Abstract
Aims: Psychosocial stress alters the hypothalamic-pituitary-adrenal axis (HPA-axis). Increasing evidence shows a link between these alterations and oxidant elevation. Oxidative stress is implicated in the stress response and in the pathogenesis of neurologic and psychiatric diseases. NADPH oxidases (NOXs) are a major source of reactive oxygen species (ROS) in the central nervous system. Here, we investigated the contributory role of NOX2-derived ROS to the development of neuroendocrine alterations in a rat model of chronic psychosocial stress, the social isolation. Results: Significant elevations in the hypothalamic levels of corticotropin-releasing factor and plasmatic adrenocorticotropic hormone were observed from 4 weeks of social isolation. Increased levels of peripheral markers of the HPA-axis (plasmatic and salivary corticosterone) were observed at a later time point of social isolation (7 weeks). Alteration in the exploratory activity of isolated rats followed the same time course. Increased expression of markers of oxidative stress (8-hydroxy-2-deoxyguanosine [8OhdG] and nitrotyrosine) and NOX2 mRNA was early detectable in the hypothalamus of isolated rats (after 2 weeks), but later (after 7 weeks) in the adrenal gland. A 3-week treatment with the antioxidant/NOX inhibitor apocynin stopped the progression of isolation-induced alterations of the HPA-axis. Rats with a loss-of-function mutation in the NOX2 subunit p47phox were totally protected from the alterations of the neuroendocrine profile, behavior, and increased NOX2 mRNA expression induced by social isolation. Innovation: We demonstrate that psychosocial stress induces early elevation of NOX2-derived oxidative stress in the hypothalamus and consequent alterations of the HPA-axis, leading ultimately to an altered behavior. Conclusion: Pharmacological targeting of NOX2 might be of crucial importance for the treatment of psychosocial stress-induced psychosis. Antioxid. Redox Signal. 18, 1385–1399.
Introduction
Psychosocial stress is known to determine the alterations of the physiological functioning of the hypothalamic-pituitary-adrenal axis (HPA-axis) (36) and to play a key role in the development of psychiatric diseases, such as psychosis (65). The HPA-axis represents the main neuroendocrine system for the regulation of the stress response (24). The paraventricular nucleus of the hypothalamus is the central element of this system, releasing mainly vasopressin and corticotropin-releasing factor (CRF). These two hormones act on the pituitary gland, stimulating the secretion of adrenocorticotropic hormone (ACTH), which, in turn, induces the production of glucocorticoid hormones (mainly cortisol in humans and corticosterone in rodents) from the adrenal gland. Alterations of the HPA-axis (mainly elevations in stress-related hormones) have been observed in psychotic patients (13, 21, 34) and in animal models of psychosis (8, 33). Increasing evidence has shown a role of oxidative stress in the control of the stress–response system, via several molecular mechanisms, including altered translocation of the glucocorticoid receptors (9), elevation in the glutamate excitotoxicity (5), and alterations of RNA synthesis and stability (52). NADPH oxidase (NOX) enzymes are proteins that transfer electrons across the biological membranes to catalyze the reduction of molecular oxygen and generate the superoxide anion O2− (10). In the central nervous system (CNS), NOX isoforms are heterogeneously distributed in different regions and cell types, and thought to be involved in the regulation of cell fate and neuronal activity (55). From a pathologic point of view, NOX enzymes have been implicated in the generation of oxidative stress seen in a variety of brain disorders (55).
Innovation.
Oxidative stress is involved in the neuroendocrine response to psychosocial stress and in the pathogenesis of psychiatric diseases. We demonstrate for the first time that psychosocial stress leads to early elevation ofNADPHoxidase 2 (NOX2)-derived oxidative stress in the hypothalamus, determining alterations of the hypothalamic-pituitary-adrenal axis and leading ultimately to an altered behavior, reminiscent of psychotic symptoms in humans. Thus, pharmacological targeting of NOX2 might be of crucial importance for treatment of psychosocial stress-induced psychosis.
