Abstract
Gastrointestinal (GI) motility function and its regulation is a complex process involving collaboration and communication of multiple cell types such as enteric neurons, interstitial cells of Cajal (ICC), and smooth muscle cells. Recent advances in GI research made a better understanding of ICC function and their role in the GI tract, and studies based on different types of techniques have shown that ICC, as an integral part of the GI neuromuscular apparatus, transduce inputs from enteric motor neurons, generate intrinsic electrical rhythmicity in phasic smooth muscles, and have a mechanical sensation ability. Absence or improper function of these cells has been linked to some GI tract disorders. This paper provides a general overview of ICC; their discovery, subtypes, function, locations in the GI tract, and some disorders associated with their loss or disease, and highlights some controversial issues with regard to the importance of ICC in the GI tract.
Keywords: Gastrointestinal, interstitial cells of Cajal, motility, pacemaker, slow waves
Interstitial cells of Cajal (ICC) were initially identified by Cajal[1] who first described nerve-like cells at the ends of motor neurons in organs innervated by peripheral nerves, and on the basis of staining characteristics with methylene blue and silver chromate, he classified them as primitive neurons. Following Cajal's first descriptions, these cells were identified using different names till Dogiel gave them the name, Cajal'sche zellen, i.e., ICC, and for more than 100 years after their discovery the name of ICC is still used. Taxi,[2,3] on the other hand, using light and electron microscopy, referred these cells as neuronoids in an attempt to distinguish them from neurons, Schwann cells, smooth muscle cells, fibroblasts, and macrophages, although these cells co-stained with nerves. Later on, ultrastructural studies suggested that ICC were either primitive muscle cells[4,5] or fibroblast-like cells.[6,7] Langton et al.,[8] showed for the first time that these cells are electrically rhythmic.
ICC MARKERS
ICC were previously characterized by morphological criteria till the discovery that ICC express the receptor tyrosine kinase, Kit (CD117), for the ligand steel factor stem cell factor or SCF and that the majority of the Kit-positive cells in the GI tract are indeed ICC.[9] This discovery was the turning point in the research of ICC and pushed it into a great advancement. Significantly not all ICC do express c-Kit, such as those in the deep muscular plexus in the human small intestine[10] and still other different cell types besides ICC do also express c-Kit, such as mast cells, melanocytes, neurons, and glia.[11] ICC also express CD34[12] and stain for Wilm's tumor gene protein 1 in a cytoplasmic pattern as well as for calretinin.[13] Other proteins such as Na+/K+/2Cl– co-transporter, NKCC1,[14] neurokinin-1 receptor,[15] and CD44[16] are expressed selectively on some or all sub-types of ICC, but further studies need to be conducted to confirm their use as selective markers of ICC in the GI tract. Other recent studies have identified anoctamin 1 (Ano1), a calcium activated chloride channel, as a new and selective molecular marker for all classes of ICC in the human and mouse GI tract that permits the immunochemical identification of these cells independent of Kit.[17]
ICC ORIGIN AND DEVELOPMENT
ICC develop independent of neural crest-derived enteric neurons or glia and originate mainly from Kit-positive mesenchymal mesodermal precursors.[18,19] In the chick, some ICC in the stomach and duodenum may also develop from multipotent, ventrally emigrating neural tube cells.[20] In the small intestine of mice, ICC emerge from a population of Kit-positive precursor cells at about embryonic day 15.[21] Kit signaling is critical during the late gestational period and after birth for the development and maintenance of ICC populations.[22,23] Kit-positive precursor cells that receive Kit signaling retain Kit expression and develop into functional ICC, whereas Kit-positive precursors that are not signaled via this pathway become smooth muscle cells.
ICC ULTRASTRUCTURE
ICC have small cell bodies and several elongated processes. Researchers have well-characterized ICC ultrastructural features[24] that are regarded as a valuable tool for their identification.[25] These include the presence of: (a) Numerous mitochondria and caveolae; (b) a basal lamina, although discontinuous; (c) abundant intermediate filaments; (d) moderately developed Golgi apparatus, few ribosomes, and rough and smooth endoplasmic reticula; and (e) close contacts established with nerve varicosities and the formation of numerous gap junctions, both with each other, forming a network through the bowel wall, and with smooth muscle cells.
ICC TYPES
Several criteria have been used in classification of ICC. For example, ICC have been grouped according to either their localization within the muscle layers (submuscular, intramuscular, myenteric, and subserosal), their basic morphology (stellate and bipolar), or their primary function (pacemakers and cells that mediate neuromuscular neurotransmission and mechanoreception). Moreover, reports have shown that there is some accordance between morphology, localization, and function of the various types of ICC.[26,27]
Several morphological types of ICC have been described based on their anatomical locations [Figure 1].[28,29] In the GI tract, most ICC occur around the circumference of the myenteric plexus (Auerbach's plexus) and these are called interstitial cells of Cajal of the myenteric plexus (ICC-MY or ICC-MP) or interstitial cells of Cajal of the Auerbach's plexus [ICC-AP]). These are multipolar cells with branched processes connecting to each other and forming a network around the myenteric plexus in the space between the circular and longitudinal muscle layers.
Figure 1.
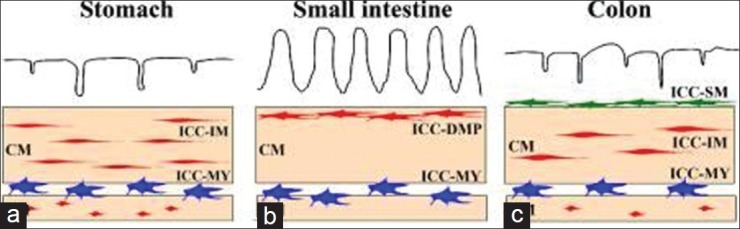
Overview of the types of interstitial cells of cajal in the gastrointestinal tract. (a-c): Schematic representations of ICC in the stomach (a), small intestine (b), and colon (c). ICC-MY (blue) are located between circular (CM) and longitudinal (LM) muscle layers. ICC-IM (red) and ICC-DMP (red) are located within circular and longitudinal muscles. ICC-SM (green) are located at the submucosal surface of the circular muscle layer in the colon. Redrawn from Satoshi Iino and Kazuhide Horiguchi, Acta Histochem Cytochem. 2006 December 28; 39(6): 145-153
Interstitial cells of Cajal of the circular muscle (ICC-CM) are the cell types found in the circular muscle. These are mainly bipolar cells or spindle-shaped cells associated with the long axis of the surrounding smooth muscle cells. These cells do not form their own network. These ICC also occur in the connective tissue septa and are being referred there as interstitial cells of Cajal of the connective tissue septa (ICC-SEP). Interstitial cells of Cajal of the longitudinal muscle (ICC-LM) are the cell types found in the longitudinal muscle. They are similar to ICC-CM but are usually less numerous. A collective term for ICC-CM and ICC-LM is intramuscular interstitial cells of Cajal (ICC-IM). Interstitial cells of Cajal of the deep muscular plexus (ICC-DMP) are the cell types found in the deep muscular plexus. These multipolar cells are associated closely with the nerve bundles of the deep muscular plexus. Interstitial cells of Cajal of the submucosa (ICC-SM) and interstitial cells of Cajal of the submucosal plexus (ICC-SMP) are the cell types found in the submucosa and submucosal plexus at the interface between the submucosal connective tissue and the innermost circular muscle layer. These cells form a loose network with each other. ICC of the subserosa are the class of cells found in the subserosa.
ICC LOCATIONS ALONG THE GI TRACT
Esophagus
ICC in the smooth muscle of the esophagus and within the lower esophageal sphincter (LOS) are of the ICC-IM subtype. They are in close contact with nerve terminals and make specific junctions (including nexuses) with smooth muscle cells.[10] There is no aggregation of ICC-IM around the myenteric plexus or at the submucosal border, as in the small and large intestines.[10]
Stomach
ICC are more densely located in the corpus and antrum than in the fundus; the antrum contains both ICC-MY and ICC-IM networks, whereas in the fundus only ICC-IM are found.[30,31] Along the circumferential axis of the antrum, ICC-MY are distributed more densely in the greater curvature than in the lesser curvature.[30,31] In the pylorus, numerous c-Kit-positive ICC-IM are identified throughout the circular and longitudinal muscle layers and around the myenteric plexus.[32] An important characteristic feature of the pylorus is the presence of ICC-SM at the submucosal border of the circular muscle layer in a confined area directly adjacent to the sphincter.[33]
Small intestine
In the small intestine, ICC-MY are organized in bundles of up to five cells, with overlapping processes.[34] These bundles extend into the adjacent portions of the longitudinal and circular muscle layers as well as the interlamellar septa.[10] These ICC-MY have close associations with myenteric nerves and have receptors for various neurotransmitters[35] and circulating hormones (e.g., cholecystokinin).[36] The density of ICC-MY network has been reported to be higher in ileum compared to other parts of the small intestine.[37] ICC-DMP have specialized contacts with both nerves and smooth muscle cells, with synapse-like contacts with nitrergic and cholinergic nerves and gap junctions with muscle cells, allowing them to relay information from nerves to muscles.[38] The first part of duodenum has a distinctive ICC distribution that is different from the rest of the intestine; ICC-MY and numerous ICC in circular muscle layer are present but no ICC-DMP are observed.[39] Moreover, ICC-IM are found predominantly in the inner one-third of the muscularis propria.[38]
Large intestine
The colon has a submucosal nerve plexus (SMP) that is present in the submucosa just beneath the innermost circular layer but unlike the small intestine it lacks the DMP.[40] The ICC in the colon include subtypes of ICC-MY, ICC-IM, and ICC-SMP. ICC-IM are dispersed throughout the musculature and run parallel to smooth muscle cells in both muscle layers.[41] Close approximations to nerves are frequent, while synapse-like close contacts are relatively infrequent.[37]
There is a big controversy regarding the regional differences of ICC distribution in the human colon. One pediatric study found no ICC-MP or ICC-SMP in caecum with the highest density of ICC present in the descending colon. Similar distribution was found in sigmoid colon but with less conspicuous ICC-MY.[42] In the anorectal region, spindle-shaped ICC are present in both muscle layers, parallel to the smooth muscle cells and are abundant, surrounding the myenteric ganglia. ICC at the submucosal plexus are less dense.[32] In the internal anal sphincter (IAS) in adults, the density of ICC seems to be significantly lower than that observed in the rectum.[43]
Pancreas
Cajal reported initially that ICC were independent elements scattered throughout the pancreas. Recently, researchers, using non-conventional light microscopy, immunohistochemistry, and transmission electron microscopy, have confirmed the presence of ICC in pancreas pancreatic interstitial cells of Cajal.[44]
Outside the GI tract
ICC have also been found in many locations outside the GI tract such as the upper urinary tract,[45] urethra,[46] myometrium,[47] myocardium,[48] uterus, fallopian tube,[49] human placenta,[50] and the ciliary muscle in monkeys.[51]
ICC FUNCTIONS
GI motility is essential for life and is a highly regulated and coordinated process. Research in GI motility started early in the history from the observations of gastric contractions[52] to the discovery of spontaneous colonic contractions in the cat tract using X-rays.[53] Bayliss and Starling in 1899[54] found that myogenic contractions causing effective peristaltic activity occurred even after neural activity was blockaded, giving an evidence of an internal gut pacemaker. Cajal[55] proposed ICC as an important player in GI motility via mediating enteric transmission, and later on, Keith[56] proposed ICC as pacemakers. Now, we know that ICC contribute to several important functions in the GI tract including: (a) Generation of electrical slow wave activity, (b) coordination of pacemaker activity and active propagation of slow waves, (c) transduction of motor neural inputs from the enteric nervous system, and (d) mechanosensation to stretch of GI muscles.[27] The following sections describe these functions and their related controversies.
