Abstract
Significance: Schizophrenia is a complex neuropsychiatric disorder affecting around 1% of the population worldwide. Its mode of inheritance suggests a multigenic neurodevelopmental disorder with symptoms appearing during late adolescence/early adulthood, with its onset strongly influenced by environmental stimuli. Many neurotransmitter systems, including dopamine, glutamate, and gamma-aminobutyric acid, show alterations in affected individuals, and the behavioral and physiological characteristics of the disease can be mimicked by drugs that produce blockade of N-methyl-d-aspartate glutamate receptors (NMDARs). Recent Advances: Mounting evidence suggests that drugs that block NMDARs specifically impair the inhibitory capacity of parvalbumin-expressing (PV+) fast-spiking neurons in adult and developing rodents, and alterations in these inhibitory neurons is one of the most consistent findings in the schizophrenic postmortem brain. Disruption of the inhibitory capacity of PV+ inhibitory neurons will alter the functional balance between excitation and inhibition in prefrontal cortical circuits producing impairment of working memory processes such as those observed in schizophrenia. Critical Issues: Mechanistically, the effect of NMDAR antagonists can be attributed to the activation of the Nox2-dependent reduced form of nicotinamide adenine dinucleotide phosphate oxidase pathway in cortical neurons, which is consistent with the emerging role of oxidative stress in the pathogenesis of mental disorders, specifically schizophrenia. Here we review the mechanisms by which NMDAR antagonists produce lasting impairment of the cortical PV+ neuronal system and the roles played by Nox2-dependent oxidative stress mechanisms. Future Directions: The discovery of the pathways by which oxidative stress leads to unbalanced excitation and inhibition in cortical neural circuits opens a new perspective toward understanding the biological underpinnings of schizophrenia. Antioxid. Redox Signal. 18, 1444–1462.
Introduction
Schizophrenia is a common psychiatric disorder with a genetic basis, but the pattern of inheritance is complex. The onset of the disease occurs generally during late adolescence or early adulthood with a lifetime morbidity of ∼1%–2% in the general population. The symptoms of schizophrenia are classified as (i) positive, including hallucinations and delusions, (ii) negative, including anhedonia and social withdrawal, and (iii) cognitive, which include working memory deficits, preservative behaviors, and poor attention. At present, no common genetic variants have been found, though a large number of genes have been associated with the disorder (69, 132).
Hypofunction of the glutamatergic system, in particular of the N-methyl-d-aspartate (NMDA)-type receptor complex (NMDAR), was directly implicated in the etiology of schizophrenia (96, 176). This association was based on original reports showing that the dissociative anesthetics phencyclidine (PCP) and ketamine had propsychotic effects in healthy humans when used at concentrations that antagonize NMDARs (8, 52, 94, 95). Moreover, these drugs also produced outbreaks in previously stabilized schizophrenia patients (123). NMDAR antagonists, such as PCP and ketamine, as well as MK-801 (see below), are now widely used in research studies as a strategy to understand the origins of schizophrenia, in both animals and humans. These studies provide new insights into the disruptions in brain circuitry that may be relevant to schizophrenia and other psychiatric diseases.
Investigations using positron-emission tomography (PET) have shown that ketamine administration acutely increased metabolism in the frontal regions of the brain, and that this increase was correlated with psychotic-like symptoms in healthy volunteers (231) as well as in rodents (43). Principal neurons in the prefrontal cortex (PFC) receive inputs from glutamatergic afferents projecting not only from intracortical structures but also from the thalamus, as well as they receive dopaminergic afferents from centers in the midbrain (73, 201); postsynaptically, principal neurons express dopamine receptors and NMDARs that make synapses bidirectionally (32, 61, 87, 130, 199), with relevant consequences for neuronal output (see 249). Dopaminergic neurons are in turn downstream to glutamatergic neurons, either directly or via GABAergic interneurons, mechanisms through which NMDAR antagonists can influence dopamine release (see 31). Thus, it has been suggested that elevated baseline levels of dopamine observed in schizophrenia may be secondary to hypoglutamatergia. In support of this hypothesis, NMDAR antagonists can enhance spontaneous and amphetamine-induced release of dopamine (159). In addition, acute application of NMDAR antagonists to nonhuman primates was shown to increase glutamate and dopamine release in PFC, leading to cortical disinhibition (220, 226). This is due to an enhanced sensitivity to antagonists of inhibitory GABAergic cells, specifically parvalbumin-positive (PV+) fast-spiking interneurons (85, 184). In sum, these facts support a multifactorial view of schizophrenia, involving interactions among the glutamatergic, GABAergic, and dopaminergic systems.
In the cerebral cortex, multiple types of GABAergic inhibitory interneurons are present that differ in their morphology, electrophysiological properties (e.g., fast-spiking and low-threshold spiking), synaptic connectivity, and expression of specific markers (e.g., parvalbumin [PV], somatostatin, and vasointestinal peptide) (103, 151). One type of GABAergic neurons, the PV-expressing fast-spiking interneurons, provides strong perisomatic inputs onto pyramidal neurons, and therefore can strongly suppress their activity (41, 158, 244). These cells are critical for the synchronization of the neural network through generation and synchronization of gamma-frequency oscillations (28, 210), which are critical for cognitive processes and are impaired in neuropsychiatric disorders (reviewed in 12, 149).
In rodents and nonhuman primates, prolonged treatment with NMDAR antagonists leads to significant alterations of the PV+ neuron phenotype, including decreased expression of PV and glutamic acid decarboxylase GAD67, the major gamma-aminobutyric acid (GABA)-synthesizing enzyme in the cortex (14, 15, 42, 107, 193). A similar loss of GAD67 expression in PV+ neurons has been consistently observed in the PFC of schizophrenic patients (222, 230), supporting the hypothesis that a dysfunction of the PV+ fast-spiking interneurons may be a core feature of the disease (135).
Thus, insights into the origins of schizophrenia could arise from understanding the mechanisms by which the exposure to NMDAR antagonists leads to prolonged alterations of PV+ neurons and their functions in cortical circuits. A key discovery was that ketamine exposures produce increased oxidative stress in the brain through activation of Nox2-dependent reduced form of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (Nox2) (14, 212), which activates a cascade of events that ultimately leads to the sustained alteration of PV+ neuronal function. Oxidative stress has been also found to be a mediator of neurochemical and behavioral alterations in other rodent models of schizophrenia, such as social isolation from rearing (198), and in rodents with diminished glutathione (GSH) production (reviewed in 48).
In this review, we will first briefly summarize the literature describing the presence of Nox2 in neurons. We will then discuss the function of NMDARs, their expression and development in the brain, and the acute and chronic consequences of exposure to NMDA antagonists. Next, we will focus on the role of Nox2 in the propsychotic effects of NMDAR antagonists and redox dysregulation. Finally, we will explore possible consequences of oxidative dysregulation of the inhibitory system for the pathophysiology of schizophrenia.
Expression of Nox2-Dependent NADPH Oxidase in Neurons
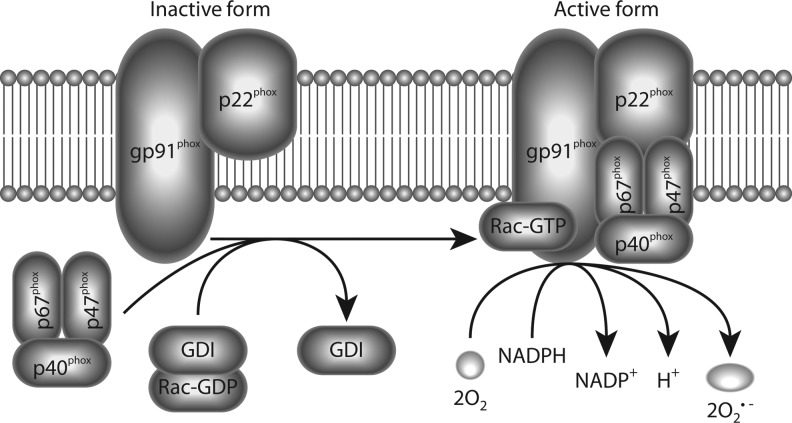
The first NADPH oxidase described, gp91phox or Nox2-dependent NADPH oxidase (Nox2), was identified in phagocytic cells as the enzyme complex responsible for the respiratory burst, an essential host response to microbial invasion (reviewed in 125). Nox2 and other members of the family are expressed in numerous tissues and cells types, including neurons (211). At least six proteins form part of the active Nox2-complex: the membrane flavocytochrome b588 core complex consisting of gp91phox and p22phox subunits and four cytoplasmic components (p40phox, p47phox, p67phox, and Rac1/2) (Fig. 1). Nox2 is inactive in resting cells, but becomes activated to generate superoxide upon assembly of the core complex with the cytoplasmic components, which occurs upon phosphorylation of p47phox (125). All the subunits required for an active Nox2 complex are expressed in neurons (reviewed in 152, 211), where activation of this complex seems to follow similar mechanisms as those described in non-neuronal cells (221). Interestingly, a specific effect of interleukin-6 (IL-6) in the activation and induction of Nox2 expression in neurons was recently demonstrated (15).
FIG. 1.
Activation and production of superoxide by reduced form of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 2 (Nox2). Catalytically functional Nox2 complex requires the assembly of membrane-bound gp91phox and p22phox, with cytosolic subunits p67phox, p47phox, p40phox, and Rac1/2. Active Nox2 complex catalyzes the oxidation of NADPH, releasing electrons that convert oxygen molecules to superoxide. Activation of several signaling kinases can promote the assembly of the Nox2 complex through phosphorylation of gp47phox subunits.
NMDAR and Its Antagonists
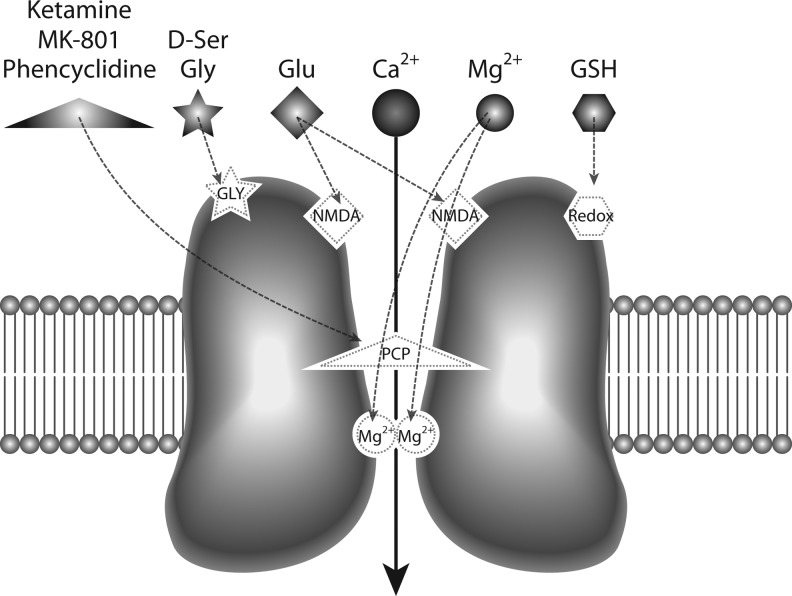
Glutamate, the main excitatory neurotransmitter, activates three different classes of ion-gated channels: α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors, kainate receptors, and NMDARs, named after their preferred synthetic agonists (142). The NMDAR has unique features that distinguish it from other ion-gated glutamate receptors (as illustrated in Fig. 2): first, it requires the binding of a co-agonist (glycine or d-serine) in addition to glutamate for maximum activation (46, 101, 110), and second, it shows a high sensitivity to magnesium blockade at rest that makes its activation strongly voltage dependent (118, 154). In addition, the NMDAR exhibits slow activation and deactivation kinetics compared to kainate and AMPA receptors, and its activity can be modulated by small extracellular molecules and ions in the CNS, in particular H+, NO, and Zn2+, and oxidizing agents (197).
FIG. 2.
Functional anatomy of the N-methyl-d-aspartate glutamate receptor (NMDAR). The NMDAR is a voltage-gated cross-membrane channel permeable to calcium. Under physiological conditions, glutamate (Glu) binds to the agonist site (labeled NMDA) and activates the channel, provided that the co-agonist site (labeled GLY) is occupied by glycine (Gly) or d-serine (D-Ser). Magnesium ions block the channel pore in a voltage-dependent manner, which is also unconditionally blocked by noncompetitive antagonists, ketamine, MK-801, or phencyclidine. A redox regulatory site (labeled Redox), especially in the NR2A subunit, provides modulation by reducing agents such as dithiothreitol (DTT) or glutathione (GSH).
The NMDAR is composed of several subunits, namely the glycine-binding core NR1, the glutamate-binding NR2, including NR2A-D, and the recently discovered glycine-binding NR3, which includes NR3A and NR3B (35, 48, 56, 144). The presence of NR1 is required for forming functional NMDARs, and this subunit combines with several NR2 and/or NR3 subunits to form heteromeric receptors (2, 40, 218). The biophysical and pharmacological properties of NMDARs depend on the subunit composition, which varies across brain regions and is subject to change during development (164).
The NMDAR is highly sensitive to redox modulation through a redox-sensitive site (Fig. 2) (1, 38, 39, 112) and decreases in the main antioxidant in the brain, GSH, or reduced activity of GSH-peroxidase can lead to oxidized hypofunctional NMDARs (125, 211). Mutagenesis studies have revealed three distinct pairs of cysteine residues on NMDAR subunits—Cys79/Cys308 and Cys744/Cys798 on the NR1 subunit and Cys87/Cys320 on the NR2A subunit—which can be specifically oxidized or reduced by agents that affect the redox state of the NMDAR (38, 112). As a result, the formation and destruction of disulfide bonds have direct consequences upon NMDAR function: while reducing agents, such as dithiothreitol and GSH increase NMDA-evoked current, oxidizing agents that promote disulfide formation, such as 5,5′-dithio-bis(2-bisnitrobenzoic acid), attenuate it (112). The oxidation status of this redox site can affect the regulation of these receptors by spermine and protons, as well as the inhibition mediated by the high-affinity Zn2+ site (139). In particular, NMDARs composed of NR1/NR2A subunits were shown to undergo a rapid and highly reversible current potentiation by sulfhydryl redox agents, including GSH, acting on a specific redox site in NR2A (112). In contrast, the effects of reducing agents on NMDARs containing NR2B and NR2C are very slow and may not be reversible unless exposed to oxidizing agents (112).
Several selective and nonselective antagonists have been used to modify NMDAR function (Table 1). Some of these drugs act as competitive antagonists, competing with glutamate or glycine at the NMDAR agonist-binding sites, and therefore can be rapidly displaced from the receptors by high local concentrations of glutamate and glycine. On the other hand, noncompetitive antagonists, such as PCP, ketamine, and MK-801, directly block the NMDAR channel when it opens (Fig. 2), and thus can affect normal physiological functions when administered to animals or humans, regardless of local concentrations of glutamate and glycine, resulting in robust cognitive alterations and psychotic events.
Table 1.
Compounds Acting at N-Methyl-d-Aspartate Glutamate Receptors As Agonists and Antagonists
| Selective agonists (glutamate site) | NMDA, aspartate, D,L(tetrazol-5-yl)glycine, homoquinolinic acid (partial agonist) |
| Selective antagonists (glutamate site) | D-AP5, CGS19755, CGP37849, LY233053, d-CCPene (NR2A=NR2B>NR2C=NR2D); PPDA (NR2C=NR2D>NR2B=NR2A); NVP-AAM077 (NR2A>NR2C>NR2D>NR2B); conantokin-G (NR2B>NR2D=NR2C=NR2A) |
| Selective agonists (glycine site) | Glycine, D-serine, (+)-HA966 (partial agonist) |
| Selective antagonists (glycine site) | 5,7-Dichlorokynurenate, L689560, L701324, GV196771A |
| Channel blockers | Mg2+, MK-801, ketamine, phencyclidine, memantine, amantidine, N1-dansylspermine (NR2A=NR2B>> NR2C=NR2D) |
Listed are some of the available compounds that have been used to modify NMDAR activity in vitro and in vivo [modified from (252)]. Relative affinities are specified in parenthesis.
NMDAR, N-methyl-d-aspartate glutamate receptor.
