Abstract
Significance: Severe life stress (SLS), as opposed to trivial everyday stress, is defined as a serious psychosocial event with the potential of causing an impacting psychological traumatism. Recent Advances: Numerous studies have attempted to understand how the central nervous system (CNS) responds to SLS. This response includes a variety of morphological and neurochemical modifications; among them, oxidative stress is almost invariably observed. Oxidative stress is defined as disequilibrium between oxidant generation and the antioxidant response. Critical Issues: In this review, we discuss how SLS leads to oxidative stress in the CNS, and how the latter impacts pathophysiological outcomes. We also critically discuss experimental methods that measure oxidative stress in the CNS. The review covers animal models and human observations. Animal models of SLS include sleep deprivation, maternal separation, and social isolation in rodents, and the establishment of hierarchy in non-human primates. In humans, SLS, which is caused by traumatic events such as child abuse, war, and divorce, is also accompanied by oxidative stress in the CNS. Future Directions: The outcome of SLS in humans ranges from resilience, over post-traumatic stress disorder, to development of chronic mental disorders. Defining the sources of oxidative stress in SLS might in the long run provide new therapeutic avenues. Antioxid. Redox Signal. 18, 1475–1490.
Introduction
“Stress” is a general term that was first employed in a biological context by the endocrinologist Hans Selye in 1936 to describe the inadequate physiological response of an organism, human or other animal, to any mental, emotional, or physical demands, whether actual or imagined (148). This response follows a typical three-stage pattern that is common in human and animals, known as general adaptation syndrome (147): (i) the alarm reaction: the body initially defends itself against adverse circumstances by activating the sympathetic nervous system. It mobilizes the body for the “fight or flight” response, which can be seen phylogenetically as an adaptive short-term reaction to emergency situations. In many cases, the stress episode is mastered during the alarm reaction stage; (ii) the resistance stage: the body adapts more or less successfully to the stressor; (iii) the exhaustion stage: the organism's adaptation is depleted or becomes detrimental, and a breakdown occurs; this is associated with illness, burnout, depression, or even death (147).
Oxidative stress has been implicated in the response to stress (136) and in the pathogenesis of neurologic and psychiatric diseases (155). Production of reactive oxygen species (ROS) by mitochondria is often considered the main cause of oxidative stress, but other sources of ROS are emerging, in particular, NADPH oxidase (NOX) enzymes (90), a family of membrane proteins with a sole known function to generate ROS. They function as a transmembrane electron transport chain using cytoplasmic NADPH as an electron donor to molecular O2 to generate superoxide anion in the extracellular space or in the lumen of intracellular organelles. Superoxide anion is generally considered the primary product of the electron transfer, but other ROS, in particular hydrogen peroxide, are also generated (10). Seven NOX genes have been identified: NOX1 to 5 and DUOX1 and 2. The best described isoform NOX2 requires an interaction with another trans-membrane protein, p22phox, as well as the cytosolic subunits, p47phox, p67phox, p40phox, and one of the small Rho GTP-binding proteins, Rac1 or 2. Other NOX isoforms require p22phox for activity, but have a different mechanism of activation. NOX1 and NOX3 enzymes require interaction with cytosolic subunits, NOXA1 and NOXO1, and with Rac1 or 2. (73). In contrast, NOX4 seems to be constitutively active; NOX5 and DUOX enzymes are mostly regulated by increased intracellular Ca2+ (10).
NOX enzymes are widely distributed in a variety of tissues, but very high expression levels can be found in specific organs or cell types (e.g., NOX1 in the colon, NOX2 in phagocytes, NOX3 in the inner ear, and NOX4 in the kidney) (10).
The presence of NOX1, NOX2, NOX3, and NOX4 transcripts has been identified in total brain samples (9, 79, 171) and in specific brain cellular subpopulations. In particular, it appears that NOX1, NOX2, and NOX4 are present in neurons, astrocytes, and microglia, whereas the localization of NOX3 is not known (155).
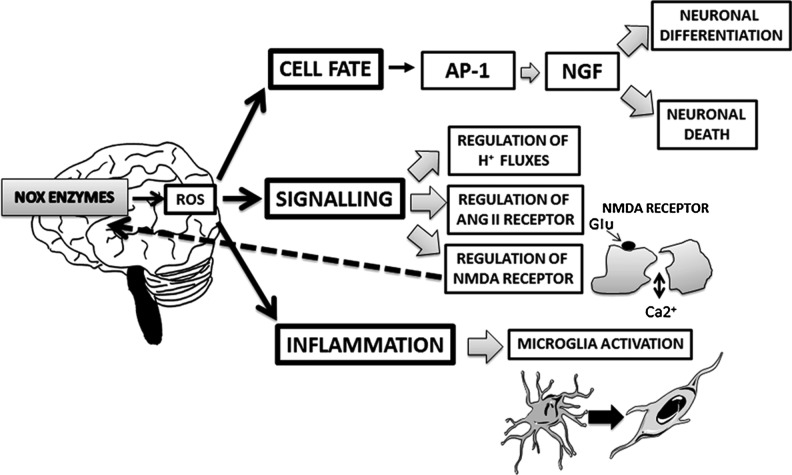
ROS produced by NOX enzymes can directly influence cellular functions, by inducing the oxidation of proteins and, subsequently, their structural and functional changes. In the CNS, NOX enzymes are key players of several physiological functions (such as neuronal differentiation and signaling) (155) (Fig. 1), but they also contribute to the development of neurodegenerative and psychiatric diseases (11, 146, 155).
FIG. 1.
Physiological roles of NOX-derived ROS in the CNS. NOX-derived ROS play key physiological functions in the CNS. They regulate neuronal fate (differentiation and death) through the activation of the AP-1 transcription factor, which, in turn, mediates the NGF pathways. They are also involved in crucial signaling pathways in the CNS, such as the regulation of the membrane potential and cellular H+ fluxes, the control of the cardiovascular homeostasis and blood pressure through the angiotensin II (ANG II) receptor, and the regulation of the glutamatergic neurotransmission through the NMDA receptor. Two possible connections exist between NOX enzymes and this receptor: NOX enzymes may regulate the receptor and, in turn, the NMDA receptor may regulate these enzymes. NOX function in microglia is important for health and normal physiology of the CNS. Thus, NOX enzymes are also involved in the neuroinflammatory response through the activation of microglia. CNS, central nervous system; ROS, reactive oxygen species; AP-1, activator protein 1; NGF, nerve growth factor; NMDA, N-methyl-d-aspartate.
Since the biological definition of stress proposed by Selye covers an enormous number of concepts, it is useful to make a distinction between the psychological definition of daily life stress and severe life stress. Daily life stress refers to several minor stressors such as time pressures, minor family problems, and professional misunderstandings or noisy neighbors. Although this type of stress may induce a temporary sense of frustration, miscontent, and irritability associated with physical symptoms (i.e., headache), it is generally successfully managed. On the other hand, a life stress is defined as severe “when it is able to produce severe strain” (29). Exposure to SLS may be acute, leading to short-lasting serious psychological reactions such as concentration problems, depression, and nervous breakdown. If exposure to SLS becomes long-lasting, stress-induced psychological disorders may turn into a chronic prolonged state that may even lead to psychiatric disorders in extreme cases (113). In this review, we will solely focus on SLS.
The Central Role of the CNS in Response to SLS
SLS induces alterations in the hypothalamic–pituitary–adrenal axis functioning
The main neuroendocrine system to be activated in response to SLS is the hypothalamic–pituitary–adrenal (HPA) axis (39).
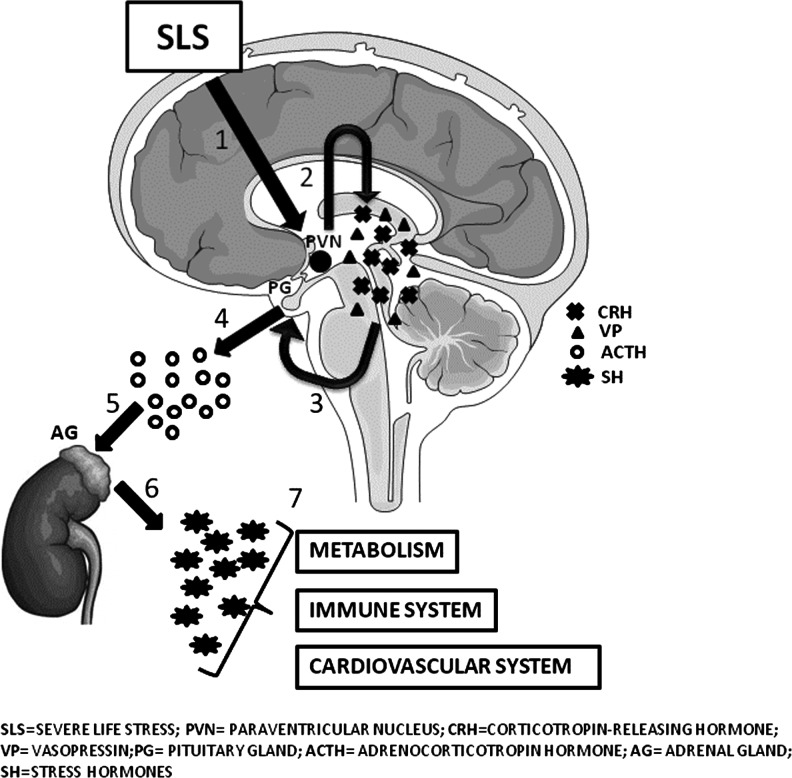
Physiologically, the HPA axis regulates diverse body functions (such as digestion, sexual behavior, etc.) and controls the reactions to stress. Anatomically, the key element of this axis is the paraventricular nucleus of the hypothalamus, which contains neuroendocrine neurons synthesizing and secreting vasopressin and corticotropin-releasing hormone (CRH). These two peptides mainly act on the anterior lobe of the pituitary gland, stimulating the secretion of the adrenocorticotropic hormone (ACTH) (69). ACTH, in turn, acts on the adrenal cortices, which respond to ACTH stimulation producing glucocorticoid hormones (mainly cortisol in humans) (Fig. 2). Cortisol is considered the main stress hormone that targets a variety of both peripheral and central systems, including metabolic, cardiovascular, and immune responses (104).
FIG. 2.
The HPA-axis functioning. After an SLS, an increase in the production of CRH and VP by the paraventricular nucleus of the hypothalamus occurs. These two peptides mainly act on the anterior lobe of the PG, stimulating the secretion of the ACTH, which, in turn, acts on the cortices of adrenal glands. The main consequence of the stimulation of the adrenal cortices is the production of SH (mainly cortisol in humans), which is a key player in the regulation of metabolism (in particular, lipidic and glicemic metabolic pathways), the immune system, and cardiovascular functions. ACTH, adrenocorticotropic hormone; CRH, corticotropin-releasing hormone; HPA, hypothalamic–pituitary–adrenal; SLS, severe life stress; VP, vasopressin.
Increasing evidence exists for a link between the HPA axis and oxidative stress (33). However, the molecular relationship between these elements needs to be clarified. Indeed, it is possible that the impact of corticosteroids on NOX-dependent ROS production is cell-type dependent (Table 1). In phagocytes, an inhibitory effect of the HPA axis on the NADPH-derived oxidative stress has been reported. Thus, hydrocortisone has been shown to inhibit the generation of NOX-derived superoxide and to suppress the expression of the p47phox subunit (78, 117, 169). Similarly, in human aortic smooth muscle cells, an inhibitory effect of glucocorticoids on superoxide production and on the mRNA expression of the p22phox subunit has been described in (111). In microglia, dexamethasone inhibits the NOX-dependent ROS production (30, 76), probably via the suppression of MKP-1-dependent MAPK pathways (76). In hippocampal neurons, both inhibition and induction of glucocorticoids on NOX-dependent ROS production have been reported (84, 182).
Table 1.
Effects of Corticosteroids on Reactive Oxygen Species Production
| Cell type | Compound | Effect | Reference |
|---|---|---|---|
| Phagocytes | Hydrocortisone | Inhibition of NOX-derived superoxide; Suppression of the expression of p47phox | Umeki and Soejima (169); Dandona and coworkers (117); Ignacchiti et al. (78) |
| Human aortic smooth muscle cells | Glucocorticoids | Inhibition of NOX-derived superoxide; Suppression of the expression of p22phox | Marumo et al. (111) |
| Microglia | Dexamethasone | Inhibition of NOX-dependent ROS production; Suppression of MKP-1-dependent MAPK pathways | Huo et al. (76); Condino-Neto et al. (30) |
| Neurons (hippocampus) | Glucocorticoids | Inhibition of NOX-dependent ROS production; Induction of NOX-dependent ROS production | You et al. (182); Kawakami-Mori et al. (84) |
ROS, reactive oxygen species; NOX, NADPH oxidase.