Animal models of mental disorders are essential tools to understand the molecular link between oxidative stress, alterations of the HPA-axis, and the development of psychiatric diseases. Recent evidence has shown that NOX2 is a major source of oxidative stress in the CNS, controlling alterations in neurotransmission and behavior (11, 53, 56) and the loss of phenotype of GABAergic interneurons (11, 53).
The social isolation rearing of rats is a model of chronic psychosocial stress that allows to study long-term alterations, reminiscent of symptoms of schizophrenic patients (23). A possible involvement of NOX2 in isolation-induced neuropathology and altered behavior has been recently shown (53).
A natural polymorphism of the Ncf1 gene (referred in the text as a loss-of-function mutation), controlling the production of reactive oxygen species (ROS) by NOX2, is known in rats (46, 47). Importantly, a single-nucleotide polymorphism determines the functional effects. Indeed DA.Ncf1DA rats with a lower capacity for ROS production (30, 46) differ only in the Ncf1 gene from the congenic strain DA.Ncf1E3. Ncf1, coding for the p47phox protein, is an essential component of the NOX2/NOX complex, and a methionine instead of a threonine at position 153 reduces the capacity of oxidative burst by 40% (30). Importantly, the Ncf1 polymorphism is widely occurring in wild rats and is therefore likely to result from natural selection (34).
Here, we investigate the role of NOX2-derived oxidative stress in the development of neuroendocrine alterations induced by psychosocial stress. We demonstrate a crucial early role of NOX2 in the disturbances of the HPA-axis, leading ultimately to psychotic diseases.
Results
Neuroendocrine and behavioral alterations induced by social isolation are time dependent
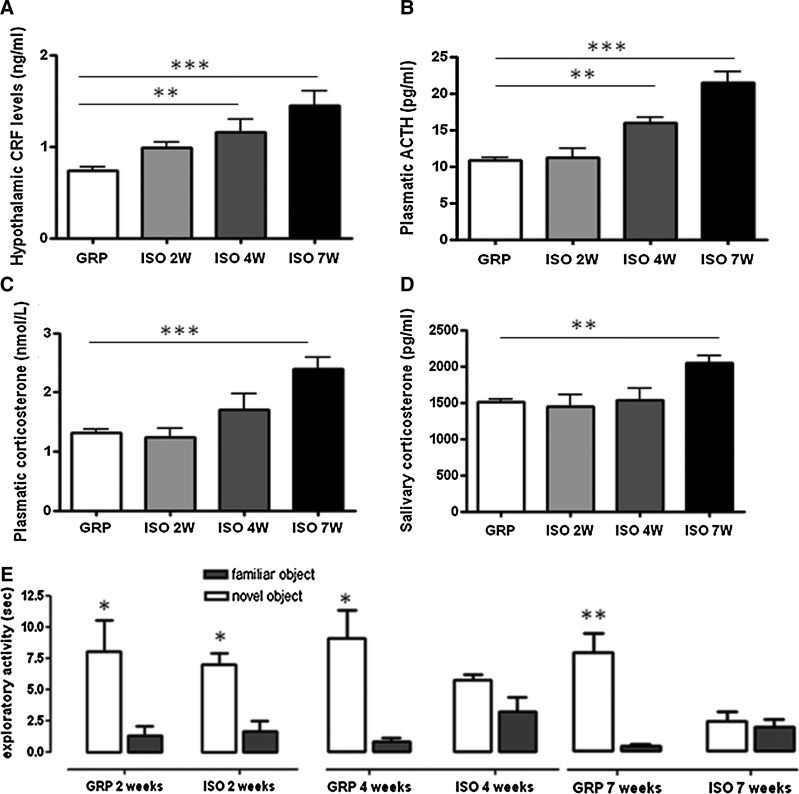
In control animals, hypothalamic levels of CRF, the plasmatic amount of ACTH, and the levels of salivary and plasmatic corticosterone were stable over the 7-week period (Fig. 1A–D). In contrast, in isolated animals, a significant increase of the hypothalamic CRF and plasmatic ACTH levels was detected from 4 weeks of social isolation (Fig. 1A, B). While no changes in the levels of plasmatic and salivary corticosterone were found in control animals over the 7-week period, a significant increase of these markers was found in isolated animals at a later time point (after 7 weeks of social isolation) (Fig. 1C, D).