Expression and Function of NMDARs in Cortical Neurons
Excitatory synaptic transmission among neurons is achieved by release of glutamate from presynaptic neurons, which generates excitatory postsynaptic potentials (EPSPs) in postsynaptic neurons through activation of glutamate receptors. NMDARs are known to mediate EPSPs in multiple regions and cell types in the brain. Therefore, it is not surprising that NMDAR antagonists inhibit EPSPs in cortical pyramidal neurons in vitro and in anesthetized preparations (37, 84, 100). Interestingly, however, when administered in vivo, these drugs cause cortical activation instead of suppression in human subjects (122, 231) and rodents (92). Recent studies have suggested that these apparent contradictory effects may result from decreases in inhibitory neuron activity, due to a predominant effect of the antagonists on NMDARs present in cortical inhibitory neurons (85, 184). Disinhibition, in turn, leads to an increase in the firing rate of the majority of pyramidal neurons. The higher sensitivity of NMDARs expressed in interneurons to NMDAR antagonists may be due to a different composition in NR2 subunits (107, 246), a key factor for voltage-dependent Mg2+ blockade (90, 119, 161, 162). Although Mg2+ dependence of NR2 subunits is determined by a shared element located in the M2 domain of NR2 subunits (26, 163, 194), it has been shown that the M1–M4 segment contains subunit-specific determinants for Mg2+ block (117). This is possibly the reason why NR2A or NR2B is more sensitive to Mg2+ block as compared to NR2C- or NR2D-containing channels (161), and thus less prone to blockade by NMDAR antagonists. In the following sections, we summarize what is known about the subunit composition of NMDARs in the different neuronal types present in the cortex.
NMDARs in excitatory neurons
The NR1 subunit of NMDARs is widely expressed across all brain regions in rodents from birth (44, 161, 166), but expression of the NR2 subunit changes throughout postnatal life (127, 161, 203). In situ hybridization studies and protein analyses have shown that the newborn rodent cortex is enriched in NR2B and NR2D subunits, and that the expression of these two subunits, especially NR2D, progressively decreases throughout postnatal life. These studies have also shown that the expression of NR2A and NR2C increases during postnatal development. Although these results were obtained at the tissue level, the high proportion of excitatory neurons in the cortex (∼80%) suggests that they relate to the pattern of subunit expression in principal neurons.
The most visible consequence of this developmental modification is the progressive change from synaptic NMDARs containing predominantly NR1/NR2B/NR2D to those containing NR1/NR2A subunits (11, 243). Such changes in the subunit composition may crucially affect the permeability of NMDARs, since NR2A or NR2B subunits have larger conductance and higher sensitivity to blockade by Mg2+ than receptors containing NR2C or NR2D subunits (44, 48).
NMDARs in inhibitory neurons
Even within the same brain region, different cell types can express different combinations of NMDAR subunits, reflecting different roles in the neuronal network. Cortical inhibitory neurons expressing the neurotransmitter GABA comprise diverse subtypes that can be grouped according to the expression of calcium-binding proteins and specific peptides, as well as by their morphology and electrophysiological properties (103, 248). The expression of NMDARs is evident in several types of inhibitory neurons (72), where they control subthreshold calcium dynamics and participate in long-term synaptic plasticity (116). One particular anatomical subtype of cortical inhibitory neurons, those expressing PV, tightly regulates the activity of principal cells by providing them with strong perisomatic inhibition and can thus control the activity of neural networks physiologically, including the generation and synchrony of network rhythms in the gamma-frequency band (28, 210). Because physiological gamma-oscillations are correlated with cognitive mechanisms, including attention and working memory, it is currently thought that the perturbation of NMDAR function in PV+ neurons may be responsible for cognitive impairments associated with psychiatric disorders (185, 228).
PV+ neurons in the rodent PFC express high levels of functional NMDARs during the first 3–4 postnatal weeks, and the activity of these receptors is necessary for the normal development of their characteristic fast-spiking (107, 236, 246, 252). Interestingly, NMDARs in these neurons have a different subunit composition than that found in neighboring pyramidal neurons, with NR2A/NR2B ratios of 5:1 in PV+ neurons and 1:1 in pyramidal neurons (107, 246). PV+ neurons also express NR2C subunits, which differentiate them from principal neurons at the same developmental age (246).
In fully matured PFC, the number of NMDAR-expressing PV+ neurons is considerably reduced, due to a dramatic downregulation of functional NMDARs in these neurons during late adolescence (191, 236). Although this seems in contradiction to the findings that NMDAR antagonism in adults produced a disinhibited state with hypoactive PV+ neurons and hyperactive pyramidal neurons (85, 184), there is evidence suggesting that a residual ∼30% of the PV+ neuron population still expresses NMDARs in the PFC when animals reach adulthood (236). Thus, it is possible that this subpopulation of NMDAR-expressing PV+ neurons could be sufficient to lead to disinhibition upon NMDAR antagonism, as suggested by computational modeling of cortical networks (232).
These studies suggested that different subtypes of PV+ neurons might be present in the adult PFC (for example, subtypes of basket and/or chandelier neurons) as occurs in the entorhinal cortex, where PV+ neurons in layer 2 express NMDARs and respond to NMDAR antagonists with altered gamma-oscillatory activity in adults (45, 102). Thus, alterations in the normal maturation of PV+ neurons in different brain regions at different times could lead to the differential maturation of the neuronal circuits involved in working memory and selective attention.
Use of NMDAR Antagonists to Model Schizophrenia-Related Behavior
The hypoglutamatergic theory of schizophrenia is based on the effects of exposure to the NMDAR noncompetitive antagonists PCP, ketamine, and MK-801, which constitute the best pharmacological models of schizophrenia in rodents, nonhuman primates, and humans (96, 173, 176). Acute exposures to these substances produce a classic psychotic episode in humans and hyperdopaminergia/hyperglutamatergia in animals as well as cognitive deficits, reminiscent of both positive and negative symptoms of schizophrenia. However, these effects are reversible and short lasting. Chronic or developmental exposures, on the other hand, reproduce enduring cognitive, neurochemical, and physiological alterations, even in absence of psychosis, as observed in schizophrenia.
Acute exposures
The discovery that the dissociative anesthetics PCP and ketamine are noncompetitive antagonists of the NMDAR (8, 95) brought new insights into the study of schizophrenia (reviewed in 96). These two drugs were known to produce a psychotic state in humans emerging from anesthesia (reviewed in 52), and were later shown to exacerbate psychosis in schizophrenic patients (91, 145). The undesirable propsychotic side effects of PCP prevented its use as an anesthetic, and its abuse as a street drug led to its prohibition [see (52) for recent review of the clinical history of PCP]. The synthesis of ketamine, an analog of PCP, and the demonstration of its potency as an anesthetic and its more manageable side effects have led to its continued use in humans (52). Nonetheless, the propsychotic effects are still observed in healthy volunteers (115, 124), and ketamine exacerbates psychosis in schizophrenia patients (123, 148).
Subanesthetic concentrations of ketamine produce psychotic-like positive symptoms in humans, as well as reductions in working memory and sustained attention performance, similar to the cognitive deficits observed in schizophrenia patients (115, 124, 229). Acute ketamine exposure can impair performance on tasks testing executive function in humans (114) and nonhuman primates (217), resembling executive functioning deficits that are associated with treatment-refractory aspects of schizophrenia (105).
Similar to its effects in humans, systemic administration of NMDAR antagonists in rodents produces deficits in spatial working memory, in reversal learning, and in sustained attention (99, 213). Exposure to PCP, ketamine, and MK-801 is therefore widely used in adult animals as acute pharmacological models to study behavioral and neurochemical disruptions relevant to schizophrenia (reviewed in 169). At subanesthetic doses, ketamine also induces a wide range of behavioral alterations in rodents, including disruption of prepulse inhibition of the startle reflex (150), working memory deficits (33, 182), reversal learning deficits (64), and alterations in social behavior (205) that further resemble aspects of the cognitive and behavioral alterations observed in schizophrenia patients.
Chronic exposures
Although acute exposures to NMDAR antagonists can produce some symptoms of schizophrenia in healthy subjects, these are transient and disappear after drug washout. On the other hand, chronic exposure, as it occurs in drug addiction, can produce, in some cases, a syndrome that is indistinguishable from schizophrenia (171), and substance abuse of NMDAR antagonists can, in fact, hinder its diagnosis (192).
Repetitive NMDAR antagonist treatment in animals produces more persistent effects on stereotypy, locomotor activity, and social withdrawal (169), as well as enduring cognitive deficits and neurochemical changes that resemble more accurately the alterations observed in schizophrenia (99, 167, 169).
Developmental exposures
Alteration of glutamatergic transmission by blockade of NMDAR during the postnatal period leads to a range of behavioral abnormalities in adults that are relevant to schizophrenia, from enhancement of exploration and psychomotor agitation, to impaired working memory (169). Prenatal or perinatal NMDAR antagonist exposures also lead to impairments in sensorimotor gating, spatial memory, social interaction behavior, and cognitive flexibility in adulthood (21, 23, 24, 169, 198, 234). In addition to cognitive deficits typical of schizophrenia, rodents treated during the perinatal period with NMDAR antagonists show alterations in conditioned fear responses (89, 238).
Functional Consequences of NMDAR Blockade
Neurochemistry
Acute exposure to NMDAR antagonists in adults is believed to result in acute disinhibition (85, 140, 157, 177), as measured by increased excitatory activity in the frontal and anterior cingulate cortex (220). PET studies have shown that hypermetabolic responses after ketamine administration were more severe in schizophrenic patients as compared to control subjects, even under resting conditions (68).
In rodents, the initial hypermetabolism observed after acute NMDAR antagonist exposure shifts to a decreased metabolic activity in several brain regions if repetitive exposures occur (43). While acute exposures to PCP or ketamine increase dopamine and glutamate release in the frontal cortex in rodents and primates (4, 98, 212), repetitive exposures decrease dopamine release and leads to a hypofunctional state (99). This repetitive regimen also elicits alterations in N-acetyl-aspartate and N-acetyl-aspartylglutamate in the temporal cortex and hippocampus as assessed by high-performance liquid chromatography (189), and decreases 5HT2A receptor binding in PFC (216), resembling schizophrenia pathology (126, 174).
Although the behavioral and neurochemical effects of acute exposures to NMDAR do not lead to enduring changes in PV+ neurons observed in schizophrenia (15, 135, 212), repetitive exposures to NMDAR antagonists produce a lasting reduction in GAD67 and PV expression in PV+ neurons of rodents (15) as well as decreased expression of PV in rodents and nonhuman primates (42, 104, 168, 193). Unlike the acute effects of NMDAR antagonists, which include hyperglutamatergia, these neurochemical changes eventually lead to a hypofunctional state through a homeostatic pathway (as discussed in the following sections), and thus reflect more faithfully the alterations observed in postmortem samples collected from patients with schizophrenia (reviewed in 135, 183).
Early postnatal NMDAR blockade produces a decrease in the number of PV+-labeled neurons and principal neuron spine density in the frontal cortex, nucleus accumbens, and hippocampus in rodents when these are analyzed in adulthood (21, 170, 235). Direct confirmation of the role of NMDAR function during postnatal development in the expression of schizophrenia-like behaviors comes from results showing that genetic ablation of these receptors from PV+ neurons decreases the expression of PV, produces disinhibition of pyramidal neurons, and leads to schizophrenia-related behaviors when mice reach adulthood (18, 29, 113). Interestingly, a more profound effect in behavior was observed in animals in which the disruption of NMDAR function in PV+ neurons occurred earlier in life—around the second postnatal week (18). This period coincides with the initiation of the maturational program of the cortical PV+ network (51, 71, 175, 195), which suggests that the normal development of PV+ neurons may depend critically on a well-preserved NMDAR function.
Electrophysiology
Dynamical activities of neural circuits, especially oscillations in the gamma-frequency range, accompany many important executive functions of the brain (66, 206), and thus constitute a crucial link between the neurochemical alterations and behavioral deficits in studies of schizophrenia (for review see 67, 219, 228). The hypo-NMDA hypothesis of schizophrenia (70, 94, 177) predicted electrophysiological consequences of acute and chronic noncompetitive NMDAR blockade, providing insights into distinct circuit mechanisms that contribute to the schizophrenic brain.
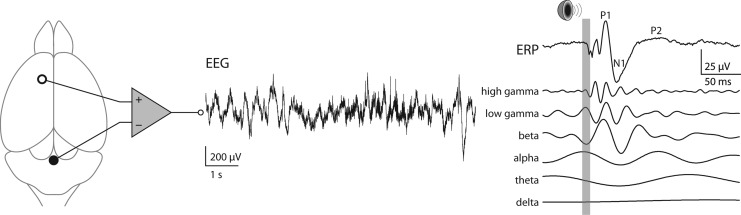
Acute application of subanesthetic concentrations of NMDAR antagonists, ketamine, MK-801, or PCP, resulted in an increase, instead of a decrease, of baseline power of gamma-frequency activity in electroencephalogram (EEG) and local field potential recorded from various cerebral cortical, hippocampal, and basal brain structures in awake, behaving rodents (Fig. 3) (55, 77, 129, 133, 146, 147, 180, 204). Later experimental work using in vivo single-unit recording suggested that the paradoxical increase of gamma-activity in response to NMDAR antagonists is due to a differential effect of these drugs on PV+ inhibitory neurons versus on pyramidal (excitatory) neurons, leading to disinhibition of excitatory activity (85, 184). Sensory-evoked activities, for example, measured auditory event-related potentials (ERPs) (Fig. 3), were observed to vary in peak amplitude and latency in the time domain (3, 153) and to have stronger gamma-frequency components in the frequency domain (55, 129, 204).
FIG. 3.
In vivo electrophysiological characterization of brain state. Illustrated is an example trace (middle) of epidural electroencephalographic (EEG) recording from frontal regions in an awake mouse. Sensory gating, a widely recognized endophenotype of schizophrenia, can be studied by quantifying the auditory event-related potential (ERP). An example ERP (right) is shown with the duration of auditory stimulus marked by a gray bar. Peaks in the time domain (labeled on the ERP trace) and power in different frequency components (traces below the ERP) are used for characterizing normal versus pathological brain states.
Consistent with the observations in rodent models, a rise in baseline gamma-power induced by acute NMDAR blockade was also found in healthy human subjects (86). Antipsychotic-treated schizophrenic patients usually have a lower level of gamma-activity in EEG, which correlates with their negative and cognitive symptoms (120, 136) and are better modeled by the enduring alterations caused by chronic or developmental NMDAR blockade, though a transient increase in gamma-power was associated with positive symptoms in psychosis (131, 136).
These results led to the hypothesis that NMDARs in PV+ neurons may serve as sensors of activity in a homeostatic control loop that goes awry in response to NMDAR antagonists (140). In contrast to transiently enhanced glutamate transmission and gamma-oscillations associated with reversible disinhibition caused by acute NMDAR blockade, lasting increase in extracellular glutamate release is a consequence of activated Nox2-dependent NADPH oxidase triggered by prolonged or repetitive NMDAR antagonism (212). This is more than likely to alter neural circuit dynamics further, which suggests that an oxidative stress mechanism is involved in the alterations in gamma-oscillatory activity observed after repetitive NMDAR antagonist exposures (183).
In contrast to in vivo investigations, in vitro studies using slice preparations yielded diverse results in terms of pharmacologically or electrically induced gamma-oscillations after acute NMDAR blockade. Amplitude of the gamma-rhythm was reported to increase in certain cortical regions, for example, the primary auditory cortex, as observed universally in vivo, but to significantly decrease in others, for example, the entorhinal cortex (45, 157, 190). Although NMDAR blockade causes enhanced gamma-activity globally in the brain, local circuit mechanisms are possibly distinct to specific regions (240, 241).
Recent studies using genetically engineered mice have revealed the importance of PV+ neurons in generating the electrophysiological phenotypes of NMDAR hypofunction. Global NR1 hypomorphic mice did not completely reproduce the alterations of auditory ERPs in schizophrenia, though they exhibited a number of behavioral deficits relevant to the disease (22). Interestingly, however, mice lacking NMDARs specifically in PV+ neurons showed electrophysiological phenotypes consistent with schizophrenia (18, 29, 113), which essentially included a raised baseline gamma-power and reduced gamma-activity evoked or induced by sensory inputs. Pharmacological studies further suggested that such aberrant gamma-oscillations were primarily due to the block of the NR2A subunit (111). Interestingly, blockade of NR2A containing NMDARs was also responsible for the activation of Nox2-mediated superoxide production after ketamine exposure (183). It is also worth noting that the manifestations of these neural circuit and behavioral abnormalities are determined within a specific developmental time window (18) that coincides with the delayed maturation of PV+ neurons (51, 71, 175). During this time window, NMDAR-blockade-triggered oxidative stress is sufficient to cause loss of function in PV+ neurons (14, 183).