SLS-induced mental disorders
Studies of extreme situations provide strong evidence of the general relationship between SLS and mental illness (131) (Fig. 3). Historically, research on health effects of SLS began with clinical records of individual reactions to war. Disasters unrelated to war have been investigated by psychologists since the 1960s. Thus, in 1963, Langner published a large-scale investigation of mental disorders (both treated and untreated) among 1660 adults in an urban community (93). In this fascinating work, the effects on children and parent mental health of some stressful factors (such as poor physical health, economic deprivation, work worries, and parents' quarrel) were investigated. However, these factors are not unequivocally leading to mental disease. Indeed in the context of SLS-induced mental disorders, resilience is of primary importance. Fundamentally, resilience refers to the positive adaptation or the ability to maintain or regain mental health, despite experiencing severe adversity (70). In short, the term resilience refers to why and how some individuals withstand SLS without developing mental illness. This can be explained by diversity among individuals in term of biological and genetic factors (28, 52, 82) along with environmental factors (70). In the context of these diversities, it is crucial to understand the physiological and biochemical mechanisms underlying resilience.
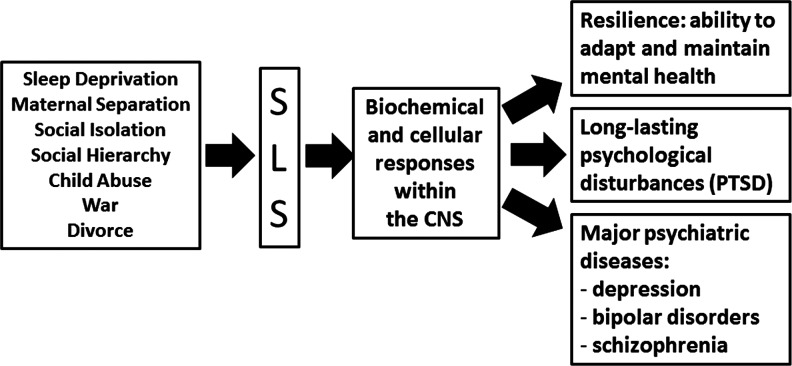
FIG. 3.
The link between SLS and mental disorders. Several external events (such as sleep deprivation, maternal separation and social isolation in rodents, the establishment of hierarchy in non-human primates, child abuse, and war and divorce in humans) might represent an SLS. The CNS reacts immediately to SLS with specific biochemical and cellular responses, which, most of the time, lead to resilience, for example, adaptation to the SLS. However, in some cases, resilience does not occur, and SLS has a broad range of psychological consequences, ranging from long-lasting psychological disturbances (such as PTSD) to major psychiatric diseases (such as depression, bipolar disorders, and schizophrenia). PTSD, post-traumatic stress disorders.
“Post-traumatic stress disorders” (PTSD) are the most studied SLS-induced mental disorders. PTSD can have multiple origins, occurring as psychological consequences of physical, emotional, or sexual abuse (50). Another disorder often induced by SLS is major depression (58). SLS, which are known to induce depression, are caused due to the loss of a job or the death of a loved one (159). SLS, such as early maternal separation, maternal neglecting, prolonged loneliness, and social isolation, may induce psychotic symptoms, such as schizophrenia and bipolar disorders (110, 145).
SLS and alterations of brain morphology
Brain plasticity and morphology can be modified by SLS (112). Numerous rodent studies have shown morphological alterations in the brain following different stressors. Most changes are observed in the hippocampus (replacement of neurons, remodeling of dendrites and synapses), but other adaptive changes are also observed in the prefrontal cortex and the amygdala of rodents (112). In humans, neuroimaging studies have greatly enriched the understanding of the neuroanatomical changes induced by SLS in humans. Data on emotional processes show that brain morphological changes mainly involve neurons of the prefrontal cortex, amygdala, insula, basal ganglia, and anterior cingulate cortex (36, 37). MRI measurements in patients with PTSD (14, 16, 64) and in female survivors of early sexual or physical abuse (17, 158) have revealed smaller volumes of the brain (from 10% to 40%). In subjects with a history of traumatic childhood experiences who developed bipolar disorders later in life, smaller frontal lobes compared with healthy controls have been described (105). Other brain volumetric studies show significant reductions in the volume of hippocampus and amygdala core nuclei volumes in patients with major depression induced by SLS (15, 152, 153). Driessen et al. also demonstrated by MRI that a reduction in the hippocampus and amygdala in women with a borderline personality developed after different episodes of early traumatization (41).
The Link Between SLS, Brain Oxidative Stress, and the Development of Mental Disorders
Oxidative stress consists of an imbalance between the amount of ROS and the capacity of antioxidant systems to neutralize them.
Oxidative stress is a known feature of numerous CNS disorders. Thus, clear evidence of the involvement of increased brain oxidative damage in the development of CNS pathologies has been reported for neurodegenerative diseases (such as Alzheimer's disease, Parkinson's disease, and amyotrophic lateral sclerosis), cerebrovascular disorders, demyelinating diseases, and psychiatric disorders, in both animal models and patients [reviewed in Ref. (155)].
In the CNS, ROS play a crucial pathophysiological role (23). The accumulation of ROS is known to increase the susceptibility of brain tissue to damage. They also trigger numerous molecular cascades, leading to increased blood-brain barrier permeability (through activation of matrix metalloproteinases and subsequently degradation of tight junctions), alterations of brain morphology, neuroinflammation, and neuronal death (61).
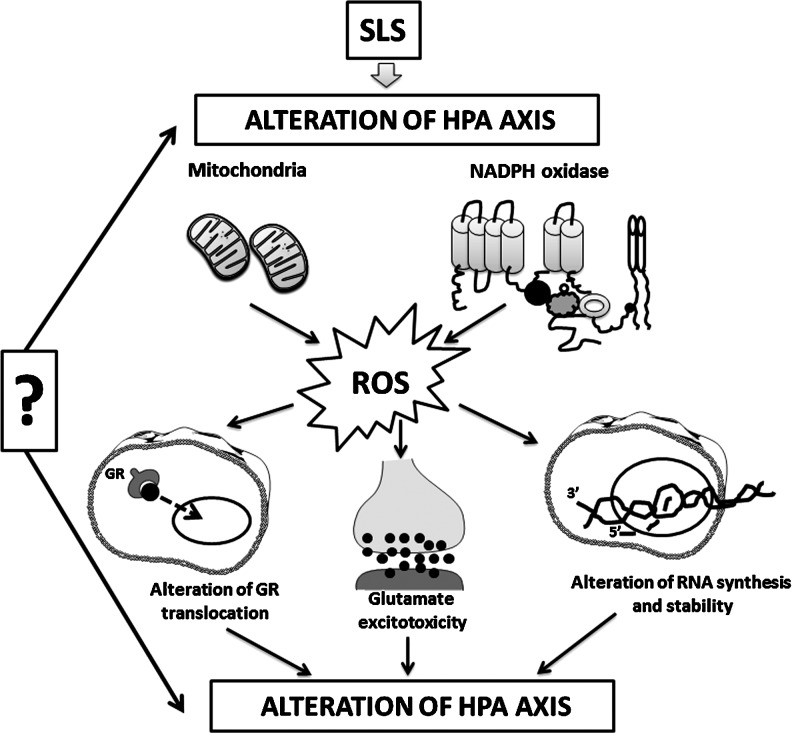
Although the exact mechanisms linking oxidative stress to the HPA axis is relatively unknown, ROS may affect the HPA axis via several molecular mechanisms (Fig. 4):
(i) alteration of the normal translocation of the glucocorticoid receptors from the cytoplasm to the nucleus: Recent studies have suggested an impaired function of the glucocorticoid receptor during oxidative stress through redox regulation. In addition, an impaired nuclear translocation of the glucocorticoid receptor has been observed in corticotrophs under high hydroxyradical generation, which may account for the reduced or eliminated effect of glucocorticoid on the expression of the pro-opiomelanocortin gene that encodes ACTH (109, 124, 164);
(ii) increase in the stress-induced glutamate toxicity: In this context, a new molecular mechanism has been recently proposed. Thus, not only glutamate excitotoxicity and/or oxidative stress alters mitochondrial activity, but also an imbalance in mitochondrial activity, in turn, leads to NMDA receptor up-regulation and increased oxidative stress, finally leading to a neurodetrimental vicious cycle (4, 122);
(iii) modulation of kinases and cysteine-rich, redox-sensitive proteins [such as ASK1, activated by the hydrogen peroxide (57) or the redox-dependent protein kinase C (55, 161)]; and
(iv) alterations of RNA synthesis and stability (154).
FIG. 4.
Mechanisms of ROS action on the HPA axis. The effects of ROS on the HPA axis might be explained as follows: SLS may directly alter the physiological functioning of the HPA axis, leading to an increased production of ROS by mitochondria and NADPH oxidase. This elevation in oxidative stress affects the translocation of the GR, induces an increase in glutamate release leading to glutamate excitotoxicity, and alters RNA synthesis and stability. Alternatively, the alteration of the physiological functioning of the HPA axis might be observed later, as a consequence of the altered GR translocation, glutamate excitotoxicity, and impaired RNA synthesis. GR, glucocorticoid receptor.
In turn, in the brain, ROS production by NOX enzymes (and, in particular, by NOX2) can be induced by various cellular stressors [reviewed in Ref. (81)]:
(i) organic and inorganic chemical compounds, such as heavy metals (i.e., zinc and cadmium), nonmetallic poison arsenic in the form of arsenite, organic solvents (i.e., ethyl or butyl alcohol), and environmental cytotoxic substances, such as cigarette smoke and diesel exhaust particles;
(ii) physical challenges such as ionizing radiation, both UVA and UVB, physical forces, changes in temperature, osmotic pressure, and pH;
(iii) adverse cellular environments such as altered nutritional conditions, hypoxia and/or hyperoxia;
(iv) inflammatory stimuli such as bacterial endotoxin lipopolysaccharide. Thus, in microglia and astrocytes, this factor is known to stimulate rapid NOX-dependent ROS production, and the expression of inducible nitric-oxide synthase and cytokines (127).
(v) stress-related humoral and neural factors, such as (e.g., norepinephrine and endogenous opioid peptides).
Stimulation of the NOX activity results in the activation of ERK1,2, p38 MAPK, Jun Kinase, and Akt pathways, the best studied cell stress signaling network (91). Therefore, the activation of NOX-mediated redox signaling may play a critical role in coordinating the responses of the cell to deal with adverse effects, either by activating stress kinases and promoting stress tolerance or by removing the seriously damaged cells by inducing apoptosis (81).
Detection of Oxidative Stress in the Brain
Oxidative stress has been found to be related to the onset and progression of numerous CNS pathologies (146). However, the measurement of oxidative stress in the brain is a major challenge. Several approaches have been used to detect oxidative stress in the brain (Table 2). Next, we summarize some of the approaches used for this purpose.
Table 2.
Methods of Detection of Biomarkers of Oxidative Stress in the Brain
| Method of detection | Biomarker | Reference |
|---|---|---|
| Physicochemical methods | Isoprostanes | Violi et al. (174) |
| Immunochemistry (ELISA) | ||
| Radio-immunoassay | ||
| Immunohistochemistry (ELISA) | 8-Hydroxideoxiguanosine | Schiavone et al. (146); Sorce et al. (156) |
| Systemical injection of HE | Superoxide | Hall et al. (2012) |
| Proteomic | Carbonylated proteins | Linares et al. (100) |
| In situ methods | Lipoxidation products; MDA | Violi et al. (2009) |
| CE-fluorescence | MDA | Cooley and Lunte (32) |
| Salycilate trapping | Free radicals | Grienberger et al. (60) |
HE, hydroethidine; MDA, malondialdehyde.
Analysis of postmortem brain samples
Immunohistochemical analysis includes the use of antibodies that detect oxidized nucleic acids, proteins, or lipids within the brain, or detect cellular responses to oxidative stress. In our hands, a useful antibody that detects the oxidation of DNA in brain tissue is the anti-8-hydroxydeoxyguanosine (anti-8OHdG) (146, 156). Indeed, for chemical reasons, guanosin is more sensitive than other nucleotides to oxidative stress (85). Nitrotyrosine antibodies detect the chemical reaction of amino acids with peroxynitrate, which is the reaction product of superoxide and nitric oxide. Strong nitrotyrosine staining has been detected in numerous neurodegenerative disorders (20) and in cerebral ischemia (157); anti-nitrotyrosine immunoreactivity in SLS brain samples has so far not been documented.
Several other antibodies have been used to detect cellular responses to oxidative stress in the brain (146, 174): (i) antibodies raised against c-fos, an immediate early gene and redox-sensitive transcription factor (10); (ii) antibodies raised against the hypoxia-inducible factor 1 alpha, a protein that is known to be up-regulated under oxidative stress conditions (10); and (iii) antibodies raised against the phosphorylated form of the MAP kinases JNK, ERK, and p38 (6). Note that, although oxidative stress undoubtedly leads to increased HIF-1alpha, cfos, and MAP kinase phosphorylation, other stimuli also do so. Thus, these parameters represent a rather nonspecific response to stress and, therefore, need careful interpretation.
