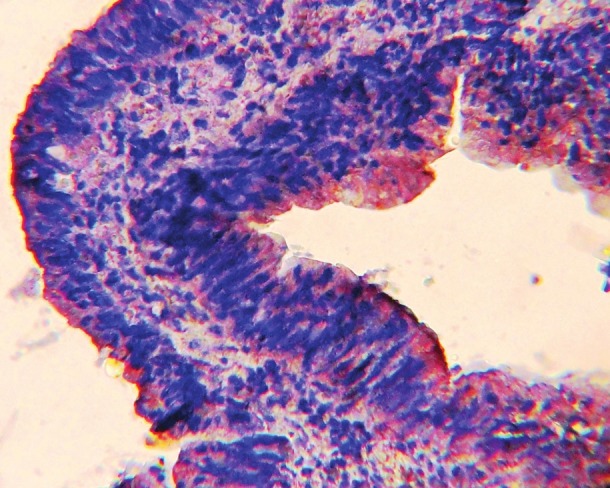
Tubulo-villous colonic adenoma with severe dysplasia showing MMP-7-positive brown cytoplasmic and membranous staining with strong intensity (×40)
Table 3.
Immunohistochemical expression of matrix metalloproteinase-7 in patients and control groups
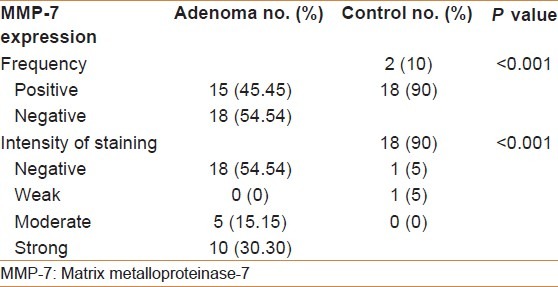
Figure 2.
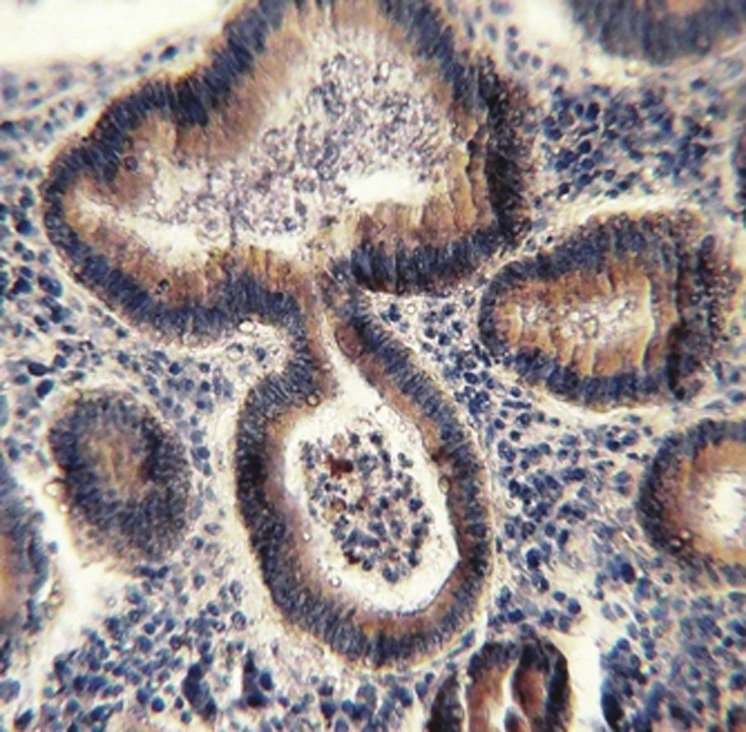
Tubular colonic adenoma with mild dysplasia showing MMP-7- positive brown cytoplasmic staining with moderate intensity (×40)
The three digital parameters of MMP-7 immunohistochemical expression (Area (A), Number of objects (N), and intensity (I)) were significantly higher in adenoma than control group (P < 0.05).