In sum, important electrophysiological endophenotypes of schizophrenia, especially baseline and sensory-driven gamma-oscillations, can be recapitulated in rodent models of noncompetitive NMDAR blockade using ketamine, MK-801, or PCP. These antagonists specifically target PV+ neurons, either through acute suppression of their activity or induction of lasting functional alteration by redox regulation of the NR2A subunit, leading to disruption of neural circuits that generate aberrant levels of functional gamma-oscillation as observed in schizophrenia. Recent computational studies that incorporate the neurochemical changes observed after NMDAR antagonist exposures corroborate these alterations in gamma-oscillatory activity (232).
The Role of Nox2 in the Propsychotic Effects of NMDAR Antagonists
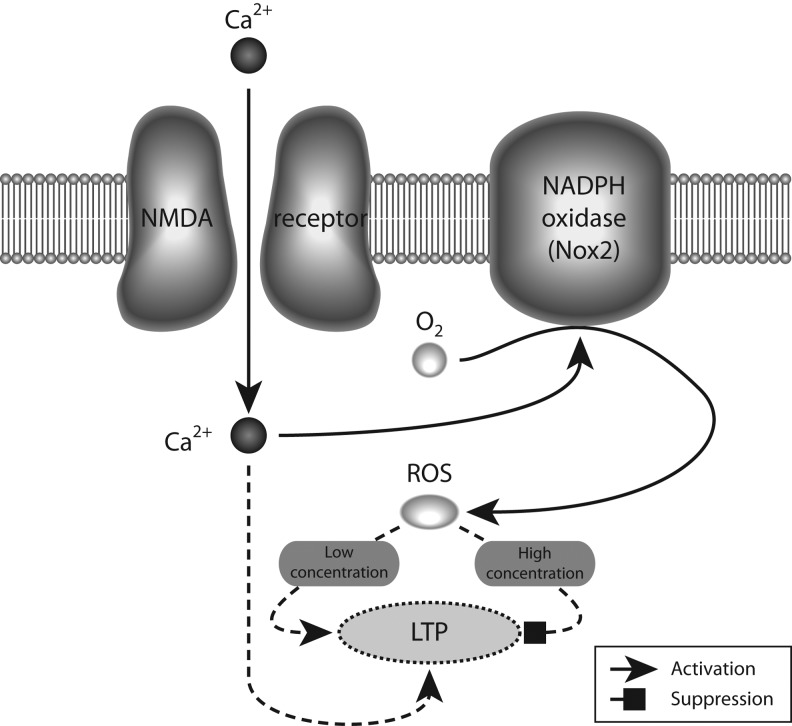
Each of the steps involved in neurotransmission, including receptor binding, opening of ion-channel neurotransmitter receptors, membrane fusion, presynaptic neurotransmitter uptake, and synaptic vesicle refilling, are influenced by the redox state of regulatory sulfhydryl groups on cysteine residues in the proteins involved in each of those steps. Alterations in the neuronal redox milieu would be thus expected to have an important impact on neurotransmission. Several lines of evidence also suggest that reactive oxygen species (ROS), specifically superoxide and hydrogen peroxide, which are normally involved in age-dependent neurodegenerative processes, may be involved in learning and memory in normal brain function (reviewed in 152) (summarized in Fig. 4).
FIG. 4.
Low level of reactive oxygen species is required for normal synaptic plasticity. Activation of NADPH oxidases by calcium entering through NMDARs leads to low concentration (in the micromolar range) of reactive oxygen species (ROS), which facilitates local field potential (LTP) by activating the PKC and ERK pathways, whereas a high concentration (in the millimolar range) of ROS inhibits LTP. For the sake of clarity, the Nox2 complex is illustrated as a single component.
Mitochondria had been traditionally regarded as the site of formation of ROS (78). However, recent data show expression of the different subunits forming the NADPH (Nox2)-oxidase complex in the cell membrane of neurons (14, 106, 200, 221), suggesting that this enzyme plays an important role in normal brain physiology. Indeed, there is a requirement for Nox2-dependent superoxide production in NMDAR-mediated long-term signaling mechanisms, such as activation of the ERK-kinase system (109). Furthermore, a similar signaling role for Nox2-produced superoxide is suggested by experiments showing decreased long-term potentiation (LTP) and altered memory in animals lacking one of the subunits that forms the Nox2 oxidase (108). In addition, several studies have shown that blockade of NMDARs also produces an increase in superoxide production, which leads to detrimental effects in neuronal network performance (14, 212, 254). The superoxide produced by Nox2 oxidase is short lived, being rapidly converted to hydrogen peroxide by the action of superoxide dismutases. Then, most actions of Nox2 oxidase activation should be mediated by hydrogen peroxide. Indeed, several lines of evidence have suggested a dual role for hydrogen peroxide levels in learning and memory processes, where low-level increases are necessary for the functional changes associated with LTP, but higher doses are detrimental for its induction (152) (Fig. 4).
Several studies have reported an altered oxidative state in schizophrenia patients (reviewed in (49). GSH, responsible for detoxification of ROS and other radical species, is consistently decreased in the cerebrospinal fluid of drug-naïve schizophrenia patients (25, 50, 138, 185), as well as in postmortem tissue (250). Polymorphisms in genes coding for enzymes that participate in GSH synthesis have been linked to the risk of schizophrenia (76, 223), and recent results show that genetically compromised GSH synthesis affects the morphological and functional integrity of hippocampal PV+ neurons (215). Furthermore, results showing that treatment with N-acetylcysteine, a precursor of GSH, improves negative symptoms and corrects mismatch negativity in schizophrenic patients provide strong support for a redox imbalance in schizophrenia (19, 128).
A role for oxidative stress mechanisms in the propsychotic effects of NMDAR antagonists was recently suggested by studies showing that there is a rapid increase in brain ROS and reactive nitrogen species as a consequence of the exposure of adult animals to those drugs (14, 15, 60, 212, 254). It was also found that the acute exposure to ketamine increased extracellular glutamate and dopamine in the prelimbic region of wild-type mice, but failed to do so in Nox2-deficient animals (212). The specific role of Nox2 was confirmed in this study by a normal response to amphetamine in Nox2 animals. These results suggest the interesting possibility that activation of superoxide production by Nox2 is required for the acute disinhibition of pyramidal neurons (17). However, such a direct role for Nox2 in glutamatergic transmission has not been clearly elucidated. It is possible that the acute oxidative stress produced by activation of Nox2 is sufficient to produce the functional inactivation of PV+ neurons, and that this is the mechanism by which disinhibition occurs. Indeed, the fast-spiking characteristic of PV+ neurons entails a high metabolic cost (79), which could render these neurons highly sensitive to oxidative stress.
Elevated synaptic activity through NMDAR activation enhances thioredoxin activity, facilitates the reduction of hyperoxidized peroxiredoxins, and promotes resistance to oxidative stress (178). However, overactivation of NMDARs, such as it occurs in excitotoxic insults, can lead to overproduction of ROS and oxidative damage (54, 121, 134, 188). Thus, a tight control of antioxidant systems must exist in neurons, and an imbalance in redox regulatory systems would be expected to alter neuronal function.
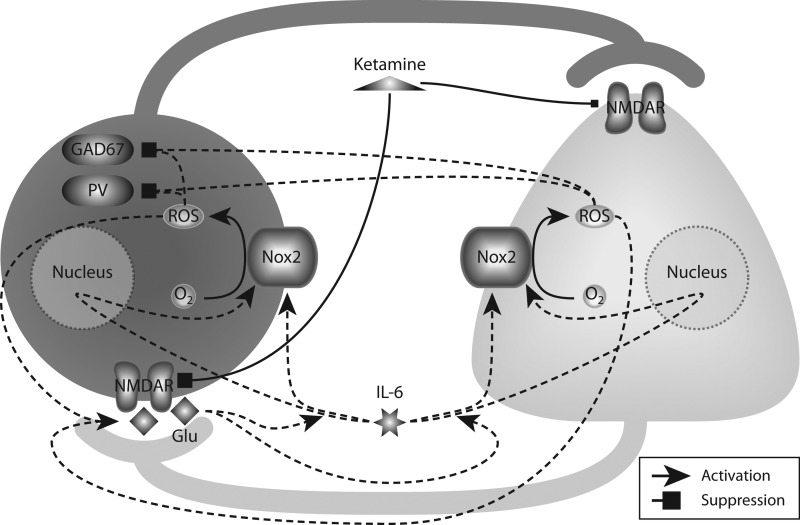
Repetitive exposures of adult mice to NMDAR antagonists showed that injections of ketamine on two consecutive days induced an increased oxidative state in the brain, which was sufficient to produce the loss of GAD67 and PV expression in PV+ neurons (14), as well as an enduring inhibitory dysfunction in the rat prelimbic region (251). This altered oxidative state produced by ketamine was due to a sustained increase in the proinflammatory cytokine IL-6 and activation of the superoxide-producing enzyme NADPH oxidase-2 (Nox2). Such effects of ketamine were absent in Nox2-deficient and IL-6-deficient animals, confirming the role of the IL-6/Nox2 pathway as mediator of ketamine effects (15) (Fig. 5).
FIG. 5.
Oxidative stress induced by repetitive NMDAR blockade leads to loss of phenotype in parvalbumin-positive (PV+) neurons. Exposure of NMDARs to noncompetitive antagonists, such as ketamine, induces a preferential blockade of NMDARs in PV+ neurons (left), which disinhibits pyramidal neurons (right). Resultant excess of glutamate (Glu) release facilitates the production of interleukin-6 (IL-6), which in turn activates the NADPH oxidase Nox2 directly (short-term), as well as upregulates the expression of Nox2 (long-term). Elevated activity of Nox2 produces ROS that further promote glutamate release and eventually cause a dramatic downregulation of parvalbumin (PV) and GAD67 in PV+ neurons.
A similar increase in superoxide production as that produced by ketamine was observed using an NR2A-preferring antagonist (NVP-AAM007), at concentrations known to preferentially affect NR2A-containing receptors (57, 183). These results give support to a specific role for NR2A-containing NMDARs in mediating the increased oxidative stress in the adult brain, as was suggested by earlier results showing that NR2A-containing NMDARs are involved in the maintenance of the inhibitory phenotype of PV+ neurons in primary cultures (107).
Oxidative Dysregulation of the Inhibitory System and Its Possible Causative Relation to the Pathophysiology of Schizophrenia
There is increasing evidence that schizophrenia, with symptoms typically appearing during late adolescence or early adulthood, is a consequence of alterations in early brain development (58, 186). Several animal models have been used to understand neurobiological processes relevant to the developmental hypothesis of schizophrenia (58, 138, 155). These models have provided insight into the vulnerability of the developing embryo and the importance of the late-prenatal/early-postnatal environment for normal maturation of brain function.
Developmental models specific to schizophrenia have focused on epidemiological and pharmacological risk factors that include, but are not limited to, prenatal/postnatal infections, NMDAR antagonist administration, and neonatal ventral hippocampal lesions (183). In all of these models, the development of behavioral and anatomical alterations resembling those found in schizophrenia has been analyzed when animals reach adulthood. Common features include alterations in gating response, decreased working memory, alterations in dopaminergic responses, and diminished inhibitory activity, specifically of PV+ neurons (183).
Starting at the end of the first postnatal week in rodents, inhibitory neuronal networks are critically involved in experience-dependent refinement of neural networks. During this period, cortical inhibition is fundamental to the formation of critical periods for sensory plasticity (81). The levels of the inhibitory neurotransmitter GABA profoundly influence inhibitory neuron axon growth and synapse formation during brain postnatal development, and alterations in GAD67, the main enzyme producing GABA, can have profound effects on the proper development of inhibitory circuits (reviewed in 88). Among all inhibitory neurons in the cortex, and across species, the last to mature are the PV+ inhibitory neurons (74, 82, 196). In rodents, the maturational process of these neurons starts at the end of the first postnatal week (47, 51, 175). Throughout the next 3 weeks, they slowly mature into fast-spiking inhibitory neurons (51, 71, 175, 195). This period of maturation occurs concomitantly with a striking transcriptional change (71, 175) in which most of the genes characteristically expressed in mature PV+ interneurons are turned on in an orchestrated fashion between the second and fourth postnatal weeks, coinciding with their electrophysiological maturation (51, 71, 175).
Among all interneuron subtypes, PV+ interneurons receive the highest number of glutamatergic synapses from thalamic afferents and intracortical networks in the adult rodent brain (75). Glutamatergic neurotransmission preferentially activates Ca2+-permeable AMPA receptors on mature PV+ interneurons, and these receptors appear slowly during postnatal development (72, 237). The expression and function of NMDARs in PV+ neurons change during postnatal development, with high levels being expressed early during postnatal development and profound functional changes occurring during adolescence (21, 107, 236, 252).
The high NMDA/AMPA receptor ratio during postnatal development might make PV+ neurons extremely sensitive to alterations in glutamatergic transmission, specifically on NMDAR function. Such effect could be detrimental for the transcriptional program that controls the maturation of the PV+ neuronal system, leading to structural alterations of the cortical network. Accumulating evidence shows that embryonic and perinatal NMDAR antagonist exposures, contrary to the reversible effects observed in adults (15), can induce a loss of PV+ neurons in several regions, and produce persistent behavioral and neurochemical deficits when animals reach adulthood (7, 21, 53, 170, 207, 214, 235, 242). High doses of NMDA antagonists during the perinatal period were previously shown to lead to diminished numbers of PV-expressing neurons when animals reached adulthood. Since exposure to these same antagonists was shown to trigger cell death, through apoptotic mechanisms in several brain regions (6, 20, 208, 233), it was believed that the reduced number of PV+ neurons observed was due to their death (177, 234, 235). However, the lack of expression of a cell marker such as PV does not necessarily incur cell death. Using a mouse line expressing GFP exclusively in PV+ neurons in the cortex (G42 line, 34), we recently demonstrated that perinatal exposures to low doses of ketamine do not lead to the death of PV+ neurons, but to the absence of PV expression in around 35% of the neurons (183). These results suggest that while the cells are still alive, their developmental maturation may be affected. Similar results were recently obtained using a mouse line expressing GFP in all GABAergic neurons (252). In this study, using an NR2A-preferring antagonist, the authors showed that prolonged blockade of NR2A-containing receptors in vivo during the critical period of plasticity in the barrel cortex produced a decrease in PV expression and an alteration of fast-spiking-mediated inhibitory postsynaptic currents onto principal neurons.
Alterations upon brain development during specific periods of pre- or postnatal life produce a decrease in the expression of PV in several brain regions (13, 65, 97, 135, 137, 143, 156, 179, 187, 209, 222, 225). Interestingly, reverse translational models using schizophrenia risk genes such as DISC1, NRG1/ErbB4, and Reelin show similar alterations of PV expression (5, 10, 59, 62, 83, 172, 202, 239).
Several animal models with disruptions in genes involved in the development and maturation of PV+ neurons are now available. From these studies, we have learned the key roles played by schizophrenia risk gene products such as TrkB, ErbB4, (receptors for brain-derived neurotrophic factor and neuregulin, respectively), dysbindin, and cannabinoid receptor 1 in the maturation process of PV+ neurons (30, 36, 63, 239, 253). Interestingly, it was recently shown that deletion of selenoprotein expression or of the Se-dependent glutathione peroxidase 4 severely affects the maturation of PV+ neurons (245). Furthermore, specific deletion of the catalytic subunit of glutathione synthase from PV+ neurons leads to a cell autonomous increased oxidative stress and pronounced decrease in PV+ synaptic contacts (165). These results suggest that PV+ neurons show a heightened sensitivity to oxidative stress conditions that could be due, in part, to their high metabolic demand (80).