Other interesting methods of detection of oxidative stress on postmortem brain samples are emerging. Recently, Linares et al. have proposed proteomic approaches for identifying carbonylated proteins in brain tissue as one of the most appropriate methods for measuring oxidative stress in the brain (100). An alternative approach that is used for postmortem samples is the expression of redox-sensitive genes (7). However, the limited postmortem stability of mRNA is a problem for this approach.
Detection of direct and indirect biomarkers of oxidative stress in cells and biological fluids
Biomarkers of oxidative stress in biological fluids include oxidized proteins (protein carbonyls, thiols) and oxidized lipids such as hydroxynonenal, malondialdeyde (MDA), and isoprostanes (isomers resulting from the oxidation of arachidonic acid), which appear to be the best candidates for molecular signatures of excessive oxidation in biological fluids (174). Neuroprostanes are also emerging as specific biomarkers of oxidative stress in the CNS (174).
The detection of indirect biomarkers of oxidative stress in cells (such as hematopoietic cells or fibroblasts) includes measurement of telomere shortening (74) by quantitative polymerase chain reaction (18) or fluorescence activated cell sorting (42). Indeed, telomeres require the enzyme telomerase for whole length restoration after cell division. Since most somatic cells do not express telomerase, many cells show telomere shortening with age. However, for at least two reasons, telomere shortening can be markedly accelerated by oxidative stress. First, due to their high content of guanines (nucleic acid is most sensitive to oxidative stress, see above), telomeres are highly sensitive to damage by oxidative stress (85). Second, ROS, especially hydroxyl radicals, produce single-strand breaks, either directly or as an intermediate step in the repair of oxidative base modifications. In contrast to the bulk part of genomic DNA, telomeric DNA lacks efficient repair of single-strand breaks (128). Thus, through the combination of increased sensitivity to oxidation and the decreased repair of oxidative damage, telomeres are particularly sensitive to the accumulation of ROS-induced 8-oxodG DNA-strand breaks (85, 123, 175). However, it should be kept in mind that the regulation of telomere length is a complex process, with oxidative stress being only a modifier. Thus, shortened telomeres are suggestive of, but not proving, oxidative stress.
Imaging studies
At present, relevant imaging studies conducted on SLS are limited to rodent work. These studies will be discussed here. Note that in our opinion, the imaging of oxidative stress in humans is a point of major importance for future work on SLS and represents an exciting emerging approach.
An injection of hydroethidine (HE or DHE), a redox-sensitive probe (46, 47), has recently been used to measure ROS levels in the live mouse brain after ketamine administration (66). Indeed, when systemically injected, HE is rapidly distributed into the brain. Nonoxidized HE is excreted in the urine, while the oxidation products of HE are retained in tissues, including the brain, for a long period. Note, however, that—as opposed to what is frequently stated—HE is not only oxidized by superoxide, but also by other ROS. High-performance liquid chromatography (HPLC) and electrochemical detection analysis is able to distinguish between different HE oxidation products (108). Capillary electrophoresis associated to fluorescence detection (CE-fluorescence) for the determination of MDA in cerebral dyalisate (obtained by in vivo microdialysis) has been proposed as an alternative method for detecting ROS in the brain (32).
A novel approach that is used for detecting the presence of oxidants in the brain is the salicylate trapping method, which is based on the hydroxylation of salicylic acid into dihydroxybenzoic acid during oxidative stress. The latter is then quantified in venous eluate by using HPLC (60).
Although these imaging methods are very important, they need further development to be suitable for routine measurements of brain oxidative stress. Further on, these methods usually cannot identify the source of ROS. Thus, the use of knock-out, transgenic, and mutant animals is crucial to specifically identify the sources of oxidants in animal models. However, despite relevant methodological developments, the field of detection of oxidative stress in the CNS remains plagued with pitfalls (53). Markers of oxidative stress in brain pathologies have shown extremely large variations among studies. Although there are obviously intrinsic variations in clinical studies, these discrepancies emerge from the fact that the choice of analytical methods is frequently determined by simplicity, rapidity, and commercial availability rather than by reliability. In addition, the exact source of oxidative stress is often not clearly identified and specifically tested.
Evidence from Animal Studies
Rodents and primates are the best suited species for conducting studies on the link between SLS, oxidative stress, and the development of psychiatric illnesses (97, 176).
Rodents
In the next section, we will describe three models of SLS that study the link between SLS, oxidative stress in the brain, and mental disorders (Table 3):
Table 3.
Effects of Severe Life Stress on HPA Axis, Brain Morphology, and Oxidative Stress in Rodent Models
| Models | HPA axis | Brain morphology | Biomarkers of brain oxidative stress | Involvement of NOX |
|---|---|---|---|---|
| Sleep deprivation | HPA hyperactivity ↑ CRH ↑ corticosterone |
Structural remodeling of hippocampus, amygdala and prefrontal cortex Alterations in neurotransmission |
↓ glutathione levels ↓ SOD activity ↓ CAT activity ↑ SOD activity No changes |
ND |
| Maternal separation | ↑ ACTH ↑ corticosterone ↑ CRF |
Alterations of GR and MCR expression and distribution Alterations in 5-HT and GLU systems |
↓ LP and↑GPx activity (12) ↑ LP,↑SOD activity, no changes in GPx activity No changes in females |
ND |
| Social isolation | ↓ ACTH ↑ sensitivity of the pituitary gland to CRF ↑ secretion of corticosteroids |
Loss of phenotype of PV interneurons ↓ GR |
↑ SOD and CAT activity ↓ GPx activity ↓ GLR ↓ GSG:GSH ↑ lipid peroxidation ↑ indirect markers of oxidative stress (8OHdG; c-fos and HIF-1α) |
↑ expression of NOX2 in NACC and PFC |
For references, see text.
ACTH, adrenocorticotropic hormone; HPA, hypothalamic–pituitary–adrenal; CRH, corticotropin-releasing hormone; CRF, corticotropin-releasing factor; GR, glucocorticoid receptors; MCR, mineralcorticoid receptors; 5-HT, serotonin; GLU, glutamate; PV, parvalbumin; SOD, superoxide dismutase; CAT, catalase; LP, lipid peroxidation; GPx, glutathione peroxidase; GLR, glutathione reductase; GSG, reduced glutathione; GSH, oxidized glutathione; 8OHdG, 8-hydroxydeoxyguanosine; HIF-1α, hypoxia-inducible factor 1α; NACC, nucleus accumbens; PFC, prefrontal cortex; ND, not determined.
The sleep deprivation model: increase of glucocorticoids and decrease of antioxidant enzymes
Sleep is a universal, dynamic brain process that is present in organisms ranging from invertebrates to mammals, which has an important homeostatic function (19). Physiological sleep rhythm plays a crucial role in the normal development of several CNS functions, such as neurogenesis, learning, and memory (65). Prolonged sleep deprivation is a well-known SLS. In rodents, chronic sleep deprivation causes the structural remodeling of brain regions (such as hippocampus, amygdala, and prefrontal cortex) (160) that are involved in the formation and consolidation of memory (59, 62).
Brain antioxidant capacity is strongly decreased in sleep-deprived animals (44): glutathione levels are reduced in whole rat brains (34), and significant reductions in the activity of superoxide dismutase (SOD) have been found in the hippocampus and brain stem (135). A recent study conducted by Alzoubi et al. has shown a protective effect of chronic administration of vitamin E, a strong antioxidant, on chronic sleep deprivation-induced cognitive impairment. In this study, the chronic administration of vitamin E was able to normalize chronic sleep deprivation-induced reduction of the GSH/GSSG ratio in the hippocampus, and the activity of catalase (CAT), SOD, and glutathione peroxidase (GPx) (5).
However, changes in antioxidant enzymes can vary depending on the model: in a study conducted on short-term (8 h) or long-term (3–14 days) sleep-deprived rodents (56), an increase in total SOD activity in rats allowed a recovery of sleep after they had been selectively sleep deprived during REM sleep; this increase was absent in the total sleep-deprived animals (3–14 days). Another group reported no increase in antioxidant enzymes SOD, CAT, GPx, or MDA activity after chronic sleep deprivation in rats (34).
The maternal separation model
In most mammals, mother–infant relationship is essential for a normal development, and the early loss of maternal care represents an SLS, affecting the vulnerability of infants over a lifespan. The quality of the mother–pup interactions, such as maternal licking, grooming, and arched-back nursing, is an important factor in maintaining a blunted HPA axis response (22, 49). In rodents, this early period is crucial for neuronal growth and myelination (120) and is characterized by a dampened activity of the HPA axis known as the stress hyporesponsive period (SHRP). This period extends from postnatal day 4 to 14 and consists of low activity of the adrenocortical system and decreased responsiveness to stressors that would usually induce a robust stress response in adult animals (140). As a consequence of maternal separation, separation anxiety-related behaviors are observed later in life (134).
Repeated periods of maternal separation in rats have repercussions in the adult age, resulting in increased corticosterone levels (130) and the expression of corticotropin releasing factor (CRF) in the hypothalamus (132). Other consequences of maternal separation in the adult brain include elevated ACTH levels in response to a stressor (35), altered CRF binding sites in several brain areas (92, 133, 172), dysregulated glucocorticoid and mineralocorticoid receptor expression (92, 137, 179), and alterations in the serotonergic (35, 162, 173) and glutamatergic systems (129). In a model of repeated maternal deprivation, it has been shown that alterations of brain lipid peroxidation and the antioxidant enzymes activities are age- and sex-dependent (170). Thus, the maternal deprivation in SHRP causes a significant decrease in brain lipid peroxidation and a significant increase in GPx activity in the hippocampus, prefrontal cortex, and striatum; whereas maternal deprivation in post-SHRP causes a significant increase in brain lipid peroxidation and a significant increase in SOD activity (with no effect on GPx) in the three considered regions of the brain (170). In addition, although an increase in SOD enzyme activity and lipid peroxidation in the brain of maternal-separated male rats can be observed, these parameters are stable in female rats (170), suggesting that female hormones are protective, possibly through a scavenging action of estrogens (116).
The social isolation model: evidence of the NOX2 enzyme as a source of oxidative stress in the brain
Social isolation and loneliness represent an SLS for both humans and rodents (68, 83). Social interactions are required for the normal development of the brain, neurotransmission, and cognitive abilities (95, 178). When deprived of social interactions, rats are aggressive, neophobic, nervous, and anxious (149). They also show hyper-reactivity to novel environments, marked cognitive impairments (51, 94), and a change of phenotype of specific subpopulations of cortical neurons, such as the parvalbumin-positive neurons (67). These alterations are reminiscent of what is observed in humans with psychosis (94). Therefore, rearing rats in social isolation immediately after weaning is a well-established tool that is used for studying the brain response to an intense stressful life event (95). Social isolation induces important alterations in the functioning and regulation of the HPA axis (149) as evidenced by low ACTH concentration and increased sensitivity of the pituitary gland to CRF, thereby blocking the negative feedback regulation of the HPA axis (149). This leads to enhanced corticosteroid secretion and a marked increase in brain steroid levels in response to novel applied stress such as foot shock or restraint (150, 151). The inhibition of the negative feedback regulation of the HPA axis in socially isolated animals also causes a gradual decrease in the number of glucocorticoid receptors in specific brain areas (e.g., hippocampus and hypothalamus) (149).
Increasing evidence indicates that brain oxidative stress is a crucial contributor to the development of neuropathological alterations induced by social isolation. Pajovic et al. showed an increased activity of SOD and CAT in the hippocampus of rats that are exposed to long-term social isolation (125). These findings have not been confirmed by another group that has rather described a decreased activity of GPx and compromised glutathione reductase in the hippocampus of long-term isolated rats (40). Another study showed increased SOD activity, a decreased GSH/GSSG ratio, and increased lipid peroxidation in both striatum and cortex after 8 weeks of social isolation (118). These alterations in brain oxidative stress parameters are associated to deficits in prepulse inhibition and social and self-directed interactive behaviors. Both behavioral and cortico-striatal redox disturbances were corrected by the antipsychotic clozapine (118).
Our group has recently investigated the role of the NOX2 enzyme in rats exposed to social isolation. A tremendous increase in NOX2 expression was observed in both prefrontal cortex and nucleus accumbens. This increase is associated with elevations in markers of oxidative stress, such as the oxidized nucleic acid 8-hydroxy-2′-deoxyguanosine, the redox-sensitive transcription factor c-fos, and the hypoxia-inducible factor-1alpha (146). Remarkably, chronic treatment with apocynin, an antioxidant compound that can block NOX2 activity under some circumstances (71), completely prevented behavioral and histopathological alterations induced by social isolation (146). These results strongly support the idea that pharmacological targeting of NOX2 activity might reverse behavioral alterations.
These findings highlight the role of NOX2 in the field of brain oxidative stress (155). However, nowadays, the link between NOX2 and the HPA axis remains elusive. In previous studies, the direct action of corticosteroids on NOX2 is rather inhibitory (30). How prolonged stress leads to such a strong increase in NOX2 remains a matter of further studies.
