Abstract
Significance and Recent Advances: Ischemic stroke is the leading cause of disability and third in mortality in industrialized nations. Immediate restoration of cerebral blood flow is crucial to salvage brain tissue, but only few patients are eligible for recanalization therapy. Thus, the need for alternative neuroprotective strategies is huge, and antioxidant interventions have long been studied in this context. Reactive oxygen species (ROS) physiologically serve as signaling molecules, but excessive amounts of ROS, as generated during ischemia/reperfusion (I/R), contribute to tissue injury. Critical Issues: Nevertheless and despite a strong rational of ROS being a pharmacological target, all antioxidant interventions failed to improve functional outcome in human clinical trials. Antioxidants may interfere with physiological functions of ROS or do not reach the crucial target structures of ROS-induced injury effectively. Future Directions: Thus, a potentially more promising approach is the inhibition of the source of disease-promoting ROS. Within recent years, NADPH oxidases (Nox) of the Nox family have been identified as mediators of neuronal pathology. As, however, several Nox homologs are expressed in neuronal tissue, and as many of the pharmacological inhibitors employed are rather unspecific, the concept of Nox as mediators of brain damage is far from being settled. In this review, we will discuss the contribution of Nox homologs to I/R injury at large as well as to neuronal damage in particular. We will illustrate that the current data provide evidence for Nox2 as the most important NADPH oxidase mediating cerebral injury. Antioxid. Redox Signal. 18, 1400–1417.
Introduction
Following heart disease and cancer, stroke is the third leading cause of death in industrialized nations. The World Health Organization reported that estimated 15 million people suffer from an acute cerebrovascular event each year, taking the life of 5.5 million/year and accounting for 5 million permanently disabled patients/year (176).
Ischemic stroke is caused by a sudden cessation of cerebral perfusion due to cerebral artery occlusion, whereas rupture of an intracranial vessel results in hemorrhagic stroke or subarachnoidal hemorrhage. Ischemic stroke accounts for more than 80% of overall strokes. Obviously, rapid and early restoration of cerebral blood flow is pivotal to minimize persistent brain damage in this situation. Currently, reperfusion is mostly achieved by thrombolytic therapy with recombinant tissue plasminogen activator, which has proved to be beneficial within the first 4.5 h after the onset of symptoms (72). Alternatively, mechanical recanalization strategies have been developed, but it is currently open whether this approach is superior to thrombolysis (119). No matter which method is used, rapid reperfusion is the goal of all therapies, and a lack of reperfusion results in bad outcome by all means. It is however assumed that supportive neuroprotective measures might help to extend the narrow time window for clinical beneficial reperfusion therapy (141).
With the restoration of cerebral blood flow and thus supply of glucose and oxygen (O2), oxidation-promoting enzymes and mitochondria are activated and generate an extensive surge of reactive oxygen species (ROS). At low concentrations, ROS act as signaling molecules and contribute to physiological cell functions (42, 92, 101). The excessive ROS formation during reperfusion after ischemic injury, however, leads to overt oxidative stress and contributes to tissue damage (23, 108, 146). Interestingly, the brain appears to be exceptionally vulnerable to oxidative stress due to its already-restricted antioxidant capacity (3). Thus, lowering ROS seemed to be a promising supportive approach in stroke therapy (71). In patients suffering from acute ischemic stroke, however, not a single substance has proved to be effective in improving functional outcome in clinical trials so far (67, 91). Just recently, NXY-059, a spin-trap agent, even though promising in preclinical experiments and in a smaller pilot study, failed to be protective in the confirmatory phase III clinical trial (45, 151). Potential reasons for the failure of antioxidant therapy include interferences with physiological, required functions of ROS, lack of access to target regions and cells of the brain, and failure to reach sufficient concentrations at the site of ROS-mediated damage in cellular subcompartments, the latter being the consequence of low reactivity of the antioxidant or insufficient accumulation within cells as reviewed by (177).
Most of these problems could be overcome if ROS-scavenging therapies would be replaced by approaches lowering ROS generation with specific and effective inhibitors of ROS-generating enzymes. Pathology-associated ROS potentially derive from several enzymatic sources, including xanthine oxidase, cyclooxygenases, or mitochondria (165). ROS-producing NADPH oxidases of the Nox family have been studied extensively in vascular physiological and pathophysiological processes, but also other organs, including the brain. More and more evidence suggests that NADPH oxidases of the Nox family play a major role in function and also in dysfunction of various cell types of the brain (cf. 5.2) (101, 153). In particular, during reoxygenation, NADPH oxidases (Nox) appear to be a main source of ROS production (2).
This review highlights the findings of NADPH oxidase of the Nox family as mediators of cerebral disease in general and in particular of cerebral ischemia/reperfusion (I/R) injury. We particularly will focus on the contribution of individual Nox homologs in this context and analyze the data on pharmacological Nox inhibition regarding their isoform-specific actions.
NADPH Oxidase-Mediated ROS Production
Nox structure and regulation
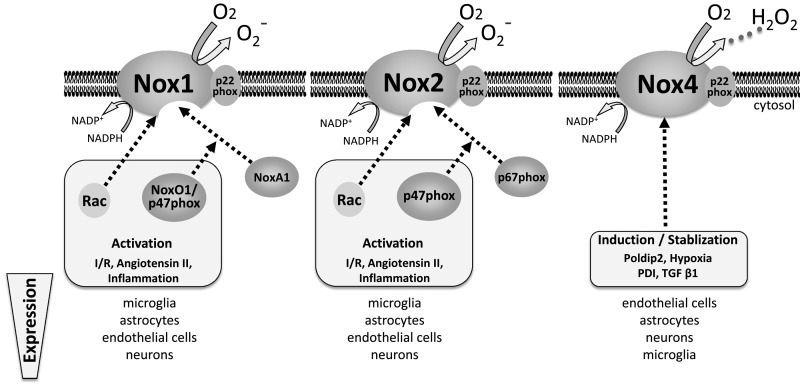
The mammalian Nox family of NADPH oxidases comprises seven members, denominated Nox1 to Nox5, dual oxidase (Duox)-1, and Duox-2. Duox-1 and 2 appear to be expressed predominantly in epithelial cells (15), whereupon disease processes, they might also be induced in other cells. Tissue-specific expression was mainly found for Nox3, which is almost exclusively expressed in the inner ear. The least in vivo studied Nox homolog is Nox5, which due to a gene deletion is missing in mice or rats (63), the working horses of today's animal research. Similar as the Duox enzymes, Nox5 is activated by calcium, and several recent studies suggest that in human cells, Nox5 might be an important source of ROS, for instance, in vascular cells [(121), reviewed by (13)]. As however almost nothing is known regarding possible functions of Duox enzymes, Nox3, and Nox5 in the brain, we will focus this review on Nox1, Nox2, and Nox4 (Fig. 1), which are all expressed in the cardiovascular system and in the brain, and which have all been linked to cerebral disease processes (36, 83, 89). As stroke is also to some extent a vascular disease, analysis of vascular NADPH oxidases is essential for its understanding.
FIG. 1.
NADPH oxidases in the brain: Structure, activation, and expression. Only data for Nox1, Nox2, and Nox4 are shown, as the other Nox protein have basically not been studied in nervous tissue. H2O2, hydrogen peroxide; I/R, ischemia/reperfusion; Nox, NADPH oxidases; O2, oxygen; O2−, superoxide; PDI, protein disulfide isomerase; TGF β1, transforming growth factor β1.
The Nox homologs are named by the large catalytic Nox protein. To achieve catalytic activity, Nox1, Nox2 as well as Nox4 have to form an integral membrane complex with p22phox. Nox2 (formerly known as gp91phox) and Nox1 require additional cytosolic subunits for their activation: the small GTPase Rac, p40phox, p47phox, and p67phox as well as NoxA1 and NoxO1, respectively. Activation of Nox1 and Nox2 usually occurs by agonist stimulation, for instance, with tumor necrosis factor α or angiotensin II (Ang II) through assembly and translocation of the regulatory cytosolic subunits to the membrane. The prototype NADPH oxidase is Nox2, which was first identified in phagocytes as a mediator of host defense. Although data on cell-specific distributions of Nox homologs have become more and more diverse and conflicting, most evidence supports that Nox2 is the predominant Nox homolog of endothelial cells (ECs), cardiomyocytes, and fibroblasts and cells derived from hematopoietic linages, whereas Nox1 dominates in epithelial cells and cancer, but is also expressed in smooth muscle cells (SMCs). In contrast, Nox4, which to our current knowledge does not require cytosolic subunits, is constitutively active and ubiquitously expressed in differentiated cells of all embryonic germ layers. Induction of Nox4 has been demonstrated during differentiation, healing, and in response to hypoxia and transforming growth factor β1 in several cell types, but also after application of cannabidiol in human leukemia cells [reviewed by (92, 120)]. While Nox1 and Nox2 are expressed in the plasma membrane, the subcellular localization of Nox4 has been a matter of longstanding debate. In addition to the nucleus, the plasma membrane, focal adhesions, the cytoskeleton, and the endoplasmic reticulum, Nox4 was also spotted in mitochondria (69, 99), and suggested to crosstalk with ROS production of the respiratory chain (46, 179).
In general, Nox enzymes of the NADPH oxidase family facilitate transport of electrons from NADPH onto O2, resulting in the generation of superoxide (O2−). Duox enzymes and Nox4, however, predominantly produce hydrogen peroxide (H2O2) (47, 109, 148, 158, 160). Although the mechanisms underlying the potentially functional important difference are still unclear, these different products might hint at different functions of Nox1/2 compared to Nox4. For instance, only O2−, but not H2O2, reacts with nitric oxide (NO) to form peroxynitrite (ONOO−), which is a much more powerful oxidant than O2− or H2O2. In contrast, H2O2 was reported to even increase the expression and activity of endothelial nitric oxide synthase (28, 53), and thus some recent data support a protective function of Nox4 in the vascular system (40, 138, 186).
Physiological Functions of Nox Proteins Outside the Brain
Physiologically, Nox-derived ROS either serve as signaling modulators and second messengers [for review see (14, 25)] or fulfill specific functions as reactive intermediates in highly defined specific reactions. The latter comprised Nox2 in host defense (136, 155), Nox3 in otoconia formation of the inner ear (127), or Duox-2 in thyroid hormone synthesis (113). Nox proteins have also been implicated in O2 sensing, but this aspect has not been settled sufficiently, and is a matter of ongoing debate (9, 62, 175, 184). In any case, a link between O2 and Nox proteins has long been sought, in particular, due to the fact that the enzyme itself requires O2, and it is still debated whether only hyperoxia or also hypoxia is accompanied by an increased formation of ROS (62). Biochemically, hypoxia is a rather reducing situation, but not only the redox potential but also in the case of NADPH oxidases, and the pathways leading to ROS formation are subject to alterations under this condition (137). Nevertheless, the evidence that Nox activation occurs at the onset of reperfusion is much stronger than that in response to hypoxia (2).
Nox Proteins and I/R Outside the Brain
A large number of studies demonstrated increased ROS formation during hypoxia/ischemia in general, and there is ample evidence for a surge in ROS generation with reoxygenation/reperfusion. Traditionally, mitochondria have been considered to be the major source of ROS. In addition, a continuously growing number of studies identified Nox proteins as contributors of ROS generation during I/R.
Lung
Several studies reported a role for Nox in I/R injury to the lungs. Al-Mehdi et al. (6) found that I/R-mediated ROS increase in lungs involves the activation of Nox2 in the pulmonary endothelium, and bone marrow transplant experiments and tissue-specific Nox2-deficient mice have meanwhile established a central role of endothelial Nox2 in I/R injury in this organ (174), also obviously and expected, leukocytic Nox2 is also not irrelevant. The fact that I/R lung injury was attenuated, and that the induction of proinflammatory cytokines was reduced in wild-type (WT) mice transplanted with bone marrow from p47phox-deficient mice, but not vice versa, supported this view (180). Furthermore, in the isolated saline-perfused lung, ischemia led to membrane depolarization and ROS generation in pulmonary microvascular ECs mediated by the phosphoinositide-3-kinase/protein kinase B, protein kinase C (PKC) pathway, which is known to activate Nox2 (30). Accordingly, apocynin, an antioxidant and relatively unspecific Nox2 inhibitor, prevented I/R lung injury (133) and decreased vascular permeability in a sheep model of pulmonary injury (50).
Heart
Potentially as the ratio of endothelium to nonendothelial tissue is much smaller, a role of Nox proteins in myocardial I/R injury is less well established than for the lung and rather controversial. Hoffmeyer et al. (78) did not detect a significant difference in the myocardial area at risk or left ventricular function in acute coronary artery occlusion and reperfusion in mice with a mutation of the p47phox subunit of Nox as compared to heterozygous controls. Additional data were derived from models of permanent coronary artery occlusion. Deletion of Nox2 did not affect left ventricular remodeling. In fact, lipid peroxidation products as a marker of oxidative stress and mortality increased in mice lacking a functional Nox2 (61). On the contrary, a significant contribution to adverse cardiac remodeling and contractile dysfunction was found for Nox2 in the murine coronary artery ligation model (106). Moreover, another study suggested a critical role for p47phox after experimental myocardial infarction for left ventricular remodeling/dysfunction and survival (51). Data on the contribution of other Nox isoforms to myocardial dysfunction after infarction or I/R are missing. Moreover, Nox proteins appear to be involved in ischemic preconditioning, which is in itself a situation of I/R: similar as in the brain (98), Nox2 is required to elicit protection by preconditioning in experimental models of myocardial I/R (19, 164). However, without preconditioning, Nox2 deficiency alone failed to affect the infarct size.
Gastrointestinal tract
Data on knockout (KO) mice are still spare for this anatomical region. Pharmacological Nox inhibition with the unspecific apocynin attenuated necrotic and apoptotic cell death, O2− production, and lipid peroxidation in the murine and rat models of hepatic I/R (104, 150). Moreover, apocynin and the Nox2-inhibitory peptide gp91ds-tat (41) prevented remote liver damage in a murine model of bilateral hind limb I/R (52). Similarly, inactivation of Rac-1, a mandatory subunit for Nox2 and Nox1 activation, protected the liver from I/R injury in rats (74). Application of dominant negative Rac in Nox2−/− mice, however, demonstrated that the beneficial effect of Rac inhibition extends beyond the blockade of Nox activation (126), which is conceivable, giving the central role of Rac for cytoskeletal functions. Finally, in the splanchnic artery occlusion and reperfusion model in rats, apocynin attenuated tissue injury, proinflammatory cytokine production, adhesion molecule expression, nitrotyrosine formation, nuclear factor kappa B expression, and apoptosis (132).
Evidence of contribution of Nox2 to human I/R
Although plenty of data on Nox-mediated I/R alterations in various organ systems in rodents or in cultured cells are available, it was just recently that Loukogeorgakis et al. (107) demonstrated that I/R-induced endothelial dysfunction of the brachial circulation is absent in patients carrying inactivating mutations of the Nox2 or p47phox. Accordingly, in vitro anoxia and reoxygenation of human platelets promoted the production of isoprostanes and thromboxane B2 via Nox2-dependent ROS formation and vascular function, as well as NO production by platelets was higher in patients with inactive NADPH oxidase due to mutations (12, 171). These data provided the needed strong support for a role of Nox2 in human I/R damage.