ICC AS PACEMAKERS
After providing an evidence of having an internal gut pace-making action,[54] Arthur Keith[56] made the first suggestion that ICC may act as physiological pacemakers. Then, Ambache[57] was the first to show that electrical slow waves control intestinal contractions and to relate these slow waves to ICC.
Slow waves in smooth muscle tissues are periodic oscillations of the cell membrane potential consisting of a rapid upstroke and a longer plateau phase followed by repolarization. These fluctuations have characteristic frequencies in each organ and in each animal, between 3 and 50 cycles per minute. Slow waves cause alternation of brief periods of high and low excitability; the period of high excitability corresponds to the plateau phase of the slow wave. Upon excitatory stimulation, the plateau phase of the slow wave rises above threshold for activation of L-type calcium channels, and consequently, action potentials are generated. Slow waves determine the maximum frequency and propagation characteristics of contractions and exhibit an intrinsic frequency gradient and found to propagate in an aboral direction (i.e., away from the mouth or oral region).[58,59] In the stomach, the fundus is believed to be electrically quiescent (i.e., without slow waves) and the corpus is believed to be the source of slow waves generation. The small intestinal slow waves originate from a region in the proximal 1 cm of the duodenum and propagate as an annular wavefront in an aboral direction with a diminishing frequency gradient along the small bowel. The colonic slow waves are not well characterized and have multiple frequencies without a dominant rhythmicity.
Peristalses are waves of contraction propagating along the GI tract for various distances as a means of mixing and propelling its content distally. The rhythmicity of peristaltic motor activity is determined by the electrical slow wave activity in the musculature, which is believed to be generated by ICC.[60]
A large number of studies using different models have demonstrated the role of ICC as pacemakers as we will see in the following sections.
In intact muscles
Early studies on intact muscle strips, using methylene blue followed by illumination to induce photochemical ablation of ICC, resulted in a significant block of slow-wave activity and thus provided a strong evidence for the involvement of ICC in the generation of slow waves.[61] Other experiments used rhodamine 123, a cytotoxic fluorescent dye that specifically accumulates in the ICC, although it might also accumulate in enteric neurons; it was found that uptake of rhodamine 123 by ICC was associated with an alteration in electrical rhythmicity.[62] In another study, strips of gastric muscle tissues were incubated with the neutralizing antibody for the receptor tyrosine kinase, Kit, (anti-c-kit antibody or ACK 2) for 31-50 days. This procedure obliterated all ICC, including ICC-MY, and abolished slow waves in the circular muscle cells. Moreover, in this study, with the absence of ICC-MY, electrical field stimulation was unable to phase advance or pace gastric slow waves.[63]
In isolated and cultured cells
The first direct test for the pacemaker function of ICC came when these cells were isolated from the submucosal pacemaker area of the canine colon. These cells were found to be spontaneously active and generating electrical depolarizations similar to slow waves recorded in intact smooth muscle cells.[8] Later on, electrical recordings that were obtained from cultured murine ICC with the whole-cell patch clamp technique have shown that ICC are rhythmically active, producing regular slow wave depolarizations with waveforms, and properties similar to slow waves in intact tissues.[64]
Pacemaker regions
Slow waves are largest in amplitude in specific areas, and using multiple intracellular electrodes for recording, they always occur first near the region of the myenteric plexus of the stomach and small bowel,[65] or near the submucosal border of the colon.[66]
Mutant animal models
Three mutant models have been used to investigate the roles of ICC in the regulation of GI motility; W/Wv mice (c-Kit loss-of-function mutants), Sl/Sld mice (steel-Dickie mutant mice, in which the gene encoding the c-Kit ligand [SCF] is defective), and Ws/Ws rats (containing c-Kit gene mutation).[27]
In mutant animals
In W/Wv mice, the networks of ICC-MY are grossly underdeveloped in the small intestine where pacemaker activity is lacking even though ICC are present in the DMP [Figure 2].[67,68] In the same mouse model, ICC-MY are evident in the stomach, where slow-wave activity can be recorded.[69] These data give a strong suggestion that ICC-MY but not ICC-DMP are crucial for generating slow-wave activity.
Figure 2.
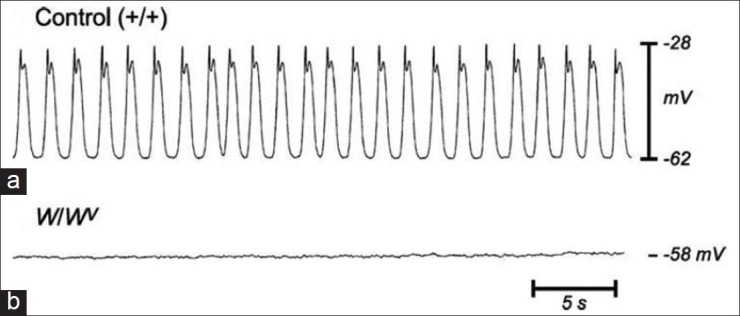
Absence of slow waves in W/Wv mice. Normal slow waves recorded in wild-type control mice small intestine smooth muscles (a) are lost in W/WV mutant mice; (b) Ward et al., 1994. J. Physiol. London 480:91-97
Similar experiments in Ws/Ws rats and steel-Dickie mutant mice (Sl/Sld) confirmed these data. In one ultrastructural study, features of ICC-DMP and ICC-MY corresponding to c-Kit-expressing cells in each location were studied by comparing Ws/Ws rats to their normal siblings and suggested that ICC in the rat intestine are heterogeneous in ultrastructure, c-Kit dependency in the cell maturation, and functional role.[70] Another study group tested the effect of steel factor mutation on ICC development and intestinal electrical rhythmicity using Sl/Sld mice and suggested that the c-Kit signaling pathway, including stimulation by SCF, is an important factor in the development of functional ICC networks in the small intestine.[71] Smooth muscles of these animals are apparently unaffected by Kit or SCF mutations, and the muscles are capable of generating Ca2+ action potentials, contractile responses, and responses to agonists,[67,69] and so excluding the possibility that these differences between mutant and control animals are due to smooth muscle changes.
With regard to the role of ICC in regulating peristaltic contractions along the gut by their generation of slow waves, one study monitored movement of barium sulfate in the small intestine of W/Wv mice using radiography. Regular peristaltic waves were observed and distal movement of intestinal contents was noted in the control mice, but these were absent in the mutant ones.[72] The action potentials and contractions appeared random, the contents of the small intestine moved back and forth in an irregular manner in the W/Wv mice, and the net propulsive effect of the contractile activity in the mutant mice was much weaker than that in the control mice. In support of these findings, Ishii et al,[73] reported that transplantation of bone marrow-derived cells that serve as potential sources of gut ICC may incorporate into ICC networks and improve dysmotility in W/Wv mice.