Primates
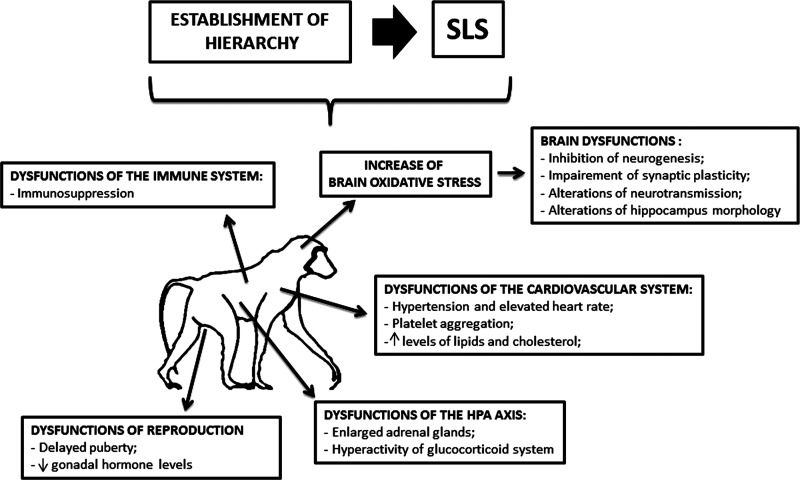
Studies of both feral and captive animal populations show that animals with specific dominance ranks, such as some species of non-human primates, tend to show characteristic stress-related physiological profiles (119). Although it is not an SLS per se, as it is a key aspect of their social life, the establishment of a social hierarchy is a source of intense stress, inducing profound changes in their physiology (143) (Fig. 5). Some species of non-human primates (such as gorillas) accept dominance of the leader once the hierarchy has been established, whereas others (such as chimpanzees and baboons) are continuously struggling at improving their social rank (143).
FIG. 5.
The effects of the establishment of hierarchy in non-human primates. The establishment of hierarchy might represent an SLS for non-human primates. Physical and emotional reactions to this type of SLS are represented by dysfunctions of the immune, cardiovascular, and reproductive systems. Alterations in the HPA axis functioning (in particular, hyperactivity of the glucocorticoid system) might be also frequently observed. The CNS may react with the establishment of the hierarchy by increased oxidative stress, which, in turn, may lead to several brain dysfunctions, such as decreased neurogenesis and alterations in synaptic plasticity and neurotransmission.
Several stress-related physiological endpoints have been found to be sensitive to rank, such as the blood level of glucocorticoids and the adrenal steroid hormones (e.g., hydrocortisone in primates). Primates that are stressed by their hierarchy show hyperactivity of the glucocorticoid system which is associated to enlarged adrenal glands and impaired sensitivity of the system to negative feedback regulation (26, 142).
However in some cases, it is the dominant individuals who show this profile. This occurs, for example, in chimpanzees where dominant individuals have to repeatedly and physically reassert their rank (26) and in species enduring frequent transient periods of rank instability (feral baboons and rhesus monkeys) (142).
Non-human primates who are socially stressed by the dominance hierarchy for prolonged periods undergo strong cerebral changes (163). These alterations might be due to the increased brain oxidative stress during the hierarchy establishment (48). Elevation of brain oxidation associated with hierarchy in non-human primates leads to inhibition of neurogenesis, impairment of synaptic plasticity (89, 107), altered patterns of apoptotic cell death (103), alterations in dopaminergic and serotonergic neurotransmission (144, 163), and alterations in neuronal morphology (such as decreases in dendritic branching or dendritic length) in the hippocampal CA3 pyramidal neurons (106, 181). The degree of remodeling is somewhat more pronounced in the dominant group compared with controls, while subordinate non-human primates show marked hippocampal neuronal degeneration, primarily of the pyramidal neurons of the CA1 and CA3 regions (115).
Evidence from human studies
The life path of an individual is marked by plentiful events and occurrences that vary in their magnitude, duration, and the meaning they have for the person. Exposure to SLS can impact forever the way an individual perceives themselves and the world around them (166).
In this part of the review, we will discuss the impact of SLS such as child abuse, war, and divorce on the brain function and the contribution of oxidative stress to the development of mental disorders.
Child abuse
A crucial step in understanding the nature and effects of life events entails the study of SLS during childhood and adolescence. Studies on SLS during childhood and adolescence mainly began in the 1980s and aimed at connecting SLS events and subsequent psychological and/or physiological disorders (141).
Child abuse is defined as “neglect, physical abuse, sexual abuse and emotional maltreatment” (45). Several studies have investigated the link between child abuse and the HPA axis. Physiologically, the HPA axis is not fully mature at birth. Indeed, there are several developmental changes during childhood in both basal HPA activity and cortisol release (63). Significantly, the activity of the HPA axis in early human development is under strong social regulation. Thus, children in deprived rearing environments show strong disturbances in basal and diurnal cortisol rhythm (24, 165).
A recent review provides a summary of the psychiatric and social disorders to which children who have experienced maltreatment, in particular sexual abuse, are predisposed throughout their entire life: (i) behavioral dysfunction (ii) emotional alterations, (iii) pain disorders, and (iv) systemic and cognitive disorders (180).
A very strong link exists between sexual abuse during childhood and the development of mental illnesses (PTSD, depression, border line personality disorders, attempted suicide, and eating disorders) (25, 139, 183). Important brain development alterations were described in a cohort of 28 naïve children and adolescents with maltreatment-related PTSD compared with 66 sociodemographically similar healthy control subjects. These included decreased volumes of prefrontal cortex, prefrontal cortical white matter, right temporal lobe volumes, and corpus callosum, while larger frontal lobes and increased cerebrospinal fluid (CSF) quantity were measured (38).
Oxidative stress has been proposed as being an important contributor in the brain alterations and mental disorders derived from child abuse (43). However, the majority of studies did not measure oxidative stress during childhood or adolescence, but indirect biomarkers of oxidative stress in adults who have experienced maltreatments when they were children. The usual parameter is telomere shortening (168). Adults who experienced maltreatment during childhood have significantly shorter telomeres. Interestingly, shorter telomeres are associated with physical and emotional neglect but not with physical or emotional abuse (168). However, as of today, no studies investigated this correlation in a larger population. Thus, the molecular pathways linking psychological stress to telomere shortening remain to be elucidated. One of the proposed mechanisms involves an increase in glucocorticoid-associated oxidative stress damage as a mediator of telomere shortening (27, 114).
War
The negative impact of war on the physical status and quality of life of survivors has been widely investigated (13) and is mostly related to physical consequences of war, such as severe physical injury or loss of specific functions of the body. However, since the Gulf War, the relationship between war and the development of physical and psychological problems has been addressed. A recent longitudinal study of American Gulf War veterans has shown alterations of the HPA axis (such as impairment in the response to ACTH to CRF and increased cortisol levels after stimulation) and of the neuroendocrine profile of the veterans (54, 98). In addition, chronic and persistent abnormalities of hippocampal blood flow and alterations of levels of the neuronal markers N-acetylaspartate in the basal ganglia and pons have also been documented in Gulf War Veterans (99, 102, 177).
Studies of war survivors (both soldiers and civilians) even led to the description of a new syndrome, PTSD (101). Indeed, before 1982, the word PTSD did not exist and, therefore, was not introduced in the psychiatric lexicon. The constellation of persistent physiological and psychological symptoms observed in American veterans who were involved in the war in Vietnam led to the definition of the term PTSD and its inclusion in the DSM-III.
Increasing evidence indicates that neuropathological and psychological alterations induced by war experiences are related to brain oxidative stress (126). Increased levels of nitric oxide/peroxynitrite in the CSF of Gulf War veterans have been reported (126). In addition, PTSD observed in American soldiers returning from Iraq has been associated with ROS-mediated brain changes (72). In Gulf War veterans affected by PTSD, elevation in the levels of 8-OhdG and the induction of 3-nitrotyrosine in the CSF have been reported (1).
In addition, modern war leads to exposure to toxic agents. An interesting article (2) investigated the effects of sarin, a highly toxic nerve agent to which people were exposed during the Gulf War (77). Neurotoxicity of sarin is mainly mediated by the inhibition of acetylcholinesterase (86) but also leads to increased oxidative stress in the CNS (2). In addition, the use of depleted uranium raised concern about a link with PTSD and brain oxidative stress in Gulf War veterans. Several studies conducted on rodents showed that depleted uranium crosses the blood–brain barrier and accumulates in a specific brain region, leading to increased brain oxidative stress, altered behavior and emotionality and sensorimotor deficits (8, 96). Thus, similar mechanisms may occur in humans exposed to depleted uranium during war. Studies conducted on the civilians who have been chronically exposed to depleted uranium may 1 day answer this question.
Divorce
Among stressful life events, divorce is considered one of the most impacting ones because of its strong physical and psychological consequences on men, women, and children (88). It is largely known that divorce affects the physiological functioning of the HPA axis of the couple (12, 87). However, data are missing for children, as most of the studies measured cortisol levels in adults who experienced parents' divorce during childhood. Higher basal cortisol levels in healthy adults who had experienced parents' divorce have been found (12, 87, 121).
From a psychological point of view, although nowadays divorce does not have the social stigma attached to it in the past, divorcing couples often feel a sense of guilt or shame, anger, and resentment, especially when young children are in the household (167). The persistence of these feelings may lead to depression and anxiety, which can manifest in many forms. In particular, symptoms for women going through a divorce depression include overeating, insomnia, or hypersomnia, excessive drinking, or substance abuse (3).
Children whose parents are going through a divorce engage in anxious behaviors and can typically experience denial, abandonment, preoccupation, anger and hostility, immaturity or hyper maturity, blaming, and acting out (138). If these emotions persist, children can sometimes develop mental disturbances (138).
Recent evidence suggests that there is a possible link between the severe mental consequences of divorce and increased peripheral and central oxidative stress (75). A study conducted at the University of New Mexico showed that divorced men have a lower anti-oxidant defense system in the CNS and decreased resistance to brain oxidative damage than nondivorced men (http://hdl.handle.net/1928/11173). In children who experienced parents' divorce or in adults who experienced divorce during childhood, increased susceptibility to ROS (in terms of decreased antioxidant defenses) of several tissues such as the brain (21) or the lung (80) has been described. Moreover, in children exposed to severe psycho-emotional stress after the divorce of parents, an increased incidence of brain cancer has been reported as well as elevations of reactive oxygen and nitrogen species in the CNS (21). Although the current number of studies on this subject is not yet very consistent, existing data encourage further research in this field.
Conclusion and Outlook
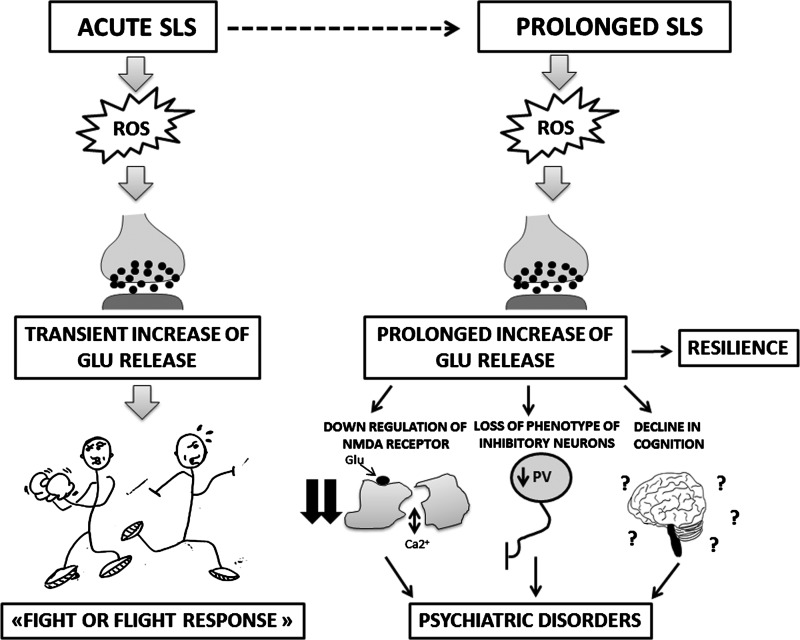
This review summarizes current knowledge on the link between SLS, brain oxidative stress, and neuropsychological responses. According to the literature, oxidative stress in response to SLS occurs in both animal models (rodents and primates) and humans. What might be the hypothetical evolutionary advantages of such a conserved pathway? There is now increasing evidence that ROS generation is involved in the regulation of neurotransmission, in particular glutamate release, which most likely plays an important role in the “fight or flight response.” Thus, a unifying, although speculative, hypothesis of the observations summarized here would be as follows:
Short lasting, acute SLS requires a high level of excitatory neurotransmission in order to allow rapid reactions of the organism in order to fight the stress situation or to escape. The activation of ROS generation through external stress facilitates and/or enhances the release of excitatory neurotransmitters. Thus, in its simplest form, this might represent a healthy response that contributes to survival. However, in the case of long-lasting chronic SLS, the neurophysiological impact is very different. Excessive release of excitatory neurotransmitters leads to exaggerated neurophysiological responses that are reminiscent of alterations observed in patients with psychosis: NMDA receptor down-regulation, loss of phenotype inhibitory neurons, behavioral alterations, and a decline in cognition (Fig. 6).