Nox Function in the Brain
Nox expression in the nervous system
Nox homolog expression was observed in all parts of the central and peripheral nervous system. Interestingly, several Nox isoforms appear to be coexpressed in the same cell, but execute different functions. Nox1, Nox2, Nox3, and Nox4 messenger ribonucleic acids (mRNAs) were detected in whole-brain extracts by PCR (10, 83), which obviously provides little information regarding functional significant protein levels. To our knowledge, no data on Nox5 expression in the brain have been published so far, but this might be a consequence of the limited availability of nonrodent brain preparations. A few studies determined Nox1, Nox2, and Nox4 protein and/or mRNA expression in defined cerebral regions, of which most of them focused on Nox2 [reviewed by (153)]; these analyses, however, are far from being exhaustive, and are rather erratic than systemic.
Interestingly, cerebral arteries have been studied in detail, and Nox1, Nox2, Nox4, p22phox, p47phox, p67phox, NoxA1 and NoxO1 were observed to be expressed at the mRNA level, as well as Nox1, Nox2, Nox4 and p22phox on the protein level in mice and rats. Interestingly, Nox4 expression was 10-times higher in the rat basilar artery compared to the aorta, and likewise, Nox2 content as well as Nox activity of cerebral arteries were higher than in systemic arteries. Given that these arteries, however, have a different tissue composition and ratio of endothelium to SMCs and fibroblasts, it is still open whether the difference is functionally important or just a consequence of the different cellularity. Nox4 expression in the basilar artery of male rats exceeded that of female rats (116), and gender differences have previously been noted for the expression of other Nox homologs, too. Thus, care should be taken to study male and female mice separately.
Endothelial cells
Cerebral vascular ECs exhibit high Nox expression: Nox1, Nox2, Nox4, p47phox, p67phox, NoxO1, and NoxA1 transcripts were all observed in cerebral ECs of the rodent brain. Ischemia or Ang II induced Nox2 protein in these cells, whereas endothelial Nox4 protein expression increased in cerebral ECs after stroke or in response to angiogenesis (Table 1) [reviewed by (88)].
Table 1.
NADPH Oxidase Subunit Expression in Cerebral Arteries
| Condition | mRNA/protein | Tissue | Species | Nox subunits | Ref. |
|---|---|---|---|---|---|
| Basal | mRNA | ||||
| Basilar artery | R | Nox1, Nox2, Nox4 p22phox, p47phox | (128) | ||
| Cerebral arteries | R | Nox2, Nox4 p22phox, p47phox, p67phox | (58) | ||
| Microvessels | M | Nox1, Nox2, Nox4 p22phox, p47phox, p67phox | (68) | ||
| ECs | R | Nox1, Nox2, Nox4 p22phox, p47phox, p67phox NoxA1, NoxO1 | (5) | ||
| EC | M | Nox1, Nox2, Nox4 | (89) | ||
| Basal | Protein | ||||
| Basilar artery | R | Nox1, Nox2, Nox4 | (116) | ||
| Basilar artery | R | Nox4 | (117) | ||
| Cerebral arteries | M | Nox2, p47phox | (114) | ||
| Cerebral arteries | M | Nox2 | (115) | ||
| Microvessel | R | Nox2, p22phox | (95) | ||
| ECs | R | Nox2 | (93) | ||
| ECs | M | Nox2 | (93) | ||
| Stimulation | Protein | ||||
| I/R | Microvessel | R | Nox2 | (105) | |
| I/R | Microvessel | M | Nox4 | (96) | |
| I/R | Newly formed capillaries | M | Nox4 | (167) | |
| I/R | ECs | M | Nox2, p47phox | (183) | |
| I/R | ECs | H | Nox4 | (96) |
ECs, endothelial cells; I/R, cerebral ischemia/reperfusion; NADPH, nicotinamide adenine dinucleotide phosphate; Nox, NADPH oxidases; M, mouse; R, rat; H, human; Ref., references
Pericytes
In small cerebral arterioles and capillaries, endothelial arteries are surrounded by pericytes. The cells contribute to the control of vascular tone and contract during stroke and resemble many features of SMCs. Pericytes are thought to mediate small-vessel contraction after reperfusion as a consequence of oxidative/nitrosative stress, leading to calcium overload and Rho kinase activation (181). Cerebral pericytes have, however, not been characterized regarding Nox expression, but data on retina pericytes are available, which potentially could be translated to the brain. Retinal pericytes express Nox2 and p47phox (123, 159), and inhibition of NADPH oxidases reduces ROS formation in these cells (43, 66). Stimulation of isolated rat retinal microvessels with endothelin-1 (ET-1) increased the ROS formation in the surrounding vascular pericytes, an effect, which was blocked by apocynin or PKC inhibition, but not by the mitochondrial complex I inhibitor rotenone, the xanthine oxidase inhibitor allopurinol, or the NO synthase inhibitor N-nitro-l-arginine methyl ester (110). In cultured bovine retinal pericytes, the saturated free fatty acid palmitate, which is frequently elevated in diabetes, increased oxidative stress and apoptosis. Overexpression of dominant negative forms of p47phox, p67phox, or Rac-1 under these conditions attenuated ROS formation and caspase-3 activity, whereas overexpression of a constitutively active Rac-1 enhanced caspase-3 activity (27). Moreover, hyperglycemia increases Nox2 protein levels in pericytes, and apocynin decreased Nox activity, ROS production, and caspase-3 activity in these cells (123). Similarly, cyclic stretch in porcine retinal vascular pericytes, a model for hypertension, induced an apocynin- and diphenylene iodonium (DPI)-sensitive ROS generation. These inhibitors also decreased the stretch-induced phosphorylation of the stress activity kinase c-Jun N-terminal kinase, caspase-3 activity, and apoptosis.
Astrocytes
Astrocytes are attached to the basal vascular membrane in close vicinity to ECs, and data on cultured astrocytes suggest that also these cells express Nox proteins: cultured rat astrocytes contain transcripts of Nox1, Nox2, Nox4, and p47phox, and upon hypo-osmotic stimulation, rapidly produced large amounts of ROS, by a mechanism sensitive to apocynin (140). Also, murine astrocytes express Nox1, Nox2, and Nox4 mRNA (89). On the protein level, Abramov et al. (1) demonstrated the presence of Nox2 and p22phox in membrane fractions of whole-cell lysates from rat primary astrocytes and p40phox, p47phox, p67phox, and Rac in the cytosolic fraction by Western blots. In addition, the presence of p67phox and Nox2 protein in astrocytes was illustrated by immunohistochemistry in primary cultured rat astrocytes and in situ in freshly prepared tissue sections of mice by co-localization staining with the astroglial marker glial fibrillary acidic protein (1). After stimulation with the PKC activator phorbol ester, astrocytic ROS production increased notably by a pathway involving intracellular calcium. Importantly, phorbol ester-induced responses were almost absent in astrocyte cultures of mice lacking a functional Nox2 homolog.
Microglia
As microglial cells are basically tissue-resident macrophages, at least the expression of Nox2 can be inferred. Indeed, microglial cells of adult mice express Nox1, Nox2, and Nox4 mRNA. As compared to ECs, Nox2 expression was greater, whereas Nox4 expression was clearly lower in these myeloid-derived cells (89), and similar observations were made in rat microglia (75). In isolated microglia of newborn mice, Cheret et al. (34) identified Nox1, Nox2, and p22phox mRNA expression as well as that of the cytosolic subunits NoxO1, NoxA1, Rac-1, Rac-2, p40phox, p47phox, and p67phox. Interestingly, Nox4 transcripts were not detectable in microglia of newborn mice. Similar as to rodents, microglial cells isolated from the human fetal brain expressed Nox2, p22phox, p40phox, p47phox, and p67phox transcripts (70).
Not surprisingly, Nox expression, which is controlled by multiple factors, including the tissue environment and the differentiation state of the cell and the organ, differs considerably between primary brain microglial cells and microglia cell lines. For example, the human microglial cell line clone 3 (HMC3) exhibits high expression of Nox4, but basically no Nox2 expression, which is truly the opposite to native microglia (102). Thus, data on Nox in cell lines should be handled with caution.
Neurons
Nox1, Nox2, and Nox4 are also contained in neurons. While investigating the role of NADPH oxidases in apoptotic death of cerebellar granule neurons of rats, Coyoy et al. (39) reported protein expression of p22phox, Nox1, Nox2, and Nox4 and the cytosolic p47phox and p67phox. At the mRNA level, also the expression of p40phox was detected. In primary cultures of murine neurons, at least mRNA expression for Nox1, Nox2, and Nox4 is apparent (89). By immunohistology, Nox2 and p22phox as well as the cytosolic subunits p40, p47phox, and p67phox were detected throughout the brain with particularly high expression in the cortex, the hippocampus, the amygdala, striatum, and the thalamus (149). In resting primary embryonic cortical neurons, the Nox organizer p47phox resided in the cytosol and translocates to the membrane upon N-methyl d-aspartic acid (NMDA) stimulation (24). Also in human neurons, Nox homologs and associated proteins are expressed, as shown for human neuron-enriched hippocampal cultures (129) and human primary cortical cultures of fetal brain tissue (73).
Nox1 and Nox4 were less well studied in these cells: Nox1 transcripts are found in neuronal tissue adjacent to the central nervous system (CNS) like in dorsal nerve roots (82) or in pheochromocytoma PC-12 cell lines (81). Investigating various types of murine CNS tissue, Nox4 expression on the transcriptional and protein level was demonstrated in cortical and hippocampal neurons and cerebellar Purkinje cells. Of note is the fact that not all cortical neurons exhibit colabeling with a Nox4 antibody pointing to differential expression levels in the same cell type possibly due to different functions within the subpopulations (167). In total, however, Nox4 protein expression appears to be low in the healthy human cortex (96).
Collectively, these data demonstrate that all cell types of the brain express Nox homologs and cytosolic activator proteins. Many of the data, however, were obtained by PCR or with the help of human antibodies in rodent tissue, which frequently yield false-positive stainings. The true level of cerebral expression of most Nox-associated proteins and the Nox homologs themselves is therefore still under debate. Eventually, only functional analyses will help understanding whether the detected elements are of physiological importance.
Nox and neuronal dys-/function in diseases of the nervous system
Given the wide distribution of Nox homologs in the brain and the multitude of effects elicited by ROS, it is not surprising that Nox enzymes have been implicated not only in cerebral I/R injury but also in a broad spectrum of very diverse neurological afflictions, which range from physiological signaling of, for example, pain to neurodegeneration like in amyotrophic lateral sclerosis (ALS) or in Alzheimer's disease (AD). Exemplarily, we describe the role of NADPH oxidases in the psychiatric disorder schizophrenia and in the neurodegenerative AD, the latter being associated with neurovascular dysregulation.
Nox and excitation: schizophrenia
In an experimental model of schizophrenia, the repetitive application of ketamine, an NMDA receptor antagonist, leads to dysfunction of a subpopulation of cortical fast-spiking inhibitory interneurons with a loss of parvalbumin and the γ-aminobutyric acid (GABA)-producing enzyme glutamic acid decarboxylase 67 (GAD67) and an increase in O2− production along with augmented p22phox and Nox2 protein. Cotreatment with apocynin prevented these effects and significantly reduced Nox activity (16). The pathophysiological relevance of these findings was further supported by a postmortem study of patients suffering from schizophrenia showing a decrease in GAD67 and NMDA receptor subunit 2A (NR2A), the latter being a regulator of the physiological activity of the NMDA receptor, in a subset of GABAergic interneurons (178). Ketamine-mediated Nox activation and O2− production in the brain with a subsequent loss of interneurons require neuronal production of interleukin-6 (IL-6). Genetic deletion of IL-6 in mice or the removal of IL-6 from the culture medium abolished O2− production and preserved the interneurons (17). The effect elicited by the IL-6/Nox/interneuron pathway is reversible in the adult brain, but not in the developing cortex. Thus, it can alter the postnatal formation of neuronal circuits and might increase the vulnerability to permanent maturational process deficits, resulting in the evolution of schizophrenic symptoms in adolescence [reviewed by (18)]. Recently, Sorce et al. (154) contributed data on the relevance of NADPH oxidases after repetitive ketamine application using Nox2-deficient mice. The KO animals were resistant to ketamine with respect to behavioral alterations and increase in tissue markers of stress. They preserved normal extracellular levels of glutamate and dopamine and did not lose the functionally relevant NR2A subunit of the NMDA receptor in the prefrontal cortex. Thus, Nox2 promotes the development of psychotic symptoms after ketamine application by increasing the excitatory mediators glutamate and dopamine.
Nox and neurodegenerative diseases
Protein aggregation is an important pathomechanism implicated in AD, ALS, and Parkinson's disease (143). As oxidation alters the solubility of proteins, oxidative stress has almost traditionally been linked to neurodegenerative diseases, although the source of ROS has been a matter of longstanding debates. Conversely, some of the oxidized proteins themselves activate and induce Nox proteins as observed in the brains of AD patients (8, 26) or in response to the amyloid β (Aβ) and amyloid precursor protein (APP). In these cases, Nox-derived ROS mediate cerebrovascular dysfunction, activate microglia, and damage neurons [reviewed by (21)]. In mouse models of AD, genetic deletion of Nox2 prevents oxidative stress, improves behavioral deficits and outcome, but does not affect Aβ accumulation. These observations suggest Nox-derived ROS as enhancers of AD plaque toxicity, but not mediators of plaque formation [reviewed by (153)]. Accordingly, in WT, but not Nox2 KO mice, the Nox2 inhibitory peptide gp91ds-tat prevented Aβ-induced vascular ROS production and reversed vascular dysregulation (130, 131). A final aspect leading to neurodegeneration is cerebrovascular dysfunction. Pharmacological inhibition or genetic deletion of Nox2 reduced cerebrovascular oxidative stress and rescued endothelium-dependent relaxation or functional hyperemia in models of Ang II, Aβ, APP, or aging-induced cerebrovascular dysfunction [reviewed by (80, 131)]. Furthermore, Nox2 deficiency restored cerebrovascular reactivity and cognitive function in mice overexpressing APP.
Collectively, all these data suggest that Nox proteins are involved in oxidative stress during neurodegeneration. The data obtained largely in mice may even suggest that Nox proteins contribute to disease progression, but whether this is also the case in humans is currently undetermined.
Nox in Cerebral Ischemia
The role of Nox in cerebral I/R has been studied in several model systems ranging from ex vivo cell culture studies to a broad spectrum of animal experiments. In rodents, the vast majority of researchers use the middle cerebral artery occlusion (MCAO) model, which allows an effective reperfusion and timely as well as locally very defined ischemia.