FIG. 1.
Effects of social isolation on the HPA-axis functioning. (A) Hypothalamic levels of CRF (ng/ml) in GRP and ISO rats after 2 weeks (2W), 4 weeks (4W), and 7 weeks (7W) of social isolation (n=5 for each group in each time point). Statistical analysis: Two-way analysis of variance followed by Tukey post hoc test, r=rearing, t=time Fr(1,24)=27.463 p<0.001; Ft(2,24)=2.480 p=0.105; Ftxr(2,24)=2.213 p=0.131; **p<0.01; ***p<0.001, not significant. p=0.126 ISO 2W versus GRP 2W; p=0.969, p=0.998, p=0.982 within GRP. (B) Plasmatic levels of ACTH (pg/ml) in GRP and ISO rats after 2W, 4W, and 7W of social isolation (n=5 for each group in each time point). Statistical analysis: Two-way analysis of variance followed by Tukey post hoc test, r=rearing, t=time Fr(1,24)=40.128 p<0.001; Ft(2,24)=10.147 p<0.001; Ftxr(2,24)=13.890 p<0.001; **p<0.01; ***p<0.001, not significant p=1 ISO 2W versus GRP 2W; p=0.852, p=0.961, p=0.961 within GRP. (C) Plasmatic levels of corticosterone (nM) in GRP and ISO rats after 2W, 4W, and 7W of social isolation (n=5 for each group in each time point). Statistical analysis: Two-way analysis of variance followed by Tukey post hoc test, r=rearing, t=time Fr(1,24)=11.962 p=0.002; Ft(2,24)=6.362 p=0.006; Ftxr(2,24)=6.543 p=0.005; ***p<0.001, not significant p=0.797 ISO 2W versus GRP 2W; p=0.153 ISO 4W versus GRP 4W, p=0.963, p=0.963, p=1; within GRP. (D) Salivary levels of corticosterone (pg/ml) in GRP and ISO rats after 2W, 4W, and 7W of social isolation (n=5 for each group in each time point). Statistical analysis: Two-way analysis of variance followed by Tukey post hoc test, r=rearing, t=time Fr(1,24)=2.869 p=0.103; Ft(2,24)=3.574 p=0.044; Ftxr(2,24)=4.618 p=0.02; **p<0.01, not significant p=0.580 ISO 2W versus GRP 2W; p=0.951 ISO 4W versus GRP 4W, p=0.966, p=0.998, p=0.981; within GRP. (E) Exploratory activity (sec) of the familiar and novel object in the Novel Object Recognition test of GRP and ISO rats after 2W, 4W, and 7W of social isolation (n=8–10 for each group in each time point). Statistical analysis: Student's t-test, *p<0.05 novel object versus familiar object within GRP 2W, ISO 2W, GRP 4W, and GRP 7W. not significant p=0.896 novel object versus familiar object within ISO 4W; p=0.994 novel object versus familiar object within ISO 7W. ACTH, adrenocorticotropic hormone; CRF, corticotropin-releasing factor; GRP, control rats (reared in group); HPA-axis, hypothalamic-pituitary-adrenal axis.
Pathological activation of the stress response in rodents is associated to an altered exploratory behavior (41, 59). While control animals were able to recognize the novel object from the familiar one over the 7-week period, the exploratory activity of isolated animals was compromised starting from 4 weeks of social isolation and worsened after 7 weeks (Fig. 1E).
Neuroendocrine and behavioral alterations induced by social isolation are associated to an early increase of oxidative stress in the hypothalamus
The presence of biomarkers of oxidative stress [e.g., 8-OHdG, one of the most abundant markers of DNA oxidation (14), and nitrotyrosine, an end product of NO-toxic species] was evaluated in the hypothalamus and in the adrenal glands. Thus, these two tissues have been associated with the central and peripheral control of the HPA-axis, respectively (45).