In the colon, impaired colonic contractions were recorded in Ws/Ws rats. The wild-type, but not Ws/Ws rats showed low- and high-frequency cyclic depolarization that was associated with highly regular myogenic motor patterns at the same frequencies. In Ws/Ws rats, irregular patterns of action potentials triggered irregular muscle contractions.[74]
Although big evidence from literature supports the role of ICC as pacemakers, conflicting results have been reported in some in vivo studies suggesting that ICC may not be necessary for the generation of slow waves. For example, in one study, both wild-type and W/Wv mice were implanted with two pairs of electrodes in the stomach and small intestine and GI slow waves were recorded both under anesthesia and in the conscious state. This study has shown that in the stomach, regular slow waves were recorded with no difference between the two groups of animals. The in vivo slow waves from the small intestine of the W/Wv mice were, however, impaired compared to those recorded from the control mice, reflected as a decrease in the slow wave frequency and rhythmicity.[75] Some other in vitro experiments paralleled these results and found that muscle strips taken from small intestine of W/Wv mice in a tissue bath continued to generate slow waves and rhythmic phasic contractions, but both were more irregular in frequency and smaller in amplitude.[76] In another study, the role of ICC in small intestinal transit and its response to exogenous pacing were investigated in W/Wv mice and found that both gastric and intestinal slow waves were completely entrained in both control and W/Wv mice. Additionally, there was no significant difference in small intestinal transit between the controls and the W/Wv mice, and pacing showed no effects on it in either group of mice.[77] Although ICC-MY are regarded as the dominant pacemakers in gastric muscles, still other types of ICC (e.g., ICC-IM or ICC-SEP) can also generate spontaneous depolarizations, referred to as unitary potentials[78] or spontaneous transient depolarizations.[79] In the absence of ICC-MY, this basic rhythm of activity of ICC-IM or ICC-SEP in the antrum or pyloric region of the stomach can drive low-frequency slow waves (also referred to as regenerative potentials, slow potentials, or slow wave-like action potentials, see Refs[78,79] ). A recent study suggests that ICC-IM or ICC-SEP might provide dominant pacemaker activity in the orad corpus of the guinea pig stomach because this area of the stomach generates high-frequency slow waves in the absence of ICC-MY.[80]
Pacemaker mechanism
Some hypotheses were suggested to explain the mechanism of slow wave generation by ICC. One mechanism proposed that a chloride channel is responsible for the rhythmic depolarizations that lead to the upstroke of the slow wave.[81] Another alternative mechanism proposed that Ether-à-go-go-related gene (ERG) potassium channels may function as the pacemaker channel.[82] Yet the most plausible hypothesis for slow wave generation proposes an increase in intracellular calcium through release from the smooth endoplasmic reticulum, which precedes the upstroke of the slow wave, and this is used frequently to determine the origin and spread of the slow wave.[83] The trigger for pacemaker activity is a localized drop in calcium levels in the much smaller pacemaker unit caused by the uptake of calcium by mitochondria resulting in the activation of a non-selective cation channel in the cell membrane that is normally inhibited by the higher calcium levels. The ensuing current through the non-selective cation channel is the pacemaker current, and the summation of multiple of pacemaker currents produces the unitary currents which are the basic pacemaker events in ICC.[84] Coordination and propagation of the pacemaker currents are achieved by voltage-dependent calcium channels that are not L-type (i.e., dihydropyridine resistant) but have T-channel-like properties.[85]
ICC-smooth muscle coupling
Hirst and his colleagues performed double electrode recording experiments and showed that ICC and smooth muscle cells in the stomach are electrically coupled in situ, and that slow waves originate in ICC and conduct to smooth muscle cells.[86] Kito and Suzuki made similar recordings from the small intestine and found that ICC-MY generate pacemaker potentials that spread electrotonically to circular smooth muscles.[87] Optical experiments using fluorescent Ca2+ dyes have also confirmed the temporal sequence of activation in ICC and smooth muscle cells.[88] Pacemaker cells may be coupled to smooth muscle cells by gap junctions as in the submucosal plexus of the canine colon,[89] but at other sites, such as between ICC and the muscle layers of the mouse's small intestine, gap junctions are not found and coupling appears to be mediated by an abundance of close apposition contacts.[90] Within certain muscle layers, no gap junctions can be recognized by electron microscopic techniques, such as in the longitudinal muscle of the intestine and in the colon of a variety of species.[91] Other mechanisms such as peg and socket connections have been proposed that might, under appropriate conditions, provide electrical coupling through the accumulation of potassium in the narrow cleft between the peg and the socket.[92]
ICC IN NEUROTRANSMISSION
In spite of the structural evidence for direct enteric nerve to smooth muscle communication,[93] a number of studies, based upon the close and the functional relationship between ICC and enteric nerve fibers, have strongly suggested the role of ICC in neurotransmission [Figure 3].[55,94]
Figure 3.
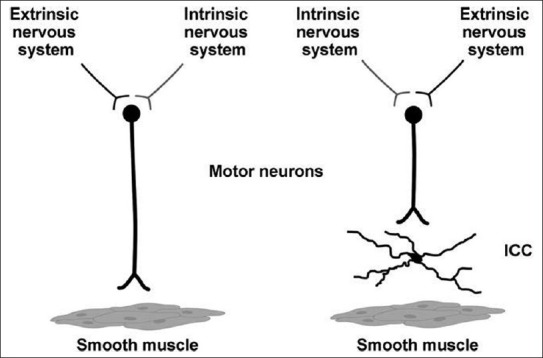
Innervation of smooth muscle cells. Two mechanisms for neuronal innervation of gastrointestinal smooth muscle exist. Most innervation occurs through interstitial cells of Cajal. Neurons can also directly innervate intestinal smooth muscle cells. Redrawn from Mazzone A, Farrugia G. Evolving concepts in the cellular control of gastrointestinal motility: Neurogastroenterology and enteric sciences. Gastroenterol Clin North Am 36: 499-513, 2007
Immunohistochemical studies have revealed that nerve fibers stained with the primary transmitters of excitatory motor neurons, such as vesicular acetylcholine (ACh) transporter and substance P, are closely associated with cell bodies and processes of ICC-IM in the stomach,[95–97] and with ICC-DMP in the small intestine.[98,95,99]
Many inhibitory motor neurons that contain nitric oxide synthase (NOS), vasoactive intestinal polypeptide (VIP), or adenosine triphosphate are also closely associated with both the cell bodies and processes of ICC-IM.[95–97] Moreover, it was found that different ICC types express various receptors for different neurotransmitters such as M2 and M3 receptors for muscarinic ACh, and VPAC1 receptors for VIP,[100] suggesting with the previous data, that ICC are densely innervated by excitatory and inhibitory enteric motor neurons.
In W/Wv mice, ICC-IM were reported to be absent in gastric fundus, LOS, and pyloric sphincter without affecting the distribution of inhibitory nerves or the density of cholinergic nerve bundles. These mice showed that NO-dependent inhibitory neurotransmission was reduced, hyperpolarizations to sodium nitroprusside were attenuated,[69,101] and cholinergic responses were also lost.[102]
In small intestine, ICC-DMP replace ICC-IM, and in one ultrastructural study, ICC-DMP were found to be innervated by both cholinergic and nitrergic nerves, and were the only cells to possess specialized synapse-like junctions with nerve varicosities and gap junction contacts with smooth muscle cells.[38] Loss of ICC-DMP by blocking Kit was shown to cause loss of cholinergic and nitrergic neural responses.[103] Previous studies suggest that ICC-IM in the stomach and ICC-DMP in the small intestine have important roles in mediating the enteric neural input to the GI muscle cells.
Despite the previous in vitro evidence for ICC role in neurotransmission, a study was done on isolated whole stomach of wild-type and W/Wv mice and found that normal gastric distension-induced adaptive relaxation occurred in both mice groups.[104]
In another study, intraluminal manometry was performed under anesthesia in neuronal NOS knockout mice (nNOS-/-), W/Wv mice, and control mice.[105] The LOS in the nNOS-/- mice was significantly hypertensive and its relaxation to swallowing and efferent vagal stimulation was markedly impaired. In contrast, the LOS in the W/Wv mice is hypotensive and relaxes normally to swallowing and efferent vagal activation. These results negate the role for ICC in neural transmission.
In a recent study, circular smooth muscles from LOS of wild type and W/Wv mutant mice were studied using intracellular and tension recordings in vitro. It was suggested that there was a significant variability in the generation of nitrergic neurotransmission in the LOS of W/Wv mutant mice, whereas purinergic and cholinergic neurotransmissions are intact and the altered nitrergic responses appear to be associated with abnormal Ca2+ -dependent signaling initiated by spontaneous Ca2+ release from sarcoplasmic reticulum in smooth muscle cells, and so c-Kit-positive ICC are not essential for nitrergic neurotransmission in mouse LOS smooth muscle.[106]
In the colon, no role was found for ICC in nitrergic neurotransmission. In one study, spontaneous activity of nitrergic nerves caused sustained inhibition of muscle activity in both wild-type and Ws/Ws rats. Electrical field stimulation of enteric nerves, after blockade of cholinergic and adrenergic activities, elicited inhibition of mechanical activity and biphasic inhibitory junction potentials both in wild-type and mutant rats.[74] Moreover, one group examined neural reflexes in the distal colonic segments prepared from the wild type and W/Wv mice by applying a localized distension on the segments from both mice groups. In the segments from the mutant mice, localized distension induced neural reflexes similar to those observed in the wild type mice, suggesting that ICC have no important role in the ascending and descending neural reflexes in the mouse's distal colon.[107]
In the anal sphincter, one group assessed the basal IAS tone and the rectoanal inhibitory reflex in vivo by a purpose-built solid-state manometric probe and by using wild-type, nNOS-deficient (nNOS-/-), eNOS-deficient (eNOS-/-), and W/Wv mice and showed a reduced basal pressure and normal relaxation to rectal distention, a phenomenon called rectoanal inhibitory reflex mediated by the inhibitory nitrergic pathway.[108] In another in vivo study, distention of the rectum elicited a volume-dependent relaxation of the anal sphincter in W/Wv mice; the degree of relaxation was lower than that in the control mice at low distention volume but comparable to the control mice at high distention volume.[109] These experiments suggest limited or no roles of ICC in the nitrergic relaxation of the anal sphincter.