Mechanisms involving oxidative stress during development are hypothesized to underlie the origin of schizophrenia pathophysiology. Decreases in antioxidant capacity during early postnatal periods in rodents, as well as genetic deficiencies in GSH, produce a loss in PV+ neurons and induce cognitive derangements relevant to the disease (27, 215). Acute GSH depletion potentiates the release of dopamine produced by amphetamine in the striatum and enhances the behavioral effects of NMDAR antagonists, as a well as those of amphetamine (93).
Antioxidants can prevent the appearance of behavioral disruptions in adult animals that were treated with PCP during the perinatal period, suggesting that oxidative mechanisms in the perinatal NMDAR antagonist model were involved (234). Exposures to PCP and to more selective NMDAR antagonists, such as MK-801 and CPP, were shown to produce a rapid increase in ROS and reactive nitrogen species in vitro (247) and in vivo (60, 254), and repetitive exposures in vivo led to a substantial elevation of baseline levels of free radicals, suggesting that this treatment results in a persistent change in the oxidative state of the cortex (254). The lack of effects of ketamine on Nox2-deficient animals supports the role of Nox2 in the loss of PV+ interneurons in the perinatal ketamine model (183). Furthermore, recent studies have implicated Nox2-dependent oxidative mechanisms in the loss of PV+ neurons and development of schizophrenia-like behavior in the isolation- rearing model (79, 198), a model that was positively associated with oxidative stress as measured by increased superoxide dismutase, decrease oxidized/reduced GSH ratio, and increased concentrations of malondialdehyde (160).
So far, the targets of oxidative stress in PV+ neurons that lead to their enduring dysfunction in neurodevelopmental models of schizophrenia are unknown. One possible hypothesis is that glutamatergic synapses onto these neurons might be particularly sensitive to oxidative stress (17). Such increased sensitivity could be due to the fundamental role that glutamatergic transmission has on the maturation of these interneurons, where both NMDA and AMPA receptors slowly appear during the second postnatal week, and show a developmental switch in subunit expression during the next weeks of development (51, 71, 175, 236, 252). Oxidative modification of neurotransmitter transporters (such as glutamate transporters) and ligand-gated ion-channels (such as GABAA and NMDARs) and consequent decrease of their activity might be another target by which oxidative stress alters the development of PV+ neurons. In neurons, cysteine, the limiting substrate in the synthesis of GSH, the antioxidant that neutralizes ROS, is taken up from the extracellular space by EAAC1, the main neuronal glutamate transporter (9). EAAC1 as well as EAAC2 have been shown to be highly sensitive to oxidative conditions, where reducing agents activate, and oxidation inactivates glutamate transport (9, 224). Regulatory redox sites have also been found in proteins that are key to glutamatergic neurotransmission through NMDARs, including the enzyme serine racemase, which is responsible for the synthesis of glycine co-agonist at NMDARs (169a), and glutamine synthase, which is responsible for glutamate synthesis (181). As discussed above, the NMDAR itself is highly sensitive to redox modulation through redox-sensitive sites (Fig. 2).
Redox-mediated changes in transcriptional activity might affect the maturational process of PV+ neurons. Under physiological conditions, nuclear antioxidants are critical for maintaining the environment needed for proper gene transcription. A number of transcription factors contain redox-sensitive residues, and in most cases, oxidation inhibits their deoxyribonucleic acid (DNA)-binding activities (227). The transcriptional activation responsible for PV+ neuronal maturation occurring during the perinatal period could thus be the target of oxidative stress that results in enduring disruption of PV+ neuronal function later in life (Fig. 6). Indeed, recent data show alterations in several frontal cortex messenger ribonucleic acid (mRNAs) after perinatal exposure to PCP (141). Whether any or all of these mRNA changes occur in PV+ neurons remains to be elucidated.
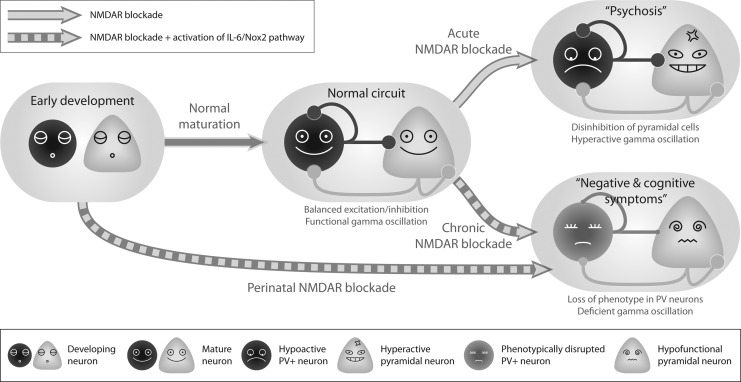
FIG. 6.
Summary of the changes induced to neural circuits as a consequence of NMDAR blockade. PV+ and pyramidal neuronal populations are represented by the dark-colored circles and light-colored triangles, respectively. In normally developed circuits, balanced excitatory and inhibitory connections between and within the two types of neurons give rise to gamma-oscillations required for cognitive functions. Acute blockade of NMDARs, without causing structural alterations to the circuit, preferentially suppresses the activity of PV+ neurons, leading to disinhibition of pyramidal neurons, which produces elevated gamma-activities and models psychotic episodes of schizophrenia. Chronic and developmental blockade of NMDARs, on the other hand, activate the IL-6/Nox2 pathway illustrated in Figure 5, rendering the PV+ neurons hypofunctional, and eventually leading to abnormal weights of synaptic connections in the network. This, in turn, generates a phenotype that recapitulates some negative and cognitive symptoms of schizophrenia, such as deficient gamma activities.
Conclusions
The possibility that PV+ inhibitory neurons are not just one of the many types of neurons that are affected in schizophrenia, but are at the nexus of the disease, with alterations starting early in development, is supported by mounting experimental evidence summarized in this review. In particular, the evidence points toward the early involvement of oxidative stress mechanisms and proinflammatory cytokines in derailing the normal development of PV+ neurons. Disruption of one of the main inhibitory circuits in the cortex would affect the way excitatory neurons develop throughout the same developmental period, through a homeostatic process that restores the excitatory/inhibitory balance. Although compensatory mechanisms may restore this balance, the altered state of the cortex could remain vulnerable to further episodes of oxidative stress (Fig. 7).
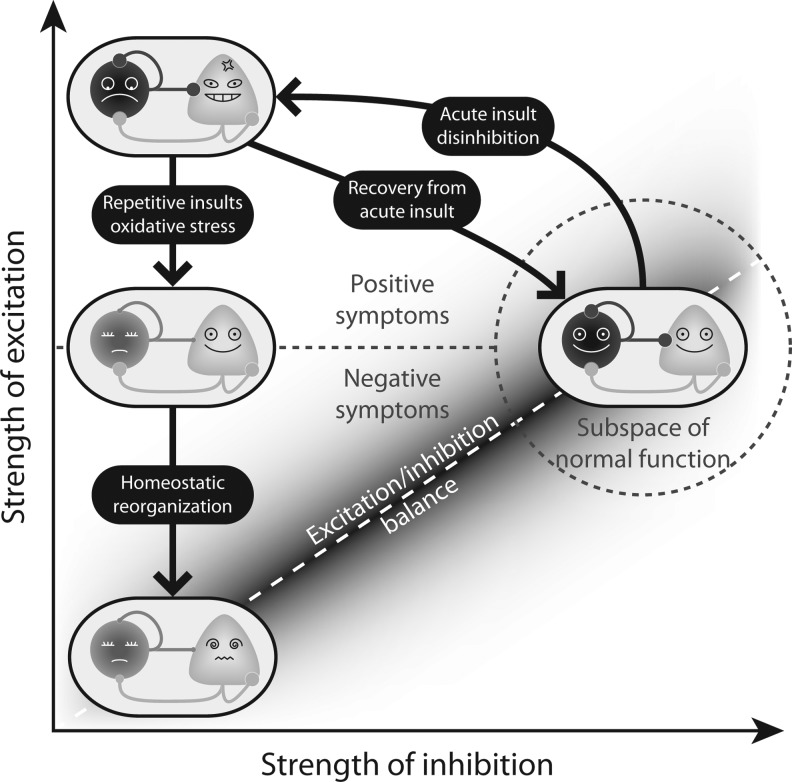
FIG. 7.
A speculative explanation of how oxidative stress produces abnormal states in the schizophrenic brain. Normal brain circuits (right) have balanced excitation and inhibition between PV+ and pyramidal neurons and thus are capable of generating functional gamma-rhythms. A single, acute insult caused by NMDAR blockade tips the excitation/inhibition balance and thus knocks the brain state out of the range of normal function (dashed gray circle) and into a disinhibited state (top left) that resembles psychosis. Although the effect of an acute insult is reversible, repetitive NMDAR blockade leads to a permanent loss of phenotype and weakened synaptic connections involving PV+ inhibitory neurons. Subsequent to this change, the circuit undergoes a homeostatic reorganization to restore the excitation/inhibition balance by downregulating the excitatory connection weights, which finally results in a state (bottom left) incapable of generating functional gamma-oscillations, reminiscent of cognitive deficits in schizophrenia.
The ultimate mechanism by which oxidative stress produces long-term effects in the maturation of cortical circuits could be related to epigenetic alterations during sensitive periods of neurodevelopment, as suggested by recent results from our group showing that ketamine effects on PV+ neurons are prevented by inhibition of DNA-methylation processes (16). These new findings are exciting because they suggest that some aspects of schizophrenia may be treated by reversing the dysregulated genes in the PV+ cells, thus restoring the cortical circuits to a normal state. The next steps are underway to test this hypothesis.
Abbreviations Used
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid
- DNA
deoxyribonucleic acid
- DTT
dithiothreitol
- EEG
electroencephalogram or electroencephalography
- EPSP
excitatory postsynaptic potential
- ERP
event-related potential
- GABA
γ-aminobutyric acid
- GSH
glutathione
- IL-6
interleukin-6
- LFP
local field potential
- LTP
long-term potentiation
- mRNA
messenger ribonucleic acid
- NADPH
reduced form of nicotinamide adenine dinucleotide phosphate
- NMDA
N-methyl-d-aspartate glutamate
- NMDAR
N-methyl-d-aspartate glutamate receptor
- PCP
phencyclidine
- PET
positron-emission tomography
- PFC
prefrontal cortex
- PV
parvalbumin
- PV+
parvalbumin-positive or parvalbumin-expressing
- ROS
reactive oxygen species
Acknowledgments
The authors were supported by the grants from NARSAD (Gwill Newman Investigator, MMB), NIMH (MH091407, MMB), and Howard Hughes Medical Institute (TJS). APD is a recipient of a Calouste Gulbenkian Foundation Fellowship, and XW is a recipient of a Life Sciences Research Foundation Pfizer Fellowship.
References
- 1.Aizenman E. Lipton SA. Loring RH. Selective modulation of NMDA responses by reduction and oxidation. Neuron. 1989;2:1257–1263. doi: 10.1016/0896-6273(89)90310-3. [DOI] [PubMed] [Google Scholar]
- 2.Al-Hallaq RA. Jarabek BR. Fu Z. Vicini S. Wolfe BB. Yasuda RP. Association of NR3A with the N-methyl-D-aspartate receptor NR1 and NR2 subunits. Mol Pharmacol. 2002;62:1119–1127. doi: 10.1124/mol.62.5.1119. [DOI] [PubMed] [Google Scholar]
- 3.Amann LC. Halene TB. Ehrlichman RS. Luminais SN. Ma N. Abel T. Siegel SJ. Chronic ketamine impairs fear conditioning and produces long-lasting reductions in auditory evoked potentials. Neurobiol Dis. 2009;35:311–317. doi: 10.1016/j.nbd.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amitai N. Kuczenski R. Behrens MM. Markou A. Repeated phencyclidine administration alters glutamate release and decreases GABA markers in the prefrontal cortex of rats. Neuropharmacology. 2011;62:1422–1431. doi: 10.1016/j.neuropharm.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ammassari-Teule M. Sgobio C. Biamonte F. Marrone C. Mercuri NB. Keller F. Reelin haploinsufficiency reduces the density of PV+ neurons in circumscribed regions of the striatum and selectively alters striatal-based behaviors. Psychopharmacology (Berl) 2009;204:511–521. doi: 10.1007/s00213-009-1483-x. [DOI] [PubMed] [Google Scholar]
- 6.Anastasio NC. Xia Y. O'Connor ZR. Johnson KM. Differential role of N-methyl-D-aspartate receptor subunits 2A and 2B in mediating phencyclidine-induced perinatal neuronal apoptosis and behavioral deficits. Neuroscience. 2009;163:1181–1191. doi: 10.1016/j.neuroscience.2009.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersen JD. Pouzet B. Spatial memory deficits induced by perinatal treatment of rats with PCP and reversal effect of D-serine. Neuropsychopharmacology. 2004;29:1080–1090. doi: 10.1038/sj.npp.1300394. [DOI] [PubMed] [Google Scholar]
- 8.Anis NA. Berry SC. Burton NR. Lodge D. The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. Br J Pharmacol. 1983;79:565–575. doi: 10.1111/j.1476-5381.1983.tb11031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aoyama K. Suh SW. Hamby AM. Liu J. Chan WY. Chen Y. Swanson RA. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat Neurosci. 2006;9:119–126. doi: 10.1038/nn1609. [DOI] [PubMed] [Google Scholar]
- 10.Ayhan Y. Abazyan B. Nomura J. Kim R. Ladenheim B. Krasnova IN. Sawa A. Margolis RL. Cadet JL. Mori S. Vogel MW. Ross CA. Pletnikov MV. Differential effects of prenatal and postnatal expressions of mutant human DISC1 on neurobehavioral phenotypes in transgenic mice: evidence for neurodevelopmental origin of major psychiatric disorders. Mol Psychiatry. 2010;16:293–306. doi: 10.1038/mp.2009.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barth AL. Malenka RC. NMDAR EPSC kinetics do not regulate the critical period for LTP at thalamocortical synapses. Nat Neurosci. 2001;4:235–236. doi: 10.1038/85070. [DOI] [PubMed] [Google Scholar]
- 12.Bartos M. Vida I. Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- 13.Beasley CL. Zhang ZJ. Patten I. Reynolds GP. Selective deficits in prefrontal cortical GABAergic neurons in schizophrenia defined by the presence of calcium-binding proteins. Biol Psychiatry. 2002;52:708–715. doi: 10.1016/s0006-3223(02)01360-4. [DOI] [PubMed] [Google Scholar]
- 14.Behrens MM. Ali SS. Dao DN. Lucero J. Shekhtman G. Quick KL. Dugan LL. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318:1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- 15.Behrens MM. Ali SS. Dugan LL. Interleukin-6 mediates the increase in NADPH-oxidase in the ketamine model of schizophrenia. J Neurosci. 2008;28:13957–13966. doi: 10.1523/JNEUROSCI.4457-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Behrens MM. Hasenstaub A. Sejnowski TJ. A role for DNA methylation in the NMDA receptor antagonist-mediated loss of phenotype of parvalbumin-positive fast-spiking interneurons. Soc Neurosci Abstr. 2010;40:CC2. [Google Scholar]
- 17.Behrens MM. Sejnowski TJ. Does schizophrenia arise from oxidative dysregulation of parvalbumin-interneurons in the developing cortex? Neuropharmacology. 2009;57:193–200. doi: 10.1016/j.neuropharm.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belforte JE. Zsiros V. Sklar ER. Jiang Z. Yu G. Li Y. Quinlan EM. Nakazawa K. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13:76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berk M. Copolov D. Dean O. Lu K. Jeavons S. Schapkaitz I. Anderson-Hunt M. Judd F. Katz F. Katz P. Ording-Jespersen S. Little J. Conus P. Cuenod M. Do KQ. Bush AI. N-acetyl cysteine as a glutathione precursor for schizophrenia—a double-blind, randomized, placebo-controlled trial. Biol Psychiatry. 2008;64:361–368. doi: 10.1016/j.biopsych.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Bittigau P. Ikonomidou C. Glutamate in neurologic diseases. J Child Neurol. 1997;12:471–485. doi: 10.1177/088307389701200802. [DOI] [PubMed] [Google Scholar]