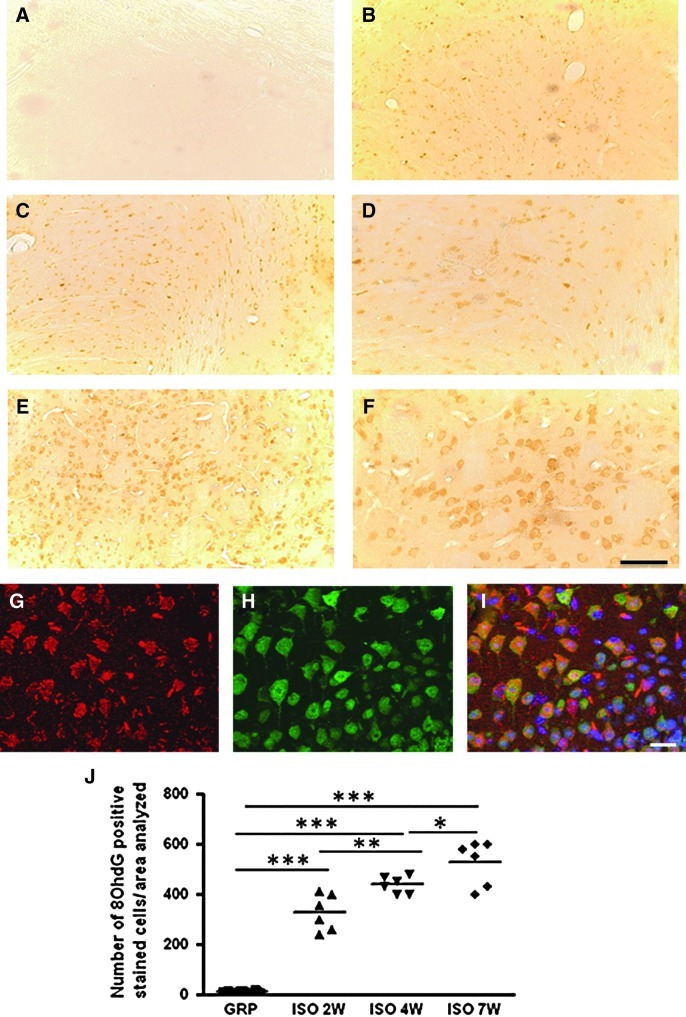
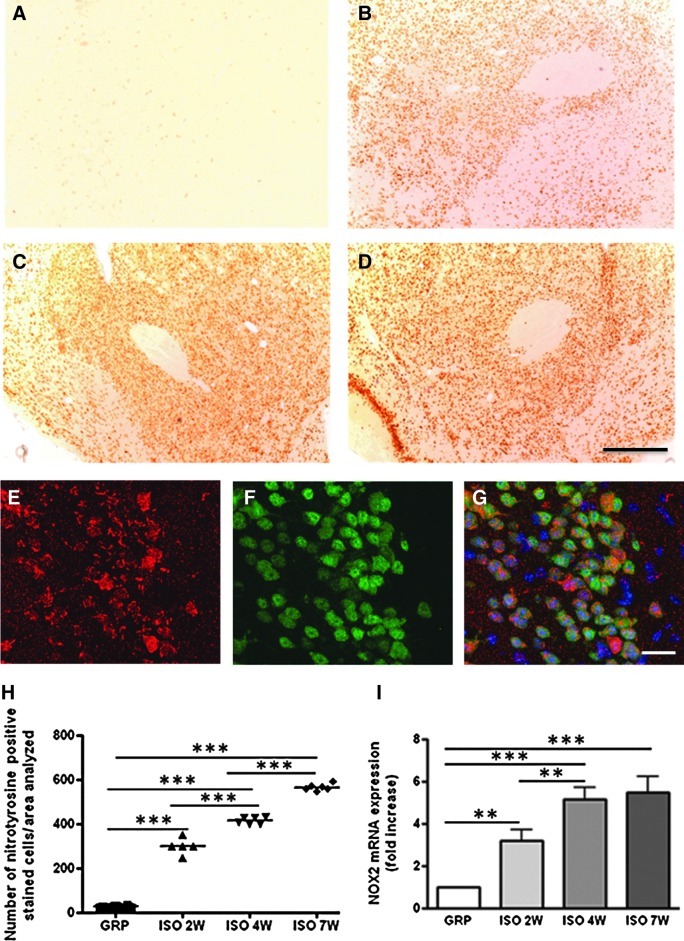
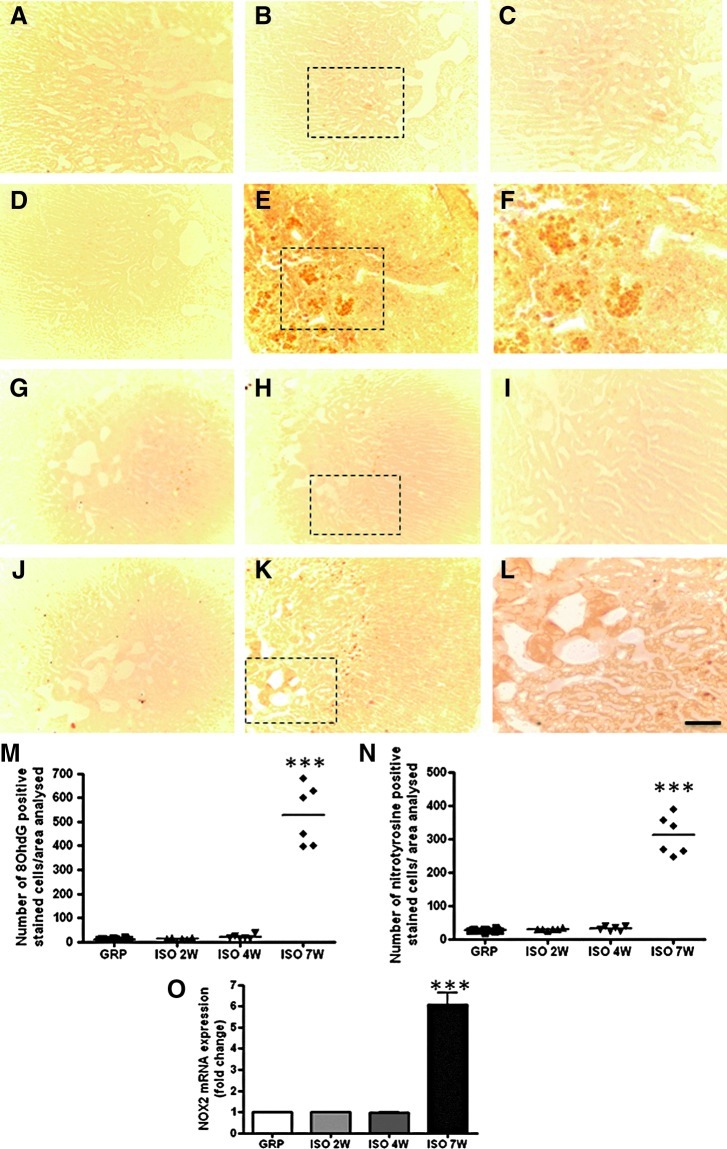
No change in the number of 8-OHdG- and nitrotyrosine-positive stained cells was detected in the hypothalamus and in the adrenal glands of control animals (Figs. 2A, G, 3A, E, and 4A, G, M, N). A significant early increase (after 2 weeks) of these two markers was detected in the hypothalamus of isolated animals (Figs. 2B, G and 3B, E), whereas the adrenal gland appeared to be affected only at later time points (from 7 weeks of social isolation) (Fig. 4B–F, H–L, M, N) both in the cortical and medullary regions (Fig. 4E, F, K, L). Confocal microscopy results for 8-OHdG and Neun, aneuronal-specific nuclear protein (44), 8OHdG and IBA-1, an ionized calcium-binding protein specifically expressed in microglia cells (2), 8OHdG and GFAP (glial fibrillary acidic protein) as a marker of astrocytes (22), nitrotyrosine and Neun, nitrotyrosine and IBA-1, and nitrotyrosine and GFAP show that in the hypothalamus of isolated rats, the large majority of 8OHdG-expressing cells were costained with Neun (Fig. 3F–H). In addition, the large majority of nitrotyrosine-expressing cells were costained with Neun (Fig. 2P–R). The large majority of both microglial cells and astrocytes were not involved in the increase of 8OHdG and nitrotyrosine (data not shown).