In short, the majority of in vitro studies establish the role for ICC-IM and ICC-DMP in mediating neural transmission between the enteric nerves and the GI smooth muscles. However, in vivo motility studies and some recent in vitro studies question the role of ICC as neural mediators.[27]
Synaptic contacts between enteric motor nerve terminals and ICC-IM
With the aid of electron microscopy, plenty of specialized junctions between ICC-IM and nerve terminals with spacing of less than 20 nm are observed.[95] Ultrastructural studies have also identified areas of electron density at junctions between enteric nerve varicosities and ICC-IM in the GI tract of several species.[95,110]
Moreover, members of the soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptors (SNAREs) that are involved in the release of neurotransmitters are present in developing enteric nerves,[111] and several of these SNARE proteins have been identified in enteric neurons of the murine stomach, including synaptotagmin, syntaxin, and synaptosomal-associated protein 25 (SNAP-25).[110] Varicose terminals of cholinergic and nitrergic motor neurons express synaptotagmin and SNAP-25, and these terminals lie in close opposition to ICC-IM, but not to smooth muscle cells.[110]
Additionally, electron-dense regions in ICC-IM opposing the presynaptic membrane specializations at enteric motor nerve terminals were observed which contain members of postsynaptic density proteins (PSDs) like PSD-95, but labeling was not resolved in smooth muscle cells.[110] These observations suggest that the ICC-IM—enteric neuron junction is functional and support the existence of a specialized kind of transmission of neural information between enteric nerves and ICC.[94]
ICC IN SETTING THE MEMBRANE POTENTIAL OF SMOOTH MUSCLE CELLS
ICC were found to hyperpolarize intestinal smooth muscle cells by generating and releasing the inhibitory gaseous neurotransmitter carbon monoxide which is a hyperpolarizing factor and helps set the intestinal smooth muscle membrane potential gradient.[112]
ICC IN STRETCH SENSING
Stretching of antral muscles by approximately 25% with precise length ramps caused depolarization and increased slow wave frequency by a non-neural mechanism.[113] The response to stretch is absent in muscles of W/Wv mice (which lack ICC-IM), suggesting that ICC-IM provide stretch sensitivity in gastric muscles. Products of arachidonic acid metabolism, such as prostaglandin E2, are likely to mediate stretch-dependent responses, and the cyclooxygenase enzyme II (COX-II) which is expressed by ICC-IM might mediate the stretch sensor mechanism associated with the ICC; since stretch-dependent responses were inhibited by the COX-II inhibitor indomethacin and they were absent in COX-II-deficient mice.[113] Some studies have suggested that a mechanosensitive Na+ channel current is present in human intestinal ICC and appears to play a role in the control of intestinal motor function.[114] In spite of the previous evidence for the role of ICC in stretch sensing, in vivo physiological tests need to be done to further confirm this function.[27]
ICC AND MOTILITY DISORDERS
There is an evidence of correlation between alterations to ICC and some motility disorders. Researchers have reported that ICC abnormalities and damage accompany various types of GI motility disorders and pathologies in almost all areas of the gut.[115] For example, in one gastric emptying disorder associated with a distended stomach full of milk, a pronounced intestinal ileus and irregular pattern of phasic contractions of intestinal muscles developed in animals treated with a neutralizing antibody for Kit.[9] Sanders and co-workers also found that animals exposed to such treatment lost ICC-MY in the small intestine and slow waves could not be recorded from the affected muscles.[22] The coming sections introduce some of the disorders in different regions of the GI tract that showed some changes in ICC.
Esophagus
Achalasia
Achalasia is a disorder of the LOS, characterized by high sphincter tone and inability to relax and lack of peristaltic contractions of esophageal body.[116] The etiology of this disorder includes paraneoplastic syndromes and autoimmunity, occasionally with antibodies directed to Auerbach's plexus, but many cases are still idiopathic.[117] The ganglia and nerve bundles are frequently reduced or absent in the area of the LOS and distal esophagus.[118] Ultrastructural studies have noted that the ICC are altered in this area as well, with loss of contacts between the ICC and nerves and reduction in mitochondria.[119] In the autosomal recessive syndrome, Allgrove's syndrome (achalasia, addisonianism, and alacrima), gastric cardiac ICC are decreased in some cases.[120]
Gastro-esophageal reflux disease
Gastro-esophageal reflux disease (GERD) is a condition in which food or liquid travels backwards from the stomach to the esophagus. This action can irritate the esophagus, causing heartburn, acid regurgitation, and other symptoms.[121] Esophagus itself may be correlated to changes in ICC and in advanced stages of GERD; inflammatory changes in the esophageal wall will also involve the ICC leading to their impairment. This destruction leads to loss of effective contraction of esophagus, maintaining reflux and thus aggravating the symptoms.[122]
Stomach
Gastroparesis
Gastroparesis is a condition that affects the ability of the stomach to empty its contents without physical blockage (obstruction). Slow wave functions are described to be abnormal in both gastroparesis and functional dyspepsia.[123,124] Examination of a stomach resected for severe gastroparesis revealed hypoganglionosis, neuronal dysplasia, and decreased myenteric and intramuscular ICC.[125] A relationship between viral infection and some cases of gastroparesis has been described; viral infections of the stomach or intestines may lead to inflammation that particularly damages the ICC.[126]
Infantile hypertrophic pyloric stenosis
This is a congenital disorder characterized by functional gastric-outlet obstruction. In this disorder, infants present with postprandial projectile vomiting and a palpable “olive” pyloric sphincter. While the pyloric lumen is not occluded, the hypertrophic muscle of the sphincter may not coordinate well with the gastric contractions, leading to the vomiting. Using both electron microscopy and immunohistochemistry, ICC were found to be absent in the hypertrophic circular muscle layer in these patients.[115,32]
Small intestine and colon
Diabetic gastroenteropathy
Gastroenteropathy causes considerable morbidity in patients with diabetes mellitus and may manifest in dysphagia, heartburn, abdominal pain or discomfort, early satiety, postprandial fullness, bloating, nausea, vomiting, constipation, diarrhea, and fecal incontinence.[127] Some studies reported a decrease in ICC, although the mechanism of this is not determined.[128] In addition, loss of c-Kit-positive ICC has been noted in the colonic muscularis propria of patients with diabetes mellitus.[129] This suggests that damage to the ICC in diabetes mellitus may be the underlying etiology of the dysmotility that accompanies the disease.
Chronic idiopathic intestinal pseudo-obstruction
Chronic idiopathic intestinal pseudo-obstruction (CIIP) is characterized by defective GI propulsion together with symptoms and signs of bowel obstruction in the absence of any lesion or mechanical obstacle.[130] CIIP is regarded as a neuropathy, myopathy, or both.[131] Several studies have indicated that ICC are decreased or absent in some idiopathic CIIP patients.[132,133] There is also a second subgroup of CIIP patients with myocyte changes suggestive of visceral myopathy in whom ICC-AP are absent in the dilated portion of the megaduodenum.[134] CIIP can also occur in the colon. Electrophysiological studies show that these patients have absent or decreased slow wave rhythms[135] and absent or decreased ICC are also demonstrable in some cases.[132,133]
Hirschsprung's disease
In this disease, the enteric nervous system is absent (aganglionosis) in a portion of the GI tract (mostly distal colon) and slow wave activity is absent in the affected area.[136] There is a lot of debate regarding the role of ICC in Hirschsprung's disease; some studies have demonstrated a decreased density of ICC in the aganglionic area,[137] while others have shown absent or sparse ICC in both the aganglionated and ganglionated portions of the resected bowels.[138] However, other studies have shown no differences in the colonic distribution of ICC between Hirschsprung's disease patients and controls.[139]
Anorectal malformations
Anorectal malformations comprise a wide spectrum of diseases, which can affect boys and girls, and involve the distal anus and rectum as well as the urinary and genital tracts. Defects range from the very minor and easily treated with an excellent functional prognosis, to those that are complex, difficult to manage, are often associated with other anomalies, and have a poor functional prognosis.[140] One study reported that colonic hypomotility seen in patients with anorectal malformations is caused by defects in distribution or density of ICC.[141] More recently, researchers found lower density of ICC in the terminal intestine of rats with anorectal malformations.[142]
ICC PLASTICITY
ICC show a high degree of plasticity, which means that loss of ICC in some disorders does not necessarily mean death of these cells.[143] Transdifferentiation and apoptosis have been proposed as mechanisms for ICC loss. Some study groups have shown that ICC can transdifferentiate (change into another adult cell type) into a fibroblast/smooth muscle phenotype not expressing Kit, and this cell in turn can transdifferentiate back into a Kit-expressing ICC following the removal of the insult.[23,144] Other studies on both normal and diseased human colonic tissues have shown ongoing apoptosis as identified by the TUNEL assay, by identifying activated caspase-3 in Kit-positive ICC, and by electron microscopy.[145]
On the other side, an increase in the number of ICC can be achieved by an increase in the survival of the remaining ICC, by development from precursor cells, and by an increase in the proliferation of ICC. A recent report describes a population of CD34-positive cells that have a low level of Kit expression in the tunica muscularis of the gut and when properly stimulated, may be capable of regenerating ICC.[146] ICC can also be induced to proliferate by several molecules including steel factor activation of the Kit receptor, neuronally derived nitric oxide, serotonin through the serotonin receptor 2B (5-HT2B receptor) and heme oxygenase-1.[147,148]
CONCLUSIONS
ICC are mesenchyme-derived cells, belonging to the family of smooth muscle cells in which the activation of Kit signaling is required for their development. The discovery that ICC express c-Kit has helped a lot in learning and understanding the morphology and physiological roles of ICC. Morphological studies have identified different phenotypic classes of ICC with different regulatory roles within the gut that contribute to both the regulation of excitation–contraction coupling and to connectivity between smooth muscle cells and the motor output of the enteric nervous system.
ICC functions include: (a) Generation of electrical slow wave activity in phasic regions of the GI tract, (b) coordination of pacemaker activity and active propagation of slow waves, (c) transduction of motor neural inputs from the enteric nervous system, (d) mechanosensation to stretch of GI muscles, and (e) setting the membrane potential gradient of gut smooth muscles. However, further in vivo studies are needed to support these proposed roles of ICC in the GI tract.