Nox expression in the course of cerebral I/R
With the aid of the MCAO model, it was observed that Nox2 protein increases from day 1 to 3 after stroke in the penumbra in ECs (183) and microglia (70). Also, the total vascular Nox activity increased in arteries of the penumbra (118). In an analysis of whole hemispheres, rapid changes in Nox expression were noted: ischemia of 2 h increased Nox2 and p22phox mRNA and protein expression in the ischemic hemisphere in rats, an effect which was enhanced in diabetic animals (100). Also, Nox4 expression changes in stroke models: Nox4 mRNA increased early after ET-1 induced stroke in the cortex of rats (112) and in neurons and in newly formed capillaries surrounding the ischemic core (167). Also in vessels, a dramatic induction of Nox4 occurs after stroke (118). Accordingly, Nox4 protein gradually increases in the ischemic cortex and basal ganglia in the hours after stroke in mice and was also found to be elevated in human cerebral vessels after stroke (96) (Table 1). Collectively, these data suggest that Nox2 and Nox4 expression increases after stroke. No data are currently available for Nox1, Nox3, Nox5, and the Duox enzymes, and thus potential expression changes in these homologs remain to be determined.
Nox homologs in stroke: Nox2 is the bad guy!
Fifteen years ago, Walder et al. (172) first studied Nox KO mice in a model of transient focal cerebral ischemia, and these animals were genetically deficient of Nox2 (Table 2). The KO mice were largely protected against the loss of cerebral tissue in an MCAO protocol employing 2 h of ischemia, followed by 22 h of reperfusion. This effect was partially dependent on Nox2 contained in tissue-resident cells, but also in circulating leukocytes, as revealed by bone marrow transplant experiments. Indeed, neutrophils, which contain the highest level of Nox2, are of special importance for O2− production and I/R injury: inhibition of leukocyte adhesion (31, 187) or depletion of neutrophils (111) resulted in smaller lesion volumes after experimental stroke (76). In a recent experiment using the bone marrow transplant approach and Nox2 KO animals, similar results were obtained, although transplantation of Nox2 KO bone marrow into WT mice yielded a much greater protection than in the previous study (163).
Table 2.
Knockout/Knockdown of Specific Nox Isoforms in Experimental Ischemic Stroke
| Genotype | Ischemic period [min] | Assessment of lesion volume after MCAO [h] | Stain | KO protective? | Ref. |
|---|---|---|---|---|---|
| siRNA Nox1 (rat) | 90 | 24 | N.D. | Yes | (35) |
| Nox1y/− | 30 | 24 | Thionin | No | (86) |
| 60 | 24 | TTC | No | (96) | |
| 60 | 24 | TTC | Yes | (89) | |
| 120 | 24 | TTC | No | (89) | |
| Permanent | 24 | TTC | No | (89) | |
| Nox2y/− | 25 | 72 | CV | Yes | (97) |
| 30 | 24 | Thionin | Yes | (44) | |
| 60 | 24 | TTC | No | (96) | |
| 75 | 24 | TTC | Yes | (33) | |
| 120 | 24 | TTC | Yes | (172) | |
| 120 | 24 | TTC | Yes | (90) | |
| 120 | 24 | HE | Yes | (163) | |
| Nox4−/− | 60 | 24 | TTC | Yes | (96) |
| Permanent | 24 | TTC | Yes | (96) |
MCAO, middle cerebral artery occlusion; KO, knockout; CV, cresyl violet; HE, hematoxylin and eosin; N.D., not determined; TTC, 2,3,5-triphenyl-tetrazolium chloride.
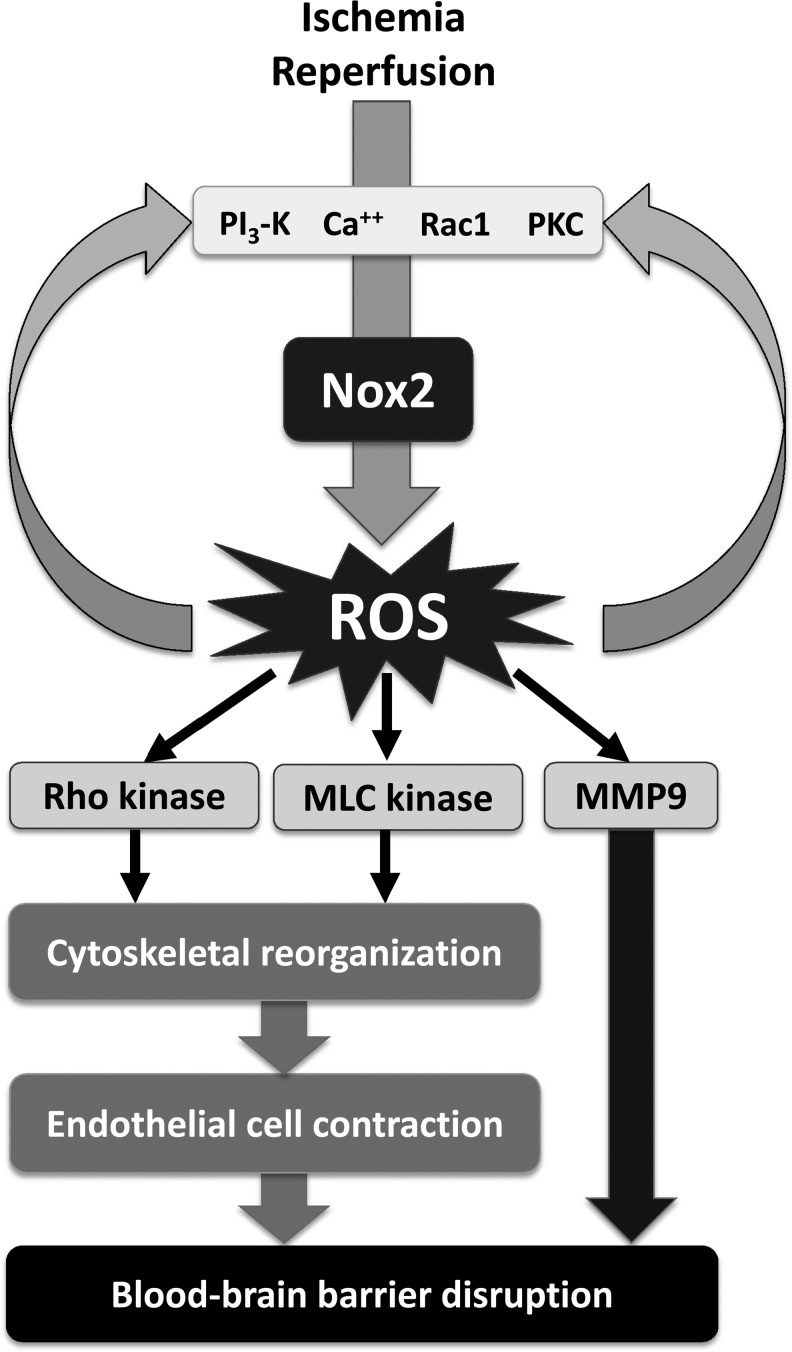
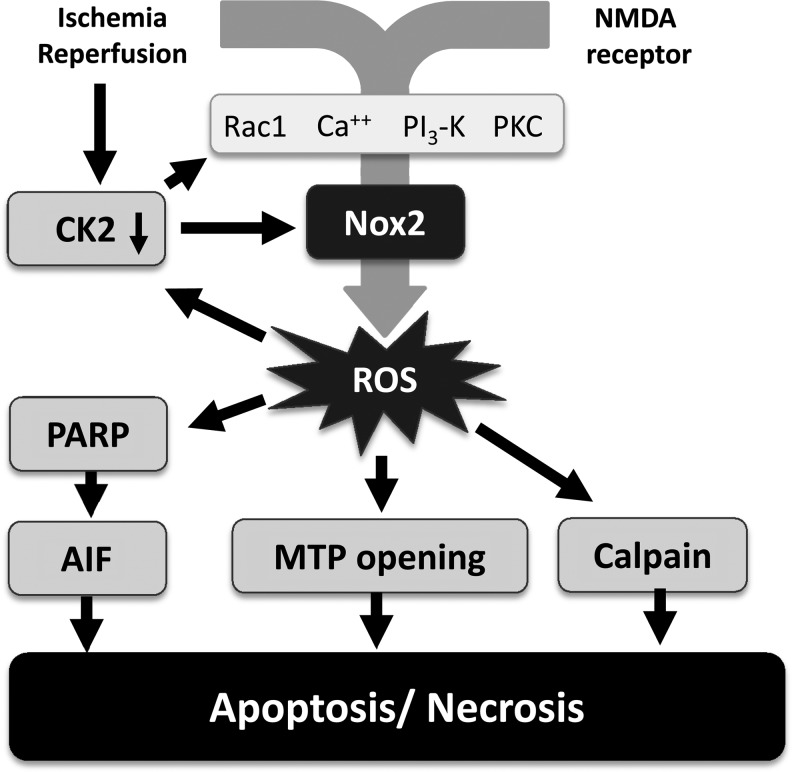
Several subsequent studies confirmed the protective effect of genetic deletion of Nox2 in cerebral I/R injury and strongly support the importance of Nox2 in stroke. In the 2-h ischemia/22-h reperfusion protocol of MCAO, lesion volume, edema formation, as well as early blood–brain barrier (BBB) leakage (Fig. 2) were dramatically attenuated in mice lacking a functional Nox2 protein, the latter most likely due to attenuated Rho kinase activation, stress fiber formation, and subsequent EC contraction (90). In line with these experiments, Chen et al. (33) also found a reduction in the lesion volume at 24 h in Nox2 KO as compared to WT mice, accompanied by an improved neurological function in a protocol using 75 min of cerebral ischemia. Furthermore, the authors also detected a reduction in oxidation products and an early increase in a 150-kDa cleaved spectrin fragment compatible with calcium homeostasis disturbances and calpain-mediated cell necrosis during early reperfusion (3 h). After 24 h of reperfusion, 150-kDa and 120-kDa cleaved spectrin fragments became apparent pointing to calpain-mediated necrosis and caspase-mediated apoptosis, also late after reperfusion (Fig. 3). Nox2 deficiency attenuated the postischemic inflammation demonstrated by an attenuated induction of intercellular adhesion molecule-1 and a reduced neutrophil infiltration into the brain as reflected by the neutrophil-specific marker myeloperoxidase (MPO). A subsequent study from the same group confirmed the neuroprotection in the MCAO model at 24 h and 72 h after 1 h of brain ischemia in Nox2 KO mice and also observed an attenuation of postischemic inflammatory gene expression and a decrease in the abundance of products of oxidative stress (32). The protective effect of Nox2 deficiency was also present with shorter ischemic periods. Similarly, a recent report stated significantly smaller infarcts at 24 h after a 30 min ischemic period in Nox2 KO mice in comparison to WT animals, and also this was associated with attenuated O2− generation, increased NO availability, and reduced nitrosative stress (44). Comparing WT and Nox2 KO mice 3 days after 25 min MCAO, Kunz et al. (97) observed a 44% reduction in ROS production and 61% smaller lesion volume in animals deficient of Nox2 compared to WT controls. Accordingly, genetic deletion of p47phox, which is required for the assembly and activation of Nox2, was neuroprotective in vivo and in vitro (157). The importance of Nox2 in the context of stroke is strongly supported by in vitro data from murine hippocampal neurons lacking a functional Nox2, which exhibit a substantial attenuated ROS generation when exposed to oxygen/glucose deprivation (OGD). Genetic deletion of Nox2 also attenuated the intermittent hypoxia-induced cognitive deficits and markers of oxidative stress in mice (125). Finally, Nox2 deficiency reduced neuronal death, oxidative injury, and microglial activation in a model of murine transient global cerebral ischemia (183) and attenuated neuronal cell death within the ganglion cell layer in a mouse model of retinal I/R (182).
FIG. 2.
Nox-mediated blood–brain barrier disruption in cerebral ischemia/reperfusion. Ischemia/reperfusion activates elements upstream of Nox2, including PI3-kinase, PKC, and calcium. The subsequent ROS formation not only activates downstream targets involved blood brain–barrier opening but also promotes further stimulation of the elements upstream of Nox2: ROS are known to increase calcium and to activate PKC, PI3-kinase, and Rac. MLC kinase, myosin light-chain kinase; MMP, matrix metalloproteinase; PKC, protein kinase C; ROS, reactive oxygen species.
FIG. 3.
Nox-mediated neuronal cell death in cerebral ischemia/reperfusion. Ischemia/reperfusion as well as NMDA receptor activation increase intracellular calcium and other elements involved in Nox2 activation, which leads to subsequent ROS formation. As illustrated for the ROS-dependent inactivation of casein kinase 2, several positive feedback loops further increase ROS formation in a vicious circle entertained by an oxidation of target molecules. Apoptosis is eventually initiated by energy breakdown through PARP and activation of apoptosis-inducing factors as well as MTP opening. Necrosis can also occur, for example, as a consequence of the calcium- and ROS-mediated activation of the protease calpain by proteolysis, which subsequently cleaves a large number of cellular proteins with high activity. MTP, mitochondrial transition pore; NMDA, N-methyl d-aspartic acid; PARP, poly-ADP ribose polymerase.
Interestingly, despite these findings, Nox2 is also required for preconditioning as shown for ethanol- and lipopolysaccharide-preconditioning protocols (98, 173). As however preconditioning is at least in part mediated by ROS, these data do not contradict the detrimental action of Nox2 in stroke.
In contrast to the reported beneficial effects of Nox2 deficiency in multiple studies of experimental stroke, a recent study was not able to reproduce the neuroprotection in mice lacking a functional Nox2 homolog after 1-h MCAO and 23 h of reperfusion (96). The reason for this discrepant result is unknown, as it would require in detail comparison of the experimental protocols side by side and a careful analysis of the health status and the genetic background of the mice, which has not been carried out. Although representing a revolutionary approach to study mechanisms in vivo, engineered mice are not without problems. As reconstitution experiments are rarely performed, it often remains unclear, whether an observed effect in such an animal is truly the consequence of the altered gene, but not of additional changes introduced into the genome (22). Moreover, the genetic background has an often underestimated influence on experimental readouts and may influence experiments even if backcrossing was performed for more than 10 generations. If a genetically altered line is bred in a homozygous fashion for a long time, genetic and epigenetic drift occurs, which influences the results. This aspect is of great importance for the Nox2y/− mice, as the immune deficit in these animals renders them susceptible to infection and thus increases the selection pressure. Indeed, we observed that the susceptibility of our Nox2y/− line to some bacteria decreased in the course of breeding and so switched our colony from a homozygous to heterozygous breeding pattern to prevent such a drift. The aspect of infection in Nox2y/− mice might also strongly influence the outcome in stroke models. Infections may have a preconditioning effect, and thus may potentially decrease the stroke size. Also, with respect to the selection bias mentioned above, the fitter animals will be selected in an unclear environment and may also experience smaller strokes. Thus, approaches that rescue the phenotype such as exogenous application of the protein produced or bone marrow transplants would provide a strong aid to control these aspects. Moreover, it is desirable to confirm data from genetically altered animals by pharmacological inhibitors or biologicals [further recommended reading: (54, 55)]. The discovery of specific Nox inhibitors in the future will substantially complement and further clarify the current, occasionally inconsistent results obtained by the use of KO mice.