- 21.Boctor SY. Ferguson SA. Neonatal NMDA receptor antagonist treatments have no effects on prepulse inhibition of postnatal day 25 Sprague-Dawley rats. Neurotoxicology. 2009;30:151–154. doi: 10.1016/j.neuro.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 22.Bodarky CL. Halene TB. Ehrlichman RS. Banerjee A. Ray R. Hahn CG. Jonak G. Siegel SJ. Novel environment and GABA agonists alter event-related potentials in N-methyl-D-aspartate NR1 hypomorphic and wild-type mice. J Pharmacol Exp Ther. 2009;331:308–318. doi: 10.1124/jpet.109.150938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Broberg BV. Dias R. Glenthoj BY. Olsen CK. Evaluation of a neurodevelopmental model of schizophrenia—early postnatal PCP treatment in attentional set-shifting. Behav Brain Res. 2008;190:160–163. doi: 10.1016/j.bbr.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 24.Broberg BV. Glenthoj BY. Dias R. Larsen DB. Olsen CK. Reversal of cognitive deficits by an ampakine (CX516) and sertindole in two animal models of schizophrenia—sub-chronic and early postnatal PCP treatment in attentional set-shifting. Psychopharmacology (Berl) 2009;206:631–640. doi: 10.1007/s00213-009-1540-5. [DOI] [PubMed] [Google Scholar]
- 25.Browne S. Clarke M. Gervin M. Waddington JL. Larkin C. O'Callaghan E. Determinants of quality of life at first presentation with schizophrenia. Br J Psychiatry. 2000;176:173–176. doi: 10.1192/bjp.176.2.173. [DOI] [PubMed] [Google Scholar]
- 26.Burnashev N. Schoepfer R. Monyer H. Ruppersberg JP. Gunther W. Seeburg PH. Sakmann B. Control by asparagine residues of calcium permeability and magnesium blockade in the NMDA receptor. Science. 1992;257:1415–1419. doi: 10.1126/science.1382314. [DOI] [PubMed] [Google Scholar]
- 27.Cabungcal JH. Preissmann D. Delseth C. Cuenod M. Do KQ. Schenk F. Transitory glutathione deficit during brain development induces cognitive impairment in juvenile and adult rats: relevance to schizophrenia. Neurobiol Dis. 2007;26:634–645. doi: 10.1016/j.nbd.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Cardin JA. Carlen M. Meletis K. Knoblich U. Zhang F. Deisseroth K. Tsai LH. Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carlen M. Meletis K. Siegle JH. Cardin JA. Futai K. Vierling-Claassen D. Ruhlmann C. Jones SR. Deisseroth K. Sheng M. Moore CI. Tsai LH. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol Psychiatry. 2011;17:537–548. doi: 10.1038/mp.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carlson GC. Talbot K. Halene TB. Gandal MJ. Kazi HA. Schlosser L. Phung QH. Gur RE. Arnold SE. Siegel SJ. Dysbindin-1 mutant mice implicate reduced fast-phasic inhibition as a final common disease mechanism in schizophrenia. Proc Natl Acad Sci U S A. 2011;108:E962–E970. doi: 10.1073/pnas.1109625108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carlsson A. Waters N. Holm-Waters S. Tedroff J. Nilsson M. Carlsson ML. Interactions between monoamines, glutamate, and GABA in schizophrenia: new evidence. Annu Rev Pharmacol Toxicol. 2001;41:237–260. doi: 10.1146/annurev.pharmtox.41.1.237. [DOI] [PubMed] [Google Scholar]
- 32.Cepeda C. Levine MS. Where do you think you are going? The NMDA-D1 receptor trap. Sci STKE. 2006;2006:pe20. doi: 10.1126/stke.3332006pe20. [DOI] [PubMed] [Google Scholar]
- 33.Chan MH. Chiu PH. Sou JH. Chen HH. Attenuation of ketamine-evoked behavioral responses by mGluR5 positive modulators in mice. Psychopharmacology (Berl) 2008;198:141–148. doi: 10.1007/s00213-008-1103-1. [DOI] [PubMed] [Google Scholar]
- 34.Chattopadhyaya B. Di Cristo G. Higashiyama H. Knott GW. Kuhlman SJ. Welker E. Huang ZJ. Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J Neurosci. 2004;24:9598–9611. doi: 10.1523/JNEUROSCI.1851-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen PE. Wyllie DJ. Pharmacological insights obtained from structure-function studies of ionotropic glutamate receptors. Br J Pharmacol. 2006;147:839–853. doi: 10.1038/sj.bjp.0706689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen YJ. Zhang M. Yin DM. Wen L. Ting A. Wang P. Lu YS. Zhu XH. Li SJ. Wu CY. Wang XM. Lai C. Xiong WC. Mei L. Gao TM. ErbB4 in parvalbumin-positive interneurons is critical for neuregulin 1 regulation of long-term potentiation. Proc Natl Acad Sci U S A. 2010;107:21818–21823. doi: 10.1073/pnas.1010669107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Z. Travers JB. Inactivation of amino acid receptors in medullary reticular formation modulates and suppresses ingestion and rejection responses in the awake rat. Am J Physiol Regul Integr Comp Physiol. 2003;285:R68–R83. doi: 10.1152/ajpregu.00054.2003. [DOI] [PubMed] [Google Scholar]
- 38.Choi Y. Chen HV. Lipton SA. Three pairs of cysteine residues mediate both redox and zn2+modulation of the nmda receptor. J Neurosci. 2001;21:392–400. doi: 10.1523/JNEUROSCI.21-02-00392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi YB. Tenneti L. Le DA. Ortiz J. Bai G. Chen HS. Lipton SA. Molecular basis of NMDA receptor-coupled ion channel modulation by S-nitrosylation. Nat Neurosci. 2000;3:15–21. doi: 10.1038/71090. [DOI] [PubMed] [Google Scholar]
- 40.Ciabarra AM. Sullivan JM. Gahn LG. Pecht G. Heinemann S. Sevarino KA. Cloning and characterization of chi-1: a developmentally regulated member of a novel class of the ionotropic glutamate receptor family. J Neurosci. 1995;15:6498–6508. doi: 10.1523/JNEUROSCI.15-10-06498.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cobb SR. Buhl EH. Halasy K. Paulsen O. Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995;378:75–78. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- 42.Cochran SM. Fujimura M. Morris BJ. Pratt JA. Acute and delayed effects of phencyclidine upon mRNA levels of markers of glutamatergic and GABAergic neurotransmitter function in the rat brain. Synapse. 2002;46:206–214. doi: 10.1002/syn.10126. [DOI] [PubMed] [Google Scholar]
- 43.Cochran SM. Kennedy M. McKerchar CE. Steward LJ. Pratt JA. Morris BJ. Induction of metabolic hypofunction and neurochemical deficits after chronic intermittent exposure to phencyclidine: differential modulation by antipsychotic drugs. Neuropsychopharmacology. 2003;28:265–275. doi: 10.1038/sj.npp.1300031. [DOI] [PubMed] [Google Scholar]
- 44.Cull-Candy S. Brickley S. Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- 45.Cunningham MO. Hunt J. Middleton S. LeBeau FE. Gillies MJ. Davies CH. Maycox PR. Whittington MA. Racca C. Region-specific reduction in entorhinal gamma oscillations and parvalbumin-immunoreactive neurons in animal models of psychiatric illness. J Neurosci. 2006;26:2767–2776. doi: 10.1523/JNEUROSCI.5054-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dalkara T. Erdemli G. Barun S. Onur R. Glycine is required for NMDA receptor activation: electrophysiological evidence from intact rat hippocampus. Brain Res. 1992;576:197–202. doi: 10.1016/0006-8993(92)90680-8. [DOI] [PubMed] [Google Scholar]
- 47.de Lecea L. del Rio JA. Soriano E. Developmental expression of parvalbumin mRNA in the cerebral cortex and hippocampus of the rat. Brain Res Mol Brain Res. 1995;32:1–13. doi: 10.1016/0169-328x(95)00056-x. [DOI] [PubMed] [Google Scholar]
- 48.Dingledine R. Borges K. Bowie D. Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- 49.Do KQ. Cabungcal JH. Frank A. Steullet P. Cuenod M. Redox dysregulation, neurodevelopment, and schizophrenia. Curr Opin Neurobiol. 2009;19:220–230. doi: 10.1016/j.conb.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 50.Do KQ. Trabesinger AH. Kirsten-Kruger M. Lauer CJ. Dydak U. Hell D. Holsboer F. Boesiger P. Cuenod M. Schizophrenia: glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. Eur J Neurosci. 2000;12:3721–3728. doi: 10.1046/j.1460-9568.2000.00229.x. [DOI] [PubMed] [Google Scholar]
- 51.Doischer D. Hosp JA. Yanagawa Y. Obata K. Jonas P. Vida I. Bartos M. Postnatal differentiation of basket cells from slow to fast signaling devices. J Neurosci. 2008;28:12956–12968. doi: 10.1523/JNEUROSCI.2890-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Domino EF. Taming the ketamine tiger. 1965. Anesthesiology. 2010;113:678–684. doi: 10.1097/ALN.0b013e3181ed09a2. [DOI] [PubMed] [Google Scholar]
- 53.du Bois TM. Newell KA. Han M. Deng C. Huang XF. Perinatal PCP treatment alters the developmental expression of prefrontal and hippocampal muscarinic receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:37–40. doi: 10.1016/j.pnpbp.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 54.Dugan LL. Sensi SL. Canzoniero LM. Handran SD. Rothman SM. Lin TS. Goldberg MP. Choi DW. Mitochondrial production of reactive oxygen species in cortical neurons following exposure to N-methyl-D-asparate. J Neurosci. 1995;15:6377–6388. doi: 10.1523/JNEUROSCI.15-10-06377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ehrlichman RS. Gandal MJ. Maxwell CR. Lazarewicz MT. Finkel LH. Contreras D. Turetsky BI. Siegel SJ. N-methyl-d-aspartic acid receptor antagonist-induced frequency oscillations in mice recreate pattern of electrophysiological deficits in schizophrenia. Neuroscience. 2009;158:705–712. doi: 10.1016/j.neuroscience.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 56.Erreger K. Chen PE. Wyllie DJ. Traynelis SF. Glutamate receptor gating. Crit Rev Neurobiol. 2004;16:187–224. doi: 10.1615/critrevneurobiol.v16.i3.10. [DOI] [PubMed] [Google Scholar]
- 57.Fantin M. Marti M. Auberson YP. Morari M. NR2A and NR2B subunit containing NMDA receptors differentially regulate striatal output pathways. J Neurochem. 2007;103:2200–2211. doi: 10.1111/j.1471-4159.2007.04966.x. [DOI] [PubMed] [Google Scholar]
- 58.Fatemi SH. Folsom TD. The neurodevelopmental hypothesis of schizophrenia, revisited. Schizophr Bull. 2009;35:528–548. doi: 10.1093/schbul/sbn187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fazzari P. Paternain AV. Valiente M. Pla R. Lujan R. Lloyd K. Lerma J. Marin O. Rico B. Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature. 2010;464:1376–1380. doi: 10.1038/nature08928. [DOI] [PubMed] [Google Scholar]
- 60.Fejgin K. Palsson E. Wass C. Svensson L. Klamer D. Nitric oxide signaling in the medial prefrontal cortex is involved in the biochemical and behavioral effects of phencyclidine. Neuropsychopharmacology. 2008;33:1874–1883. doi: 10.1038/sj.npp.1301587. [DOI] [PubMed] [Google Scholar]
- 61.Fiorentini C. Gardoni F. Spano P. Di Luca M. Missale C. Regulation of dopamine D1 receptor trafficking and desensitization by oligomerization with glutamate N-methyl-D-aspartate receptors. J Biol Chem. 2003;278:20196–20202. doi: 10.1074/jbc.M213140200. [DOI] [PubMed] [Google Scholar]
- 62.Fisahn A. Neddens J. Yan L. Buonanno A. Neuregulin-1 modulates hippocampal gamma oscillations: implications for schizophrenia. Cereb Cortex. 2009;19:612–618. doi: 10.1093/cercor/bhn107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fitzgerald ML. Lupica CR. Pickel VM. Decreased parvalbumin immunoreactivity in the cortex and striatum of mice lacking the CB1 receptor. Synapse. 2011;65:827–831. doi: 10.1002/syn.20911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Floresco SB. Zhang Y. Enomoto T. Neural circuits subserving behavioral flexibility and their relevance to schizophrenia. Behav Brain Res. 2009;204:396–409. doi: 10.1016/j.bbr.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 65.Francois J. Ferrandon A. Koning E. Angst MJ. Sandner G. Nehlig A. Selective reorganization of GABAergic transmission in neonatal ventral hippocampal-lesioned rats. Int J Neuropsychopharmacol. 2009;12:1097–1110. doi: 10.1017/S1461145709009985. [DOI] [PubMed] [Google Scholar]
- 66.Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci. 2009;32:209–224. doi: 10.1146/annurev.neuro.051508.135603. [DOI] [PubMed] [Google Scholar]
- 67.Gandal MJ. Edgar JC. Klook K. Siegel SJ. Gamma synchrony: towards a translational biomarker for the treatment-resistant symptoms of schizophrenia. Neuropharmacology. 2011;62:1504–1518. doi: 10.1016/j.neuropharm.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garrity AG. Pearlson GD. McKiernan K. Lloyd D. Kiehl KA. Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry. 2007;164:450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- 69.Girard SL. Xiong L. Dion PA. Rouleau GA. Where are the missing pieces of the schizophrenia genetics puzzle? Curr Opin Genet Dev. 2011;21:310–316. doi: 10.1016/j.gde.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 70.Goff DC. Coyle JT. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry. 2001;158:1367–1377. doi: 10.1176/appi.ajp.158.9.1367. [DOI] [PubMed] [Google Scholar]
- 71.Goldberg EM. Jeong HY. Kruglikov I. Tremblay R. Lazarenko RM. Rudy B. Rapid developmental maturation of neocortical FS cell intrinsic excitability. Cereb Cortex. 2011;21:666–682. doi: 10.1093/cercor/bhq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goldberg JH. Yuste R. Tamas G. Ca2+imaging of mouse neocortical interneurone dendrites: contribution of Ca2+-permeable AMPA and NMDA receptors to subthreshold Ca2+dynamics. J Physiol. 2003;551:67–78. doi: 10.1113/jphysiol.2003.042598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goldman-Rakic PS. Leranth C. Williams SM. Mons N. Geffard M. Dopamine synaptic complex with pyramidal neurons in primate cerebral cortex. Proc Natl Acad Sci U S A. 1989;86:9015–9019. doi: 10.1073/pnas.86.22.9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grateron L. Cebada-Sanchez S. Marcos P. Mohedano-Moriano A. Insausti AM. Munoz M. Arroyo-Jimenez MM. Martinez-Marcos A. Artacho-Perula E. Blaizot X. Insausti R. Postnatal development of calcium-binding proteins immunoreactivity (parvalbumin, calbindin, calretinin) in the human entorhinal cortex. J Chem Neuroanat. 2003;26:311–316. doi: 10.1016/j.jchemneu.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 75.Gulyas AI. Megias M. Emri Z. Freund TF. Total number and ratio of excitatory and inhibitory synapses converging onto single interneurons of different types in the CA1 area of the rat hippocampus. J Neurosci. 1999;19:10082–10097. doi: 10.1523/JNEUROSCI.19-22-10082.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gysin R. Kraftsik R. Sandell J. Bovet P. Chappuis C. Conus P. Deppen P. Preisig M. Ruiz V. Steullet P. Tosic M. Werge T. Cuenod M. Do KQ. Impaired glutathione synthesis in schizophrenia: convergent genetic and functional evidence. Proc Natl Acad Sci U S A. 2007;104:16621–16626. doi: 10.1073/pnas.0706778104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hakami T. Jones NC. Tolmacheva EA. Gaudias J. Chaumont J. Salzberg M. O'Brien TJ. Pinault D. NMDA receptor hypofunction leads to generalized and persistent aberrant gamma oscillations independent of hyperlocomotion and the state of consciousness. PLoS One. 2009;4:e6755. doi: 10.1371/journal.pone.0006755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Handy DE. Loscalzo J. Redox regulation of mitochondrial function. Antioxid Redox Signal. 2011;16:1323–1367. doi: 10.1089/ars.2011.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Harte MK. Powell SB. Swerdlow NR. Geyer MA. Reynolds GP. Deficits in parvalbumin and calbindin immunoreactive cells in the hippocampus of isolation reared rats. J Neural Transm. 2007;114:893–898. doi: 10.1007/s00702-007-0627-6. [DOI] [PubMed] [Google Scholar]