ICC abnormality or decrease in number has been reported in some GI tract diseases. However, understanding the nature of the inter-relationship between ICC and the generation of human gut motility disorders and the underlying mechanism for ICC loss is still in progress. Future effort has to be spent to better understand and characterize the nature and function of ICC by looking for more specific markers that will help in quantifying ICC in normal versus diseased tissues, which will in turn clarify the importance of these cells and elucidate the basis of some GI tract disorders.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Cajal SR. Vol. 2. Paris: Maloine; 1911. Histologie du système nerveux de l’homme et des vertébrés; pp. 891–942. [Google Scholar]
- 2.Taxi J. On the existence of ciliated neurons in the sympathetic ganglia of certain vertebrates. C R Seances Soc Biol Fil. 1961;155:1860–3. [PubMed] [Google Scholar]
- 3.Taxi J. Electron microscope study of the innervation of intestinal smooth muscle, compared to that of some other mammalian smooth muscles. Arch Biol (Liege) 1964;75:301–28. [PubMed] [Google Scholar]
- 4.Imaizumi M, Hama K. An electron microscopic study on the interstitial cells of the gizzard in the love-bird (Uroloncha domestica) Z Zellforsch Mikrosk Anat. 1969;97:351–7. doi: 10.1007/BF00968841. [DOI] [PubMed] [Google Scholar]
- 5.Faussone Pellegrini MS, Cortesini C, Romagnoli P. Ultrastructure of the tunica muscularis of the cardial portion of the human esophagus and stomach, with special reference to the so-called Cajal's interstitial cells. Arch Ital Anat Embriol. 1977;82:157–77. [PubMed] [Google Scholar]
- 6.Richardson KC. Electronmicroscopic observations on Auerbach's plexus in the rabbit, with special reference to the problem of smooth muscle innervation. Am J Anat. 1958;103:99–135. doi: 10.1002/aja.1001030105. [DOI] [PubMed] [Google Scholar]
- 7.Komuro T. Three-dimensional observation of the fibroblast-like cells associated with the rat myenteric plexus, with special reference to the interstitial cells of Cajal. Cell Tissue Res. 1989;255:343–51. doi: 10.1007/BF00224117. [DOI] [PubMed] [Google Scholar]
- 8.Langton P, Ward SM, Carl A, Norell MA, Sanders KM. Spontaneous electrical activity of interstitial cells of Cajal isolated from canine proximal colon. Proc Natl Acad Sci U S A. 1989;86:7280–4. doi: 10.1073/pnas.86.18.7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maeda H, Yamagata A, Nishikawa S, Yoshinaga K, Kobayashi S, Nishi K, et al. Requirement of c-kit for development of intestinal pacemaker system. Development. 1992;116:369–75. doi: 10.1242/dev.116.2.369. [DOI] [PubMed] [Google Scholar]
- 10.Torihashi S, Horisawa M, Watanabe Y. c-Kit immunoreactive interstitial cells in the human gastrointestinal tract. J Auton Nerv Syst. 1999;75:38–50. doi: 10.1016/s0165-1838(98)00174-x. [DOI] [PubMed] [Google Scholar]
- 11.Zhang SC, Fedoroff S. Cellular localization of stem cell factor and c-kit receptor in the mouse nervous system. J Neurosci Res. 1997;47:1–15. [PubMed] [Google Scholar]
- 12.Robinson TL, Sircar K, Hewlett BR, Chorneyko K, Riddell RH, Huizinga JD. Gastrointestinal stromal tumors may originate from a subset of CD34-positive interstitial cells of Cajal. Am J Pathol. 2000;156:1157–63. doi: 10.1016/S0002-9440(10)64984-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsuzuki TH, Takahashi E, Maeda N. Wilm's tumour gene protein (WT-1) and Calretinin (Cal) immunoreactivity in gastrointestinal stromal tumour (GIST) Modern Pathol. 2005;18:121. [Google Scholar]
- 14.Wouters M, De Laet A, Donck LV, Delpire E, van Bogaert PP, Timmermans JP, et al. Subtractive hybridization unravels a role for the ion cotransporter NKCC1 in the murine intestinal pacemaker. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1219–27. doi: 10.1152/ajpgi.00032.2005. [DOI] [PubMed] [Google Scholar]
- 15.Chen H, Redelman D, Ro S, Ward SM, Ordög T, Sanders KM. Selective labeling and isolation of functional classes of interstitial cells of Cajal of human and murine small intestine. Am J Physiol Cell Physiol. 2007;292:C497–507. doi: 10.1152/ajpcell.00147.2006. [DOI] [PubMed] [Google Scholar]
- 16.Lorincz A, Redelman D, Horváth VJ, Bardsley MR, Chen H, Ordög T. Progenitors of interstitial cells of Cajal in the postnatal murine stomach. Gastroenterology. 2008;134:1083–93. doi: 10.1053/j.gastro.2008.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomez-Pinilla PJ, Gibbons SJ, Bardsley MR, Lorincz A, Pozo MJ, Pasricha PJ, et al. Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1370–81. doi: 10.1152/ajpgi.00074.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young HM, Ciampoli D, Southwell BR, Newgreen DF. Origin of interstitial cells of Cajal in the mouse intestine. Dev Biol. 1996;180:97–107. doi: 10.1006/dbio.1996.0287. [DOI] [PubMed] [Google Scholar]
- 19.Sanders KM, Ordög T, Koh SD, Torihashi S, Ward SM. Development and plasticity of interstitial cells of Cajal. Neurogastroenterol Motil. 1999;11:311–38. doi: 10.1046/j.1365-2982.1999.00164.x. [DOI] [PubMed] [Google Scholar]
- 20.Sohal GS, Ali MM, Farooqui FA. A second source of precursor cells for the developing enteric nervous system and interstitial cells of Cajal. Int J Dev Neurosci. 2002;20:619–26. doi: 10.1016/s0736-5748(02)00103-x. [DOI] [PubMed] [Google Scholar]
- 21.Torihashi S, Ward SM, Sanders KM. Development of c-Kit-positive cells and the onset of electrical rhythmicity in murine small intestine. Gastroenterology. 1997;112:144–55. doi: 10.1016/s0016-5085(97)70229-4. [DOI] [PubMed] [Google Scholar]
- 22.Torihashi S, Ward SM, Nishikawa S, Nishi K, Kobayashi S, Sanders KM. c-kit-dependent development of interstitial cells and electrical activity in the murine gastrointestinal tract. Cell Tissue Res. 1995;280:97–111. doi: 10.1007/BF00304515. [DOI] [PubMed] [Google Scholar]
- 23.Torihashi S, Nishi K, Tokutomi Y, Nishi T, Ward S, Sanders KM. Blockade of kit signaling induces transdifferentiation of interstitial cells of Cajal to a smooth muscle phenotype. Gastroenterology. 1999;117:140–8. doi: 10.1016/s0016-5085(99)70560-3. [DOI] [PubMed] [Google Scholar]
- 24.Komuro T. Comparative morphology of interstitial cells of Cajal: Ultrastructural characterization. Microsc Res Tech. 1999;47:267–85. doi: 10.1002/(SICI)1097-0029(19991115)47:4<267::AID-JEMT5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 25.Huizinga JD, Thuneberg L, Vanderwinden JM, Rumessen JJ. Interstitial cells of Cajal as targets for pharmacological intervention in gastrointestinal motor disorders. Trends Pharmacol Sci. 1997;18:393–403. doi: 10.1016/s0165-6147(97)01108-5. [DOI] [PubMed] [Google Scholar]
- 26.Sanders KM, Ward SM. Kit mutants and gastrointestinal physiology. J Physiol. 2007;578:33–42. doi: 10.1113/jphysiol.2006.122473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin J, Chen JD. Roles of interstitial cells of Cajal in regulating gastrointestinal motility: In vitro versus in vivo studies. J Cell Mol Med. 2008;12:1118–29. doi: 10.1111/j.1582-4934.2008.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanani M, Farrugia G, Komuro T. Intercellular coupling of interstitial cells of Cajal in the digestive tract. Int Rev Cytol. 2005;242:249–82. doi: 10.1016/S0074-7696(04)42006-3. [DOI] [PubMed] [Google Scholar]
- 29.Komuro T. Structure and organization of interstitial cells of Cajal in the gastrointestinal tract. J Physiol. 2006;576:653–8. doi: 10.1113/jphysiol.2006.116624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirst GD, Beckett EA, Sanders KM, Ward SM. Regional variation in contribution of myenteric and intramuscular interstitial cells of Cajal to generation of slow waves in mouse gastric antrum. J Physiol. 2002;540:1003–12. doi: 10.1113/jphysiol.2001.013672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mazet B, Raynier C. Interstitial cells of Cajal in the guinea pig gastric antrum: Distribution and regional density. Cell Tissue Res. 2004;316:23–34. doi: 10.1007/s00441-003-0835-9. [DOI] [PubMed] [Google Scholar]
- 32.Vanderwinden JM, Liu H, De Laet MH, Vanderhaeghen JJ. Study of the interstitial cells of Cajal in infantile hypertrophic pyloric stenosis. Gastroenterology. 1996;111:279–88. doi: 10.1053/gast.1996.v111.pm8690192. [DOI] [PubMed] [Google Scholar]
- 33.Horiguchi K, Semple GS, Sanders KM, Ward SM. Distribution of pacemaker function through the tunica muscularis of the canine gastric antrum. J Physiol. 2001;537:237–50. doi: 10.1111/j.1469-7793.2001.0237k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rumessen JJ, Mikkelsen HB, Qvortrup K, Thuneberg L. Ultrastructure of interstitial cells of Cajal in circular muscle of human small intestine. Gastroenterology. 1993;104:343–50. doi: 10.1016/0016-5085(93)90400-7. [DOI] [PubMed] [Google Scholar]