Even though expression levels of Nox1 and Nox4 have been detected in ECs of cerebral arteries (4) and the brain at large, only few studies investigated the role of Nox1 in experimental stroke. Two studies in Nox1-deficient mice failed to show neuroprotection in models of 0.5 h and 1 h of MCAO and reperfusion at 24 h after induction of ischemia (86, 96), whereas in a rat transient MCAO (1 h) model, knockdown of Nox1 by adenovirus-mediated transduction of Nox1 small hairpin RNA significantly reduced the lesion size and attenuated neuronal cell death. Likewise, ROS generation, Nox1 expression, 8-hydroxy-2′-deoxyguanosine immunoreactivity, and cell death were reduced when applying the same knockdown approach to primary rat cortical neuron cultures exposed to OGD and reoxygenation (35). In our hands, 1 h MCAO followed by 23 h of reperfusion resulted in an better neurological functional outcome and in a moderate reduction in lesion volume, cerebral edema, and BBB leakage in Nox1 KO compared to WT mice. Whether this neuroprotection also remains stable in the days following the stroke, or whether the lesion volume of KO and WT controls converges later, needs to be established. However, the neuroprotective effect of Nox1 deficiency disappears if ischemia extends beyond 1 h (89). Given the narrow window for a potential pharmacological intervention, Nox1 therefore does not appear to be a promising target for stroke treatment.
Also, the role of Nox4 is still obscure. An observational study in a mouse model of permanent focal cerebral ischemia identified a dramatic increase in cortical neuronal Nox4 expression detectable at 24 h and persisting throughout the 30-day follow-up period with maximum days ranging 7–15. Nox4 protein was detected in newly formed capillaries corresponding to the peak around day 7 (167) pointing to a potential role for Nox4 in stroke repair. The only experimental stroke study in Nox4-deficient mice to date (96) revealed an impressive protection at 24 h after stroke induction in the 1-h and permanent MCAO model. This effect was independent of age or gender. Restoring oxidative stress by adding H2O2 abolished the stroke protective phenotype in Nox4 KO mice. In a different Nox4 KO strain, we failed to observe any protection in the MCAO model (Kahles 2008 unpublished observation), and indeed, the rational for Nox4 being an important mediator of acute stroke is weak: on the protein level, Nox4 is basically undetectable in the brain and has to be induced in response to stroke first (96). Thus, at time of reperfusion when the vast of the damage occurs, Nox2 expression largely dominates over that of Nox4. In vascular and cardiac injury models, more and more studies suggest that Nox4 can be protective (40, 96, 138) as it is unable of generating ONOO− (160), an important mediator of tissue damage and rather promotes NO formation. Thus, additional studies have to be carried out to address the potential of Nox4 as a mediator of cerebral injury in stroke.
Collectively, these data provide a strong basis of an important role of Nox2 in I/R damage of the brain. Although Nox1 and Nox4 might not be unimportant under certain experimental situations, their overall impact appears to be much less, and confirmatory data supporting the functional importance of these Nox homologs still have to be sought.
Nox inhibition in stroke: a quest of specificity?
Although genetically modified mice considerably broadened our understanding of the contribution of NADPH oxidases to stroke pathology, specific pharmacological inhibition of Nox proteins would not only confirm and complement the findings, but it would also open up the field to translational studies. Obviously, specific Nox inhibitors, once developed, would be potential candidates for stroke therapy. Several drugs, which are used to treat common cardiovascular/stroke risk factors like hypertension or hyperlipidemia, interfere with NADPH oxidase induction or activation. Apart from their primary blood pressure- or lipid-lowering effects, these agents appear to elicit beneficial, pleiotropic effects in stroke possibly linked to inhibition of NADPH oxidases and have some effect in cerebrovascular diseases.
Lipid-lowering HMG-CoA reductase inhibitors, also termed statins, act on small GTPases like Rac-1. Statin treatment abolishes the geranylgeranylation of Rac-1 and prevents the translocation of Rac-1 from the cytosol to the membrane, which is an essential step in Nox activation. A multitude of experimental stroke studies demonstrated a neuroprotective effect of statins independent of whether treatment is initiated before (57, 79, 90, 166) or after the cerebrovascular event [reviewed by (11)] and also independent of the lipid-lowering potential (pleiotropic effects). In line with the experimental data, statins are beneficial in the primary and secondary prevention of ischemic stroke in humans (7, 37). Their effect, however, is rather modest, and future trials have to investigate whether the protection occurs class wide with all statins or is substance specific. Of note is the fact that withdrawal of pre-existing statin treatment led to impairment of endothelial function (169) and resulted in increased mortality and morbidity in patients with acute coronary syndrome and ischemic stroke (20, 56). Furthermore, statin use early in stroke hospitalization was strongly associated with improved survival after stroke (60). Despite these positive observations, statins act on multiple targets, and thus the observed effects are probably not just a consequence of NADPH oxidase inhibition.
The renin-angiotensin-aldosterone-system (RAAS) also is a potential pharmacological target for the treatment of ischemic stroke with a link to NADPH oxidases: Ang II and aldosterone induce and activate Nox proteins in vessels and even in defined regions of the brain (29, 134, 189). In a model of cerebral I/R in rats, candesartan, a representative of the angiotensin II type-1 receptor blockers, reduced products of oxidative stress, p22phox, and Nox2 mRNA induction as well as tissue injury and concomitantly improved functional outcome (100). Furthermore, candesartan attenuated the prothrombotic platelet-leukocyte-EC interaction in cerebral vessels after bilateral common carotid artery occlusion and reperfusion and attenuated the I/R-associated increase in ROS (84). Aliskiren, a new renin inhibitor, appears to be as effective as candesartan in cerebral I/R in rats, as it significantly reduced mortality, improving functional outcome as compared to WT controls. This neuroprotective effect was independent of blood pressure and might be related to a decrease in proinflammatory gene expression in the ischemic core (147). So far, the clinical data on RAAS inhibition yielded conflicting results ranging from beneficial to no effect [reviewed by (144), (145, 185)]. Additional studies are therefore needed to further evaluate the concept of RAAS inhibition in stroke and to better establish the link to Nox proteins.
DPI is a widely used potent, yet unspecific, NADPH oxidase inhibitor for in vitro experiments. Data of DPI in animal models of stroke are scarce due to its toxicity (e.g., hypoglycemia and cardiomyopathy), irreversible binding to the flavin adenine nucleotide of Nox, broad range of unspecific effects, and its low solubility (87). In a rat model of focal cerebral I/R (124), dimethyl sulfoxide (DMSO) was therefore used as a solvent for DPI, and structural and functional outcome was assessed up to 48 h after stroke in DMSO-treated, DMSO/DPI-treated, and untreated animals. Lesion volume and functional outcome were significantly reduced in all the treated animals. I/R injury at 24 h and 48 h following stroke, however, was not different between DMSO- and DMSO/DPI-treated animals, pointing to the well-known protective effect of the antioxidant DMSO, which was obviously not further outperformed by DPI.
A large number of Nox inhibitor studies in experimental stroke have been undertaken with the natural organic compound apocynin. Experimental settings differ considerably in terms of time and route of administration, dose, model of focal I/R, ischemic period, species, and age of animals (Table 3). Overall, apocynin showed to be protective if applied before reperfusion or at the latest with reperfusion. Post-treatment revealed no beneficial effect. Even with pretreatment, some authors observed an increase in mortality when higher doses of apocynin were used (85), or when specific experimental conditions different from the conventional MCAO model were applied: in a pharmacological recanalization model, where external tissue plasminogen activator dissolves an intravascular clot, apocynin had no positive effect when aged female rats were studied, whereas in young female animals, neuroprotection occurred (94). In addition, Tang et al. (162) reported an increase in intracerebral hemorrhage with higher doses of apocynin. The interpretation of these studies is further complicated by the varying modes of action, application, and dose of apocynin, as well as the specific pathophysiology associated with the disease model used: at low concentrations, apocynin appears to be relatively specific as an inhibitor for the interaction of Nox2 and p47phox in cells containing MPO, thus primarily neutrophils (156, 168). The mode of action of apocynin in other cells is still debated controversially. It is not known whether glycocalix-bound MPO is sufficiently active to activate apocynin in the vascular system in the absence of leukocytes, or whether other peroxidases like cyclooxygenase can activate apocynin. Apocynin also has complex unspecific effects. In high concentrations, it acts as an antioxidant (77), and once activated, can even act as a pro-oxidant and induces oxidative stress (142, 170). With respect to stroke, this could result in a protective preconditioning effect. Importantly, apocynin also lowers intracellular calcium (59, 137), which is a key factor in mediating excitotoxicity (49). Nevertheless, the protective effect of apocynin was not observed in Nox2 KO mice (85), and this at least provides a strong support for a central role of Nox2 in I/R-induced brain damage. Taken together, apocynin is a potent neuroprotecting agent if applied before or at time of reperfusion. Importantly, in relation to stroke onset, apocynin was effective even when given as long as 2 h after induction of ischemia (163). A standardized approach comparing various ischemic periods and doses and addressing the safety issues related to gender and age (94) is mandatory before these experimental data can be translated into the clinical setting.
Table 3.
Pharmacological Nox Inhibition with Apocynin in Focal Cerebral Ischemia/Reperfusion
| Time of admin.a | Route of admin. | Dose [mg/kg] | Ischemic period [min] | Spec. | Geno-type | Time of lesion assessment [h] | Prot.? | Ref. |
|---|---|---|---|---|---|---|---|---|
| −1 month period | po | 7.5 daily | 120 | Rb | n/a | 24 | No | (188) |
| −3 h | iv | 0.4/4.0 | 120 | M | WT | 24c | No | (90) |
| −3 h | iv | 40.0 | 120 | M | WT | 24c | Yes | (90) |
| ≤−2.5 h | ip | 5.0 | ≥120d | R | Young | 24 | Yes | (94) |
| ≤−2.5 h | ip | 5.0 | ≥120d | R | Aged | 24 | No | (94) |
| −2.5 h | ip | 30.0 | 90 | R | N/A | 24c | Yes | (105) |
| −2.5 h | ip | 5.0b | 120 | R | N/A | 24 | Yes | (188) |
| −2 h &+5 min | ip | 5.0 | 90 | R | N/A | 24 | Yes | (122) |
| −1 h | ip | 2.5 | 30 | M | WT | 24 | Yes | (85) |
| −1 h | ip | 5.0 | 30 | M | WT | 24 | No | (85) |
| −1 h | ip | 2.5 | 30 | M | Nox2y/− | 24 | No | (85) |
| −1 h | iv | 1.0/5.0 | 30e | R | N/A | 6 | No | (38) |
| −1 h | iv | 10.0/20.0 | 30e | R | N/A | 6 | Yes | (38) |
| −0.5 h | ip | 50.0 | 90 | R | N/A | 24 | Yes | (161) |
| −0.5 h | iv | 2.5 | 120 | M | WT | 24 | Yes | (162) |
| −0.5 h | iv | 3.75/5.0 | 120 | M | WT | 24 | No | (162) |
| −5 min | ip | 5.0 | 120 | R | N/A | 24 | Yes | (65) |
| −5 min | ip | 4.0 | 75 | M | WT | 24 & 72 | Yes | (33) |
| 0 | iv | 2.5 | 120 | M | WT | 24 | Yes | (163) |
| 0 | iv | 2.5 | 120 | M | Nox2y/− | 24 | No | (163) |
| 0 | iv | 20.0 | 30e | R | N/A | 6 | Yes | (38) |
| 0 | iv | 0.1mg | 60 | M | WT | 24 | No | (96) |
| +0.5 h | iv | 20 | 30e | R | N/A | 6 | No | (38) |
| +1 h | ip | 2.5 | 30 | M | WT | 24 | No | (85) |
Relative to start of reperfusion (− is before, + is after, and 0 is at the time of reperfusion).
Fed a liquid diet for 8 weeks.
Blood–brain barrier damage/brain edema.
Clot MCAO model in female rats: tPA at 2 h.
Distal transient MCAO.
Admin., administration; iv, intravenous; ip, intraperitoneal; KO, knockout; MCAO, middle cerebral artery occlusion; Prot., protection; Spec., species; tPA, tissue plasminogen activator; WT, wild type.
Other natural compounds like honokiol or plumbagin also inhibited Nox activity or expression and attenuated tissue injury after experimental stroke (48, 103, 152). As these compounds are, however, not well characterized, a link between Nox inhibition and neuroprotection cannot be inferred, so far. Nevertheless, these data suggest that in rodent models of cerebral I/R, salvage of tissue is possible with inhibitors at least acting on the redox status of the cell and potentially on the Nox system.
Due to the tremendous clinical potential of specific Nox inhibitors in several disease indications, including cardiovascular diseases, several initiatives currently develop small-molecule inhibitors. Data on these new, more specific Nox inhibitors, like the compounds from GenKyoTex, are not available for stroke, yet although a peptide inhibitor against Nox2 conferred some protection (139) in a model of global cerebral ischemia. Most of the stroke experimental studies pretreated animals before the onset of ischemia, as this approach overcomes the significant problem of drug delivery during stroke. It also however puts the experimental treatment in a situation more favorable than the clinical scenario. Already in the past, this consideration was used to explain why so many compounds exert protection in animal experiments, but not in humans. Indeed, in those studies in which Nox inhibitors were applied at time of reperfusion or even later, the protective effect was greatly attenuated as, for example, shown for the beneficial effect of statin treatment (135). Nevertheless, apocynin appears to be an extraordinary promising drug in acute stroke treatment, even when given after stroke onset, but these observations still await confirmation in future standardized trials. In addition, just recently, a strong protective effect against stroke in the MCAO model was observed with intrathecal application of the compound VAS2870 (96). Unfortunately, up to now, it is not known how VAS2870 works. In HEK293 cells overexpressing Nox4, the compound does not inhibit ROS production (Brandes RP 2010, unpublished observation) and in a recent analysis, it was concluded that the compound does not work at all on the enzymatic Nox complex, but rather on upstream signaling (64).
Conclusion
ROS profoundly contribute to tissue damage after I/R in several organs, including the brain. A large number of experimental studies provide evidence that, in particular, Nox2 is a major source of ROS in the brain, although Nox1 and Nox4 are not unimportant. Specific Nox inhibitors and high-quality Nox antibodies are not readily available, and data on Nox function in ischemic stroke are predominantly derived from mouse experiments. Given the small brain of the mouse and therefore the relatively large penumbra in these animals, it is questionable whether these data can be readily translated into the human situation. Most in vivo studies investigated the role of Nox2. Overall, genetic deletion of Nox2 elicited strong neuroprotection with a reduced lesion volume, attenuated BBB breakdown, and improved functional outcome. In contrast, only few studies dealt with Nox1 in stroke; the results are heterogeneous and point to an effect restricted to a small time window at the most. Just recently, Nox4 has been suggested to be involved in the pathophysiology of stroke; the particular study however could be criticized for the failure to reproduce previous data on Nox2 and stroke and for the uncertainties regarding the molecular mechanism of action and specificity of VAS2870. Thus, for the time being, any Nox inhibitory strategy for the treatment of stroke should focus on Nox2.