FIG. 6.
Effects of acute and prolonged SLS: the link with glutamate neurotransmission. The activation of ROS generation through external acute stress facilitates and/or enhances the release of glutamate. High levels of this neurotransmitter are physiologically required in order to allow a rapid healthy reaction of the organism to the acute SLS (fight or flight response), which contributes to survival. In the case of long-lasting chronic SLS, the activation of ROS leads to a prolonged increase in glutamate release. The reactions to this long-lasting excitotoxicity might be adaptation and resilience. However, exaggerated pathological responses, reminiscent of what is observed in patients with psychosis, might occur: NMDA receptor down-regulation, loss of phenotype of inhibitory neurons (mainly represented by the loss of PV), behavioral alterations, and cognitive decline. PV, parvalbumin.
In humans, there is strong evidence that the responses to SLS show large inter-individual variations. Indeed, while some individuals show resilience, others show severe long-lasting damage, ranging from post-traumatic disorders to full-blown psychosis.
We think that the improved understanding of SLS responses will ultimately help prevent and/or treat post-traumatic disorders. At least in some animal models, the source of oxidative stress has been identified and—if confirmed in humans—this might provide interesting targets for drug development (146). However, translational research into SLS is still hampered by the lack of tools that allow the detection of oxidative stress in the human brain. Thus, future development in imaging technologies will be crucial. In addition, an understanding of resilience is of utmost importance. Thus, further studies are needed to clarify to what extent genetic elements or environmentally determined personality traits account for resilience.
Abbreviations Used
- 5-HT
serotonin
- 8OHdG
8-hydroxydeoxyguanosine
- ACTH
adrenocorticotropic hormone
- AP-1
activator protein 1
- CAT
catalase
- CNS
central nervous system
- CRF
corticotropin-releasing factor
- CRH
corticotropin-releasing hormone
- CSF
cerebrospinal fluid
- GLR
glutathione reductase
- GLU
glutamate
- GPx
glutathione peroxidase
- GR
glucocorticoid receptors
- GSG
reduced glutathione
- GSH
oxidized glutathione
- HE
hydroethidine
- HPA
hypothalamic–pituitary–adrenal
- HPLC
high-performance liquid chromatography
- MCR
mineralcorticoid receptors
- MDA
malondialdeyde
- NACC
nucleus accumbens
- ND
not determined
- NGF
nerve growth factor
- NMDA
N-methyl-d-aspartate
- NOX
NADPH oxidase
- PFC
prefrontal cortex
- PTSD
post-traumatic stress disorders
- PV
parvalbumin
- ROS
reactive oxygen species
- SHRP
stress hyporesponsive period
- SLS
severe life stress
- SOD
superoxide dismutase
- VP
vasopressin
Acknowledgments
The authors were supported by the Swiss National Foundation (Grant No. 3200A0-103725) and the Italian PRIN to L.T.
Author Disclosure Statement
K.H.K. and V.J. are founding members of the start-up company GenKyoTex, which develops NOX inhibitors.
References
- 1.Abu-Qare A. Abou-Donia M. Increased 8-hydroxy-2-deoxyguanosine, a biomarker of oxidative DNA damage in rat urine following a single dermal dose of DEET (N,N-diethyl-m-toluamide), and permethrin, alone and in combination. Toxicol Lett. 2000;117:151–160. doi: 10.1016/s0378-4274(00)00257-5. [DOI] [PubMed] [Google Scholar]
- 2.Abu-Qare AW. Abou-Donia MB. Combined exposure to sarin and pyridostigmine bromide increased levels of rat urinary 3-nitrotyrosine and 8-hydroxy-2-deoxyguanosine, biomarkers of oxidative stress. Toxicol Lett. 2001;123:51–58. doi: 10.1016/s0378-4274(01)00380-0. [DOI] [PubMed] [Google Scholar]
- 3.Achen T. A Woman's Guide to Divorce. Bloomington, IN: AuthorHouse; 2004. [Google Scholar]
- 4.Albrecht P. Lewerenz J. Dittmer S. Noack R. Maher P. Methner A. Mechanisms of oxidative glutamate toxicity: the glutamate/cystine antiporter system xc- as a neuroprotective drug target. CNS Neurol Disord Drug Targets. 2010;9:973–982. doi: 10.2174/187152710791292567. [DOI] [PubMed] [Google Scholar]
- 5.Alzoubi KH. Khabour OF. Rashid BA. Damaj IM. Salah HA. The neuroprotective effect of vitamin E on chronic sleep deprivation-induced memory impairment: the role of oxidative stress. Behav Brain Res. 2012;226:205–210. doi: 10.1016/j.bbr.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 6.Anand SS. Babu PP. c-Jun N terminal kinases (JNK) are activated in the brain during the pathology of experimental cerebral malaria. Neurosci Lett. 2011;488:118–122. doi: 10.1016/j.neulet.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Anantharam V. Lehrmann E. Kanthasamy A. Yang Y. Banerjee P. Becker KG. Freed WJ. Kanthasamy AG. Microarray analysis of oxidative stress regulated genes in mesencephalic dopaminergic neuronal cells: relevance to oxidative damage in Parkinson's disease. Neurochem Int. 2007;50:834–847. doi: 10.1016/j.neuint.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aschner M. Jiang GCT. Toxicity studies on depleted uranium in primary rat cortical neurons and in caenorhabditis elegans: what have we learned? J Toxicol Environ Health Part B. 2009;12:525–539. doi: 10.1080/10937400903358942. [DOI] [PubMed] [Google Scholar]
- 9.Bánfi B. Malgrange B. Knisz J. Steger K. Dubois-Dauphin M. Krause K. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J Biol Chem. 2004;279:46065–46072. doi: 10.1074/jbc.M403046200. [DOI] [PubMed] [Google Scholar]
- 10.Bedard K. Krause K. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 11.Behrens M. Ali S. Dao D. Lucero J. Shekhtman G. Quick K. Dugan L. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318:1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- 12.Bloch M. Peleg I. Koren D. Aner H. Klein E. Long-term effects of early parental loss due to divorce on the HPA axis. Hormones Behav. 2007;51:516–523. doi: 10.1016/j.yhbeh.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Bras M. Milunovic V. Boban M. Mickovic V. Loncar Z. Gregurek R. Laco M. A quality of life in chronic combat related posttraumatic stress disorder—a study on Croatian War veterans. Coll Antropol. 2011;35:681–686. [PubMed] [Google Scholar]
- 14.Bremner J. Functional neuroanatomical correlates of traumatic stress revisited 7 years later, this time with data. Psychopharmacol Bull. 2003;37:6–25. [PubMed] [Google Scholar]
- 15.Bremner J. Brain imaging in anxiety disorders. Expert Rev Neurother. 2004;4:275–284. doi: 10.1586/14737175.4.2.275. [DOI] [PubMed] [Google Scholar]
- 16.Bremner J. Krystal J. Southwick S. Charney D. Functional neuroanatomical correlates of the effects of stress on memory. J Trauma Stress. 1995;8:527–553. doi: 10.1007/BF02102888. [DOI] [PubMed] [Google Scholar]
- 17.Bremner JD. Randall P. Vermetten E. Staib L. Bronen RA. Mazure C. Capelli S. McCarthy G. Innis RB. Charney DS. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse—a preliminary report. Biol Psychiatry. 1997;41:23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Broberg K. Björk J. Paulsson K. Höglund M. Albin M. Constitutional short telomeres are strong genetic susceptibility markers for bladder cancer. Carcinogenesis. 2005;26:1263–1271. doi: 10.1093/carcin/bgi063. [DOI] [PubMed] [Google Scholar]
- 19.Brown M. Naidoo N. The UPR and the anti-oxidant response: relevance to sleep and sleep loss. Mol Neurobiol. 2010;42:103–113. doi: 10.1007/s12035-010-8114-8. [DOI] [PubMed] [Google Scholar]
- 20.Bruijn LI. Becher MW. Lee MK. Anderson KL. Jenkins NA. Copeland NG. Sisodia SS. Rothstein JD. Borchelt DR. Price DL. Cleveland DW. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron. 1997;18:327–338. doi: 10.1016/s0896-6273(00)80272-x. [DOI] [PubMed] [Google Scholar]
- 21.Bukhtoyarov OV. Samarin DM. Psychogenic carcinogenesis: carcinogenesis is without exogenic carcinogens. Med Hypotheses. 2009;73:531–536. doi: 10.1016/j.mehy.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Caldji C. Tannenbaum B. Sharma S. Francis D. Plotsky PM. Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci U S A. 1998;95:5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campese VM. Ye S. Zhong H. Yanamadala V. Ye Z. Chiu J. Reactive oxygen species stimulate central and peripheral sympathetic nervous system activity. Am J Physiol Heart Circ Physiol. 2004;287:H695–H703. doi: 10.1152/ajpheart.00619.2003. [DOI] [PubMed] [Google Scholar]
- 24.Carlson M. Earls F. Psychological and neuroendocrinological sequelae of early social deprivation in institutionalized children in Romania. Ann N Y Acad Sci. 1997;807:419–428. doi: 10.1111/j.1749-6632.1997.tb51936.x. [DOI] [PubMed] [Google Scholar]
- 25.Carol G. A representational perspective of child abuse and prevention: internal working models of attachment and caregiving. Child Abuse Neglect. 1996;20:411–424. doi: 10.1016/0145-2134(96)00016-6. [DOI] [PubMed] [Google Scholar]
- 26.Cavigelli S. Behavioural patterns associated with faecal cortisol levels in free-ranging female ring-tailed lemurs, Lemur catta. Anim Behav. 1999;57:935–944. doi: 10.1006/anbe.1998.1054. [DOI] [PubMed] [Google Scholar]
- 27.Ceccatelli S. Tamm C. Zhang Q. Chen M. Mechanisms and modulation of neural cell damage induced by oxidative stress. Physiol Behav. 2007;92:87–92. doi: 10.1016/j.physbeh.2007.05.048. [DOI] [PubMed] [Google Scholar]
- 28.Cicchetti D. Curtis W. Multilevel perspectives on pathways to resilient functioning. Dev Psychopathol. 2007;19:627–629. doi: 10.1017/s0954579407000314. [DOI] [PubMed] [Google Scholar]
- 29.Company HM. Boston, MA: Houghton Mifflin Company; 2010. The American Heritage Medical Dictionary. [Google Scholar]
- 30.Condino-Neto A. Whitney C. Newburger P. Dexamethasone but not indomethacin inhibits human phagocyte nicotinamide adenine dinucleotide phosphate oxidase activity by down-regulating expression of genes encoding oxidase components. J Immunol. 1998;161:4960–4967. [PubMed] [Google Scholar]
- 31.This reference has been deleted.