- 35.Vannucchi MG, De Giorgio R, Faussone-Pellegrini MS. NK1 receptor expression in the interstitial cells of Cajal and neurons and tachykinins distribution in rat ileum during development. J Comp Neurol. 1997;383:153–62. doi: 10.1002/(sici)1096-9861(19970630)383:2<153::aid-cne3>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 36.Patterson LM, Zheng H, Ward SM, Berthoud HR. Immunohistochemical identification of cholecystokinin A receptors on interstitial cells of Cajal, smooth muscle, and enteric neurons in rat pylorus. Cell Tissue Res. 2001;305:11–23. doi: 10.1007/s004410100402. [DOI] [PubMed] [Google Scholar]
- 37.Rømert P, Mikkelsen HB. c-Kit immunoreactive interstitial cells of Cajal in the human small and large intestine. Histochem Cell Biol. 1998;109:195–202. doi: 10.1007/s004180050218. [DOI] [PubMed] [Google Scholar]
- 38.Wang XY, Paterson C, Huizinga JD. Cholinergic and nitrergic innervation of ICC-DMP and ICC-IM in the human small intestine. Neurogastroenterol Motil. 2003;15:531–43. doi: 10.1046/j.1365-2982.2003.00429.x. [DOI] [PubMed] [Google Scholar]
- 39.Vanderwinden JM, De Laet MH, Vanderhaeghen JJ. Distribution of interstitial cells of Cajal in human duodenum and intestine. Neurogastroenterol Motil. 1998;10:435. (Abstract) [Google Scholar]
- 40.Rumessen JJ, Peters S, Thuneberg L. Light- and electron microscopical studies of interstitial cells of Cajal and muscle cells at the submucosal border of human colon. Lab Invest. 1993;68:481–95. [PubMed] [Google Scholar]
- 41.Mazzia C, Porcher C, Julé Y, Christen MO, Henry M. Ultrastructural study of relationships between c-kit immunoreactive interstitial cells and other cellular elements in the human colon. Histochem Cell Biol. 2000;113:401–11. doi: 10.1007/s004180000154. [DOI] [PubMed] [Google Scholar]
- 42.Horisawa M, Watanabe Y, Torihashi S. Distribution of c-Kit immunopositive cells in normal human colon and in Hirschsprung's disease. J Pediatr Surg. 1998;33:1209–14. doi: 10.1016/s0022-3468(98)90152-x. [DOI] [PubMed] [Google Scholar]
- 43.Hagger R, Gharaie S, Finlayson C, Kumar D. Distribution of the interstitial cells of Cajal in the human anorectum. J Auton Nerv Syst. 1998;73:75–9. doi: 10.1016/s0165-1838(98)00038-1. [DOI] [PubMed] [Google Scholar]
- 44.Popescu LM, Hinescu ME, Ionescu N, Ciontea SM, Cretoiu D, Ardelean C. Interstitial cells of Cajal in pancreas. J Cell Mol Med. 2005;9:169–90. doi: 10.1111/j.1582-4934.2005.tb00347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lang RJ, Klemm MF. Interstitial cell of Cajal-like cells in the upper urinary tract. J Cell Mol Med. 2005;9:543–56. doi: 10.1111/j.1582-4934.2005.tb00487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sergeant GP, Hollywood MA, McHale NG, Thornbury KD. Ca2+ signalling in urethral interstitial cells of Cajal. J Physiol. 2006;576:715–20. doi: 10.1113/jphysiol.2006.115956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ciontea SM, Radu E, Regalia T, Ceafalan L, Cretoiu D, Gherghiceanu M, et al. C-kit immunopositive interstitial cells (Cajal-type) in human myometrium. J Cell Mol Med. 2005;9:407–20. doi: 10.1111/j.1582-4934.2005.tb00366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Popescu LM, Gherghiceanu M, Hinescu ME, Cretoiu D, Ceafalan L, Regalia T, et al. Insights into the interstitium of ventricular myocardium: Interstitial Cajal-like cells (ICLC) J Cell Mol Med. 2006;10:429–58. doi: 10.1111/j.1582-4934.2006.tb00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Popescu LM, Ciontea SM, Cretoiu D. Interstitial Cajal-like cells in human uterus and fallopian tube. Ann N Y Acad Sci. 2007;1101:139–65. doi: 10.1196/annals.1389.022. [DOI] [PubMed] [Google Scholar]
- 50.Suciu L, Popescu LM, Gherghiceanu M. Human placenta: De visu demonstration of interstitial Cajal-like cells. J Cell Mol Med. 2007;11:590–7. doi: 10.1111/j.1582-4934.2007.00058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paula JS, Souza ES, Oliveira MB, Rodrigues Mde L, Garcia SB. Is the positive c-kit immunostaining associated with the presence of cells analogous to the intersticial cells of Cajal in the ciliary muscle? Arq Bras Oftalmol. 2009;72:43–6. doi: 10.1590/s0004-27492009000100009. [DOI] [PubMed] [Google Scholar]
- 52.Beaumont W. Experiments and observations on the gastric juice and the physiology of digestion. By William Beaumont. Plattsburgh. Printed by F. P. Allen. 1833. Nutr Rev. 1977;35:144–5. doi: 10.1111/j.1753-4887.1977.tb06570.x. [DOI] [PubMed] [Google Scholar]
- 53.Cannon WB. The movements of the intestines studied by means of the Röntgen rays. J Med Res. 1902;7:72–5. [PMC free article] [PubMed] [Google Scholar]
- 54.Bayliss WM, Starling EH. The movements and innervation of the small intestine. J Physiol. 1899;24:99–143. doi: 10.1113/jphysiol.1899.sp000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cajal SR. Vol. 2. Madrid: Imprenta y librería de Nicolás Moya; 1899. Textura del sistema nervioso del hombre y de los vertebrados; p. 1. [Google Scholar]
- 56.Keith A. A new theory of the causation of enterostasis. Lancet. 1915;2:371–5. [Google Scholar]
- 57.Ambache N. The electrical activity of isolated mammalian intestines. J Physiol. 1947;106:139–53. [PubMed] [Google Scholar]
- 58.Diamant NE, Bortoff A. Nature of the intestinal low-wave frequency gradient. Am J Physiol. 1969;216:301–7. doi: 10.1152/ajplegacy.1969.216.2.301. [DOI] [PubMed] [Google Scholar]
- 59.Szurszewski . Electrophysiological basis of gastrointestinal motility. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. New York: Raven Press; 1987. pp. 383–422. [Google Scholar]
- 60.Huizinga JD, Lammers WJ. Gut peristalsis is governed by a multitude of cooperating mechanisms. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1–8. doi: 10.1152/ajpgi.90380.2008. [DOI] [PubMed] [Google Scholar]
- 61.Thuneberg L, Johansen V, Rumessen JJ, Andersen BG. Interstitial cells of cajal ICC: Selective uptake of methylene blue inhibits slow wave activity. In: Roman C, editor. Gastrointestinal Motility E. (Lancaster, PA): MTP Press; 1983. pp. 495–502. [Google Scholar]
- 62.Ward SM, Burke EP, Sanders KM. Use of rhodamine 123 to label and lesion interstitial cells of Cajal in canine colonic circular muscle. Anat Embryol (Berl) 1990;182:215–24. doi: 10.1007/BF00185515. [DOI] [PubMed] [Google Scholar]
- 63.Ordög T, Ward SM, Sanders KM. Interstitial cells of Cajal generate electrical slow waves in the murine stomach. J Physiol. 1999;518:257–69. doi: 10.1111/j.1469-7793.1999.0257r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koh SD, Sanders KM, Ward SM. Spontaneous electrical rhythmicity in cultured interstitial cells of Cajal from the murine small intestine. J Physiol. 1998;513:203–13. doi: 10.1111/j.1469-7793.1998.203by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Suzuki N, Prosser CL, Dahms V. Boundary cells between longitudinal and circular layers: Essential for electrical slow waves in cat intestine. Am J Physiol. 1986;250:G287–94. doi: 10.1152/ajpgi.1986.250.3.G287. [DOI] [PubMed] [Google Scholar]
- 66.Smith TK, Reed JB, Sanders KM. Interaction of two electrical pacemakers in muscularis of canine proximal colon. Am J Physiol. 1987;252:C290–9. doi: 10.1152/ajpcell.1987.252.3.C290. [DOI] [PubMed] [Google Scholar]
- 67.Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 1994;480:91–7. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huizinga JD, Thuneberg L, Klüppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–9. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- 69.Burns AJ, Lomax AE, Torihashi S, Sanders KM, Ward SM. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc Natl Acad Sci U S A. 1996;93:12008–13. doi: 10.1073/pnas.93.21.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Horiguchi K, Komuro T. Ultrastructural characterization of interstitial cells of Cajal in the rat small intestine using control and Ws/Ws mutant rats. Cell Tissue Res. 1998;293:277–84. doi: 10.1007/s004410051119. [DOI] [PubMed] [Google Scholar]
- 71.Ward SM, Burns AJ, Torihashi S, Harney SC, Sanders KM. Impaired development of interstitial cells and intestinal electrical rhythmicity in steel mutants. Am J Physiol. 1995;269:C1577–85. doi: 10.1152/ajpcell.1995.269.6.C1577. [DOI] [PubMed] [Google Scholar]
- 72.Der-Silaphet T, Malysz J, Hagel S, Larry Arsenault A, Huizinga JD. Interstitial cells of Cajal direct normal propulsive contractile activity in the mouse small intestine. Gastroenterology. 1998;114:724–36. doi: 10.1016/s0016-5085(98)70586-4. [DOI] [PubMed] [Google Scholar]
- 73.Ishii S, Tsuji S, Tsujii M, Nishida T, Watabe K, Iijima H, et al. Restoration of gut motility in Kit-deficient mice by bone marrow transplantation. J Gastroenterol. 2009;44:834–41. doi: 10.1007/s00535-009-0077-z. [DOI] [PubMed] [Google Scholar]
- 74.Albertí E, Mikkelsen HB, Wang XY, Díaz M, Larsen JO, Huizinga JD, et al. Pacemaker activity and inhibitory neurotransmission in the colon of Ws/Ws mutant rats. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1499–510. doi: 10.1152/ajpgi.00136.2006. [DOI] [PubMed] [Google Scholar]
- 75.Hou X, Yin J, Liu J, Pasricha PJ, Chen JD. In vivo gastric and intestinal slow waves in W/WV mice. Dig Dis Sci. 2005;50:1335–41. doi: 10.1007/s10620-005-2783-6. [DOI] [PubMed] [Google Scholar]