FIG. 2.
Social isolation induces an early increase of oxidative stress (8OhdG) in the rat hypothalamus. (A–F) Representative images of DAB immunohistochemistry for 8OhdG in control (A) and isolated rats for a period of 2W (B), 4W (C, D), and 7W (E, F) of social isolation, (n=6 for each group in each time point). (D, F) are magnified views of (C, E), respectively. Scale bar: 55 μm. (G–I) Representative images of immunofluorescence (analyzed by confocal microscopy) for 8OhdG (red staining, G), NeuN (green staining, H), and merged with DAPI staining (I) in isolated animals (n=6 for each group in each time point). Scale bar: 54 μm. (J) Quantification of 8OhdG-immunoreactive cells in the hypothalamus of GRP and ISO rats after a period of 2W, 4W, and 7W (n=6 for each group in each time point). Statistical analysis: Two-way analysis of variance followed by Tukey post hoc test. Fr(1,30)=663.015 p<0.001; Ft(2,30)=12.160 p<0.001; Ftxr(2,30)=12.864 *p<0.05, **p<0.01, ***p<0.001. 8OhdG, 8-hydroxy-2-deoxyguanosine.
FIG. 3.
Social isolation induces an early increase of oxidative stress (nitrotyrosine and NOX2) in the rat hypothalamus. (A–G) Representative images of DAB immunohistochemistry for nitrotyrosine in control (A) and isolated rats for a period of 2W (B), 4W (C), and 7W (D) of social isolation, (n=6 for each group in each time point). Scale bar: 50 μm. (E–G) Representative images of immunofluorescence (analyzed by confocal microscopy) for nitrotyrosine (red staining, E), NeuN (green staining, F), and a merged with DAPI (G) in isolated animals (n=6 for each group in each time point). (H) Quantification of nitrotyrosine immunoreactive cells in the hypothalamus of GRP and ISO rats after a period of 2W, 4W, and 7W (n=6 for each group in each time point). Statistical analysis: Two-way analysis of variance followed by Tukey post hoc test. Fr(1,30)=586.675 p<0.001; Ft(2,30)=199.945 p<0.001; Ftxr(2,30)=211.987 p<0.001; ***p<0.001. (I) Real-time PCR for NOX2 mRNA expression (fold change) in the hypothalamus of GRP and ISO rats after a period of 2W, 4W, and 7W (n=6 for each group in each time point). Statistical analysis: Two-way analysis of variance followed by Tukey post hoc test. Fr(1,30)=101, 428 p<0.001; Ft(2,30)=4.116 p=0.026; Ftxr(2,30)=4.079 p=0.027; **p<0.01; ***p<0.001. NOX2, NADPH oxidase 2; PCR, polymerase chain reaction.
FIG. 4.
Social isolation induces a late increase of oxidative stress in the rat adrenal gland. (A–F) Representative images of DAB immunohistochemistry for 8OhdG in control (A) and isolated rats for a period of 2W (B), 4W (D), and 7W (E) of social isolation, (n=6 for each group in each time point). (C, F) are magnified views of the dotted box in (B, E), respectively. (G–L) Representative images of DAB immunohistochemistry for nitrotyrosine in control (G) and isolated rats for a period of 2W (H), 4W (J), and 7W (K) of social isolation (n=6 for each group in each time point). (I, L) are magnified views of the dotted box in (H, K), respectively. (M) Quantification of 8OhdG-immunoreactive cells in the adrenal glands of GRP and ISO rats after a period of 2W, 4W, and 7W (n=6 for each group in each time point). Statistical analysis: Two-way analysis of variance followed by Tukey post hoc test. Fr(1,30)=105.918 p<0.001; Ft(2,30)=98.500 p<0.001; Ftxr(2,30)=99.244 p<0.001 ***p<0.001, not significant; rearing within 2W p=0.996, rearing within 4W p=0.701. (N) Quantification of nitrotyrosine-immunoreactive cells in the adrenal glands of GRP and ISO rats after a period of 2W, 4W, and 7W (n=6 for each group in each time point). Statistical analysis: Two-way analysis of variance followed by Tukey post hoc test. Fr(1,30)=149.028 p<0.001; Ft(2,30)=132.718 p<0.001; Ftxr(2,30)=137.412 p<0.001; ***p<0.001. (O) Real-time PCR for NOX2 mRNA expression (fold change) in the adrenal glands of GRP and ISO rats after a period of 2W, 4W, and 7W (n=6 for each group in each time point). Statistical analysis: Two-way analysis of variance followed by Tukey post hoc test. Fr(1,30)=81.995 p<0.001; Ft(2,30)=81.996 p<0.001; Ftxr(2,30)=81.995p<0.001; ***p<0.001. Scale bar: 58 μM.