Abbreviations Used
- Aβ
amyloid β
- AD
Alzheimer`s disease
- ALS
amyotrophic lateral sclerosis
- Ang II
angiotensin II
- APP
amyloid precursor protein
- BBB
blood–brain barrier
- CNS
central nervous system
- CV
cresyl violet
- DMSO
dimethyl sulfoxide
- DPI
diphenylene iodonium
- Duox
dual oxidase
- ECs
endothelial cells
- ET-1
endothelin
- GABA
γ-aminobutyric acid
- GAD67
glutamic acid decarboxylase 67
- HE
hematoxylin eosin
- HMC3
human microglial cell line clone 3
- H2O2
hydrogen peroxide
- IL-6
interleukin-6
- ip
intraperitoneal
- I/R
ischemia/reperfusion
- iv
intravenous
- KO
knockout
- MCAO
middle cerebral artery occlusion
- MLC kinase
myosin light-chain kinase
- MMP
matrix metalloproteinase
- MPO
myeloperoxidase
- mRNA
messenger ribonucleic acid
- MTP
mitochondrial transition pore
- NADPH
nicotinamide adenine dinucleotide phosphate
- NMDA
N-methyl d-aspartic acid
- NO
nitric oxide
- Nox
NADPH oxidases
- NR2A
NMDA receptor subunit 2A
- O2
oxygen
- O2−
superoxide
- OGD
oxygen/glucose deprivation
- ONOO−
peroxynitrite
- PARP
poly-ADP ribose-polymerase
- PKC
protein kinase C
- RAAS
renin-angiotensin-aldosterone-system
- ROS
reactive oxygen species
- SMCs
smooth muscle cells
- TGF-β1
transforming growth factor-β1
- tPA
tissue plasminogen activator
- TTC
2,3,5-triphenyl-tetrazolium chloride
- WT
wild type
Acknowledgments
This work was supported by the German Research Foundation (DFG) (grants KA2279/4-1, SFB834, and SFB815) as well as the DFG cluster of excellence ECCPS.
References
- 1.Abramov AY. Jacobson J. Wientjes F. Hothersall J. Canevari L. Duchen MR. Expression and modulation of an NADPH oxidase in mammalian astrocytes. J Neurosci. 2005;25:9176–9184. doi: 10.1523/JNEUROSCI.1632-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abramov AY. Scorziello A. Duchen MR. Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J Neurosci. 2007;27:1129–1138. doi: 10.1523/JNEUROSCI.4468-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adibhatla RM. Hatcher JF. Lipid oxidation and peroxidation in CNS health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2010;12:125–169. doi: 10.1089/ars.2009.2668. [DOI] [PubMed] [Google Scholar]
- 4.Ago T. Kitazono T. Kuroda J. Kumai Y. Kamouchi M. Ooboshi H. Wakisaka M. Kawahara T. Rokutan K. Ibayashi S. Iida M. NAD(P)H oxidases in rat basilar arterial endothelial cells. Stroke. 2005;36:1040–1046. doi: 10.1161/01.STR.0000163111.05825.0b. [DOI] [PubMed] [Google Scholar]
- 5.Ago T. Kitazono T. Ooboshi H. Iyama T. Han YH. Takada J. Wakisaka M. Ibayashi S. Utsumi H. Iida M. Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation. 2004;109:227–233. doi: 10.1161/01.CIR.0000105680.92873.70. [DOI] [PubMed] [Google Scholar]
- 6.Al-Mehdi AB. Zhao G. Dodia C. Tozawa K. Costa K. Muzykantov V. Ross C. Blecha F. Dinauer M. Fisher AB. Endothelial NADPH oxidase as the source of oxidants in lungs exposed to ischemia or high K+ Circ Res. 1998;83:730–737. doi: 10.1161/01.res.83.7.730. [DOI] [PubMed] [Google Scholar]
- 7.Amarenco P. Benavente O. Goldstein LB. Callahan A., 3rd Sillesen H. Hennerici MG. Gilbert S. Rudolph AE. Simunovic L. Zivin JA. Welch KM. Results of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial by stroke subtypes. Stroke. 2009;40:1405–1409. doi: 10.1161/STROKEAHA.108.534107. [DOI] [PubMed] [Google Scholar]
- 8.Ansari MA. Scheff SW. NADPH-oxidase activation and cognition in Alzheimer disease progression. Free Radic Biol Med. 2011;51:171–178. doi: 10.1016/j.freeradbiomed.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Archer SL. Reeve HL. Michelakis E. Puttagunta L. Waite R. Nelson DP. Dinauer MC. Weir EK. O2 sensing is preserved in mice lacking the gp91 phox subunit of NADPH oxidase. Proc Natl Acad Sci U S A. 1999;96:7944–7949. doi: 10.1073/pnas.96.14.7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banfi B. Malgrange B. Knisz J. Steger K. Dubois-Dauphin M. Krause KH. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J Biol Chem. 2004;279:46065–46072. doi: 10.1074/jbc.M403046200. [DOI] [PubMed] [Google Scholar]
- 11.Baryan HK. Allan SM. Vail A. Smith CJ. Systematic review and meta-analysis of the efficacy of statins in experimental stroke. Int J Stroke. 2012;7:150–156. doi: 10.1111/j.1747-4949.2011.00740.x. [DOI] [PubMed] [Google Scholar]
- 12.Basili S. Pignatelli P. Tanzilli G. Mangieri E. Carnevale R. Nocella C. Di Santo S. Pastori D. Ferroni P. Violi F. Anoxia-reoxygenation enhances platelet thromboxane A2 production via reactive oxygen species-generated NOX2: effect in patients undergoing elective percutaneous coronary intervention. Arterioscler Thromb Vasc Biol. 2011;31:1766–1771. doi: 10.1161/ATVBAHA.111.227959. [DOI] [PubMed] [Google Scholar]
- 13.Bedard K. Jaquet V. Krause KH. NOX5: from basic biology to signaling and disease. Free Radic Biol Med. 2012;52:725–734. doi: 10.1016/j.freeradbiomed.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 14.Bedard K. Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 15.Bedard K. Lardy B. Krause KH. NOX family NADPH oxidases: not just in mammals. Biochimie. 2007;89:1107–1112. doi: 10.1016/j.biochi.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Behrens MM. Ali SS. Dao DN. Lucero J. Shekhtman G. Quick KL. Dugan LL. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318:1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- 17.Behrens MM. Ali SS. Dugan LL. Interleukin-6 mediates the increase in NADPH-oxidase in the ketamine model of schizophrenia. J Neurosci. 2008;28:13957–13966. doi: 10.1523/JNEUROSCI.4457-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Behrens MM. Sejnowski TJ. Does schizophrenia arise from oxidative dysregulation of parvalbumin-interneurons in the developing cortex? Neuropharmacology. 2009;57:193–200. doi: 10.1016/j.neuropharm.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bell RM. Cave AC. Johar S. Hearse DJ. Shah AM. Shattock MJ. Pivotal role of NOX-2-containing NADPH oxidase in early ischemic preconditioning. FASEB J. 2005;19:2037–2039. doi: 10.1096/fj.04-2774fje. [DOI] [PubMed] [Google Scholar]
- 20.Blanco M. Nombela F. Castellanos M. Rodriguez-Yanez M. Garcia-Gil M. Leira R. Lizasoain I. Serena J. Vivancos J. Moro MA. Davalos A. Castillo J. Statin treatment withdrawal in ischemic stroke: a controlled randomized study. Neurology. 2007;69:904–910. doi: 10.1212/01.wnl.0000269789.09277.47. [DOI] [PubMed] [Google Scholar]
- 21.Block ML. NADPH oxidase as a therapeutic target in Alzheimer's disease. BMC Neurosci. 2008;9(Suppl 2):S8. doi: 10.1186/1471-2202-9-S2-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bolivar VJ. Cook MN. Flaherty L. Mapping of quantitative trait loci with knockout/congenic strains. Genome research. 2001;11:1549–1552. doi: 10.1101/gr.194001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brandes RP. Role of NADPH oxidases in the control of vascular gene expression. Antioxid Redox Signal. 2003;5:803–811. doi: 10.1089/152308603770380115. [DOI] [PubMed] [Google Scholar]
- 24.Brennan AM. Suh SW. Won SJ. Narasimhan P. Kauppinen TM. Lee H. Edling Y. Chan PH. Swanson RA. NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nat Neurosci. 2009;12:857–863. doi: 10.1038/nn.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown DI. Griendling KK. Nox proteins in signal transduction. Free Radic Biol Med. 2009;47:1239–1253. doi: 10.1016/j.freeradbiomed.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruce-Keller AJ. Gupta S. Parrino TE. Knight AG. Ebenezer PJ. Weidner AM. LeVine H., 3rd Keller JN. Markesbery WR. NOX activity is increased in mild cognitive impairment. Antioxid Redox Signal. 2010;12:1371–1382. doi: 10.1089/ars.2009.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cacicedo JM. Benjachareowong S. Chou E. Ruderman NB. Ido Y. Palmitate-induced apoptosis in cultured bovine retinal pericytes: roles of NAD(P)H oxidase, oxidant stress, and ceramide. Diabetes. 2005;54:1838–1845. doi: 10.2337/diabetes.54.6.1838. [DOI] [PubMed] [Google Scholar]
- 28.Cai H. Davis ME. Drummond GR. Harrison DG. Induction of endothelial NO synthase by hydrogen peroxide via a Ca(2+)/calmodulin-dependent protein kinase II/janus kinase 2-dependent pathway. Arterioscler Thromb Vasc Biol. 2001;21:1571–1576. doi: 10.1161/hq1001.097028. [DOI] [PubMed] [Google Scholar]
- 29.Capone C. Faraco G. Park L. Cao X. Davisson RL. Iadecola C. The cerebrovascular dysfunction induced by slow pressor doses of angiotensin II precedes the development of hypertension. Am J Physiol Heart Circ Physiol. 2011;300:H397–H407. doi: 10.1152/ajpheart.00679.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chatterjee S. Browning EA. Hong N. Debolt K. Sorokina EM. Liu W. Birnbaum MJ. Fisher AB. Membrane depolarization is the trigger for PI3K/Akt activation and leads to the generation of ROS. Am J Physiol Heart Circ Physiol. 2012;302:H105–H114. doi: 10.1152/ajpheart.00298.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen H. Chopp M. Zhang RL. Bodzin G. Chen Q. Rusche JR. Todd RF., III Anti-CD11b monoclonal antibody reduces ischemic cell damage after transient focal cerebral ischemia in rat. Ann Neurol. 1994;35:458–463. doi: 10.1002/ana.410350414. [DOI] [PubMed] [Google Scholar]
- 32.Chen H. Kim GS. Okami N. Narasimhan P. Chan PH. NADPH oxidase is involved in post-ischemic brain inflammation. Neurobiol Dis. 2011;42:341–348. doi: 10.1016/j.nbd.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen H. Song YS. Chan PH. Inhibition of NADPH oxidase is neuroprotective after ischemia-reperfusion. J Cereb Blood Flow Metab. 2009;29:1262–1272. doi: 10.1038/jcbfm.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheret C. Gervais A. Lelli A. Colin C. Amar L. Ravassard P. Mallet J. Cumano A. Krause KH. Mallat M. Neurotoxic activation of microglia is promoted by a nox1-dependent NADPH oxidase. J Neurosci. 2008;28:12039–12051. doi: 10.1523/JNEUROSCI.3568-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi DH. Lee KH. Kim MY. Han SH. Lee J. Role of NADPH oxidase 1 in ischemia/reperfusion- induced brain injury after stroke. Abstract presented at 25th International Symposium on Cerebral Blood Flow, Metabolism and Function; Barcelona, Spain. 2011. [Google Scholar]
- 36.Chrissobolis S. Faraci FM. The role of oxidative stress and NADPH oxidase in cerebrovascular disease. Trends Mol Med. 2008;14:495–502. doi: 10.1016/j.molmed.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collins R. Armitage J. Parish S. Sleight P. Peto R. Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high-risk conditions. Lancet. 2004;363:757–767. doi: 10.1016/S0140-6736(04)15690-0. [DOI] [PubMed] [Google Scholar]
- 38.Connell BJ. Saleh MC. Khan BV. Saleh TM. Apocynin may limit total cell death following cerebral ischemia and reperfusion by enhancing apoptosis. Food Chem Toxicol. 2011;49:3063–3069. doi: 10.1016/j.fct.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 39.Coyoy A. Valencia A. Guemez-Gamboa A. Moran J. Role of NADPH oxidase in the apoptotic death of cultured cerebellar granule neurons. Free Radic Biol Med. 2008;45:1056–1064. doi: 10.1016/j.freeradbiomed.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 40.Craige SM. Chen K. Pei Y. Li C. Huang X. Chen C. Shibata R. Sato K. Walsh K. Keaney JF., Jr NADPH oxidase 4 promotes endothelial angiogenesis through endothelial nitric oxide synthase activation. Circulation. 2011;124:731–740. doi: 10.1161/CIRCULATIONAHA.111.030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Csanyi G. Cifuentes-Pagano E. Al GI. Ranayhossaini DJ. Egana L. Lopes LR. Jackson HM. Kelley EE. Pagano PJ. Nox2 B-loop peptide, Nox2ds, specifically inhibits the NADPH oxidase Nox2. Free Radic Biol Med. 2011;51:1116–1125. doi: 10.1016/j.freeradbiomed.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.D'Autreaux B. Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 43.Dalkara T. Gursoy-Ozdemir Y. Yemisci M. Brain microvascular pericytes in health and disease. Acta Neuropathol. 2011;122:1–9. doi: 10.1007/s00401-011-0847-6. [DOI] [PubMed] [Google Scholar]
- 44.De Silva TM. Brait VH. Drummond GR. Sobey CG. Miller AA. Nox2 oxidase activity accounts for the oxidative stress and vasomotor dysfunction in mouse cerebral arteries following ischemic stroke. PLoS One. 2011;6:e28393. doi: 10.1371/journal.pone.0028393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diener HC. Lees KR. Lyden P. Grotta J. Davalos A. Davis SM. Shuaib A. Ashwood T. Wasiewski W. Alderfer V. Hardemark HG. Rodichok L. NXY-059 for the treatment of acute stroke: pooled analysis of the SAINT I and II Trials. Stroke. 2008;39:1751–1758. doi: 10.1161/STROKEAHA.107.503334. [DOI] [PubMed] [Google Scholar]
- 46.Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Radic Biol Med. 2011;51:1289–1301. doi: 10.1016/j.freeradbiomed.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dikalov SI. Dikalova AE. Bikineyeva AT. Schmidt HH. Harrison DG. Griendling KK. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med. 2008;45:1340–1351. doi: 10.1016/j.freeradbiomed.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ding Y. Chen ZJ. Liu S. Che D. Vetter M. Chang CH. Inhibition of Nox-4 activity by plumbagin, a plant-derived bioactive naphthoquinone. J Pharm Pharmacol. 2005;57:111–116. doi: 10.1211/0022357055119. [DOI] [PubMed] [Google Scholar]