- 76.Malysz J, Thuneberg L, Mikkelsen HB, Huizinga JD. Action potential generation in the small intestine of W mutant mice that lack interstitial cells of Cajal. Am J Physiol. 1996;271:G387–99. doi: 10.1152/ajpgi.1996.271.3.G387. [DOI] [PubMed] [Google Scholar]
- 77.Yin J, Hou X, Chen JD. Roles of interstitial cells of Cajal in intestinal transit and exogenous electrical pacing. Dig Dis Sci. 2006;51:1818–23. doi: 10.1007/s10620-006-9313-z. [DOI] [PubMed] [Google Scholar]
- 78.Edwards FR, Hirst GD, Suzuki H. Unitary nature of regenerative potentials recorded from circular smooth muscle of guinea-pig antrum. J Physiol. 1999;519:235–50. doi: 10.1111/j.1469-7793.1999.0235o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van Helden DF, Imtiaz MS, Nurgaliyeva K, von der Weid P, Dosen PJ. Role of calcium stores and membrane voltage in the generation of slow wave action potentials in guinea-pig gastric pylorus. J Physiol. 2000;524:245–65. doi: 10.1111/j.1469-7793.2000.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hashitani H, Garcia-Londoño AP, Hirst GD, Edwards FR. Atypical slow waves generated in gastric corpus provide dominant pacemaker activity in guinea pig stomach. J Physiol. 2005;569:459–65. doi: 10.1113/jphysiol.2005.097907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huizinga JD, Zhu Y, Ye J, Molleman A. High-conductance chloride channels generate pacemaker currents in interstitial cells of Cajal. Gastroenterology. 2002;123:1627–36. doi: 10.1053/gast.2002.36549. [DOI] [PubMed] [Google Scholar]
- 82.Zhu Y, Golden CM, Ye J, Wang XY, Akbarali HI, Huizinga JD. ERG K+ currents regulate pacemaker activity in ICC. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1249–58. doi: 10.1152/ajpgi.00149.2003. [DOI] [PubMed] [Google Scholar]
- 83.Torihashi S, Fujimoto T, Trost C, Nakayama S. Calcium oscillation linked to pacemaking of interstitial cells of Cajal: Requirement of calcium influx and localization of TRP4 in caveolae. J Biol Chem. 2002;277:19191–7. doi: 10.1074/jbc.M201728200. [DOI] [PubMed] [Google Scholar]
- 84.Sanders KM, Koh SD, Ward SM. Interstitial cells of Cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006;68:307–43. doi: 10.1146/annurev.physiol.68.040504.094718. [DOI] [PubMed] [Google Scholar]
- 85.Bayguinov O, Ward SM, Kenyon JL, Sanders KM. Voltage-gated Ca2+ currents are necessary for slow-wave propagation in the canine gastric antrum. Am J Physiol Cell Physiol. 2007;293:C1645–59. doi: 10.1152/ajpcell.00165.2007. [DOI] [PubMed] [Google Scholar]
- 86.Cousins HM, Edwards FR, Hickey H, Hill CE, Hirst GD. Electrical coupling between the myenteric interstitial cells of Cajal and adjacent muscle layers in the guinea-pig gastric antrum. J Physiol. 2003;550:829–44. doi: 10.1113/jphysiol.2003.042176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kito Y, Suzuki H. Properties of pacemaker potentials recorded from myenteric interstitial cells of Cajal distributed in the mouse small intestine. J Physiol. 2003;553:803–18. doi: 10.1113/jphysiol.2003.051334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hennig GW, Hirst GD, Park KJ, Smith CB, Sanders KM, Ward SM, et al. Propagation of pacemaker activity in the guinea-pig antrum. J Physiol. 2004;556:585–99. doi: 10.1113/jphysiol.2003.059055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Berezin I, Huizinga JD, Daniel EE. Interstitial cells of Cajal in the canine colon: A special communication network at the inner border of the circular muscle. J Comp Neurol. 1988;273:42–51. doi: 10.1002/cne.902730105. [DOI] [PubMed] [Google Scholar]
- 90.Thuneberg L. Interstitial cells of Cajal: Intestinal pacemaker cells? Adv Anat Embryol Cell Biol. 1982;71:1–130. [PubMed] [Google Scholar]
- 91.Liu LW, Farraway L, Berezin I, Huizinga JD. Interstitial cells of Cajal: Mediators of communication between circular and longitudinal muscle layers of canine colon. Cell Tissue Res. 1998;294:69–79. doi: 10.1007/s004410051157. [DOI] [PubMed] [Google Scholar]
- 92.Vigmond EJ, Bardakjian BL, Thuneberg L, Huizinga JD. Intercellular coupling mediated by potassium accumulation in peg-and-socket junctions. IEEE Trans Biomed Eng. 2000;47:1576–83. doi: 10.1109/10.887938. [DOI] [PubMed] [Google Scholar]
- 93.Sarna SK. Are interstitial cells of Cajal plurifunction cells in the gut? Am J Physiol Gastrointest Liver Physiol. 2008;294:G372–90. doi: 10.1152/ajpgi.00344.2007. [DOI] [PubMed] [Google Scholar]
- 94.Daniel EE, Posey-Daniel V. Neuromuscular structures in opossum esophagus: Role of interstitial cells of Cajal. Am J Physiol. 1984;246:G305–15. doi: 10.1152/ajpgi.1984.246.3.G305. [DOI] [PubMed] [Google Scholar]
- 95.Wang XY, Sanders KM, Ward SM. Intimate relationship between interstitial cells of Cajal and enteric nerves in the guinea-pig small intestine. Cell Tissue Res. 1999;295:247–56. doi: 10.1007/s004410051231. [DOI] [PubMed] [Google Scholar]
- 96.Ward SM. Interstitial cells of Cajal in enteric neurotransmission. Gut. 2000;47:iv40–3. doi: 10.1136/gut.47.suppl_4.iv40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Beckett EA, Horiguchi K, Khoyi M, Sanders KM, Ward SM. Loss of enteric motor neurotransmission in the gastric fundus of Sl/Sl (d) mice. J Physiol. 2002;543:871–87. doi: 10.1113/jphysiol.2002.021915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lavin ST, Southwell BR, Murphy R, Jenkinson KM, Furness JB. Activation of neurokinin 1 receptors on interstitial cells of Cajal of the guinea-pig small intestine by substance P. Histochem Cell Biol. 1998;110:263–71. doi: 10.1007/s004180050288. [DOI] [PubMed] [Google Scholar]
- 99.Iino S, Ward SM, Sanders KM. Interstitial cells of Cajal are functionally innervated by excitatory motor neurones in the murine intestine. J Physiol. 2004;556:521–30. doi: 10.1113/jphysiol.2003.058792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Garcia-Lopez P, Garcia-Marin V, Martínez-Murillo R, Freire M. Updating old ideas and recent advances regarding the interstitial cells of Cajal. Brain Res Rev. 2009;61:154–69. doi: 10.1016/j.brainresrev.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 101.Ward SM, Morris G, Reese L, Wang XY, Sanders KM. Interstitial cells of Cajal mediate enteric inhibitory neurotransmission in the lower esophageal and pyloric sphincters. Gastroenterology. 1998;115:314–29. doi: 10.1016/s0016-5085(98)70198-2. [DOI] [PubMed] [Google Scholar]
- 102.Ward SM, Beckett EA, Wang X, Baker F, Khoyi M, Sanders KM. Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J Neurosci. 2000;20:1393–403. doi: 10.1523/JNEUROSCI.20-04-01393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ward SM, McLaren GJ, Sanders KM. Interstitial cells of Cajal in the deep muscular plexus mediate enteric motor neurotransmission in the mouse small intestine. J Physiol. 2006;573:147–59. doi: 10.1113/jphysiol.2006.105189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dixit D, Zarate N, Liu LW, Boreham DR, Huizinga JD. Interstitial cells of Cajal and adaptive relaxation in the mouse stomach. Am J Physiol Gastrointest Liver Physiol. 2006;291:G1129–36. doi: 10.1152/ajpgi.00518.2005. [DOI] [PubMed] [Google Scholar]
- 105.Sivarao DV, Mashimo HL, Thatte HS, Goyal RK. Lower esophageal sphincter is achalasic in nNOS(-/-) and hypotensive in W/W (v) mutant mice. Gastroenterology. 2001;121:34–42. doi: 10.1053/gast.2001.25541. [DOI] [PubMed] [Google Scholar]
- 106.Zhang Y, Carmichael SA, Wang XY, Huizinga JD, Paterson WG. Neurotransmission in lower esophageal sphincter of W/Wv mutant mice. Am J Physiol Gastrointest Liver Physiol. 2010;298:G14–24. doi: 10.1152/ajpgi.00266.2009. [DOI] [PubMed] [Google Scholar]
- 107.Okishio Y, Takeuchi T, Fujita A, Suenaga K, Fujinami K, Munakata S, et al. Ascending contraction and descending relaxation in the distal colon of mice lacking interstitial cells of Cajal. J Smooth Muscle Res. 2005;41:163–74. doi: 10.1540/jsmr.41.163. [DOI] [PubMed] [Google Scholar]
- 108.Terauchi A, Kobayashi D, Mashimo H. Distinct roles of nitric oxide synthases and interstitial cells of Cajal in rectoanal relaxation. Am J Physiol Gastrointest Liver Physiol. 2005;289:G291–9. doi: 10.1152/ajpgi.00005.2005. [DOI] [PubMed] [Google Scholar]
- 109.de Lorijn F, de Jonge WJ, Wedel T, Vanderwinden JM, Benninga MA, Boeckxstaens GE. Interstitial cells of Cajal are involved in the afferent limb of the rectoanal inhibitory reflex. Gut. 2005;54:1107–13. doi: 10.1136/gut.2004.051045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Beckett EA, Takeda Y, Yanase H, Sanders KM, Ward SM. Synaptic specializations exist between enteric motor nerves and interstitial cells of Cajal in the murine stomach. J Comp Neurol. 2005;493:193–206. doi: 10.1002/cne.20746. [DOI] [PubMed] [Google Scholar]