- 80.Hasenstaub A. Otte S. Callaway E. Sejnowski TJ. Metabolic cost as a unifying principle governing neuronal biophysics. Proc Natl Acad Sci U S A. 2010;107:12329–12334. doi: 10.1073/pnas.0914886107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hensch TK. Fagiolini M. Mataga N. Stryker MP. Baekkeskov S. Kash SF. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science. 1998;282:1504–1508. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Henson MA. Roberts AC. Perez-Otano I. Philpot BD. Influence of the NR3A subunit on NMDA receptor functions. Prog Neurobiol. 2010;91:23–37. doi: 10.1016/j.pneurobio.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hikida T. Jaaro-Peled H. Seshadri S. Oishi K. Hookway C. Kong S. Wu D. Xue R. Andrade M. Tankou S. Mori S. Gallagher M. Ishizuka K. Pletnikov M. Kida S. Sawa A. Dominant-negative DISC1 transgenic mice display schizophrenia-associated phenotypes detected by measures translatable to humans. Proc Natl Acad Sci U S A. 2007;104:14501–14506. doi: 10.1073/pnas.0704774104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hirsch JC. Crepel F. Blockade of NMDA receptors unmasks a long-term depression in synaptic efficacy in rat prefrontal neurons in vitro. Exp Brain Res. 1991;85:621–624. doi: 10.1007/BF00231747. [DOI] [PubMed] [Google Scholar]
- 85.Homayoun H. Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27:11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hong LE. Summerfelt A. Buchanan RW. O'Donnell P. Thaker GK. Weiler MA. Lahti AC. Gamma and delta neural oscillations and association with clinical symptoms under subanesthetic ketamine. Neuropsychopharmacology. 2010;35:632–640. doi: 10.1038/npp.2009.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hu JL. Liu G. Li YC. Gao WJ. Huang YQ. Dopamine D1 receptor-mediated NMDA receptor insertion depends on Fyn but not Src kinase pathway in prefrontal cortical neurons. Mol Brain. 2010;3:20. doi: 10.1186/1756-6606-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang ZJ. Activity-dependent development of inhibitory synapses and innervation pattern: role of GABA signalling and beyond. J Physiol. 2009;587:1881–1888. doi: 10.1113/jphysiol.2008.168211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hunt PS. Neonatal treatment with a competitive NMDA antagonist results in response-specific disruption of conditioned fear in preweanling rats. Psychopharmacology (Berl) 2006;185:179–187. doi: 10.1007/s00213-005-0291-1. [DOI] [PubMed] [Google Scholar]
- 90.Ishii T. Moriyoshi K. Sugihara H. Sakurada K. Kadotani H. Yokoi M. Akazawa C. Shigemoto R. Mizuno N. Masu M, et al. Molecular characterization of the family of the N-methyl-D-aspartate receptor subunits. J Biol Chem. 1993;268:2836–2843. [PubMed] [Google Scholar]
- 91.Itil T. Keskiner A. Kiremitci N. Holden JM. Effect of phencyclidine in chronic schizophrenics. Can Psychiatr Assoc J. 1967;12:209–212. doi: 10.1177/070674376701200217. [DOI] [PubMed] [Google Scholar]
- 92.Jackson ME. Homayoun H. Moghaddam B. NMDA receptor hypofunction produces concomitant firing rate potentiation and burst activity reduction in the prefrontal cortex. Proc Natl Acad Sci U S A. 2004;101:8467–8472. doi: 10.1073/pnas.0308455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jacobsen JP. Rodriguiz RM. Mork A. Wetsel WC. Monoaminergic dysregulation in glutathione-deficient mice: possible relevance to schizophrenia? Neuroscience. 2005;132:1055–1072. doi: 10.1016/j.neuroscience.2005.01.059. [DOI] [PubMed] [Google Scholar]
- 94.Javitt DC. Glutamatergic theories of schizophrenia. Isr J Psychiatry Relat Sci. 2010;47:4–16. [PubMed] [Google Scholar]
- 95.Javitt DC. Jotkowitz A. Sircar R. Zukin SR. Non-competitive regulation of phencyclidine/sigma-receptors by the N-methyl-D-aspartate receptor antagonist D-(-)-2-amino-5-phosphonovaleric acid. Neurosci Lett. 1987;78:193–198. doi: 10.1016/0304-3940(87)90632-x. [DOI] [PubMed] [Google Scholar]
- 96.Javitt DC. Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- 97.Jenkins TA. Harte MK. Stenson G. Reynolds GP. Neonatal lipopolysaccharide induces pathological changes in parvalbumin immunoreactivity in the hippocampus of the rat. Behav Brain Res. 2009;205:355–359. doi: 10.1016/j.bbr.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 98.Jentsch JD. Redmond DE., Jr. Elsworth JD. Taylor JR. Youngren KD. Roth RH. Enduring cognitive deficits and cortical dopamine dysfunction in monkeys after long-term administration of phencyclidine. Science. 1997;277:953–955. doi: 10.1126/science.277.5328.953. [DOI] [PubMed] [Google Scholar]
- 99.Jentsch JD. Roth RH. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1999;20:201–225. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- 100.Jiang D. Akopian G. Ho YS. Walsh JP. Andersen JK. Chronic brain oxidation in a glutathione peroxidase knockout mouse model results in increased resistance to induced epileptic seizures. Exp Neurol. 2000;164:257–268. doi: 10.1006/exnr.2000.7431. [DOI] [PubMed] [Google Scholar]
- 101.Johnson JW. Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- 102.Jones RS. Buhl EH. Basket-like interneurones in layer II of the entorhinal cortex exhibit a powerful NMDA-mediated synaptic excitation. Neurosci Lett. 1993;149:35–39. doi: 10.1016/0304-3940(93)90341-h. [DOI] [PubMed] [Google Scholar]
- 103.Kawaguchi Y. Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- 104.Keilhoff G. Becker A. Grecksch G. Wolf G. Bernstein HG. Repeated application of ketamine to rats induces changes in the hippocampal expression of parvalbumin, neuronal nitric oxide synthase and cFOS similar to those found in human schizophrenia. Neuroscience. 2004;126:591–598. doi: 10.1016/j.neuroscience.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 105.Kerns JG. Nuechterlein KH. Braver TS. Barch DM. Executive functioning component mechanisms and schizophrenia. Biol Psychiatry. 2008;64:26–33. doi: 10.1016/j.biopsych.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 106.Kim MJ. Shin KS. Chung YB. Jung KW. Cha CI. Shin DH. Immunohistochemical study of p47Phox and gp91Phox distributions in rat brain. Brain Res. 2005;1040:178–186. doi: 10.1016/j.brainres.2005.01.066. [DOI] [PubMed] [Google Scholar]
- 107.Kinney JW. Davis CN. Tabarean I. Conti B. Bartfai T. Behrens MM. A specific role for NR2A-containing NMDA receptors in the maintenance of parvalbumin and GAD67 immunoreactivity in cultured interneurons. J Neurosci. 2006;26:1604–1615. doi: 10.1523/JNEUROSCI.4722-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kishida KT. Hoeffer CA. Hu D. Pao M. Holland SM. Klann E. Synaptic plasticity deficits and mild memory impairments in mouse models of chronic granulomatous disease. Mol Cell Biol. 2006;26:5908–5920. doi: 10.1128/MCB.00269-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kishida KT. Pao M. Holland SM. Klann E. NADPH oxidase is required for NMDA receptor-dependent activation of ERK in hippocampal area CA1. J Neurochem. 2005;94:299–306. doi: 10.1111/j.1471-4159.2005.03189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kleckner NW. Dingledine R. Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science. 1988;241:835–837. doi: 10.1126/science.2841759. [DOI] [PubMed] [Google Scholar]
- 111.Kocsis B. Differential role of NR2A and NR2B subunits in N-Methyl-D-aspartate receptor antagonist-induced aberrant cortical gamma oscillations. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kohr G. Eckardt S. Luddens H. Monyer H. Seeburg PH. NMDA receptor channels: subunit-specific potentiation by reducing agents. Neuron. 1994;12:1031–1040. doi: 10.1016/0896-6273(94)90311-5. [DOI] [PubMed] [Google Scholar]
- 113.Korotkova T. Fuchs EC. Ponomarenko A. von Engelhardt J. Monyer H. NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron. 2010;68:557–569. doi: 10.1016/j.neuron.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 114.Krystal JH. Abi-Saab W. Perry E. D'Souza DC. Liu N. Gueorguieva R. McDougall L. Hunsberger T. Belger A. Levine L. Breier A. Preliminary evidence of attenuation of the disruptive effects of the NMDA glutamate receptor antagonist, ketamine, on working memory by pretreatment with the group II metabotropic glutamate receptor agonist, LY354740, in healthy human subjects. Psychopharmacology (Berl) 2005;179:303–309. doi: 10.1007/s00213-004-1982-8. [DOI] [PubMed] [Google Scholar]
- 115.Krystal JH. Karper LP. Seibyl JP. Freeman GK. Delaney R. Bremner JD. Heninger GR. Bowers MB., Jr. Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 116.Kullmann DM. Lamsa KP. Long-term synaptic plasticity in hippocampal interneurons. Nat Rev Neurosci. 2007;8:687–699. doi: 10.1038/nrn2207. [DOI] [PubMed] [Google Scholar]
- 117.Kuner T. Schoepfer R. Multiple structural elements determine subunit specificity of Mg2+block in NMDA receptor channels. J Neurosci. 1996;16:3549–3558. doi: 10.1523/JNEUROSCI.16-11-03549.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kupper J. Ascher P. Neyton J. Internal Mg2+block of recombinant NMDA channels mutated within the selectivity filter and expressed in Xenopus oocytes. J Physiol. 1998;507(Pt 1):1–12. doi: 10.1111/j.1469-7793.1998.001bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kutsuwada T. Kashiwabuchi N. Mori H. Sakimura K. Kushiya E. Araki K. Meguro H. Masaki H. Kumanishi T. Arakawa M, et al. Molecular diversity of the NMDA receptor channel. Nature. 1992;358:36–41. doi: 10.1038/358036a0. [DOI] [PubMed] [Google Scholar]
- 120.Kwon JS. O'Donnell BF. Wallenstein GV. Greene RW. Hirayasu Y. Nestor PG. Hasselmo ME. Potts GF. Shenton ME. McCarley RW. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry. 1999;56:1001–1005. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lafon-Cazal M. Pietri S. Culcasi M. Bockaert J. NMDA-dependent superoxide production and neurotoxicity. Nature. 1993;364:535–537. doi: 10.1038/364535a0. [DOI] [PubMed] [Google Scholar]
- 122.Lahti AC. Holcomb HH. Medoff DR. Tamminga CA. Ketamine activates psychosis and alters limbic blood flow in schizophrenia. Neuroreport. 1995;6:869–872. doi: 10.1097/00001756-199504190-00011. [DOI] [PubMed] [Google Scholar]
- 123.Lahti AC. Koffel B. LaPorte D. Tamminga CA. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology. 1995;13:9–19. doi: 10.1016/0893-133X(94)00131-I. [DOI] [PubMed] [Google Scholar]
- 124.Lahti AC. Weiler MA. Tamara Michaelidis BA. Parwani A. Tamminga CA. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology. 2001;25:455–467. doi: 10.1016/S0893-133X(01)00243-3. [DOI] [PubMed] [Google Scholar]
- 125.Lambeth JD. Krause KH. Clark RA. NOX enzymes as novel targets for drug development. Semin Immunopathol. 2008;30:339–363. doi: 10.1007/s00281-008-0123-6. [DOI] [PubMed] [Google Scholar]
- 126.Laruelle M. Abi-Dargham A. Casanova MF. Toti R. Weinberger DR. Kleinman JE. Selective abnormalities of prefrontal serotonergic receptors in schizophrenia. A postmortem study. Arch Gen Psychiatry. 1993;50:810–818. doi: 10.1001/archpsyc.1993.01820220066007. [DOI] [PubMed] [Google Scholar]
- 127.Laurie DJ. Bartke I. Schoepfer R. Naujoks K. Seeburg PH. Regional, developmental and interspecies expression of the four NMDAR2 subunits, examined using monoclonal antibodies. Brain Res Mol Brain Res. 1997;51:23–32. doi: 10.1016/s0169-328x(97)00206-4. [DOI] [PubMed] [Google Scholar]
- 128.Lavoie S. Murray MM. Deppen P. Knyazeva MG. Berk M. Boulat O. Bovet P. Bush AI. Conus P. Copolov D. Fornari E. Meuli R. Solida A. Vianin P. Cuenod M. Buclin T. Do KQ. Glutathione precursor, N-acetyl-cysteine, improves mismatch negativity in schizophrenia patients. Neuropsychopharmacology. 2008;33:2187–2199. doi: 10.1038/sj.npp.1301624. [DOI] [PubMed] [Google Scholar]
- 129.Lazarewicz MT. Ehrlichman RS. Maxwell CR. Gandal MJ. Finkel LH. Siegel SJ. Ketamine modulates theta and gamma oscillations. J Cogn Neurosci. 2010;22:1452–1464. doi: 10.1162/jocn.2009.21305. [DOI] [PubMed] [Google Scholar]
- 130.Lee FJ. Xue S. Pei L. Vukusic B. Chery N. Wang Y. Wang YT. Niznik HB. Yu XM. Liu F. Dual regulation of NMDA receptor functions by direct protein-protein interactions with the dopamine D1 receptor. Cell. 2002;111:219–230. doi: 10.1016/s0092-8674(02)00962-5. [DOI] [PubMed] [Google Scholar]
- 131.Lee KH. Williams LM. Breakspear M. Gordon E. Synchronous gamma activity: a review and contribution to an integrative neuroscience model of schizophrenia. Brain Res Brain Res Rev. 2003;41:57–78. doi: 10.1016/s0165-0173(02)00220-5. [DOI] [PubMed] [Google Scholar]
- 132.Lee KW. Woon PS. Teo YY. Sim K. Genome wide association studies (GWAS) and copy number variation (CNV) studies of the major psychoses: What have we learnt? Neurosci Biobehav Rev. 2012;36:556–571. doi: 10.1016/j.neubiorev.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 133.Leung LW. Spectral analysis of hippocampal EEG in the freely moving rat: effects of centrally active drugs and relations to evoked potentials. Electroencephalogr Clin Neurophysiol. 1985;60:65–77. doi: 10.1016/0013-4694(85)90952-6. [DOI] [PubMed] [Google Scholar]
- 134.Leveille F. Soriano FX. Papadia S. Hardingham GE. Excitotoxic insults lead to peroxiredoxin hyperoxidation. Oxid Med Cell Longev. 2009;2:110–113. doi: 10.4161/oxim.2.2.8300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lewis DA. Hashimoto T. Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 136.Light GA. Hsu JL. Hsieh MH. Meyer-Gomes K. Sprock J. Swerdlow NR. Braff DL. Gamma band oscillations reveal neural network cortical coherence dysfunction in schizophrenia patients. Biol Psychiatry. 2006;60:1231–1240. doi: 10.1016/j.biopsych.2006.03.055. [DOI] [PubMed] [Google Scholar]
- 137.Lipska BK. Lerman DN. Khaing ZZ. Weinberger DR. The neonatal ventral hippocampal lesion model of schizophrenia: effects on dopamine and GABA mRNA markers in the rat midbrain. Eur J Neurosci. 2003;18:3097–3104. doi: 10.1111/j.1460-9568.2003.03047.x. [DOI] [PubMed] [Google Scholar]
- 138.Lipska BK. Weinberger DR. To model a psychiatric disorder in animals: schizophrenia as a reality test. Neuropsychopharmacology. 2000;23:223–239. doi: 10.1016/S0893-133X(00)00137-8. [DOI] [PubMed] [Google Scholar]
- 139.Lipton SA. Choi YB. Takahashi H. Zhang D. Li W. Godzik A. Bankston LA. Cysteine regulation of protein function—as exemplified by NMDA-receptor modulation. Trends Neurosci. 2002;25:474–480. doi: 10.1016/s0166-2236(02)02245-2. [DOI] [PubMed] [Google Scholar]