- 32.Cooley J. Lunte C. Detection of malondialdehyde in vivo using microdialysis sampling with CE-fluorescence. Electrophoresis. 2011;32:2994–2999. doi: 10.1002/elps.201100143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Costantini D. Marasco V. Møller A. A meta-analysis of glucocorticoids as modulators of oxidative stress in vertebrates. J Comp Physiol B. 2011;181:447–456. doi: 10.1007/s00360-011-0566-2. [DOI] [PubMed] [Google Scholar]
- 34.D'Almeida Vn. Lobo LcL. Hipolide DbC. de Oliveira AC. Nobrega JN. Tufilk S. Sleep deprivation induces brain region-specific decreases in glutathione levels. NeuroReport. 1998;9:2853–2856. doi: 10.1097/00001756-199808240-00031. [DOI] [PubMed] [Google Scholar]
- 35.Daniels WMU. Pietersen CY. Carstens ME. Stein DJ. Maternal separation in rats leads to anxiety-like behavior and a blunted ACTH response and altered neurotransmitter levels in response to a subsequent stressor. Metab Brain Dis. 2004;19:3–14. doi: 10.1023/b:mebr.0000027412.19664.b3. [DOI] [PubMed] [Google Scholar]
- 36.Davidson RJ. Abercrombie H. Nitschke JB. Putnam K. Regional brain function, emotion and disorders of emotion. Curr Opin Neurobiol. 1999;9:228–234. doi: 10.1016/s0959-4388(99)80032-4. [DOI] [PubMed] [Google Scholar]
- 37.Davidson RJ. Lewis DA. Alloy LB. Amaral DG. Bush G. Cohen JD. Drevets WC. Farah MJ. Kagan J. McClelland JL. Nolen-Hoeksema S. Peterson BS. Neural and behavioral substrates of mood and mood regulation. Biol Psychiatry. 2002;52:478–502. doi: 10.1016/s0006-3223(02)01458-0. [DOI] [PubMed] [Google Scholar]
- 38.De Bellis MD. Keshavan MS. Shifflett H. Iyengar S. Beers SR. Hall J. Moritz G. Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biol Psychiatry. 2002;52:1066–1078. doi: 10.1016/s0006-3223(02)01459-2. [DOI] [PubMed] [Google Scholar]
- 39.Dedovic K. Duchesne A. Andrews J. Engert V. Pruessner JC. The brain and the stress axis: the neural correlates of cortisol regulation in response to stress. NeuroImage. 2009;47:864–871. doi: 10.1016/j.neuroimage.2009.05.074. [DOI] [PubMed] [Google Scholar]
- 40.Djordjevic J. Djordjevic A. Adzic M. Radojcic M. Chronic social isolation compromises the activity of both glutathione peroxidase and catalase in hippocampus of male wistar rats. Cell Mol Neurobiol. 2010;30:693–700. doi: 10.1007/s10571-009-9493-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Driessen M. Herrmann J. Stahl K. Zwaan M. Meier S. Hill A. Osterheider M. Petersen D. Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Arch Gen Psychiatry. 2000;57:1115–1122. doi: 10.1001/archpsyc.57.12.1115. [DOI] [PubMed] [Google Scholar]
- 42.Drissi R. Wu J. Hu Y. Bockhold C. Dome JS. Telomere shortening alters the kinetics of the DNA damage response after ionizing radiation in human cells. Cancer Prev Res. 2011;4:1973–1981. doi: 10.1158/1940-6207.CAPR-11-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Epel ES. Blackburn EH. Lin J. Dhabhar FS. Adler NE. Morrow JD. Cawthon RM. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Everson CA. Laatsch CD. Hogg N. Antioxidant defense responses to sleep loss and sleep recovery. Am J Phys Regul Integr Comp Physiol. 2005;288:R374–R383. doi: 10.1152/ajpregu.00565.2004. [DOI] [PubMed] [Google Scholar]
- 45.Felitti VJ. Anda RF. Nordenberg D. Williamson DF. Spitz AM. Edwards V. Koss MP. Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 46.Fernandes DC. Wosniak Jo. Pescatore LA. Bertoline MA. Liberman M. Laurindo FRM. Santos CX. Analysis of DHE-derived oxidation products by HPLC in the assessment of superoxide production and NADPH oxidase activity in vascular systems. Am J Physiol Cell Physiol. 2007;292:C413–C422. doi: 10.1152/ajpcell.00188.2006. [DOI] [PubMed] [Google Scholar]
- 47.Fink B. Laude K. McCann L. Doughan A. Harrison DG. Dikalov S. Detection of intracellular superoxide formation in endothelial cells and intact tissues using dihydroethidium and an HPLC-based assay. Am J Physiol Cell Physiol. 2004;287:C895–C902. doi: 10.1152/ajpcell.00028.2004. [DOI] [PubMed] [Google Scholar]
- 48.Fleshner M. Maier S. Lyons D. Raskind M. The neurobiology of the stress-resistant brain. Stress. 2011;14:498–502. doi: 10.3109/10253890.2011.596865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Francis DD. Champagne FA. Liu D. Meaney MJ. Maternal care, gene expression, and the development of individual differences in stress reactivity. Ann N Y Acad Sci. 1999;896:66–84. doi: 10.1111/j.1749-6632.1999.tb08106.x. [DOI] [PubMed] [Google Scholar]
- 50.Fullerton C. Ursano R. Wang L. Acute stress disorder, posttraumatic stress disorder, and depression in disaster or rescue workers. Am J Psychiatry. 2004;161:1370–1376. doi: 10.1176/appi.ajp.161.8.1370. [DOI] [PubMed] [Google Scholar]
- 51.Geyer MA. Ellenbroek B. Animal behavior models of the mechanisms underlying antipsychotic atypicality. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1071–1079. doi: 10.1016/j.pnpbp.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 52.Gillespie C. Phifer J. Bradley B. Ressler K. Risk and resilience: genetic and environmental influences on development of the stress response. Depress Anxiety. 2009;26:984–992. doi: 10.1002/da.20605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Giustarini D. Dalle-Donne I. Tsikas D. Rossi R. Oxidative stress and human diseases: origin, link, measurement, mechanisms, and biomarkers. Crit Rev Clin Lab Sci. 2009;46:241–281. doi: 10.3109/10408360903142326. [DOI] [PubMed] [Google Scholar]
- 54.Golier JA. Caramanica K. Yehuda R. Neuroendocrine response to CRF stimulation in veterans with and without PTSD in consideration of war zone era. Psychoneuroendocrinology. 2012;37:350–357. doi: 10.1016/j.psyneuen.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 55.Gopalakrishna R. Chen Z-H. Gundimeda U. Selenocompounds induce a redox modulation of protein kinase C in the cell, compartmentally independent from cytosolic glutathione: its role in inhibition of tumor promotion. Arch Biochem Biophys. 1997;348:37–48. doi: 10.1006/abbi.1997.0335. [DOI] [PubMed] [Google Scholar]
- 56.Gopalakrishnan A. Ji L. Cirelli C. Sleep deprivation and cellular responses to oxidative stress. Sleep. 2004;27:27–35. doi: 10.1093/sleep/27.1.27. [DOI] [PubMed] [Google Scholar]
- 57.Gotoh Y. Cooper J. Reactive oxygen species- and dimerization-induced activation of apoptosis signal-regulating kinase 1 in tumor necrosis factor-alpha signal transduction. J Biol Chem. 1998;273:17477–17482. doi: 10.1074/jbc.273.28.17477. [DOI] [PubMed] [Google Scholar]
- 58.Gourion D. Events of life and links with severe depression at different ages. Encephale. 2009;35:250–256. doi: 10.1016/S0013-7006(09)73480-3. [DOI] [PubMed] [Google Scholar]
- 59.Graves L. Heller E. Pack A. Abel T. Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Learn Mem. 2003;10:168–176. doi: 10.1101/lm.48803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grienberger H. Pillai D. Schlachetzki F. Gruber M. Dittmar M. Detection of free radicals by isolated perfusion of the rat brain following hemorrhagic stroke: a novel approach to cerebrovascular biomarker research. Exp Brain Res. 2010;206:311–317. doi: 10.1007/s00221-010-2410-4. [DOI] [PubMed] [Google Scholar]
- 61.Gu Y. Dee C. Shen J. Interaction of free radicals, matrix metalloproteinases and caveolin-1 impacts blood-brain barrier permeability. Front Biosci (Schol Ed) 2011;3:1216–1231. doi: 10.2741/222. [DOI] [PubMed] [Google Scholar]
- 62.Guan Z. Peng X. Fang J. Sleep deprivation impairs spatial memory and decreases extracellular signal-regulated kinase phosphorylation in the hippocampus. Brain Res. 2004;1018:38–47. doi: 10.1016/j.brainres.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 63.Gunnar MR. Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27:199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- 64.Gurvits TV. Shenton ME. Hokama H. Ohta H. Lasko NB. Gilbertson MW. Orr SP. Kikinis R. Jolesz FA. McCarley RW. Pitman RK. Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. Biol Psychiatry. 1996;40:1091–1099. doi: 10.1016/S0006-3223(96)00229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guzmán-Marín R. Suntsova N. Stewart DR. Gong H. Szymusiak R. McGinty D. Sleep deprivation reduces proliferation of cells in the dentate gyrus of the hippocampus in rats. J Physiol. 2003;549:563–571. doi: 10.1113/jphysiol.2003.041665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hall DJ. Han S-H. Chepetan A. Inui EG. Rogers M. Dugan LL. Dynamic optical imaging of metabolic and NADPH oxidase-derived superoxide in live mouse brain using fluorescence lifetime unmixing. J Cereb Blood Flow Metab. 2012;32:23–32. doi: 10.1038/jcbfm.2011.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harte M. Powell S. Swerdlow N. Geyer M. Reynolds G. Deficits in parvalbumin and calbindin immunoreactive cells in the hippocampus of isolation reared rats. J Neural Transm. 2007;114:893–898. doi: 10.1007/s00702-007-0627-6. [DOI] [PubMed] [Google Scholar]
- 68.Hawkley L. Burleson M. Berntson G. Cacioppo J. Loneliness in everyday life: cardiovascular activity, psychosocial context, and health behaviors. J Pers Soc Psychol. 2003;85:105–120. doi: 10.1037/0022-3514.85.1.105. [DOI] [PubMed] [Google Scholar]
- 69.Herman JP. Figueiredo H. Mueller NK. Ulrich-Lai Y. Ostrander MM. Choi DC. Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamic-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 70.Herrman H. Stewart D. Diaz-Granados N. Berger E. Jackson B. Yuen T. What is resilience? Can J Psychiatry. 2011;56:258–265. doi: 10.1177/070674371105600504. [DOI] [PubMed] [Google Scholar]
- 71.Heumüller S. Wind S. Barbosa-Sicard E. Schmidt HHHW. Busse R. Schröder K. Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- 72.Hoge CW. McGurk D. Thomas JL. Cox AL. Engel CC. Castro CA. Mild traumatic brain injury in U.S. soldiers returning from Iraq. N Engl J Med. 2008;358:453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- 73.Hordijk PL. Regulation of NADPH oxidases: the role of Rac proteins. Circ Res. 2006;98:453–462. doi: 10.1161/01.RES.0000204727.46710.5e. [DOI] [PubMed] [Google Scholar]
- 74.Houben JMJ. Moonen HJJ. van Schooten FJ. Hageman GJ. Telomere length assessment: biomarker of chronic oxidative stress? Free Radic Biol Med. 2008;44:235–246. doi: 10.1016/j.freeradbiomed.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 75.Hunt N. McHale S. Psychosocial aspects of andrologic disease. Endocrinol Metab Clin North Am. 2007;36:521–531. doi: 10.1016/j.ecl.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 76.Huo Y. Rangarajan P. Ling E. Dheen S. Dexamethasone inhibits the Nox-dependent ROS production via suppression of MKP-1-dependent MAPK pathways in activated microglia. BMC Neurosci. 2011;12:49. doi: 10.1186/1471-2202-12-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Husain K. Somani S. Persistent/delayed toxic effects of low-dose sarin and pyridostigmine under physical stress (exercise) in mice. Indian J Physiol Pharmacol. 2004;48:150–164. [PubMed] [Google Scholar]
- 78.Ignacchiti M. Sesti-Costa R. Marchi L. Chedraoui-Silva S. Mantovani B. Effect of academic psychological stress in post-graduate students: the modulatory role of cortisol on superoxide release by neutrophils. Stress. 2011;14:290–300. doi: 10.3109/10253890.2010.545459. [DOI] [PubMed] [Google Scholar]
- 79.Infanger D. Sharma R. Davisson R. NADPH oxidases of the brain: distribution, regulation, and function. Antioxid Redox Signal. 2006;8:1583–1596. doi: 10.1089/ars.2006.8.1583. [DOI] [PubMed] [Google Scholar]
- 80.Islam T. Urman R. Gauderman WJ. Milam J. Lurmann F. Shankardass K. Avol E. Gilliland F. McConnell R. Parental stress increases the detrimental effect of traffic exposure on children's lung function. Am J Respir Crit Care Med. 2011;184:822–827. doi: 10.1164/rccm.201104-0720OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jiang F. Zhang Y. Dusting G. NADPH oxidase-mediated redox signaling: roles in cellular stress response, stress tolerance, and tissue repair. Pharmacol Rev. 2011;63:218–242. doi: 10.1124/pr.110.002980. [DOI] [PubMed] [Google Scholar]