- 49.Dirnagl U. Iadecola C. Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 50.Dodd OJ. Pearse DB. Effect of the NADPH oxidase inhibitor apocynin on ischemia-reperfusion lung injury. Am J Physiol Heart Circ Physiol. 2000;279:H303–H312. doi: 10.1152/ajpheart.2000.279.1.H303. [DOI] [PubMed] [Google Scholar]
- 51.Doerries C. Grote K. Hilfiker-Kleiner D. Luchtefeld M. Schaefer A. Holland SM. Sorrentino S. Manes C. Schieffer B. Drexler H. Landmesser U. Critical role of the NAD(P)H oxidase subunit p47phox for left ventricular remodeling/dysfunction and survival after myocardial infarction. Circ Res. 2007;100:894–903. doi: 10.1161/01.RES.0000261657.76299.ff. [DOI] [PubMed] [Google Scholar]
- 52.Dorman RB. Wunder C. Saba H. Shoemaker JL. MacMillan-Crow LA. Brock RW. NAD(P)H oxidase contributes to the progression of remote hepatic parenchymal injury and endothelial dysfunction, but not microvascular perfusion deficits. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1025–G1032. doi: 10.1152/ajpgi.00246.2005. [DOI] [PubMed] [Google Scholar]
- 53.Drummond GR. Cai H. Davis ME. Ramasamy S. Harrison DG. Transcriptional and posttranscriptional regulation of endothelial nitric oxide synthase expression by hydrogen peroxide. Circ Res. 2000;86:347–354. doi: 10.1161/01.res.86.3.347. [DOI] [PubMed] [Google Scholar]
- 54.Eisener-Dorman AF. Lawrence DA. Bolivar VJ. Cautionary insights on knockout mouse studies: the gene or not the gene? Brain, behavior, and immunity. 2009;23:318–324. doi: 10.1016/j.bbi.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eisener-Dorman AF. Lawrence DA. Bolivar VJ. Behavioral and genetic investigations of low exploratory behavior in Il18r1(-/-) mice: we can't always blame it on the targeted gene. Brain Behav Immun. 2010;24:1116–1125. doi: 10.1016/j.bbi.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Endres M. Laufs U. Discontinuation of statin treatment in stroke patients. Stroke. 2006;37:2640–2643. doi: 10.1161/01.STR.0000240690.69406.28. [DOI] [PubMed] [Google Scholar]
- 57.Engelhorn T. Doerfler A. Heusch G. Schulz R. Reduction of cerebral infarct size by the AT1-receptor blocker candesartan, the HMG-CoA reductase inhibitor rosuvastatin and their combination. An experimental study in rats. Neurosci Lett. 2006;406:92–96. doi: 10.1016/j.neulet.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 58.Erdos B. Snipes JA. Tulbert CD. Katakam P. Miller AW. Busija DW. Rosuvastatin improves cerebrovascular function in Zucker obese rats by inhibiting NAD(P)H oxidase-dependent superoxide production. Am J Physiol Heart Circ Physiol. 2006;290:H1264–H1270. doi: 10.1152/ajpheart.00804.2005. [DOI] [PubMed] [Google Scholar]
- 59.Espinosa A. Garcia A. Hartel S. Hidalgo C. Jaimovich E. NADPH oxidase and hydrogen peroxide mediate insulin-induced calcium increase in skeletal muscle cells. J Biol Chem. 2009;284:2568–2575. doi: 10.1074/jbc.M804249200. [DOI] [PubMed] [Google Scholar]
- 60.Flint AC. Kamel H. Navi BB. Rao VA. Faigeles BS. Conell C. Klingman JG. Sidney S. Hills NK. Sorel M. Cullen SP. Johnston SC. Statin use during ischemic stroke hospitalization is strongly associated with improved poststroke survival. Stroke. 2012;43:147–154. doi: 10.1161/STROKEAHA.111.627729. [DOI] [PubMed] [Google Scholar]
- 61.Frantz S. Brandes RP. Hu K. Rammelt K. Wolf J. Scheuermann H. Ertl G. Bauersachs J. Left ventricular remodeling after myocardial infarction in mice with targeted deletion of the NADPH oxidase subunit gp91PHOX. Basic Res Cardiol. 2006;101:127–132. doi: 10.1007/s00395-005-0568-x. [DOI] [PubMed] [Google Scholar]
- 62.Fuchs B. Sommer N. Dietrich A. Schermuly RT. Ghofrani HA. Grimminger F. Seeger W. Gudermann T. Weissmann N. Redox signaling and reactive oxygen species in hypoxic pulmonary vasoconstriction. Respir Physiol Neurobiol. 2010;174:282–291. doi: 10.1016/j.resp.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 63.Fulton DJ. Nox5 and the regulation of cellular function. Antioxid Redox Signal. 2009;11:2443–2452. doi: 10.1089/ars.2009.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gatto GJ. Ao Z. Kearse MG. Zhou M. Morales CR. Daniels E. Bradley BT. Goserud MT. Goodman KB. Douglas SA. Harpel MR. Johns DG. NADPH oxidase-dependent and -independent mechanisms of reported inhibitors of reactive oxygen generation. J Enzyme Inhib Med Chem. 2011 doi: 10.3109/14756366.2011.636360. [DOI] [PubMed] [Google Scholar]
- 65.Genovese T. Mazzon E. Paterniti I. Esposito E. Bramanti P. Cuzzocrea S. Modulation of NADPH oxidase activation in cerebral ischemia/reperfusion injury in rats. Brain Res. 2011;1372:92–102. doi: 10.1016/j.brainres.2010.11.088. [DOI] [PubMed] [Google Scholar]
- 66.Geraldes P. Hiraoka-Yamamoto J. Matsumoto M. Clermont A. Leitges M. Marette A. Aiello LP. Kern TS. King GL. Activation of PKC-delta and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat Med. 2009;15:1298–1306. doi: 10.1038/nm.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ginsberg MD. Current status of neuroprotection for cerebral ischemia: synoptic overview. Stroke. 2009;40:S111–S114. doi: 10.1161/STROKEAHA.108.528877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Girouard H. Park L. Anrather J. Zhou P. Iadecola C. Angiotensin II attenuates endothelium-dependent responses in the cerebral microcirculation through nox-2-derived radicals. Arterioscler Thromb Vasc Biol. 2006;26:826–832. doi: 10.1161/01.ATV.0000205849.22807.6e. [DOI] [PubMed] [Google Scholar]
- 69.Graham KA. Kulawiec M. Owens KM. Li X. Desouki MM. Chandra D. Singh KK. NADPH oxidase 4 is an oncoprotein localized to mitochondria. Cancer Biol Ther. 2010;10:223–231. doi: 10.4161/cbt.10.3.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Green SP. Cairns B. Rae J. Errett-Baroncini C. Hongo JA. Erickson RW. Curnutte JT. Induction of gp91-phox, a component of the phagocyte NADPH oxidase, in microglial cells during central nervous system inflammation. J Cereb Blood Flow Metab. 2001;21:374–384. doi: 10.1097/00004647-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 71.Gursoy-Ozdemir Y. Can A. Dalkara T. Reperfusion-induced oxidative/nitrative injury to neurovascular unit after focal cerebral ischemia. Stroke. 2004;35:1449–1453. doi: 10.1161/01.STR.0000126044.83777.f4. [DOI] [PubMed] [Google Scholar]
- 72.Hacke W. Kaste M. Bluhmki E. Brozman M. Davalos A. Guidetti D. Larrue V. Lees KR. Medeghri Z. Machnig T. Schneider D. von KR. Wahlgren N. Toni D. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 73.Haorah J. Ramirez SH. Floreani N. Gorantla S. Morsey B. Persidsky Y. Mechanism of alcohol-induced oxidative stress and neuronal injury. Free Radic Biol Med. 2008;45:1542–1550. doi: 10.1016/j.freeradbiomed.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Harada N. Iimuro Y. Nitta T. Yoshida M. Uchinami H. Nishio T. Hatano E. Yamamoto N. Yamamoto Y. Yamaoka Y. Inactivation of the small GTPase Rac1 protects the liver from ischemia/reperfusion injury in the rat. Surgery. 2003;134:480–491. doi: 10.1067/s0039-6060(03)00256-3. [DOI] [PubMed] [Google Scholar]
- 75.Harrigan TJ. Abdullaev IF. Jourd'heuil D. Mongin AA. Activation of microglia with zymosan promotes excitatory amino acid release via volume-regulated anion channels: the role of NADPH oxidases. J Neurochem. 2008;106:2449–2462. doi: 10.1111/j.1471-4159.2008.05553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hartl R. Schurer L. Schmid-Schonbein GW. del Zoppo GJ. Experimental antileukocyte interventions in cerebral ischemia. J Cereb Blood Flow Metab. 1996;16:1108–1119. doi: 10.1097/00004647-199611000-00004. [DOI] [PubMed] [Google Scholar]
- 77.Heumuller S. Wind S. Barbosa-Sicard E. Schmidt HH. Busse R. Schroder K. Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- 78.Hoffmeyer MR. Jones SP. Ross CR. Sharp B. Grisham MB. Laroux FS. Stalker TJ. Scalia R. Lefer DJ. Myocardial ischemia/reperfusion injury in NADPH oxidase-deficient mice. Circ Res. 2000;87:812–817. doi: 10.1161/01.res.87.9.812. [DOI] [PubMed] [Google Scholar]
- 79.Hong H. Zeng JS. Kreulen DL. Kaufman DI. Chen AF. Atorvastatin protects against cerebral infarction via inhibition of NADPH oxidase-derived superoxide in ischemic stroke. Am J Physiol Heart Circ Physiol. 2006;291:H2210–H2215. doi: 10.1152/ajpheart.01270.2005. [DOI] [PubMed] [Google Scholar]
- 80.Iadecola C. Park L. Capone C. Threats to the mind: aging, amyloid, and hypertension. Stroke. 2009;40:S40–S44. doi: 10.1161/STROKEAHA.108.533638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ibi M. Katsuyama M. Fan C. Iwata K. Nishinaka T. Yokoyama T. Yabe-Nishimura C. NOX1/NADPH oxidase negatively regulates nerve growth factor-induced neurite outgrowth. Free Radic Biol Med. 2006;40:1785–1795. doi: 10.1016/j.freeradbiomed.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 82.Ibi M. Matsuno K. Shiba D. Katsuyama M. Iwata K. Kakehi T. Nakagawa T. Sango K. Shirai Y. Yokoyama T. Kaneko S. Saito N. Yabe-Nishimura C. Reactive oxygen species derived from NOX1/NADPH oxidase enhance inflammatory pain. J Neurosci. 2008;28:9486–9494. doi: 10.1523/JNEUROSCI.1857-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Infanger DW. Sharma RV. Davisson RL. NADPH oxidases of the brain: distribution, regulation, and function. Antioxid Redox Signal. 2006;8:1583–1596. doi: 10.1089/ars.2006.8.1583. [DOI] [PubMed] [Google Scholar]
- 84.Ishikawa M. Sekizuka E. Yamaguchi N. Nakadate H. Terao S. Granger DN. Minamitani H. Angiotensin II type 1 receptor signaling contributes to platelet-leukocyte-endothelial cell interactions in the cerebral microvasculature. Am J Physiol Heart Circ Physiol. 2007;292:H2306–H2315. doi: 10.1152/ajpheart.00601.2006. [DOI] [PubMed] [Google Scholar]
- 85.Jackman KA. Miller AA. De Silva TM. Crack PJ. Drummond GR. Sobey CG. Reduction of cerebral infarct volume by apocynin requires pretreatment and is absent in Nox2-deficient mice. Br J Pharmacol. 2009;156:680–688. doi: 10.1111/j.1476-5381.2008.00073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jackman KA. Miller AA. Drummond GR. Sobey CG. Importance of NOX1 for angiotensin II-induced cerebrovascular superoxide production and cortical infarct volume following ischemic stroke. Brain Res. 2009;1286:215–220. doi: 10.1016/j.brainres.2009.06.056. [DOI] [PubMed] [Google Scholar]
- 87.Jaquet V. Scapozza L. Clark R. Krause KH. Lambeth JD. Small molecule NOX inhibitors: ROS-generating NADPH oxidases as therapeutic targets. Antioxid Redox Signal. 2009;11:2535–2552. doi: 10.1089/ars.2009.2585. [DOI] [PubMed] [Google Scholar]
- 88.Kahles T. Brandes RP. NADPH oxidases as therapeutic targets in ischemic stroke. Cell Mol Life Sci. 2012;69:2345–2363. doi: 10.1007/s00018-012-1011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kahles T. Kohnen A. Heumueller S. Rappert A. Bechmann I. Liebner S. Wittko IM. Neumann-Haefelin T. Steinmetz H. Schroeder K. Brandes RP. NADPH oxidase Nox1 contributes to ischemic injury in experimental stroke in mice. Neurobiol Dis. 2010;40:185–192. doi: 10.1016/j.nbd.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 90.Kahles T. Luedike P. Endres M. Galla HJ. Steinmetz H. Busse R. Neumann-Haefelin T. Brandes RP. NADPH oxidase plays a central role in blood-brain barrier damage in experimental stroke. Stroke. 2007;38:3000–3006. doi: 10.1161/STROKEAHA.107.489765. [DOI] [PubMed] [Google Scholar]
- 91.Kamat CD. Gadal S. Mhatre M. Williamson KS. Pye QN. Hensley K. Antioxidants in central nervous system diseases: preclinical promise and translational challenges. J Alzheimers Dis. 2008;15:473–493. doi: 10.3233/jad-2008-15314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Katsuyama M. Matsuno K. Yabe-Nishimura C. Physiological roles of NOX/NADPH oxidase, the superoxide-generating enzyme. J Clin Biochem Nutr. 2012;50:9–22. doi: 10.3164/jcbn.11-06SR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kazama K. Anrather J. Zhou P. Girouard H. Frys K. Milner TA. Iadecola C. Angiotensin II impairs neurovascular coupling in neocortex through NADPH oxidase-derived radicals. Circ Res. 2004;95:1019–1026. doi: 10.1161/01.RES.0000148637.85595.c5. [DOI] [PubMed] [Google Scholar]
- 94.Kelly KA. Li X. Tan Z. VanGilder RL. Rosen CL. Huber JD. NOX2 inhibition with apocynin worsens stroke outcome in aged rats. Brain Res. 2009;1292:165–172. doi: 10.1016/j.brainres.2009.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim DE. Suh YS. Lee MS. Kim KY. Lee JH. Lee HS. Hong KW. Kim CD. Vascular NAD(P)H oxidase triggers delayed cerebral vasospasm after subarachnoid hemorrhage in rats. Stroke. 2002;33:2687–2691. doi: 10.1161/01.str.0000033071.99143.9e. [DOI] [PubMed] [Google Scholar]