- 140.Lisman JE. Coyle JT. Green RW. Javitt DC. Benes FM. Heckers S. Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liu F. Zou X. Sadovova N. Zhang X. Shi L. Guo L. Qian F. Wen Z. Patterson TA. Hanig JP. Paule MG. Slikker W., Jr. Wang C. Changes in gene expression after phencyclidine administration in developing rats: a potential animal model for schizophrenia. Int J Dev Neurosci. 2011;29:351–358. doi: 10.1016/j.ijdevneu.2010.07.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lodge D. The history of the pharmacology and cloning of ionotropic glutamate receptors and the development of idiosyncratic nomenclature. Neuropharmacology. 2009;56:6–21. doi: 10.1016/j.neuropharm.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 143.Lodge DJ. Behrens MM. Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci. 2009;29:2344–2354. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Low CM. Wee KS. New insights into the not-so-new NR3 subunits of N-methyl-D-aspartate receptor: localization, structure, and function. Mol Pharmacol. 2010;78:1–11. doi: 10.1124/mol.110.064006. [DOI] [PubMed] [Google Scholar]
- 145.Luby ED. Cohen BD. Rosenbaum G. Gottlieb JS. Kelley R. Study of a new schizophrenomimetic drug; sernyl. AMA Arch Neurol Psychiatry. 1959;81:363–369. doi: 10.1001/archneurpsyc.1959.02340150095011. [DOI] [PubMed] [Google Scholar]
- 146.Ma J. Leung LS. Relation between hippocampal gamma waves and behavioral disturbances induced by phencyclidine and methamphetamine. Behav Brain Res. 2000;111:1–11. doi: 10.1016/s0166-4328(00)00138-8. [DOI] [PubMed] [Google Scholar]
- 147.Ma J. Leung LS. The supramammillo-septal-hippocampal pathway mediates sensorimotor gating impairment and hyperlocomotion induced by MK-801 and ketamine in rats. Psychopharmacology (Berl) 2007;191:961–974. doi: 10.1007/s00213-006-0667-x. [DOI] [PubMed] [Google Scholar]
- 148.Malhotra AK. Pinals DA. Adler CM. Elman I. Clifton A. Pickar D. Breier A. Ketamine-induced exacerbation of psychotic symptoms and cognitive impairment in neuroleptic-free schizophrenics. Neuropsychopharmacology. 1997;17:141–150. doi: 10.1016/S0893-133X(97)00036-5. [DOI] [PubMed] [Google Scholar]
- 149.Mann EO. Paulsen O. Role of GABAergic inhibition in hippocampal network oscillations. Trends Neurosci. 2007;30:343–349. doi: 10.1016/j.tins.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 150.Mansbach RS. Geyer MA. Parametric determinants in pre-stimulus modification of acoustic startle: interaction with ketamine. Psychopharmacology (Berl) 1991;105:162–168. doi: 10.1007/BF02244303. [DOI] [PubMed] [Google Scholar]
- 151.Markram H. Toledo-Rodriguez M. Wang Y. Gupta A. Silberberg G. Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 152.Massaad CA. Klann E. Reactive oxygen species in the regulation of synaptic plasticity and memory. Antioxid Redox Signal. 2011;14:2013–2054. doi: 10.1089/ars.2010.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Maxwell CR. Ehrlichman RS. Liang Y. Trief D. Kanes SJ. Karp J. Siegel SJ. Ketamine produces lasting disruptions in encoding of sensory stimuli. J Pharmacol Exp Ther. 2006;316:315–324. doi: 10.1124/jpet.105.091199. [DOI] [PubMed] [Google Scholar]
- 154.Mayer ML. Westbrook GL. Guthrie PB. Voltage-dependent block by Mg2+of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- 155.Meyer U. Feldon J. Neural basis of psychosis-related behaviour in the infection model of schizophrenia. Behav Brain Res. 2009;204:322–334. doi: 10.1016/j.bbr.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 156.Meyer U. Nyffeler M. Yee BK. Knuesel I. Feldon J. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav Immun. 2008;22:469–486. doi: 10.1016/j.bbi.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 157.Middleton S. Jalics J. Kispersky T. Lebeau FE. Roopun AK. Kopell NJ. Whittington MA. Cunningham MO. NMDA receptor-dependent switching between different gamma rhythm-generating microcircuits in entorhinal cortex. Proc Natl Acad Sci U S A. 2008;105:18572–18577. doi: 10.1073/pnas.0809302105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Miles R. Toth K. Gulyas AI. Hajos N. Freund TF. Differences between somatic and dendritic inhibition in the hippocampus. Neuron. 1996;16:815–823. doi: 10.1016/s0896-6273(00)80101-4. [DOI] [PubMed] [Google Scholar]
- 159.Miller DW. Abercrombie ED. Effects of MK-801 on spontaneous and amphetamine-stimulated dopamine release in striatum measured with in vivo microdialysis in awake rats. Brain Res Bull. 1996;40:57–62. doi: 10.1016/0361-9230(95)02144-2. [DOI] [PubMed] [Google Scholar]
- 160.Moller M. Du Preez JL. Emsley R. Harvey BH. Isolation rearing-induced deficits in sensorimotor gating and social interaction in rats are related to cortico-striatal oxidative stress, and reversed by sub-chronic clozapine administration. Eur Neuropsychopharmacol. 2011;21:471–483. doi: 10.1016/j.euroneuro.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 161.Monyer H. Burnashev N. Laurie DJ. Sakmann B. Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 162.Monyer H. Sprengel R. Schoepfer R. Herb A. Higuchi M. Lomeli H. Burnashev N. Sakmann B. Seeburg PH. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- 163.Mori H. Masaki H. Yamakura T. Mishina M. Identification by mutagenesis of a Mg(2+)-block site of the NMDA receptor channel. Nature. 1992;358:673–675. doi: 10.1038/358673a0. [DOI] [PubMed] [Google Scholar]
- 164.Mori H. Mishina M. Structure and function of the NMDA receptor channel. Neuropharmacology. 1995;34:1219–1237. doi: 10.1016/0028-3908(95)00109-j. [DOI] [PubMed] [Google Scholar]
- 165.Morishita H CY. Dalton TP. Hensch TK. Cell-autonomous redox dysregulation weakens parvalbumin circuits and allows plasticity in post-adolescent mouse visual cortex. Soc for Neurosci Abstr. 2010;40:AA1. [Google Scholar]
- 166.Moriyoshi K. Masu M. Ishii T. Shigemoto R. Mizuno N. Nakanishi S. Molecular cloning and characterization of the rat NMDA receptor. Nature. 1991;354:31–37. doi: 10.1038/354031a0. [DOI] [PubMed] [Google Scholar]
- 167.Morris BJ. Cochran SM. Pratt JA. PCP: from pharmacology to modelling schizophrenia. Curr Opin Pharmacol. 2005;5:101–106. doi: 10.1016/j.coph.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 168.Morrow BA. Elsworth JD. Roth RH. Repeated phencyclidine in monkeys results in loss of parvalbumin-containing axo-axonic projections in the prefrontal cortex. Psychopharmacology (Berl) 2007 doi: 10.1007/s00213-007-0708-0. [DOI] [PubMed] [Google Scholar]
- 169.Mouri A. Noda Y. Enomoto T. Nabeshima T. Phencyclidine animal models of schizophrenia: approaches from abnormality of glutamatergic neurotransmission and neurodevelopment. Neurochem Int. 2007;51:173–184. doi: 10.1016/j.neuint.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 169a.Mustafa AK. Kumar M. Selvakumar B. Ho GP. Ehmsen JT. Barrow RK. Amzel LM. Snyder SH. Nitric oxide S-nitrosylates serine racemase, mediating feedback inhibition of D-serine formation. Proc Natl Acad Sci USA. 2007;104:2950–2955. doi: 10.1073/pnas.0611620104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Nakatani-Pawlak A. Yamaguchi K. Tatsumi Y. Mizoguchi H. Yoneda Y. Neonatal phencyclidine treatment in mice induces behavioral, histological and neurochemical abnormalities in adulthood. Biol Pharm Bull. 2009;32:1576–1583. doi: 10.1248/bpb.32.1576. [DOI] [PubMed] [Google Scholar]
- 171.Narendran R. Frankle WG. Keefe R. Gil R. Martinez D. Slifstein M. Kegeles LS. Talbot PS. Huang Y. Hwang DR. Khenissi L. Cooper TB. Laruelle M. Abi-Dargham A. Altered prefrontal dopaminergic function in chronic recreational ketamine users. Am J Psychiatry. 2005;162:2352–2359. doi: 10.1176/appi.ajp.162.12.2352. [DOI] [PubMed] [Google Scholar]
- 172.Neddens J. Buonanno A. Selective populations of hippocampal interneurons express ErbB4 and their number and distribution is altered in ErbB4 knockout mice. Hippocampus. 2010;20:724–744. doi: 10.1002/hipo.20675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Newcomer JW. Krystal JH. NMDA receptor regulation of memory and behavior in humans. Hippocampus. 2001;11:529–542. doi: 10.1002/hipo.1069. [DOI] [PubMed] [Google Scholar]
- 174.Nudmamud S. Reynolds LM. Reynolds GP. N-acetylaspartate and N-Acetylaspartylglutamate deficits in superior temporal cortex in schizophrenia and bipolar disorder: a postmortem study. Biol Psychiatry. 2003;53:1138–1141. doi: 10.1016/s0006-3223(02)01742-0. [DOI] [PubMed] [Google Scholar]
- 175.Okaty BW. Miller MN. Sugino K. Hempel CM. Nelson SB. Transcriptional and electrophysiological maturation of neocortical fast-spiking GABAergic interneurons. J Neurosci. 2009;29:7040–7052. doi: 10.1523/JNEUROSCI.0105-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Olney JW. Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- 177.Olney JW. Newcomer JW. Farber NB. NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res. 1999;33:523–533. doi: 10.1016/s0022-3956(99)00029-1. [DOI] [PubMed] [Google Scholar]
- 178.Papadia S. Soriano FX. Leveille F. Martel MA. Dakin KA. Hansen HH. Kaindl A. Sifringer M. Fowler J. Stefovska V. McKenzie G. Craigon M. Corriveau R. Ghazal P. Horsburgh K. Yankner BA. Wyllie DJ. Ikonomidou C. Hardingham GE. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat Neurosci. 2008;11:476–487. doi: 10.1038/nn2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Penschuck S. Flagstad P. Didriksen M. Leist M. Michael-Titus AT. Decrease in parvalbumin-expressing neurons in the hippocampus and increased phencyclidine-induced locomotor activity in the rat methylazoxymethanol (MAM) model of schizophrenia. Eur J Neurosci. 2006;23:279–284. doi: 10.1111/j.1460-9568.2005.04536.x. [DOI] [PubMed] [Google Scholar]
- 180.Pinault D. N-methyl d-aspartate receptor antagonists ketamine and MK-801 induce wake-related aberrant gamma oscillations in the rat neocortex. Biol Psychiatry. 2008;63:730–735. doi: 10.1016/j.biopsych.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 181.Pinteaux E. Copin JC. Ledig M. Tholey G. Modulation of oxygen-radical-scavenging enzymes by oxidative stress in primary cultures of rat astroglial cells. Dev Neurosci. 1996;18:397–404. doi: 10.1159/000111433. [DOI] [PubMed] [Google Scholar]
- 182.Pitsikas N. Boultadakis A. Sakellaridis N. Effects of sub-anesthetic doses of ketamine on rats' spatial and non-spatial recognition memory. Neuroscience. 2008;154:454–460. doi: 10.1016/j.neuroscience.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 183.Powell SB. Sejnowski TJ. Behrens MM. Behavioral and neurochemical consequences of cortical oxidative stress on parvalbumin-interneuron maturation in rodent models of schizophrenia. Neuropharmacology. 2012;62:1322–1331. doi: 10.1016/j.neuropharm.2011.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Quirk MC. Sosulski DL. Feierstein CE. Uchida N. Mainen ZF. A defined network of fast-spiking interneurons in orbitofrontal cortex: responses to behavioral contingencies and ketamine administration. Front Syst Neurosci. 2009;3:13. doi: 10.3389/neuro.06.013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Rao SG. Williams GV. Goldman-Rakic PS. Destruction and creation of spatial tuning by disinhibition: GABA(A) blockade of prefrontal cortical neurons engaged by working memory. J Neurosci. 2000;20:485–494. doi: 10.1523/JNEUROSCI.20-01-00485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Rapoport JL. Addington AM. Frangou S. Psych MR. The neurodevelopmental model of schizophrenia: update 2005. Mol Psychiatry. 2005;10:434–449. doi: 10.1038/sj.mp.4001642. [DOI] [PubMed] [Google Scholar]
- 187.Reynolds GP. Abdul-Monim Z. Neill JC. Zhang ZJ. Calcium binding protein markers of GABA deficits in schizophrenia—postmortem studies and animal models. Neurotox Res. 2004;6:57–61. doi: 10.1007/BF03033297. [DOI] [PubMed] [Google Scholar]
- 188.Reynolds IJ. Hastings TG. Glutamate induces the production of reactive oxygen species in cultured forebrain neurons following NMDA receptor activation. J Neurosci. 1995;15:3318–3327. doi: 10.1523/JNEUROSCI.15-05-03318.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Reynolds LM. Cochran SM. Morris BJ. Pratt JA. Reynolds GP. Chronic phencyclidine administration induces schizophrenia-like changes in N-acetylaspartate and N-acetylaspartylglutamate in rat brain. Schizophr Res. 2005;73:147–152. doi: 10.1016/j.schres.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 190.Roopun AK. Cunningham MO. Racca C. Alter K. Traub RD. Whittington MA. Region-specific changes in gamma and beta2 rhythms in NMDA receptor dysfunction models of schizophrenia. Schizophr Bull. 2008;34:962–973. doi: 10.1093/schbul/sbn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Rotaru DC. Yoshino H. Lewis DA. Ermentrout GB. Gonzalez-Burgos G. Glutamate receptor subtypes mediating synaptic activation of prefrontal cortex neurons: relevance for schizophrenia. J Neurosci. 2011;31:142–156. doi: 10.1523/JNEUROSCI.1970-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Rounsaville BJ. DSM-V research agenda: substance abuse/psychosis comorbidity. Schizophr Bull. 2007;33:947–952. doi: 10.1093/schbul/sbm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Rujescu D. Bender A. Keck M. Hartmann AM. Ohl F. Raeder H. Giegling I. Genius J. McCarley RW. Moller HJ. Grunze H. A pharmacological model for psychosis based on N-methyl-D-aspartate receptor hypofunction: molecular, cellular, functional and behavioral abnormalities. Biol Psychiatry. 2006;59:721–729. doi: 10.1016/j.biopsych.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 194.Sakurada K. Masu M. Nakanishi S. Alteration of Ca2+permeability and sensitivity to Mg2+and channel blockers by a single amino acid substitution in the N-methyl-D-aspartate receptor. J Biol Chem. 1993;268:410–415. [PubMed] [Google Scholar]
- 195.Sauer JF. Bartos M. Recruitment of early postnatal parvalbumin-positive hippocampal interneurons by GABAergic excitation. J Neurosci. 2010;30:110–115. doi: 10.1523/JNEUROSCI.4125-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sauer JF. Bartos M. Postnatal differentiation of cortical interneuron signalling. Eur J Neurosci. 2011;34:1687–1696. doi: 10.1111/j.1460-9568.2011.07872.x. [DOI] [PubMed] [Google Scholar]
- 197.Scatton B. The NMDA receptor complex. Fundam Clin Pharmacol. 1993;7:389–400. doi: 10.1111/j.1472-8206.1993.tb01036.x. [DOI] [PubMed] [Google Scholar]