Correlation of MMP-7 immunohistochemical expression with different clinicopathological parameters in colorectal adenomas.
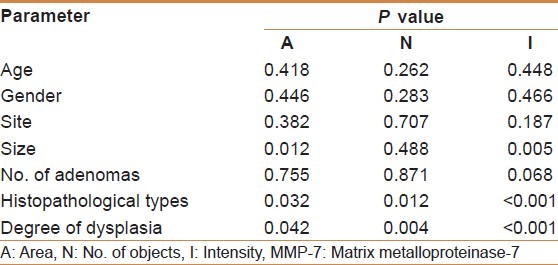
Mean (A and I) of MMP-7 showed a significant correlation with large sized adenoma (≥ 1cm) (P < 0.05), also a significant correlation of the three digital parameters (A, N, and I) of MMP-7 expression with villous configuration (tubulovillous and villous) and severe dysplasia in colorectal adenoma had been identified (P < 0.05). There was no significant correlation between age, gender, site, and number of adenomas with the mean (A, N, and I) of MMP-7 [Table 4].
Table 4.
Correlations of matrix metalloproteinase-7 immunohistochemical expression with different clinicopathological parameters in patients with colorectal adenomas
DISCUSSION
It has been proposed that aberrant crypt foci (small areas of epithelium with irregular glandular architecture without any evidence of dysplasia) are precursor lesions which give rise to adenomas. Adenomas can gradually grow in size and change from a tubular to a villous architecture. In general, colonic adenomas comprise tubular, tubulovillous, and villous adenomas, each of which has a different potential for malignancy, depending on its size. They are considered to play an important role as normal mucosa progresses to adenoma.[4]
MMPs are over expressed in a variety of premalignant tumor tissues, including colorectal adenoma and MMP-7 has been shown to be important in the growth of early colonic adenomas and their transformation into invasive cancer.[7]
The present study found that the frequency of the positive cases of MMP-7 expression was significantly higher in adenoma than in control (45.45% versus 10%, P < 0.001) and the three digital parameters of MMP-7 immunohistochemical expression (A, N, and I) were also significantly higher in adenoma than in normal colonic mucosa. Strong MMP-7 staining was mainly seen in adenoma cases 10 (30.30%) in comparison with control 0 (0%), the difference is significant (P < 0.001). Several published studies are in accordance with these data, Kirimlioglu et al., found that immunohistochemical staining for MMP-7 was identified in the cytoplasm of all types of adenoma cells and it was not expressed either in any of the control colorectal epithelia studied or in non tumoral epithelial cells next to adenomas.[4] Clapper et al., showed that strong MMP-7 expression is restricted to the neoplastic cells of small and large adenomas and is absent in the non-neoplastic colonic epithelium.[5] Nosho et al., observed a progressive increase in the expression of MMP-7 during the transition from normal to adenomatous to carcinomatous colonic mucosa.[8] Heslin et al. and Fingleton et al., recorded that MMP-7 over expression is an early event in the carcinogenetic cascade as normal colonic mucosa progresses to adenoma.[9,10] Newell et al., had shown that MMP-7 is focally over expressed in 50% of benign adenomas.[11]
Advanced colorectal adenomatous polyps are identified based on size ≥ 10 mm, high-grade dysplasia, and/or villous histology.[12] The current work found that MMP-7 over expression was significantly correlated with severe degree of dysplasia, villous configuration and large size (≥ 1cm). In keeping with these results, Kirimlioglu et al., revealed that the degree of MMP-7 over expression was significantly higher in villous type and high grade dysplasia.[4] Ishikawa et al., and Takeuchi et al., found that Matrilysin mRNA expression in adenomas was correlated with the degree of dysplasia and the size of the mass.[13,14] Hai-yan et al., also demonstrated that immunohistochemical expression of MMP-7 in colorectal adenomas was significantly correlated with the degree of epithelial dysplasia and can be used as an indicator of risk of malignant transformation in adenoma.[15]