- 96.Kleinschnitz C. Grund H. Wingler K. Armitage ME. Jones E. Mittal M. Barit D. Schwarz T. Geis C. Kraft P. Barthel K. Schuhmann MK. Herrmann AM. Meuth SG. Stoll G. Meurer S. Schrewe A. Becker L. Gailus-Durner V. Fuchs H. Klopstock T. de Angelis MH. Jandeleit-Dahm K. Shah AM. Weissmann N. Schmidt HH. Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000479. pii: e1000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kunz A. Anrather J. Zhou P. Orio M. Iadecola C. Cyclooxygenase-2 does not contribute to postischemic production of reactive oxygen species. J Cereb Blood Flow Metab. 2007;27:545–551. doi: 10.1038/sj.jcbfm.9600369. [DOI] [PubMed] [Google Scholar]
- 98.Kunz A. Park L. Abe T. Gallo EF. Anrather J. Zhou P. Iadecola C. Neurovascular protection by ischemic tolerance: role of nitric oxide and reactive oxygen species. J Neurosci. 2007;27:7083–7093. doi: 10.1523/JNEUROSCI.1645-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kuroda J. Ago T. Matsushima S. Zhai P. Schneider MD. Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci U S A. 2010;107:15565–15570. doi: 10.1073/pnas.1002178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kusaka I. Kusaka G. Zhou C. Ishikawa M. Nanda A. Granger DN. Zhang JH. Tang J. Role of AT1 receptors and NAD(P)H oxidase in diabetes-aggravated ischemic brain injury. Am J Physiol Heart Circ Physiol. 2004;286:H2442–H2451. doi: 10.1152/ajpheart.01169.2003. [DOI] [PubMed] [Google Scholar]
- 101.Lambeth JD. Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic Biol Med. 2007;43:332–347. doi: 10.1016/j.freeradbiomed.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li B. Bedard K. Sorce S. Hinz B. Dubois-Dauphin M. Krause KH. NOX4 expression in human microglia leads to constitutive generation of reactive oxygen species and to constitutive IL-6 expression. J Innate Immun. 2009;1:570–581. doi: 10.1159/000235563. [DOI] [PubMed] [Google Scholar]
- 103.Liou KT. Shen YC. Chen CF. Tsao CM. Tsai SK. Honokiol protects rat brain from focal cerebral ischemia-reperfusion injury by inhibiting neutrophil infiltration and reactive oxygen species production. Brain Res. 2003;992:159–166. doi: 10.1016/j.brainres.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 104.Liu PG. He SQ. Zhang YH. Wu J. Protective effects of apocynin and allopurinol on ischemia/reperfusion-induced liver injury in mice. World J Gastroenterol. 2008;14:2832–2837. doi: 10.3748/wjg.14.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu W. Sood R. Chen Q. Sakoglu U. Hendren J. Cetin O. Miyake M. Liu KJ. Normobaric hyperoxia inhibits NADPH oxidase-mediated matrix metalloproteinase-9 induction in cerebral microvessels in experimental stroke. J Neurochem. 2008;107:1196–1205. doi: 10.1111/j.1471-4159.2008.05664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Looi YH. Grieve DJ. Siva A. Walker SJ. Anilkumar N. Cave AC. Marber M. Monaghan MJ. Shah AM. Involvement of Nox2 NADPH oxidase in adverse cardiac remodeling after myocardial infarction. Hypertension. 2008;51:319–325. doi: 10.1161/HYPERTENSIONAHA.107.101980. [DOI] [PubMed] [Google Scholar]
- 107.Loukogeorgakis SP. van den Berg MJ. Sofat R. Nitsch D. Charakida M. Haiyee B. de Groot E. MacAllister RJ. Kuijpers TW. Deanfield JE. Role of NADPH oxidase in endothelial ischemia/reperfusion injury in humans. Circulation. 2010;121:2310–2316. doi: 10.1161/CIRCULATIONAHA.108.814731. [DOI] [PubMed] [Google Scholar]
- 108.Marnett LJ. Riggins JN. West JD. Endogenous generation of reactive oxidants and electrophiles and their reactions with DNA and protein. J Clin Invest. 2003;111:583–593. doi: 10.1172/JCI18022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Martyn KD. Frederick LM. von LK. Dinauer MC. Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 110.Matsuo J. Oku H. Kanbara Y. Kobayashi T. Sugiyama T. Ikeda T. Involvement of NADPH oxidase and protein kinase C in endothelin-1-induced superoxide production in retinal microvessels. Exp Eye Res. 2009;89:693–699. doi: 10.1016/j.exer.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 111.Matsuo Y. Kihara T. Ikeda M. Ninomiya M. Onodera H. Kogure K. Role of neutrophils in radical production during ischemia and reperfusion of the rat brain: effect of neutrophil depletion on extracellular ascorbyl radical formation. J Cereb Blood Flow Metab. 1995;15:941–947. doi: 10.1038/jcbfm.1995.119. [DOI] [PubMed] [Google Scholar]
- 112.McCann SK. Dusting GJ. Roulston CL. Early increase of Nox4 NADPH oxidase and superoxide generation following endothelin-1-induced stroke in conscious rats. J Neurosci Res. 2008;86:2524–2534. doi: 10.1002/jnr.21700. [DOI] [PubMed] [Google Scholar]
- 113.Milenkovic M. De Deken X. Jin L. De Felice M. Di Lauro R. Dumont JE. Corvilain B. Miot F. Duox expression and related H2O2 measurement in mouse thyroid: onset in embryonic development and regulation by TSH in adult. J Endocrinol. 2007;192:615–626. doi: 10.1677/JOE-06-0003. [DOI] [PubMed] [Google Scholar]
- 114.Miller AA. De Silva TM. Judkins CP. Diep H. Drummond GR. Sobey CG. Augmented superoxide production by Nox2-containing NADPH oxidase causes cerebral artery dysfunction during hypercholesterolemia. Stroke. 2010;41:784–789. doi: 10.1161/STROKEAHA.109.575365. [DOI] [PubMed] [Google Scholar]
- 115.Miller AA. Drummond GR. De Silva TM. Mast AE. Hickey H. Williams JP. Broughton BR. Sobey CG. NADPH oxidase activity is higher in cerebral versus systemic arteries of four animal species: role of Nox2. Am J Physiol Heart Circ Physiol. 2009;296:H220–H225. doi: 10.1152/ajpheart.00987.2008. [DOI] [PubMed] [Google Scholar]
- 116.Miller AA. Drummond GR. Mast AE. Schmidt HH. Sobey CG. Effect of gender on NADPH-oxidase activity, expression, and function in the cerebral circulation: role of estrogen. Stroke. 2007;38:2142–2149. doi: 10.1161/STROKEAHA.106.477406. [DOI] [PubMed] [Google Scholar]
- 117.Miller AA. Drummond GR. Schmidt HH. Sobey CG. NADPH oxidase activity and function are profoundly greater in cerebral versus systemic arteries. Circ Res. 2005;97:1055–1062. doi: 10.1161/01.RES.0000189301.10217.87. [DOI] [PubMed] [Google Scholar]
- 118.Miller AA. Dusting GJ. Roulston CL. Sobey CG. NADPH-oxidase activity is elevated in penumbral and non-ischemic cerebral arteries following stroke. Brain Res. 2006;1111:111–116. doi: 10.1016/j.brainres.2006.06.082. [DOI] [PubMed] [Google Scholar]
- 119.Molina CA. Reperfusion therapies for acute ischemic stroke: current pharmacological and mechanical approaches. Stroke. 2011;42:S16–S19. doi: 10.1161/STROKEAHA.110.598763. [DOI] [PubMed] [Google Scholar]
- 120.Montezano AC. Burger D. Ceravolo GS. Yusuf H. Montero M. Touyz RM. Novel Nox homologues in the vasculature: focusing on Nox4 and Nox5. Clin Sci (Lond) 2011;120:131–141. doi: 10.1042/CS20100384. [DOI] [PubMed] [Google Scholar]
- 121.Montezano AC. Burger D. Paravicini TM. Chignalia AZ. Yusuf H. Almasri M. He Y. Callera GE. He G. Krause KH. Lambeth D. Quinn MT. Touyz RM. Nicotinamide adenine dinucleotide phosphate reduced oxidase 5 (Nox5) regulation by angiotensin II and endothelin-1 is mediated via calcium/calmodulin-dependent, rac-1-independent pathways in human endothelial cells. Circ Res. 2010;106:1363–1373. doi: 10.1161/CIRCRESAHA.109.216036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Murotomi K. Takagi N. Takeo S. Tanonaka K. NADPH oxidase-mediated oxidative damage to proteins in the postsynaptic density after transient cerebral ischemia and reperfusion. Mol Cell Neurosci. 2011;46:681–688. doi: 10.1016/j.mcn.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 123.Mustapha NM. Tarr JM. Kohner EM. Chibber R. NADPH Oxidase versus Mitochondria-Derived ROS in Glucose-Induced Apoptosis of Pericytes in Early Diabetic Retinopathy. J Ophthalmol. 2010;2010:746978. doi: 10.1155/2010/746978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nagel S. Genius J. Heiland S. Horstmann S. Gardner H. Wagner S. Diphenyleneiodonium and dimethylsulfoxide for treatment of reperfusion injury in cerebral ischemia of the rat. Brain Res. 2007;1132:210–217. doi: 10.1016/j.brainres.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 125.Nair D. Dayyat EA. Zhang SX. Wang Y. Gozal D. Intermittent hypoxia-induced cognitive deficits are mediated by NADPH oxidase activity in a murine model of sleep apnea. PLoS One. 2011;6:e19847. doi: 10.1371/journal.pone.0019847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ozaki M. Deshpande SS. Angkeow P. Bellan J. Lowenstein CJ. Dinauer MC. Goldschmidt-Clermont PJ. Irani K. Inhibition of the Rac1 GTPase protects against nonlethal ischemia/reperfusion-induced necrosis and apoptosis in vivo. FASEB J. 2000;14:418–429. doi: 10.1096/fasebj.14.2.418. [DOI] [PubMed] [Google Scholar]
- 127.Paffenholz R. Bergstrom RA. Pasutto F. Wabnitz P. Munroe RJ. Jagla W. Heinzmann U. Marquardt A. Bareiss A. Laufs J. Russ A. Stumm G. Schimenti JC. Bergstrom DE. Vestibular defects in head-tilt mice result from mutations in Nox3, encoding an NADPH oxidase. Genes Dev. 2004;18:486–491. doi: 10.1101/gad.1172504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Paravicini TM. Chrissobolis S. Drummond GR. Sobey CG. Increased NADPH-oxidase activity and Nox4 expression during chronic hypertension is associated with enhanced cerebral vasodilatation to NADPH in vivo. Stroke. 2004;35:584–589. doi: 10.1161/01.STR.0000112974.37028.58. [DOI] [PubMed] [Google Scholar]
- 129.Park KW. Jin BK. Thrombin-induced oxidative stress contributes to the death of hippocampal neurons: role of neuronal NADPH oxidase. J Neurosci Res. 2008;86:1053–1063. doi: 10.1002/jnr.21571. [DOI] [PubMed] [Google Scholar]
- 130.Park L. Anrather J. Zhou P. Frys K. Pitstick R. Younkin S. Carlson GA. Iadecola C. NADPH-oxidase-derived reactive oxygen species mediate the cerebrovascular dysfunction induced by the amyloid beta peptide. J Neurosci. 2005;25:1769–1777. doi: 10.1523/JNEUROSCI.5207-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Park L. Zhou P. Pitstick R. Capone C. Anrather J. Norris EH. Younkin L. Younkin S. Carlson G. McEwen BS. Iadecola C. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc Natl Acad Sci U S A. 2008;105:1347–1352. doi: 10.1073/pnas.0711568105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Paterniti I. Galuppo M. Mazzon E. Impellizzeri D. Esposito E. Bramanti P. Cuzzocrea S. Protective effects of apocynin, an inhibitor of NADPH oxidase activity, in splanchnic artery occlusion and reperfusion. J Leukoc Biol. 2010;88:993–1003. doi: 10.1189/jlb.0610322. [DOI] [PubMed] [Google Scholar]
- 133.Pearse DB. Dodd JM. Ischemia-reperfusion lung injury is prevented by apocynin, a novel inhibitor of leukocyte NADPH oxidase. Chest. 1999;116:55S–56S. doi: 10.1378/chest.116.suppl_1.55s. [DOI] [PubMed] [Google Scholar]
- 134.Peterson JR. Sharma RV. Davisson RL. Reactive oxygen species in the neuropathogenesis of hypertension. Curr Hypertens Rep. 2006;8:232–241. doi: 10.1007/s11906-006-0056-1. [DOI] [PubMed] [Google Scholar]
- 135.Prinz V. Laufs U. Gertz K. Kronenberg G. Balkaya M. Leithner C. Lindauer U. Endres M. Intravenous rosuvastatin for acute stroke treatment: an animal study. Stroke. 2008;39:433–438. doi: 10.1161/STROKEAHA.107.492470. [DOI] [PubMed] [Google Scholar]
- 136.Rada B. Leto TL. Oxidative innate immune defenses by Nox/Duox family NADPH oxidases. Contrib Microbiol. 2008;15:164–187. doi: 10.1159/000136357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rathore R. Zheng YM. Niu CF. Liu QH. Korde A. Ho YS. Wang YX. Hypoxia activates NADPH oxidase to increase [ROS]i and [Ca2+]i through the mitochondrial ROS-PKCepsilon signaling axis in pulmonary artery smooth muscle cells. Free Radic Biol Med. 2008;45:1223–1231. doi: 10.1016/j.freeradbiomed.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ray R. Murdoch CE. Wang M. Santos CX. Zhang M. Alom-Ruiz S. Anilkumar N. Ouattara A. Cave AC. Walker SJ. Grieve DJ. Charles RL. Eaton P. Brewer AC. Shah AM. Endothelial nox4 NADPH oxidase enhances vasodilatation and reduces blood pressure in vivo. Arterioscler Thromb Vasc Biol. 2011;31:1368–1376. doi: 10.1161/ATVBAHA.110.219238. [DOI] [PubMed] [Google Scholar]
- 139.Raz L. Zhang QG. Zhou CF. Han D. Gulati P. Yang LC. Yang F. Wang RM. Brann DW. Role of Rac1 GTPase in NADPH oxidase activation and cognitive impairment following cerebral ischemia in the rat. PLoS One. 2010;5:e12606. doi: 10.1371/journal.pone.0012606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Reinehr R. Gorg B. Becker S. Qvartskhava N. Bidmon HJ. Selbach O. Haas HL. Schliess F. Haussinger D. Hypoosmotic swelling and ammonia increase oxidative stress by NADPH oxidase in cultured astrocytes and vital brain slices. Glia. 2007;55:758–771. doi: 10.1002/glia.20504. [DOI] [PubMed] [Google Scholar]
- 141.Rha JH. Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke. 2007;38:967–973. doi: 10.1161/01.STR.0000258112.14918.24. [DOI] [PubMed] [Google Scholar]