Social isolation induced no changes in the mRNA expression of NOX1, NOX3, and NOX4, both in the hypothalamus (Supplementary Fig. S1A–C; Supplementary Data are available online at www.liebertpub.com/ars) and in the adrenal glands (Supplementary Fig. S2A–C). Interestingly, NOX2 mRNA expression showed the same time dependency observed for the biomarkers of oxidative stress, being significantly elevated at early time points in the hypothalamus (Fig. 3I) and at later time points in the adrenal glands (Fig. 4O).
The antioxidant/NOX inhibitor apocynin stops the progression of neuroendocrine alterations induced by social isolation
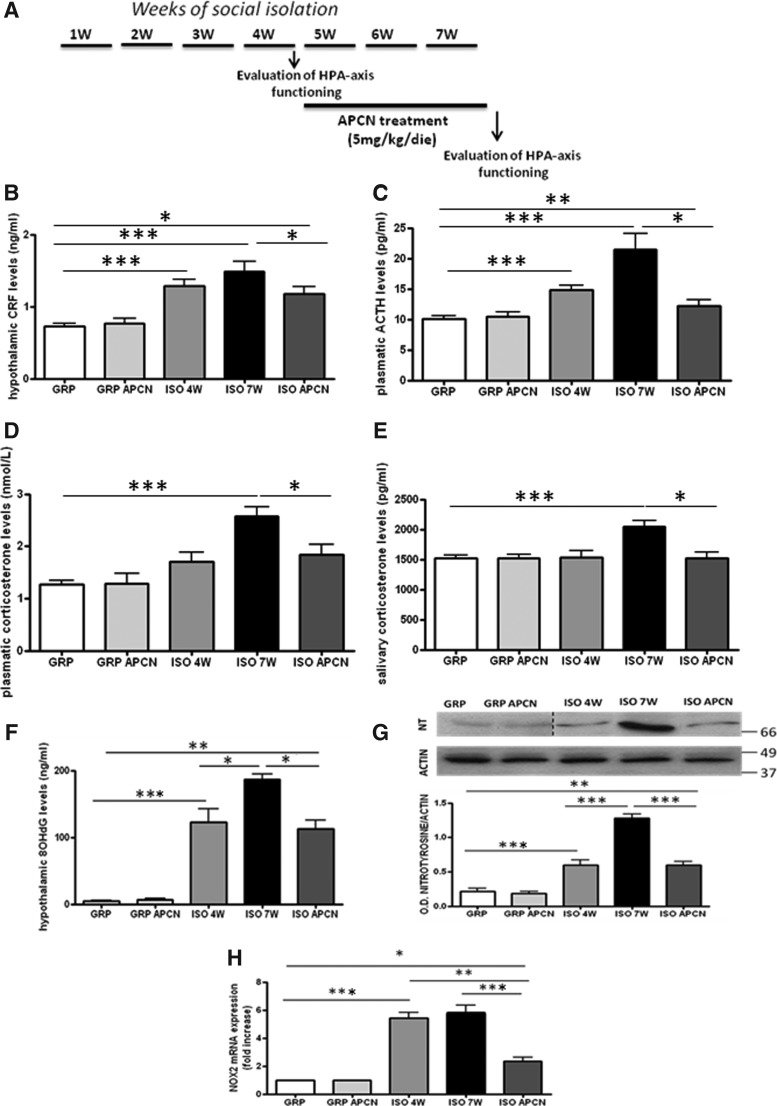
Apocynin prevents the behavioral and neuropathological alterations induced by 7 weeks of social isolation (53). Hypothalamic CRF, plasmatic ACTH, and plasmatic and salivary corticosterone were analyzed in control and isolated animals at the end of weeks 4 and 7 (Fig. 5A). While apocynin had no effects on the HPA-axis of control animals, treatment with this compound was able to stop the progression of the neuroendocrine alterations. Indeed, (i) the biomarkers of the HPA-axis of isolated animals treated with apocynin were significantly decreased compared to the ones measured in the 7-week isolated animals not treated with apocynin (Fig. 5B–E); (ii) the biomarkers of the HPA-axis of isolated animals treated with apocynin did not differ from the ones measured in the set of animals isolated for 4 weeks not treated with apocynin (Fig. 5B–E).