The foregoing data suggest that MMP-7 over expression is an early event in the adenoma-carcinoma pathway and because MMP-7 is more likely to be expressed in adenomas with a potential for malignancy, this enzyme may play a role in the malignant conversion of colorectal adenomas.[4,7,9,10,15]
Ougolkov et al., suggested that MMP-7 could be considered to function as an oncogene since colorectal tumourigenesis is suppressed in mice lacking matrilysin and tumourigenic potential is enhanced in cell lines over expressing MMP-7.[16]
In the early stages of colorectal tumorogenesis, there is direct interaction of individual MMPs, particularly MMP-7, with the genes involved in the pathways of colorectal cancer development,[17] and as adenomas progress to invasive carcinomas, MMP-7 moreover remains elevated as detected immunohistochemically and zymographically. MMP-7 protein and mRNA are consistently expressed in liver metastasis too.[17,18] The accumulation of beta-catenin leads to the up-regulation of MMP-7 gene transcription in colorectal adenomas. This indicates that the transcriptional upregulation of MMP-7 is under the control of genes that have been identified as being important in the early stages of colorectal tumourigenesis.[18]
To the best of our knowledge, this is the first clinicopatholgical study conducted in Iraq on the immunohistochemical MMP-7 expression in colorectaladenomas, and using automated cellular image analysis system. Compared with the manual immunohistochemical scoring method, there are major benefits of using an image analyzer (Digimizer) to quantify immunohistochemical staining. First, it converts a qualitative or semi-quantitative assay into a truly quantitative assay. Second, it can improve assay objectivity and reproducibility. Third, such image analyzer-assisted immunohistochemical quantification does not require much additional training and is simple to perform. Instead of adding a totally new assay to laboratory operation, it modifies only the quantifying step while keeping the previous immunohistochemical assay steps unchanged.[6]
CONCLUSION
In conclusion, MMP-7 plays an important role in the growth and malignant conversion of colorectal adenomas as it is more likely to be expressed in advanced colorectal adenomatous polyps with large size, severe dysplasia and villous histology. The use of automated cellular image analysis system (Digmizer) to quantify immunohistochemical staining yields more consistent assay results, converts semiquantitative assay to a truly quantitative assay, and improves assay objectivity and reproducibility.
Footnotes
Source of Support: College of Medicine/Al-Nahrain University Especially the Department of Pathology and Forensic Medicine, and Teaching Laboratories at Al-Khadhmiya Teaching Hospital
Conflict of Interest: None declared
REFERENCES
- 1.Morán A, Ortega P, de Juan C, Fernández-Marcelo T, Frías C, Sánchez-Pernaute A, et al. Differential colorectal carcinogenesis: Molecular basis and clinical relevance. World J Gastrointest Oncol. 2010;2:151–8. doi: 10.4251/wjgo.v2.i3.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanaka T. Colorectal carcinogenesis: Review of human and experimental animal studies. J Carcinog. 2009;8:5. doi: 10.4103/1477-3163.49014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Worthley DL, Leggett BA. Colorectal Cancer: Molecular features and clinical opportunities. Clin Biochem Rev. 2010;31:31–8. [PMC free article] [PubMed] [Google Scholar]