- 82.Joseph S. Linley PA. Growth following adversity: theoretical perspectives and implications for clinical practice. Clin Psychol Rev. 2006;26:1041–1053. doi: 10.1016/j.cpr.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 83.Karelina K. DeVries AC. Modeling social influences on human health. Psychosom Med. 2011;73:67–74. doi: 10.1097/PSY.0b013e3182002116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kawakami-Mori F. Shimosawa T. Mu SW H. Ogura S. Yatomi Y. Fujita T. NADPH oxidase-mediated Rac1 GTP activity is necessary for non-genomic actions of the mineralcorticoid receptor in the CA1 region of the rat hippocampus. Am J Physiol Endocrinol Metab. 2012;302:E425–E432. doi: 10.1152/ajpendo.00227.2011. [DOI] [PubMed] [Google Scholar]
- 85.Kawanishi S. Oikawa S. Mechanism of telomere shortening by oxidative stress. Ann N Y Acad Sci. 2004;1019:278–284. doi: 10.1196/annals.1297.047. [DOI] [PubMed] [Google Scholar]
- 86.Khan W. Dechkovskaia A. Herrick E. Jones K. Abou-Donia M. Acute sarin exposure causes differential regulation of choline acetyltransferase, acetylcholinesterase, and acetylcholine receptors in the central nervous system of the rat. Toxicol Sci. 2000;57:112–120. doi: 10.1093/toxsci/57.1.112. [DOI] [PubMed] [Google Scholar]
- 87.Kiecolt-Glaser J. Bane C. Glaser R. Malarkey W. Love, marriage, and divorce: newlyweds' stress hormones foreshadow relationship changes. J Consult Clin Psychol. 2003;71:176–188. doi: 10.1037//0022-006x.71.1.176. [DOI] [PubMed] [Google Scholar]
- 88.Kocalevent R. Hinz A. Brähler E. Klapp B. Determinants of fatigue and stress. BMC Res Notes. 2011;4:238. doi: 10.1186/1756-0500-4-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kozorovitskiy Y. Gould E. Dominance hierarchy influences adult neurogenesis in the dentate gyrus. J Neurosci. 2004;24:6755–6759. doi: 10.1523/JNEUROSCI.0345-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Krause K. Aging: A revisited theory based on free radicals generated by NOX family NADPH oxidases. Exp Gerontol. 2007;42:256–262. doi: 10.1016/j.exger.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 91.Kyriakis J. Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 92.Ladd CO. Thrivikraman KV. Huot RL. Plotsky PM. Differential neuroendocrine responses to chronic variable stress in adult Long Evans rats exposed to handling-maternal separation as neonates. Psychoneuroendocrinology. 2005;30:520–533. doi: 10.1016/j.psyneuen.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 93.Langner T. Life Stress and Mental Health: II. The Midtown Manhattan Study. Oxford, England: Free Press Glencoe; 1963. [Google Scholar]
- 94.Lapiz M. Fulford A. Muchimapura S. Mason R. Parker T. Marsden C. Influence of postweaning social isolation in the rat on brain development, conditioned behavior, and neurotransmission. Neurosci Behav Physiol. 2003;33:13–29. doi: 10.1023/a:1021171129766. [DOI] [PubMed] [Google Scholar]
- 95.Leng A. Feldon J. Ferger B. Long-term social isolation and medial prefrontal cortex: dopaminergic and cholinergic neurotransmission. Pharmacol Biochem Behav. 2004;77:371–379. doi: 10.1016/j.pbb.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 96.Lestaevel P. Romero E. Dhieux B. Ben Soussan H. Berradi H. Dublineau I. Voisin P. Gourmelon P. Different pattern of brain pro-/anti-oxidant activity between depleted and enriched uranium in chronically exposed rats. Toxicology. 2009;258:1–9. doi: 10.1016/j.tox.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 97.Levine A. Worrell TR. Zimnisky R. Schmauss C. Early life stress triggers sustained changes in histone deacetylase expression and histone H4 modifications that alter responsiveness to adolescent antidepressant treatment. Neurobiol Dis. 2012;45:488–498. doi: 10.1016/j.nbd.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li B. Mahan CM. Kang HK. Eisen SA. Engel CC. Longitudinal Health Study of US 1991 Gulf War Veterans: changes in health status at 10-year follow-up. Am J Epidemiol. 2011;174:761–768. doi: 10.1093/aje/kwr154. [DOI] [PubMed] [Google Scholar]
- 99.Li X. Spence JS. Buhner DM. Hart J. Cullum CM. Biggs MM. Hester AL. Odegard TN. Carmack PS. Briggs RW. Haley RW. Hippocampal dysfunction in Gulf war veterans: investigation with ASL perfusion MR imaging and physostigmine challenge. Radiology. 2011;261:218–225. doi: 10.1148/radiol.11101715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Linares Ma. Marin-Garcia P. Mendez Do. Puyet A. Diez A. Bautista JM. Proteomic approaches to identifying carbonylated proteins in brain tissue. J Proteome Res. 2011;10:1719–1727. doi: 10.1021/pr101014e. [DOI] [PubMed] [Google Scholar]
- 101.Linden S. Hess V. Jones E. The neurological manifestations of trauma: lessons from World War I. Eur Arch Psychiatry Clin Neurosci. 2012;262:253–264. doi: 10.1007/s00406-011-0272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu P. Aslan S. Li X. Buhner DM. Spence JS. Briggs RW. Haley RW. Lu H. Perfusion deficit to cholinergic challenge in veterans with Gulf war illness. Neurotoxicology. 2011;32:242–246. doi: 10.1016/j.neuro.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lucassen PJ. Muller MB. Holsboer F. Bauer J. Holtrop A. Wouda J. Hoogendijk WJG. De Kloet ER. Swaab DF. Hippocampal apoptosis in major depression is a minor event and absent from subareas at risk for glucocorticoid overexposure. Am J Pathol. 2001;158:453–468. doi: 10.1016/S0002-9440(10)63988-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lupien SJ. Maheu F. Tu M. Fiocco A. Schramek TE. The effects of stress and stress hormones on human cognition: implications for the field of brain and cognition. Brain Cogn. 2007;65:209–237. doi: 10.1016/j.bandc.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 105.Lyoo IK. Han MH. Cho DY. A brain MRI study in subjects with borderline personality disorder. J Affect Disord. 1998;50:235–243. doi: 10.1016/s0165-0327(98)00104-9. [DOI] [PubMed] [Google Scholar]
- 106.Magariños A. McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: comparison of stressors. Neuroscience. 1995;69:83–88. doi: 10.1016/0306-4522(95)00256-i. [DOI] [PubMed] [Google Scholar]
- 107.Magariños A. McEwen BS. Fluge G. Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci. 1996;16:3534–3540. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Maghzal GJ. Stocker R. Improved analysis of hydroethidine and 2-hydroxyethidium by HPLC and electrochemical detection. Free Radic Biol Med. 2007;43:1095–1096. doi: 10.1016/j.freeradbiomed.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 109.Makino Y. Tanaka H. Dahlman-Wright K. Makino I. Modulation of glucocorticoid-inducible gene expression by metal ions. Mol Pharmacol. 1996;49:612–620. [PubMed] [Google Scholar]
- 110.Marsden CA. King MV. Fone KCF. Influence of social isolation in the rat on serotonergic function and memory—relevance to models of schizophrenia and the role of 5-HT6 receptors. Neuropharmacology. 2011;61:400–407. doi: 10.1016/j.neuropharm.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 111.Marumo T. Schini-Kerth V. Brandes R. Busse R. Glucocorticoids inhibit superoxide anion production and p22 phox mRNA expression in human aortic smooth muscle cells. Hypertension. 1998;32:1083–1088. doi: 10.1161/01.hyp.32.6.1083. [DOI] [PubMed] [Google Scholar]
- 112.McEwen B. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 113.McGraw-Hill. Concise Dictionary of Modern Medicine. NewYork: McGraw-Hill Companies I; 2002. [Google Scholar]
- 114.McIntosh L. Sapolsky R. Glucocorticoids may enhance oxygen radical-mediated neurotoxicity. Neurotoxicology. 1996;17:873–882. [PubMed] [Google Scholar]
- 115.McKittrick C. Magariños A. Blanchard D. Blanchard R. McEwen B. Sakai R. Chronic social stress reduces dendritic arbors in CA3 of hippocampus and decreases binding to serotonin transporter sites. Synapse. 2000;36:85–94. doi: 10.1002/(SICI)1098-2396(200005)36:2<85::AID-SYN1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 116.Miura T. Muraoka S. Ogiso T. Inhibition of lipid peroxidation by estradiol and 2-hydroxyestradiol. Steroids. 1996;61:379–383. doi: 10.1016/0039-128x(96)00044-x. [DOI] [PubMed] [Google Scholar]
- 117.Mohanty P. Hamouda W. Garg R. Aljada A. Ghanim H. Dandona P. Glucose challenge stimulates reactive oxygen species (ROS) generation by leucocytes. J Clin Endocrinol Metab. 2000;85:2970–2973. doi: 10.1210/jcem.85.8.6854. [DOI] [PubMed] [Google Scholar]
- 118.Möller M. Du Preez JL. Emsley R. Harvey BH. Isolation rearing-induced deficits in sensorimotor gating and social interaction in rats are related to cortico-striatal oxidative stress, and reversed by sub-chronic clozapine administration. Eur Neuropsychopharmacol. 2011;21:471–483. doi: 10.1016/j.euroneuro.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 119.Morgan D. Grant K. Prioleau O. Nader S. Kaplan J. Nader M. Predictors of social status in cynomolgus monkeys (Macaca fascicularis) after group formation. Am J Primatol. 2000;52:115–131. doi: 10.1002/1098-2345(200011)52:3<115::AID-AJP1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 120.Morgane PJ. Mokler DJ. Galler JR. Effects of prenatal protein malnutrition on the hippocampal formation. Neurosci Biobehav Rev. 2002;26:471–483. doi: 10.1016/s0149-7634(02)00012-x. [DOI] [PubMed] [Google Scholar]
- 121.Nancy AN. Childhood parental loss and cortisol levels in adult men. Psychoneuroendocrinology. 2004;29:1012–1018. doi: 10.1016/j.psyneuen.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 122.Nguyen D. Alavi MV. Kim KY. Kang T. Scott RT. Noh YH. Lindsey JD. Wissinger B. Ellisman MH. Weinreb RN. Perkins GA. Ju WK. A new vicious cycle involving glutamate excitotoxicity, oxidative stress and mitochondrial dynamics. Cell Death Dis. 2011;2:e240. doi: 10.1038/cddis.2011.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Oikawa S. Tada-Oikawa S. Kawanishi S. Site-specific DNA damage at the GGG sequence by UVA involves acceleration of telomere shortening. Biochemistry. 2001;40:4763–4768. doi: 10.1021/bi002721g. [DOI] [PubMed] [Google Scholar]
- 124.Okamoto K. Tanaka H. Ogawa H. Makino Y. Eguchi H. Hayashi S-i. Yoshikawa N. Poellinger L. Umesono K. Makino I. Redox-dependent regulation of nuclear import of the glucocorticoid receptor. J Biol Chem. 1999;274:10363–10371. doi: 10.1074/jbc.274.15.10363. [DOI] [PubMed] [Google Scholar]
- 125.Pajovic S. Pejic S. Stojiljkovic V. Gavrilovic L. Dronjak S. Kanazir D. Alterations in hippocampal antioxidant enzyme activities and sympatho-adrenomedullary system of rats in response to different stress models. Physiol Res. 2006;55:453–460. doi: 10.33549/physiolres.930807. [DOI] [PubMed] [Google Scholar]
- 126.Pall ML. Common etiology of posttraumatic stress disorder, fibromyalgia, chronic fatigue syndrome and multiple chemical sensitivity via elevated nitric oxide/peroxynitrite. Med Hypotheses. 2001;57:139–145. doi: 10.1054/mehy.2001.1325. [DOI] [PubMed] [Google Scholar]
- 127.Pawate S. Shen Q. Fan F. Bhat NR. Redox regulation of glial inflammatory response to lipopolysaccharide and interferongamma. J Neurosci Res. 2004;77:540–551. doi: 10.1002/jnr.20180. [DOI] [PubMed] [Google Scholar]
- 128.Petersen S. Saretzki G. Zglinicki Tv. Preferential accumulation of single-stranded regions in telomeres of human fibroblasts. Exp Cell Res. 1998;239:152–160. doi: 10.1006/excr.1997.3893. [DOI] [PubMed] [Google Scholar]
- 129.Pickering C. Gustafsson L. Cebere A. Nylander I. Liljequist S. Repeated maternal separation of male Wistar rats alters glutamate receptor expression in the hippocampus but not the prefrontal cortex. Brain Res. 2006;1099:101–108. doi: 10.1016/j.brainres.2006.04.136. [DOI] [PubMed] [Google Scholar]
- 130.Pinheiro M. Ferraz-de-Paula V. Ribeiro A. Sakai M. Bernardi M. Palermo-Neto J. Long-term maternal separation differentially alters serum corticosterone levels and blood neutrophil activity in a/j and C57BL/6 mouse offspring. Neuroimmunomodulation. 2011;18:184–190. doi: 10.1159/000323516. [DOI] [PubMed] [Google Scholar]
- 131.Pirraglia P. Hampton J. Rosen A. Witt W. Psychological distress and trends in healthcare expenditures and outpatient healthcare. Am J Manag Care. 2011;17:319–328. [PMC free article] [PubMed] [Google Scholar]