- 198.Schiavone S. Sorce S. Dubois-Dauphin M. Jaquet V. Colaianna M. Zotti M. Cuomo V. Trabace L. Krause KH. Involvement of NOX2 in the development of behavioral and pathologic alterations in isolated rats. Biol Psychiatry. 2009;66:384–392. doi: 10.1016/j.biopsych.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 199.Scott L. Zelenin S. Malmersjo S. Kowalewski JM. Markus EZ. Nairn AC. Greengard P. Brismar H. Aperia A. Allosteric changes of the NMDA receptor trap diffusible dopamine 1 receptors in spines. Proc Natl Acad Sci U S A. 2006;103:762–767. doi: 10.1073/pnas.0505557103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Serrano F. Kolluri NS. Wientjes FB. Card JP. Klann E. NADPH oxidase immunoreactivity in the mouse brain. Brain Res. 2003;988:193–198. doi: 10.1016/s0006-8993(03)03364-x. [DOI] [PubMed] [Google Scholar]
- 201.Sesack SR. Carr DB. Omelchenko N. Pinto A. Anatomical substrates for glutamate-dopamine interactions: evidence for specificity of connections and extrasynaptic actions. Ann N Y Acad Sci. 2003;1003:36–52. doi: 10.1196/annals.1300.066. [DOI] [PubMed] [Google Scholar]
- 202.Shen S. Lang B. Nakamoto C. Zhang F. Pu J. Kuan SL. Chatzi C. He S. Mackie I. Brandon NJ. Marquis KL. Day M. Hurko O. McCaig CD. Riedel G. St Clair D. Schizophrenia-related neural and behavioral phenotypes in transgenic mice expressing truncated Disc1. J Neurosci. 2008;28:10893–10904. doi: 10.1523/JNEUROSCI.3299-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sheng M. Cummings J. Roldan LA. Jan YN. Jan LY. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature. 1994;368:144–147. doi: 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- 204.Siegel SJ. Connolly P. Liang Y. Lenox RH. Gur RE. Bilker WB. Kanes SJ. Turetsky BI. Effects of strain, novelty, and NMDA blockade on auditory-evoked potentials in mice. Neuropsychopharmacology. 2003;28:675–682. doi: 10.1038/sj.npp.1300087. [DOI] [PubMed] [Google Scholar]
- 205.Silvestre JS. Nadal R. Pallares M. Ferre N. Acute effects of ketamine in the holeboard, the elevated-plus maze, and the social interaction test in Wistar rats. Depress Anxiety. 1997;5:29–33. [PubMed] [Google Scholar]
- 206.Singer W. Neuronal synchrony: a versatile code for the definition of relations? Neuron. 1999;24:49–65. doi: 10.1016/s0896-6273(00)80821-1. 111–125. [DOI] [PubMed] [Google Scholar]
- 207.Sircar R. Rudy JW. Repeated neonatal phencyclidine treatment impairs performance of a spatial task in juvenile rats. Ann N Y Acad Sci. 1998;844:303–309. [PubMed] [Google Scholar]
- 208.Slikker W., Jr. Zou X. Hotchkiss CE. Divine RL. Sadovova N. Twaddle NC. Doerge DR. Scallet AC. Patterson TA. Hanig JP. Paule MG. Wang C. Ketamine-induced neuronal cell death in the perinatal rhesus monkey. Toxicol Sci. 2007;98:145–158. doi: 10.1093/toxsci/kfm084. [DOI] [PubMed] [Google Scholar]
- 209.Smith SE. Li J. Garbett K. Mirnics K. Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sohal VS. Zhang F. Yizhar O. Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sorce S. Krause KH. NOX Enzymes in the Central Nervous System: From Signaling to Disease. Antioxid Redox Signal. 2009;11:2481–2504. doi: 10.1089/ars.2009.2578. [DOI] [PubMed] [Google Scholar]
- 212.Sorce S. Schiavone S. Tucci P. Colaianna M. Jaquet V. Cuomo V. Dubois-Dauphin M. Trabace L. Krause KH. The NADPH oxidase NOX2 controls glutamate release: a novel mechanism involved in psychosis-like ketamine responses. J Neurosci. 2010;30:11317–11325. doi: 10.1523/JNEUROSCI.1491-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Stefani MR. Moghaddam B. Systemic and prefrontal cortical NMDA receptor blockade differentially affect discrimination learning and set-shift ability in rats. Behav Neurosci. 2005;119:420–428. doi: 10.1037/0735-7044.119.2.420. [DOI] [PubMed] [Google Scholar]
- 214.Stefani MR. Moghaddam B. Transient N-methyl-D-aspartate receptor blockade in early development causes lasting cognitive deficits relevant to schizophrenia. Biol Psychiatry. 2005;57:433–436. doi: 10.1016/j.biopsych.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 215.Steullet P. Cabungcal JH. Kulak A. Kraftsik R. Chen Y. Dalton TP. Cuenod M. Do KQ. Redox dysregulation affects the ventral but not dorsal hippocampus: impairment of parvalbumin neurons, gamma oscillations, and related behaviors. J Neurosci. 2010;30:2547–2558. doi: 10.1523/JNEUROSCI.3857-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Steward LJ. Kennedy MD. Morris BJ. Pratt JA. The atypical antipsychotic drug clozapine enhances chronic PCP-induced regulation of prefrontal cortex 5-HT2A receptors. Neuropharmacology. 2004;47:527–537. doi: 10.1016/j.neuropharm.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 217.Stoet G. Snyder LH. Effects of the NMDA antagonist ketamine on task-switching performance: evidence for specific impairments of executive control. Neuropsychopharmacology. 2006;31:1675–1681. doi: 10.1038/sj.npp.1300930. [DOI] [PubMed] [Google Scholar]
- 218.Sucher NJ. Akbarian S. Chi CL. Leclerc CL. Awobuluyi M. Deitcher DL. Wu MK. Yuan JP. Jones EG. Lipton SA. Developmental and regional expression pattern of a novel NMDA receptor-like subunit (NMDAR-L) in the rodent brain. J Neurosci. 1995;15:6509–6520. doi: 10.1523/JNEUROSCI.15-10-06509.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sun Y. Farzan F. Barr MS. Kirihara K. Fitzgerald PB. Light GA. Daskalakis ZJ. Gamma oscillations in schizophrenia: mechanisms and clinical significance. Brain Res. 2011;1413:98–114. doi: 10.1016/j.brainres.2011.06.065. [DOI] [PubMed] [Google Scholar]
- 220.Takahata R. Moghaddam B. Activation of glutamate neurotransmission in the prefrontal cortex sustains the motoric and dopaminergic effects of phencyclidine. Neuropsychopharmacology. 2003;28:1117–1124. doi: 10.1038/sj.npp.1300127. [DOI] [PubMed] [Google Scholar]
- 221.Tejada-Simon MV. Serrano F. Villasana LE. Kanterewicz BI. Wu GY. Quinn MT. Klann E. Synaptic localization of a functional NADPH oxidase in the mouse hippocampus. Mol Cell Neurosci. 2005;29:97–106. doi: 10.1016/j.mcn.2005.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Torrey EF. Barci BM. Webster MJ. Bartko JJ. Meador-Woodruff JH. Knable MB. Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biol Psychiatry. 2005;57:252–260. doi: 10.1016/j.biopsych.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 223.Tosic M. Ott J. Barral S. Bovet P. Deppen P. Gheorghita F. Matthey ML. Parnas J. Preisig M. Saraga M. Solida A. Timm S. Wang AG. Werge T. Cuenod M. Do KQ. Schizophrenia and oxidative stress: glutamate cysteine ligase modifier as a susceptibility gene. Am J Hum Genet. 2006;79:586–592. doi: 10.1086/507566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Trotti D. Rizzini BL. Rossi D. Haugeto O. Racagni G. Danbolt NC. Volterra A. Neuronal and glial glutamate transporters possess an SH-based redox regulatory mechanism. Eur J Neurosci. 1997;9:1236–1243. doi: 10.1111/j.1460-9568.1997.tb01478.x. [DOI] [PubMed] [Google Scholar]
- 225.Tseng KY. Lewis BL. Hashimoto T. Sesack SR. Kloc M. Lewis DA. O'Donnell P. A neonatal ventral hippocampal lesion causes functional deficits in adult prefrontal cortical interneurons. J Neurosci. 2008;28:12691–12699. doi: 10.1523/JNEUROSCI.4166-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Tsukada H. Miyasato K. Nishiyama S. Fukumoto D. Kakiuchi T. Domino EF. Nicotine normalizes increased prefrontal cortical dopamine D1 receptor binding and decreased working memory performance produced by repeated pretreatment with MK-801: a PET study in conscious monkeys. Neuropsychopharmacology. 2005;30:2144–2153. doi: 10.1038/sj.npp.1300745. [DOI] [PubMed] [Google Scholar]
- 227.Turpaev KT. Reactive oxygen species and regulation of gene expression. Biochemistry (Mosc) 2002;67:281–292. doi: 10.1023/a:1014819832003. [DOI] [PubMed] [Google Scholar]
- 228.Uhlhaas PJ. Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- 229.Umbricht D. Schmid L. Koller R. Vollenweider FX. Hell D. Javitt DC. Ketamine-induced deficits in auditory and visual context-dependent processing in healthy volunteers: implications for models of cognitive deficits in schizophrenia. Arch Gen Psychiatry. 2000;57:1139–1147. doi: 10.1001/archpsyc.57.12.1139. [DOI] [PubMed] [Google Scholar]
- 230.Volk DW. Lewis DA. Impaired prefrontal inhibition in schizophrenia: relevance for cognitive dysfunction. Physiol Behav. 2002;77:501–505. doi: 10.1016/s0031-9384(02)00936-8. [DOI] [PubMed] [Google Scholar]
- 231.Vollenweider FX. Leenders KL. Oye I. Hell D. Angst J. Differential psychopathology and patterns of cerebral glucose utilisation produced by (S)- and (R)-ketamine in healthy volunteers using positron emission tomography (PET) Eur Neuropsychopharmacol. 1997;7:25–38. doi: 10.1016/s0924-977x(96)00042-9. [DOI] [PubMed] [Google Scholar]
- 232.Volman V ST. Behrens MM. Downregulation of parvalbumin at cortical GABA synapses reduces network gamma oscillatory activity. J Neuroscience. 2011;31:18137–18148. doi: 10.1523/JNEUROSCI.3041-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Wang C. Kaufmann JA. Sanchez-Ross MG. Johnson KM. Mechanisms of N-methyl-D-aspartate-induced apoptosis in phencyclidine-treated cultured forebrain neurons. J Pharmacol Exp Ther. 2000;294:287–295. [PubMed] [Google Scholar]
- 234.Wang C. McInnis J. West JB. Bao J. Anastasio N. Guidry JA. Ye Y. Salvemini D. Johnson KM. Blockade of phencyclidine-induced cortical apoptosis and deficits in prepulse inhibition by M40403, a superoxide dismutase mimetic. J Pharmacol Exp Ther. 2003;304:266–271. doi: 10.1124/jpet.102.041798. [DOI] [PubMed] [Google Scholar]
- 235.Wang CZ. Yang SF. Xia Y. Johnson KM. Postnatal phencyclidine administration selectively reduces adult cortical parvalbumin-containing interneurons. Neuropsychopharmacology. 2008;33:2442–2455. doi: 10.1038/sj.npp.1301647. [DOI] [PubMed] [Google Scholar]
- 236.Wang HX. Gao WJ. Cell type-specific development of NMDA receptors in the interneurons of rat prefrontal cortex. Neuropsychopharmacology. 2009;34:2028–2040. doi: 10.1038/npp.2009.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Wang HX. Gao WJ. Development of calcium-permeable AMPA receptors and their correlation with NMDA receptors in fast-spiking interneurons of rat prefrontal cortex. J Physiol. 2010;588:2823–2838. doi: 10.1113/jphysiol.2010.187591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Wedzony K. Fijal K. Mackowiak M. Chocyk A. Detrimental effect of postnatal blockade of N-methyl-D-aspartate receptors on sensorimotor gating is reversed by neuroleptic drugs. Pharmacol Rep. 2008;60:856–864. [PubMed] [Google Scholar]
- 239.Wen L. Lu YS. Zhu XH. Li XM. Woo RS. Chen YJ. Yin DM. Lai C. Terry AV., Jr. Vazdarjanova A. Xiong WC. Mei L. Neuregulin 1 regulates pyramidal neuron activity via ErbB4 in parvalbumin-positive interneurons. Proc Natl Acad Sci U S A. 2010;107:1211–1216. doi: 10.1073/pnas.0910302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Whittington MA. Cunningham MO. LeBeau FE. Racca C. Traub RD. Multiple origins of the cortical gamma rhythm. Dev Neurobiol. 2011;71:92–106. doi: 10.1002/dneu.20814. [DOI] [PubMed] [Google Scholar]
- 241.Whittington MA. Roopun AK. Traub RD. Davies CH. Circuits and brain rhythms in schizophrenia: a wealth of convergent targets. Curr Opin Pharmacol. 2011;11:508–514. doi: 10.1016/j.coph.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 242.Wiley JL. Buhler KG. Lavecchia KL. Johnson KM. Pharmacological challenge reveals long-term effects of perinatal phencyclidine on delayed spatial alternation in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:867–873. doi: 10.1016/S0278-5846(03)00146-5. [DOI] [PubMed] [Google Scholar]
- 243.Williams K. Russell SL. Shen YM. Molinoff PB. Developmental switch in the expression of NMDA receptors occurs in vivo and in vitro. Neuron. 1993;10:267–278. doi: 10.1016/0896-6273(93)90317-k. [DOI] [PubMed] [Google Scholar]
- 244.Williams SM. Goldman-Rakic PS. Leranth C. The synaptology of parvalbumin-immunoreactive neurons in the primate prefrontal cortex. J Comp Neurol. 1992;320:353–369. doi: 10.1002/cne.903200307. [DOI] [PubMed] [Google Scholar]
- 245.Wirth EK. Conrad M. Winterer J. Wozny C. Carlson BA. Roth S. Schmitz D. Bornkamm GW. Coppola V. Tessarollo L. Schomburg L. Kohrle J. Hatfield DL. Schweizer U. Neuronal selenoprotein expression is required for interneuron development and prevents seizures and neurodegeneration. FASEB J. 2010;24:844–852. doi: 10.1096/fj.09-143974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Xi D. Keeler B. Zhang W. Houle JD. Gao WJ. NMDA receptor subunit expression in GABAergic interneurons in the prefrontal cortex: Application of laser microdissection technique. J Neurosci Methods. 2009;176:172–181. doi: 10.1016/j.jneumeth.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Xia S. Cai ZY. Thio LL. Kim-Han JS. Dugan LL. Covey DF. Rothman SM. The estrogen receptor is not essential for all estrogen neuroprotection: new evidence from a new analog. Neurobiol Dis. 2002;9:282–293. doi: 10.1006/nbdi.2002.0478. [DOI] [PubMed] [Google Scholar]
- 248.Xu X. Roby KD. Callaway EM. Immunochemical characterization of inhibitory mouse cortical neurons: three chemically distinct classes of inhibitory cells. J Comp Neurol. 2010;518:389–404. doi: 10.1002/cne.22229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Yang CR. Chen L. Targeting prefrontal cortical dopamine D1 and N-methyl-D-aspartate receptor interactions in schizophrenia treatment. Neuroscientist. 2005;11:452–470. doi: 10.1177/1073858405279692. [DOI] [PubMed] [Google Scholar]
- 250.Yao JK. Leonard S. Reddy R. Altered glutathione redox state in schizophrenia. Dis Markers. 2006;22:83–93. doi: 10.1155/2006/248387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Zhang Y. Behrens MM. Lisman JE. Prolonged exposure to NMDAR antagonist suppresses inhibitory synaptic transmission in prefrontal cortex. J Neurophysiol. 2008;100:959–965. doi: 10.1152/jn.00079.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Zhang Z. Sun QQ. Development of NMDA NR2 subunits and their roles in critical period maturation of neocortical GABAergic interneurons. Dev Neurobiol. 2011;71:221–245. doi: 10.1002/dneu.20844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Zheng K. An JJ. Yang F. Xu W. Xu ZQ. Wu J. Hokfelt TG. Fisahn A. Xu B. Lu B. TrkB signaling in parvalbumin-positive interneurons is critical for gamma-band network synchronization in hippocampus. Proc Natl Acad Sci U S A. 2011;108:17201–17206. doi: 10.1073/pnas.1114241108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Zuo DY. Wu YL. Yao WX. Cao Y. Wu CF. Tanaka M. Effect of MK-801 and ketamine on hydroxyl radical generation in the posterior cingulate and retrosplenial cortex of free-moving mice, as determined by in vivo microdialysis. Pharmacol Biochem Behav. 2007;86:1–7. doi: 10.1016/j.pbb.2006.05.010. [DOI] [PubMed] [Google Scholar]