- 142.Riganti C. Costamagna C. Bosia A. Ghigo D. The NADPH oxidase inhibitor apocynin (acetovanillone) induces oxidative stress. Toxicol Appl Pharmacol. 2006;212:179–187. doi: 10.1016/j.taap.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 143.Ross CA. Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004;10(Suppl):S10–S17. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- 144.Saavedra JM. Sanchez-Lemus E. Benicky J. Blockade of brain angiotensin II AT1 receptors ameliorates stress, anxiety, brain inflammation and ischemia: therapeutic implications. Psychoneuroendocrinology. 2011;36:1–18. doi: 10.1016/j.psyneuen.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sandset EC. Bath PM. Boysen G. Jatuzis D. Korv J. Luders S. Murray GD. Richter PS. Roine RO. Terent A. Thijs V. Berge E. The angiotensin-receptor blocker candesartan for treatment of acute stroke (SCAST): a randomised, placebo-controlled, double-blind trial. Lancet. 2011;377:741–750. doi: 10.1016/S0140-6736(11)60104-9. [DOI] [PubMed] [Google Scholar]
- 146.Saran M. To what end does nature produce superoxide? NADPH oxidase as an autocrine modifier of membrane phospholipids generating paracrine lipid messengers. Free Radic Res. 2003;37:1045–1059. doi: 10.1080/10715760310001594631. [DOI] [PubMed] [Google Scholar]
- 147.Schmerbach K. Pfab T. Zhao Y. Culman J. Mueller S. Villringer A. Muller DN. Hocher B. Unger T. Thoene-Reineke C. Effects of aliskiren on stroke in rats expressing human renin and angiotensinogen genes. PLoS One. 2010;5:e15052. doi: 10.1371/journal.pone.0015052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Serrander L. Cartier L. Bedard K. Banfi B. Lardy B. Plastre O. Sienkiewicz A. Forro L. Schlegel W. Krause KH. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J. 2007;406:105–114. doi: 10.1042/BJ20061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Serrano F. Kolluri NS. Wientjes FB. Card JP. Klann E. NADPH oxidase immunoreactivity in the mouse brain. Brain Res. 2003;988:193–198. doi: 10.1016/s0006-8993(03)03364-x. [DOI] [PubMed] [Google Scholar]
- 150.Shiotani S. Shimada M. Taketomi A. Soejima Y. Yoshizumi T. Hashimoto K. Shimokawa H. Maehara Y. Rho-kinase as a novel gene therapeutic target in treatment of cold ischemia/reperfusion-induced acute lethal liver injury: effect on hepatocellular NADPH oxidase system. Gene Ther. 2007;14:1425–1433. doi: 10.1038/sj.gt.3303000. [DOI] [PubMed] [Google Scholar]
- 151.Shuaib A. Lees KR. Lyden P. Grotta J. Davalos A. Davis SM. Diener HC. Ashwood T. Wasiewski WW. Emeribe U. NXY-059 for the treatment of acute ischemic stroke. N Engl J Med. 2007;357:562–571. doi: 10.1056/NEJMoa070240. [DOI] [PubMed] [Google Scholar]
- 152.Son TG. Camandola S. Arumugam TV. Cutler RG. Telljohann RS. Mughal MR. Moore TA. Luo W. Yu QS. Johnson DA. Johnson JA. Greig NH. Mattson MP. Plumbagin, a novel Nrf2/ARE activator, protects against cerebral ischemia. J Neurochem. 2010;112:1316–1326. doi: 10.1111/j.1471-4159.2009.06552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sorce S. Krause KH. NOX enzymes in the central nervous system: from signaling to disease. Antioxid Redox Signal. 2009;11:2481–2504. doi: 10.1089/ars.2009.2578. [DOI] [PubMed] [Google Scholar]
- 154.Sorce S. Schiavone S. Tucci P. Colaianna M. Jaquet V. Cuomo V. Dubois-Dauphin M. Trabace L. Krause KH. The NADPH oxidase NOX2 controls glutamate release: a novel mechanism involved in psychosis-like ketamine responses. J Neurosci. 2010;30:11317–11325. doi: 10.1523/JNEUROSCI.1491-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Soucy-Faulkner A. Mukawera E. Fink K. Martel A. Jouan L. Nzengue Y. Lamarre D. Vande Velde C. Grandvaux N. Requirement of NOX2 and reactive oxygen species for efficient RIG-I-mediated antiviral response through regulation of MAVS expression. PLoS Pathog. 2010;6:e1000930. doi: 10.1371/journal.ppat.1000930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Stolk J. Hiltermann TJ. Dijkman JH. Verhoeven AJ. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am J Respir Cell Mol Biol. 1994;11:95–102. doi: 10.1165/ajrcmb.11.1.8018341. [DOI] [PubMed] [Google Scholar]
- 157.Suh SW. Shin BS. Ma H. Van Hoecke M. Brennan AM. Yenari MA. Swanson RA. Glucose and NADPH oxidase drive neuronal superoxide formation in stroke. Ann Neurol. 2008;64:654–663. doi: 10.1002/ana.21511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Suh YA. Arnold RS. Lassegue B. Shi J. Xu X. Sorescu D. Chung AB. Griendling KK. Lambeth JD. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- 159.Suzuma I. Murakami T. Suzuma K. Kaneto H. Watanabe D. Ojima T. Honda Y. Takagi H. Yoshimura N. Cyclic stretch-induced reactive oxygen species generation enhances apoptosis in retinal pericytes through c-jun NH2-terminal kinase activation. Hypertension. 2007;49:347–354. doi: 10.1161/01.HYP.0000253535.26659.2f. [DOI] [PubMed] [Google Scholar]
- 160.Takac I. Schroder K. Zhang L. Lardy B. Anilkumar N. Lambeth JD. Shah AM. Morel F. Brandes RP. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J Biol Chem. 2011;286:13304–13313. doi: 10.1074/jbc.M110.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tang LL. Ye K. Yang XF. Zheng JS. Apocynin attenuates cerebral infarction after transient focal ischaemia in rats. J Int Med Res. 2007;35:517–522. doi: 10.1177/147323000703500411. [DOI] [PubMed] [Google Scholar]
- 162.Tang XN. Cairns B. Cairns N. Yenari MA. Apocynin improves outcome in experimental stroke with a narrow dose range. Neuroscience. 2008;154:556–562. doi: 10.1016/j.neuroscience.2008.03.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tang XN. Zheng Z. Giffard RG. Yenari MA. Significance of marrow-derived nicotinamide adenine dinucleotide phosphate oxidase in experimental ischemic stroke. Ann Neurol. 2011;70:606–615. doi: 10.1002/ana.22476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Thirunavukkarasu M. Adluri RS. Juhasz B. Samuel SM. Zhan L. Kaur A. Maulik G. Sanchez JA. Hager J. Maulik N. Novel role of NADPH oxidase in ischemic myocardium: a study with Nox2 knockout mice. Funct Integr Genomics. 2011 doi: 10.1007/s10142-011-0256-x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 165.Traystman RJ. Kirsch JR. Koehler RC. Oxygen radical mechanisms of brain injury following ischemia and reperfusion. J Appl Physiol. 1991;71:1185–1195. doi: 10.1152/jappl.1991.71.4.1185. [DOI] [PubMed] [Google Scholar]
- 166.Trinkl A. Vosko MR. Wunderlich N. Dichgans M. Hamann GF. Pravastatin reduces microvascular basal lamina damage following focal cerebral ischemia and reperfusion. Eur J Neurosci. 2006;24:520–526. doi: 10.1111/j.1460-9568.2006.04920.x. [DOI] [PubMed] [Google Scholar]
- 167.Vallet P. Charnay Y. Steger K. Ogier-Denis E. Kovari E. Herrmann F. Michel JP. Szanto I. Neuronal expression of the NADPH oxidase NOX4, and its regulation in mouse experimental brain ischemia. Neuroscience. 2005;132:233–238. doi: 10.1016/j.neuroscience.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 168.Van den Worm E. Beukelman CJ. Van den Berg AJ. Kroes BH. Labadie RP. Van Dijk H. Effects of methoxylation of apocynin and analogs on the inhibition of reactive oxygen species production by stimulated human neutrophils. Eur J Pharmacol. 2001;433:225–230. doi: 10.1016/s0014-2999(01)01516-3. [DOI] [PubMed] [Google Scholar]
- 169.Vecchione C. Brandes RP. Withdrawal of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors elicits oxidative stress and induces endothelial dysfunction in mice. Circ Res. 2002;91:173–179. doi: 10.1161/01.res.0000028004.76218.b8. [DOI] [PubMed] [Google Scholar]
- 170.Vejrazka M. Micek R. Stipek S. Apocynin inhibits NADPH oxidase in phagocytes but stimulates ROS production in non-phagocytic cells. Biochim Biophys Acta. 2005;1722:143–147. doi: 10.1016/j.bbagen.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 171.Violi F. Sanguigni V. Carnevale R. Plebani A. Rossi P. Finocchi A. Pignata C. De Mattia D. Martire B. Pietrogrande MC. Martino S. Gambineri E. Soresina AR. Pignatelli P. Martino F. Basili S. Loffredo L. Hereditary deficiency of gp91(phox) is associated with enhanced arterial dilatation: results of a multicenter study. Circulation. 2009;120:1616–1622. doi: 10.1161/CIRCULATIONAHA.109.877191. [DOI] [PubMed] [Google Scholar]
- 172.Walder CE. Green SP. Darbonne WC. Mathias J. Rae J. Dinauer MC. Curnutte JT. Thomas GR. Ischemic stroke injury is reduced in mice lacking a functional NADPH oxidase. Stroke. 1997;28:2252–2258. doi: 10.1161/01.str.28.11.2252. [DOI] [PubMed] [Google Scholar]
- 173.Wang Q. Sun AY. Simonyi A. Kalogeris TJ. Miller DK. Sun GY. Korthuis RJ. Ethanol preconditioning protects against ischemia/reperfusion-induced brain damage: role of NADPH oxidase-derived ROS. Free Radic Biol Med. 2007;43:1048–1060. doi: 10.1016/j.freeradbiomed.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Weissmann N. Sydykov A. Kalwa H. Storch U. Fuchs B. Schnitzler MMy. Brandes RP. Grimminger F. Meissner M. Freichel M. Offermanns S. Veit F. Pak O. Krause K-H. Schermuly RT. Brewer AC. Schmidt HHHW. Seeger W. Shah AM. Gudermann T. Ghofrani HA. Dietrich A. Activation of TRPC6 channels is essential for lung ischaemia-reperfusion induced oedema in mice. Nat Commun. 2012;3:649. doi: 10.1038/ncomms1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Weissmann N. Zeller S. Schafer RU. Turowski C. Ay M. Quanz K. Ghofrani HA. Schermuly RT. Fink L. Seeger W. Grimminger F. Impact of mitochondria and NADPH oxidases on acute and sustained hypoxic pulmonary vasoconstriction. Am J Respir Cell Mol Biol. 2006;34:505–513. doi: 10.1165/rcmb.2005-0337OC. [DOI] [PubMed] [Google Scholar]
- 176.Mackay J. Mensah G WHO. The Atlas of Heart Disease and Stroke. World Health Organization; 2004. ISBN 92 4 156276 5. [Google Scholar]
- 177.Wingler K. Hermans J. Schiffers P. Moens A. Paul M. Schmidt H. NOX 1, 2, 4, 5: counting out oxidative stress. Br J Pharmacol. 2011;164:866–883. doi: 10.1111/j.1476-5381.2011.01249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Woo TU. Walsh JP. Benes FM. Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-D-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2004;61:649–657. doi: 10.1001/archpsyc.61.7.649. [DOI] [PubMed] [Google Scholar]
- 179.Wosniak J., Jr. Santos CX. Kowaltowski AJ. Laurindo FR. Cross-talk between mitochondria and NADPH oxidase: effects of mild mitochondrial dysfunction on angiotensin II-mediated increase in Nox isoform expression and activity in vascular smooth muscle cells. Antioxid Redox Signal. 2009;11:1265–1278. doi: 10.1089/ars.2009.2392. [DOI] [PubMed] [Google Scholar]
- 180.Yang Z. Sharma AK. Marshall M. Kron IL. Laubach VE. NADPH oxidase in bone marrow-derived cells mediates pulmonary ischemia-reperfusion injury. Am J Respir Cell Mol Biol. 2009;40:375–381. doi: 10.1165/rcmb.2008-0300OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yemisci M. Gursoy-Ozdemir Y. Vural A. Can A. Topalkara K. Dalkara T. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med. 2009;15:1031–1037. doi: 10.1038/nm.2022. [DOI] [PubMed] [Google Scholar]
- 182.Yokota H. Narayanan SP. Zhang W. Liu H. Rojas M. Xu Z. Lemtalsi T. Nagaoka T. Yoshida A. Brooks SE. Caldwell RW. Caldwell RB. Neuroprotection from retinal ischemia/reperfusion injury by NOX2 NADPH oxidase deletion. Invest Ophthalmol Vis Sci. 2011;52:8123–8131. doi: 10.1167/iovs.11-8318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yoshioka H. Niizuma K. Katsu M. Okami N. Sakata H. Kim GS. Narasimhan P. Chan PH. NADPH oxidase mediates striatal neuronal injury after transient global cerebral ischemia. J Cereb Blood Flow Metab. 2010;31:868–880. doi: 10.1038/jcbfm.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yuan G. Khan SA. Luo W. Nanduri J. Semenza GL. Prabhakar NR. Hypoxia-inducible factor 1 mediates increased expression of NADPH oxidase-2 in response to intermittent hypoxia. J Cell Physiol. 2011;226:2925–2933. doi: 10.1002/jcp.22640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yusuf S. Diener HC. Sacco RL. Cotton D. Ounpuu S. Lawton WA. Palesch Y. Martin RH. Albers GW. Bath P. Bornstein N. Chan BP. Chen ST. Cunha L. Dahlof B. De KJ. Donnan GA. Estol C. Gorelick P. Gu V. Hermansson K. Hilbrich L. Kaste M. Lu C. Machnig T. Pais P. Roberts R. Skvortsova V. Teal P. Toni D. VanderMaelen C. Voigt T. Weber M. Yoon BW. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med. 2008;359:1225–1237. doi: 10.1056/NEJMoa0804593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhang M. Brewer AC. Schroder K. Santos CX. Grieve DJ. Wang M. Anilkumar N. Yu B. Dong X. Walker SJ. Brandes RP. Shah AM. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc Natl Acad Sci U S A. 2010;107:18121–18126. doi: 10.1073/pnas.1009700107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhang RL. Chopp M. Jiang N. Tang WX. Prostak J. Manning AM. Anderson DC. Anti-intercellular adhesion molecule-1 antibody reduces ischemic cell damage after transient but not permanent middle cerebral artery occlusion in the Wistar rat. Stroke. 1995;26:1438–1442. doi: 10.1161/01.str.26.8.1438. [DOI] [PubMed] [Google Scholar]
- 188.Zhao H. Mayhan WG. Arrick DM. Xiong W. Sun H. Alcohol-induced exacerbation of ischemic brain injury: role of NAD(P)H oxidase. Alcohol Clin Exp Res. 2010;34:1948–1955. doi: 10.1111/j.1530-0277.2010.01284.x. [DOI] [PubMed] [Google Scholar]
- 189.Zimmerman MC. Sharma RV. Davisson RL. Superoxide mediates angiotensin II-induced influx of extracellular calcium in neural cells. Hypertension. 2005;45:717–723. doi: 10.1161/01.HYP.0000153463.22621.5e. [DOI] [PubMed] [Google Scholar]