- 4.Kirimlioglu H, Kirimlioglu V, Yilmaz S, Sagir V, Coban S, Turkmen E, et al. Role of matrix metalloproteinase-7 in colorectal adenomas. Dig Dis Sci. 2006;51:2068–72. doi: 10.1007/s10620-005-9070-4. [DOI] [PubMed] [Google Scholar]
- 5.Clapper ML, Hensley HH, Chang WC, Devarajan K, Nguyen MT, Cooper HS. Detection of colorectal adenomas using a bioactivatable probe specific for matrix metalloproteinase activity. Neoplasia. 2011;13:685–91. doi: 10.1593/neo.11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qasim BJ, Ali HH, Hussein AG. Immunohistochemical expression of estrogen and progesterone receptors in human colorectal adenoma and carcinoma using specified automated cellular image analysis system: Aclinicopathological study. Oman Med J. 2011;26:307–14. doi: 10.5001/omj.2011.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lièvre A, Milet J, Carayol J, Corre DL, Milan C, Pariente A, et al. Genetic polymorphisms of MMP-1, MMP-3 and MMP-7 gene promoter and risk of colorectal adenoma. BMC Cancer. 2006;6:270. doi: 10.1186/1471-2407-6-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nosho K, Yamamoto H, Taniguchi H, Adachi Y, Yoshida Y, Arimura Y, et al. Interplay of insulin-like growth factor-II, insulin-like growth factor-I, insulin-like growth factor-I receptor, COX-2, and matrix metalloproteinase-7, play key roles in the early stage of colorectal carcinogenesis. Clin Cancer Res. 2004;10:7950–7. doi: 10.1158/1078-0432.CCR-04-0875. [DOI] [PubMed] [Google Scholar]
- 9.Heslin MJ, Yan J, Johnson MR, Weiss H, Diasio RB, Urist MM. Role of matrix metalloproteinases in colorectal carcinogenesis. Ann Surg. 2001;233:786–92. doi: 10.1097/00000658-200106000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fingleton BM, Heppner Goss KJ, Crawford HC, Matrisian LM. Matrilysin in early stage intestinal tumorigenesis. APMIS. 1999;107:102–10. doi: 10.1111/j.1699-0463.1999.tb01532.x. [DOI] [PubMed] [Google Scholar]
- 11.Newell KJ, Witty JP, Rodgers WH, Matrisian LM. Expression and localization of matrix-degrading metalloproteinases during colorectal tumorigenesis. Mol Carcinog. 1994;10:199–206. doi: 10.1002/mc.2940100404. [DOI] [PubMed] [Google Scholar]
- 12.Ishii T, Notohara K, Umapathy A, Mallitt KA, Chikuba H, Moritani Y, et al. Tubular adenomas with minor villous changes show molecular features characteristic of tubulovillous adenomas. Am J Surg Pathol. 2011;35:212–20. doi: 10.1097/PAS.0b013e318205df20. [DOI] [PubMed] [Google Scholar]
- 13.Ishikawa T, Ichikawa Y, Mitsuhashi M, Momiyama N, Chishima T, Tanaka K, et al. Matrilysin is associated with progression of colorectal tumor. Cancer Lett. 1996;107:5–10. doi: 10.1016/0304-3835(96)04336-4. [DOI] [PubMed] [Google Scholar]
- 14.Takeuchi N, Ichikawa Y, Ishikawa T, Momiyama N, Hasegawa S, Nagashima Y, et al. Matrilysin gene expression in sporadic and familial colorectal adenomas. Mol Carcinog. 1997;19:225–9. [PubMed] [Google Scholar]
- 15.Hai-yan P, Xiao-dong G, Ying-bin J. Expression and significance of matrilysin in human colorectal adenoma. J Int Med Health Guid News. 2009;18:20–2. [Google Scholar]
- 16.Ougolkov AV, Yamashita K, Mai M, Minamoto T. Oncogenic beta-catenin and MMP-7 (matrilysin) cosegregate in late-stage clinical colon cancer. Gastroenterology. 2002;122:60–71. doi: 10.1053/gast.2002.30306. [DOI] [PubMed] [Google Scholar]
- 17.Leeman MF, Curran S, Murray GI. New insights into the roles of matrix metalloproteinases in colorectal cancer development and progression. J Pathol. 2003;201:528–34. doi: 10.1002/path.1466. [DOI] [PubMed] [Google Scholar]
- 18.Zeng ZS, Shu WP, Cohen AM, Guillem JG. Matrix metalloproteinase-7 expression in colorectal cancer liver metastases: Evidence for involvement of MMP-7 activation in human cancer metastases. Clin Cancer Res. 2002;8:144–8. [PubMed] [Google Scholar]