- 111.Vohra BP, Tsuji K, Nagashimada M, Uesaka T, Wind D, Fu M, et al. Differential gene expression and functional analysis implicate novel mechanisms in enteric nervous system precursor migration and neuritogenesis. Dev Biol. 2006;298:259–71. doi: 10.1016/j.ydbio.2006.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Farrugia G, Lei S, Lin X, Miller SM, Nath KA, Ferris CD, et al. A major role for carbon monoxide as an endogenous hyperpolarizing factor in the gastrointestinal tract. Proc Natl Acad Sci U S A. 2003;100:8567–70. doi: 10.1073/pnas.1431233100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Won KJ, Sanders KM, Ward SM. Interstitial cells of Cajal mediate mechanosensitive responses in the stomach. Proc Natl Acad Sci U S A. 2005;102:14913–8. doi: 10.1073/pnas.0503628102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Strege PR, Ou Y, Sha L, Rich A, Gibbons SJ, Szurszewski JH, et al. Sodium current in human intestinal interstitial cells of Cajal. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1111–21. doi: 10.1152/ajpgi.00152.2003. [DOI] [PubMed] [Google Scholar]
- 115.Langer JC, Berezin I, Daniel EE. Hypertrophic pyloric stenosis: Ultrastructural abnormalities of enteric nerves and the interstitial cells of Cajal. J Pediatr Surg. 1995;30:1535–43. doi: 10.1016/0022-3468(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 116.Sifrim D, Janssens J, Vantrappen G. Failing deglutitive inhibition in primary esophageal motility disorders. Gastroenterology. 1994;106:875–82. doi: 10.1016/0016-5085(94)90745-5. [DOI] [PubMed] [Google Scholar]
- 117.Storch WB, Eckardt VF, Wienbeck M, Eberl T, Auer PG, Hecker A, et al. Autoantibodies to Auerbach's plexus in achalasia. Cell Mol Biol (Noisy-le-grand) 1995;41:1033–8. [PubMed] [Google Scholar]
- 118.Goldblum JR, Whyte RI, Orringer MB, Appelman HD. Achalasia. A morphologic study of 42 resected specimens. Am J Surg Pathol. 1994;18:327–37. [PubMed] [Google Scholar]
- 119.Faussone-Pellegrini MS, Cortesini C. The muscle coat of the lower esophageal sphincter in patients with achalasia and hypertensive sphincter.An electron microscopic study. J Submicrosc Cytol. 1985;17:673–85. [PubMed] [Google Scholar]
- 120.Khelif K, De Laet MH, Chaouachi B, Segers V, Vanderwinden JM. Achalasia of the cardia in Allgrove's (triple A) syndrome: Histopathologic study of 10 cases. Am J Surg Pathol. 2003;27:667–72. doi: 10.1097/00000478-200305000-00010. [DOI] [PubMed] [Google Scholar]
- 121.Richter JE. Gastrooesophageal reflux disease. Best Pract Res Clin Gastroenterol. 2007;21:609–31. doi: 10.1016/j.bpg.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 122.Shafik A, El-Sibai O, Shafik I, Shafik A. Electroesophagogram in gastroesophageal reflux disease with a new theory on the pathogenesis of its electric changes. BMC Surg. 2004;4:13. doi: 10.1186/1471-2482-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen JD, Schirmer BD, McCallum RW. Serosal and cutaneous recordings of gastric myoelectrical activity in patients with gastroparesis. Am J Physiol. 1994;266:G90–8. doi: 10.1152/ajpgi.1994.266.1.G90. [DOI] [PubMed] [Google Scholar]
- 124.Lin X, Chen JZ. Abnormal gastric slow waves in patients with functional dyspepsia assessed by multichannel electrogastrography. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1370–5. doi: 10.1152/ajpgi.2001.280.6.G1370. [DOI] [PubMed] [Google Scholar]
- 125.Zárate N, Mearin F, Wang XY, Hewlett B, Huizinga JD, Malagelada JR. Severe idiopathic gastroparesis due to neuronal and interstitial cells of Cajal degeneration: Pathological findings and management. Gut. 2003;52:966–70. doi: 10.1136/gut.52.7.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sigurdsson L, Flores A, Putnam PE, Hyman PE, Di Lorenzo C. Postviral gastroparesis: Presentation, treatment, and outcome. J Pediatr. 1997;131:751–4. doi: 10.1016/s0022-3476(97)70106-9. [DOI] [PubMed] [Google Scholar]
- 127.Camilleri M. Advances in diabetic gastroparesis. Rev Gastroenterol Disord. 2002;2:47–56. [PubMed] [Google Scholar]
- 128.He CL, Soffer EE, Ferris CD, Walsh RM, Szurszewski JH, Farrugia G. Loss of interstitial cells of Cajal and inhibitory innervation in insulin-dependent diabetes. Gastroenterology. 2001;121:427–34. doi: 10.1053/gast.2001.26264. [DOI] [PubMed] [Google Scholar]
- 129.Nakahara M, Isozaki K, Hirota S, Vanderwinden JM, Takakura R, Kinoshita K, et al. Deficiency of KIT-positive cells in the colon of patients with diabetes mellitus. J Gastroenterol Hepatol. 2002;17:666–70. doi: 10.1046/j.1440-1746.2002.02756.x. [DOI] [PubMed] [Google Scholar]
- 130.De Giorgio R, Sarnelli G, Corinaldesi R, Stanghellini V. Advances in our understanding of the pathology of chronic intestinal pseudo-obstruction. Gut. 2004;53:1549–52. doi: 10.1136/gut.2004.043968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Coulie B, Camilleri M. Intestinal pseudo-obstruction. Annu Rev Med. 1999;50:37–55. doi: 10.1146/annurev.med.50.1.37. [DOI] [PubMed] [Google Scholar]
- 132.Jain D, Moussa K, Tandon M, Culpepper-Morgan J, Proctor DD. Role of interstitial cells of Cajal in motility disorders of the bowel. Am J Gastroenterol. 2003;98:618–24. doi: 10.1111/j.1572-0241.2003.07295.x. [DOI] [PubMed] [Google Scholar]
- 133.Streutker CJ, Huizinga JD, Campbell F, Ho J, Riddell RH. Loss of CD117 (c-kit)- and CD34-positive ICC and associated CD34-positive fibroblasts defines a subpopulation of chronic intestinal pseudo-obstruction. Am J Surg Pathol. 2003;27:228–35. doi: 10.1097/00000478-200302000-00012. [DOI] [PubMed] [Google Scholar]
- 134.Boeckxstaens GE, Rumessen JJ, de Wit L, Tytgat GN, Vanderwinden JM. Abnormal distribution of the interstitial cells of Cajal in an adult patient with pseudo-obstruction and megaduodenum. Am J Gastroenterol. 2002;97:2120–6. doi: 10.1111/j.1572-0241.2002.05932.x. [DOI] [PubMed] [Google Scholar]
- 135.Shafik A, Shafik AA, El-Sibai O, Mostafa RM. Electric activity of the colon in subjects with constipation due to total colonic inertia: An electrophysiologic study. Arch Surg. 2003;138:1007–11. doi: 10.1001/archsurg.138.9.1007. [DOI] [PubMed] [Google Scholar]
- 136.Kubota M, Ito Y, Ikeda K. Membrane properties and innervation of smooth muscle cells in Hirschsprung's disease. Am J Physiol. 1983;244:G406–15. doi: 10.1152/ajpgi.1983.244.4.G406. [DOI] [PubMed] [Google Scholar]
- 137.Yamataka A, Kato Y, Tibboel D, Murata Y, Sueyoshi N, Fujimoto T, et al. A lack of intestinal pacemaker (c-kit) in aganglionic bowel of patients with Hirschsprung's disease. J Pediatr Surg. 1995;30:441–4. doi: 10.1016/0022-3468(95)90051-9. [DOI] [PubMed] [Google Scholar]
- 138.Rolle U, Piotrowska AP, Nemeth L, Puri P. Altered distribution of interstitial cells of Cajal in Hirschsprung disease. Arch Pathol Lab Med. 2002;126:928–33. doi: 10.5858/2002-126-0928-ADOICO. [DOI] [PubMed] [Google Scholar]
- 139.Newman CJ, Laurini RN, Lesbros Y, Reinberg O, Meyrat BJ. Interstitial cells of Cajal are normally distributed in both ganglionated and aganglionic bowel in Hirschsprung's disease. Pediatr Surg Int. 2003;19:662–8. doi: 10.1007/s00383-003-1026-1. [DOI] [PubMed] [Google Scholar]
- 140.Levitt MA, Peña A. Anorectal malformations. Orphanet J Rare Dis. 2007;2:33. doi: 10.1186/1750-1172-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kenny SE, Connell MG, Rintala RJ, Vaillant C, Edgar DH, Lloyd DA. Abnormal colonic interstitial cells of Cajal in children with anorectal malformations. J Pediatr Surg. 1998;33:130–2. doi: 10.1016/s0022-3468(98)90379-7. [DOI] [PubMed] [Google Scholar]
- 142.Macedo M, Martins JL, Meyer KF, Soares IC. Study of density of interstitial cells of Cajal in the terminal intestine of rats with anorectal malformation. Eur J Pediatr Surg. 2008;18:75–9. doi: 10.1055/s-2008-1038482. [DOI] [PubMed] [Google Scholar]
- 143.Huizinga JD, Zarate N, Farrugia G. Physiology, injury, and recovery of interstitial cells of Cajal: Basic and clinical science. Gastroenterology. 2009;137:1548–56. doi: 10.1053/j.gastro.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Faussone-Pellegrini MS, Vannucchi MG, Ledder O, Huang TY, Hanani M. Plasticity of interstitial cells of Cajal: A study of mouse colon. Cell Tissue Res. 2006;325:211–7. doi: 10.1007/s00441-006-0174-8. [DOI] [PubMed] [Google Scholar]
- 145.Gibbons S, De Giorgio R, Garrity M. Increased numbers of activated caspase-3 immunoreactive interstitial cells of Cajal in the colon of patients with slow-transit constipation. Neurogastroenterol Motil. 2005;17:6. [Google Scholar]
- 146.Redelman DL, Horvath VJ, Bardsley MR, Chen H, Ördög T. Turnover of interstitial cells of Cajal in the murine stomach: regeneration from local progenitors and limited survival after loss of electrical pacemaker activity and kit expression. Gastroenterology. 2006;130:A–538. [Google Scholar]
- 147.Choi KM, Gibbons SJ, Roeder JL, Lurken MS, Zhu J, Wouters MM, et al. Regulation of interstitial cells of Cajal in the mouse gastric body by neuronal nitric oxide. Neurogastroenterol Motil. 2007;19:585–95. doi: 10.1111/j.1365-2982.2007.00936.x. [DOI] [PubMed] [Google Scholar]
- 148.Wouters MM, Gibbons SJ, Roeder JL, Distad M, Ou Y, Strege PR, et al. Exogenous serotonin regulates proliferation of interstitial cells of Cajal in mouse jejunum through 5-HT2B receptors. Gastroenterology. 2007;133:897–906. doi: 10.1053/j.gastro.2007.06.017. [DOI] [PubMed] [Google Scholar]