- 132.Plotsky P. Meaney M. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- 133.Plotsky PM. Thrivikraman KV. Nemeroff CB. Caldji C. Sharma S. Meaney MJ. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology. 2005;30:2192–2204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- 134.Pryce CR. Feldon J. Long-term neurobehavioural impact of the postnatal environment in rats: manipulations, effects and mediating mechanisms. Neurosci Biobehav Rev. 2003;27:57–71. doi: 10.1016/s0149-7634(03)00009-5. [DOI] [PubMed] [Google Scholar]
- 135.Ramanathan L. Gulyani S. Nienhuis R. Siegel JM. Sleep deprivation decreases superoxide dismutase activity in rat hippocampus and brainstem. NeuroReport. 2002;13:1387–1390. doi: 10.1097/00001756-200208070-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rasheed N. Ahmad A. Al-Sheeha M. Alghasham A. Palit G. Neuroprotective and anti-stress effect of A68930 in acute and chronic unpredictable stress model in rats. Neurosci Lett. 2011;504:151–155. doi: 10.1016/j.neulet.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 137.Rivarola MaAl. Suarez MM. Early maternal separation and chronic variable stress in adulthood changes the neural activity and the expression of glucocorticoid receptor in limbic structures. Int J Dev Neurosci. 2009;27:567–574. doi: 10.1016/j.ijdevneu.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 138.Robbers S. van Oort F. Huizink A. Verhulst F. van Beijsterveldt C. Boomsma D. Bartels M. Childhood problem behavior and parental divorce: evidence for gene–environment interaction. Soc Psychiatry Psychiatr Epidemiol. 2012:1–10. doi: 10.1007/s00127-011-0470-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Romans SE. Gendall KA. Martin JL. Mullen PE. Child sexual abuse and later disordered eating: a New Zealand epidemiological study. Int J Eating Disord. 2001;29:380–392. doi: 10.1002/eat.1034. [DOI] [PubMed] [Google Scholar]
- 140.Rosenfeld P. Wetmore JB. Levine S. Effects of repeated maternal separations on the adrenocortical response to stress of preweanling rats. Physiol Behav. 1992;52:787–791. doi: 10.1016/0031-9384(92)90415-x. [DOI] [PubMed] [Google Scholar]
- 141.Rutter M. Psychological sequelae of brain damage in children. Am J Psychiatry. 1981;138:1533–1544. doi: 10.1176/ajp.138.12.1533. [DOI] [PubMed] [Google Scholar]
- 142.Sapolsky R. Potential behavioral modification of glucocorticoid damage to the hippocampus. Behav Brain Res. 1993;57:175–182. doi: 10.1016/0166-4328(93)90133-b. [DOI] [PubMed] [Google Scholar]
- 143.Sapolsky R. The influence of social hierarchy on primate health. Science. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- 144.Sapolsky RM. Is impaired neurogenesis relevant to the affective symptoms of depression? Biol Psychiatry. 2004;56:137–139. doi: 10.1016/j.biopsych.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 145.Schen C. When mothers leave their children behind. Harv Rev Psychiatry. 2005;13:233–243. doi: 10.1080/10673220500243380. [DOI] [PubMed] [Google Scholar]
- 146.Schiavone S. Sorce S. Dubois-Dauphin M. Jaquet V. Colaianna M. Zotti M. Cuomo V. Trabace L. Krause K. Involvement of NOX2 in the development of behavioral and pathologic alterations in isolated rats. Biol Psychiatry. 2009;66:384–392. doi: 10.1016/j.biopsych.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 147.Schwarzer R. The Role of Stressful Life Events. Wiley Online Library; 2001. [Google Scholar]
- 148.Selye H. A syndrome produced by diverse nocuous agents. Nature. 1936;138:32–33. doi: 10.1176/jnp.10.2.230a. [DOI] [PubMed] [Google Scholar]
- 149.Serra M. Pisu M. Floris I. Biggio G. Social isolation-induced changes in the hypothalamic-pituitary-adrenal axis in the rat. Stress. 2005;8:259–264. doi: 10.1080/10253890500495244. [DOI] [PubMed] [Google Scholar]
- 150.Serra M. Pisu MG. Floris I. Cara V. Purdy RH. Biggio G. Social isolation-induced increase in the sensitivity of rats to the steroidogenic effect of ethanol. J Neurochem. 2003;85:257–263. doi: 10.1046/j.1471-4159.2003.01680.x. [DOI] [PubMed] [Google Scholar]
- 151.Serra M. Pisu MG. Littera M. Papi G. Sanna E. Tuveri F. Usala L. Purdy RH. Biggio G. Social isolation-induced decreases in both the abundance of neuroactive steroids and GABAA receptor function in rat brain. J Neurochem. 2000;75:732–740. doi: 10.1046/j.1471-4159.2000.0750732.x. [DOI] [PubMed] [Google Scholar]
- 152.Sheline Y. Gado M. Kraemer H. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160:1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- 153.Sheline Y. Gado M. Price J. Amygdala core nuclei volumes are decreased in recurrent major depression. Neuroreport. 1998;9:2023–2028. doi: 10.1097/00001756-199806220-00021. [DOI] [PubMed] [Google Scholar]
- 154.Shen Q. McQuilkin P. Newburger P. RNA-binding proteins that specifically recognize the selenocysteine insertion sequence of human cellular glutathione peroxidase mRNA. J Biol Chem. 1995;270:30448–30452. doi: 10.1074/jbc.270.51.30448. [DOI] [PubMed] [Google Scholar]
- 155.Sorce S. Krause K-H. NOX enzymes in the central nervous system: from signaling to disease. Antioxid Redox Signal. 2009;11:2481–2504. doi: 10.1089/ars.2009.2578. [DOI] [PubMed] [Google Scholar]
- 156.Sorce S. Schiavone S. Tucci P. Colaianna M. Jaquet V. Cuomo V. Dubois-Dauphin M. Trabace L. Krause K-H. The NADPH oxidase NOX2 controls glutamate release: a novel mechanism involved in psychosis-like ketamine responses. J Neurosci. 2010;30:11317–11325. doi: 10.1523/JNEUROSCI.1491-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ste-Marie L. Hazell AS. Bémeur C. Butterworth R. Montgomery J. Immunohistochemical detection of inducible nitric oxide synthase, nitrotyrosine and manganese superoxide dismutase following hyperglycemic focal cerebral ischemia. Brain Res. 2001;918:10–19. doi: 10.1016/s0006-8993(01)02903-1. [DOI] [PubMed] [Google Scholar]
- 158.Stein MB. Yehuda R. Koverola C. Hanna C. Enhanced dexamethasone suppression of plasma cortisol in adult women traumatized by childhood sexual abuse. Biol Psychiatry. 1997;42:680–686. doi: 10.1016/s0006-3223(96)00489-1. [DOI] [PubMed] [Google Scholar]
- 159.Stroebe W. Abakoumkin G. Stroebe M. Beyond depression: yearning for the loss of a loved one. Omega. 2010;61:85–101. doi: 10.2190/OM.61.2.a. [DOI] [PubMed] [Google Scholar]
- 160.Süer C. Dolu N. Artis AS. Sahin L. Yilmaz A. Cetin A. The effects of long-term sleep deprivation on the long-term potentiation in the dentate gyrus and brain oxidation status in rats. Neurosci Res. 2011;70:71–77. doi: 10.1016/j.neures.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 161.Sundaresan M. Yu Z. Ferrans V. Sulciner D. Gutkind J. Irani K. Goldschmidt-Clermont P. Finkel T. Regulation of reactive-oxygen-species generation in fibroblasts by Rac1. Biochem J. 1996;318:379–382. doi: 10.1042/bj3180379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sung Y-H. Shin M-S. Cho S. Baik H-H. Jin B-K. Chang H-K. Lee E-K. Kim C-J. Depression-like state in maternal rats induced by repeated separation of pups is accompanied by a decrease of cell proliferation and an increase of apoptosis in the hippocampus. Neurosci Lett. 2010;470:86–90. doi: 10.1016/j.neulet.2009.12.063. [DOI] [PubMed] [Google Scholar]
- 163.Tamashiro KLK. Nguyen MMN. Sakai RR. Social stress: from rodents to primates. Front Neuroendocrinol. 2005;26:27–40. doi: 10.1016/j.yfrne.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 164.Tanaka H. Makino Y. Okamoto K. Iida T. Yan K. Yoshikawa N. Redox regulation of the glucocorticoid receptor. Antioxid Redox Signal. 1999;1:403–423. doi: 10.1089/ars.1999.1.4-403. [DOI] [PubMed] [Google Scholar]
- 165.Tarullo AR. Gunnar MR. Child maltreatment and the developing HPA axis. Hormones Behav. 2006;50:632–639. doi: 10.1016/j.yhbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 166.Taylor S. Stanek L. Ressler K. Huhman K. Differential brain-derived neurotrophic factor expression in limbic brain regions following social defeat of territorial aggression. Behav Neurosci. 2011;125:911–920. doi: 10.1037/a0026172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tesler P. Thompson P. Collaborative Divorce: The Revolutionary New Way to Restructure Your Family, Resolve Legal Issues, and Move on with Your Life. NewYork: Regan Books, Harper Collins; 2006. [Google Scholar]
- 168.Tyrka AR. Price LH. Kao H-T. Porton B. Marsella SA. Carpenter LL. Childhood maltreatment and telomere shortening: preliminary support for an effect of early stress on cellular aging. Biol Psychiatry. 2010;67:531–534. doi: 10.1016/j.biopsych.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Umeki S. Soejima R. Hydrocortisone inhibits the respiratory burst oxidase from human neutrophils in whole-cell and cell-free systems. Biochim Biophys Acta. 1990;1052:211–215. doi: 10.1016/0167-4889(90)90078-r. [DOI] [PubMed] [Google Scholar]
- 170.Uysal N. Gonenc S. Acikgoz O. Pekçetin C. Kayatekin BM. Sonmez A. Semin I. Age-dependent effects of maternal deprivation on oxidative stress in infant rat brain. Neurosci Lett. 2005;384:98–101. doi: 10.1016/j.neulet.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 171.Vallet P. Charnay Y. Steger K. Ogier-Denis E. Kovari E. Herrmann F. Michel JP. Szanto I. Neuronal expression of the NADPH oxidase NOX4, and its regulation in mouse experimental brain ischemia. Neuroscience. 2005;132:233–238. doi: 10.1016/j.neuroscience.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 172.Vazquez DM. Eskandari R. Phelka A. Lopez JF. Impact of maternal deprivation on brain corticotropin-releasing hormone circuits: prevention of CRH receptor-2 mRNA changes by desipramine treatment. Neuropsychopharmacology. 2003;28:898–909. doi: 10.1038/sj.npp.1300126. [DOI] [PubMed] [Google Scholar]
- 173.Vazquez DM. Lopez JF. Van Hoers H. Watson SJ. Levine S. Maternal deprivation regulates serotonin 1A and 2A receptors in the infant rat. Brain Res. 2000;855:76–82. doi: 10.1016/s0006-8993(99)02307-0. [DOI] [PubMed] [Google Scholar]
- 174.Violi F. Sanguigni V. Carnevale R. Plebani A. Rossi P. Finocchi A. Pignata C. De Mattia D. Martire B. Pietrogrande M. Martino S. Gambineri E. Soresina A. Pignatelli P. Martino F. Basili S. Loffredo L. Hereditary deficiency of gp91phox is associated with enhanced arterial dilatation results of a multicenter study. Vasc Med. 2009;120:1616–1622. doi: 10.1161/CIRCULATIONAHA.109.877191. [DOI] [PubMed] [Google Scholar]
- 175.von Zglinicki T. Martin-Ruiz C. Telomeres as biomarkers for ageing and age-related diseases. Curr Mol Med. 2005;5:197–203. doi: 10.2174/1566524053586545. [DOI] [PubMed] [Google Scholar]
- 176.Wei L. Simen A. Mane S. Kaffman A. Early life stress inhibits expression of a novel innate immune pathway in the developing hippocampus. Neuropsychopharmacology. 2012;37:567–580. doi: 10.1038/npp.2011.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Weiner M. Meyerhoff D. Neylan T. Hlavin J. Ramage E. Marmar C. Truran D. Chu P. Kornak J. Furlong C. McCarthy C. The relationship between Gulf War illness, brain N-acetylaspartate, and post-traumatic stress disorder. Mil Med. 2011;176:896–902. doi: 10.7205/milmed-d-10-00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Weiss I. Feldon J. Environmental animal models for sensorimotor gating deficiencies in schizophrenia: a review. Psychopharmacology (Berl) 2001;156:305–326. doi: 10.1007/s002130100800. [DOI] [PubMed] [Google Scholar]
- 179.Wilber AA. Wellman CL. Neonatal maternal separation alters the development of glucocorticoid receptor expression in the interpositus nucleus of the cerebellum. Int J Dev Neurosci. 2009;27:649–654. doi: 10.1016/j.ijdevneu.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 180.Wilson DR. Health consequences of childhood sexual abuse. Perspect Psychiatr Care. 2010;46:56–64. doi: 10.1111/j.1744-6163.2009.00238.x. [DOI] [PubMed] [Google Scholar]
- 181.Woolley CS. Gould E. McEwen BS. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990;531:225–231. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- 182.You J. Yun S. Nam K. Kang C. Won R. Lee E. Mechanism of glucocorticoid-induced oxidative stress in rat hippocampal slice cultures. Can J Physiol Pharmacol. 2009;87:440–447. doi: 10.1139/y09-027. [DOI] [PubMed] [Google Scholar]
- 183.Zlotnick C. Mattia J. Zimmerman M. Clinical features of survivors of sexual abuse with major depression. Child Abuse Neglect. 2001;25:357–367. doi: 10.1016/s0145-2134(00)00251-9. [DOI] [PubMed] [Google Scholar]