FIG. 5.
Effects of 3W of apocynin (APCN) treatment on the alterations of the HPA-axis functioning induced by social isolation. (A) Schematic representation of the treatment protocol with APCN treatment (5 mg/kg/die) during the last 3W of social isolation. (B–E) Effects of 3-week APCN treatment on the levels of the four markers of HPA-axis functioning in GRP and ISO rats. n=10 for GRP 4W; 5 for GRP 7W; 5 for GRP 4W+3W APCN; 10 for ISO 4W; 5 for ISO 7W; 5 for ISO 4W+3W APCN. Three-way ANOVA followed by Tukey post hoc test; r=rearing, t=time, tr=treatment. For CRF, Fr(1,40)=118.71 p<0.001; Ftr(1,40)=1.863 p=0.180; Ft(1,40)=0.295 p=0.590; Frxtr(1,40)=3.470 p=0.070; Frxt(1,40)=1.084 p=0.304; Ftrxt(1,40)=1.134 p=0.293; Frxtrxt(1,40)=4.679 p=0.037; *p<0.05; ***p<0.001. For plasmatic ACTH, Fr(1,40)=70.105 p<0.001; Ftr(1,40)=11.246 p<0.01; Ft(1,40)=2.491 p=0.122; Frxtr(1,40)=11.246 p<0.01; Frxt(1,40)=0.233 p=0.632; Ftrxt(1,40)=11.246 p<0.01; Frxtrxt(1,40)=11.246 p<0.01; *p<0.05; **p<0.01; ***p<0.001. For plasmatic corticosterone, Fr(1,40)=124.987 p<0.001; Ftr(1,40)=4.414 p<0.05; Ft(1,40)=0.002 p=0.963; Frxtr(1,40)=4.414 p<0.05; Frxt(1,40)=3.046 p=0.089; Ftrxt(1,40)=4.414 p<0.05; Frxtrxt(1,40)=4.414 p<0.05; *p<0.05; ***p<0.001. For salivary corticosterone, Fr(1,40)=17.823 p<0.001; Ftr(1,40)=5.284 p<0.05; Ft(1,40)=0.0676 p=0.796; Frxtr(1,40)=5.284 p<0.05; Frxt(1,40)=3.047 p=0.089; Ftrxt(1,40)=5.284 p<0.05; Frxtrxt(1,40)=5.284 p<0.05; *p<0.05; ***p<0.001. (F) Hypothalamic 8OhdG levels (ng/ml) assessed by ELISA in GRP and ISO animals treated according to the protocol shown in Figure 4A. Fr(1,40)=289.252 p<0.001; Ftr(1,40)=6.083 p<0.05; Ft(1,40)=3.922 p=0.054; Frxtr(1,40)=5.473 p<0.05; Frxt(1,40)=2.406 p=0.128; Ftrxt(1,40)=5.551 p<0.05; Frxtrxt(1,40)=6.001 p<0.05; *p<0.05; **p<0.01; ***p<0.001. (G) Representative images of western blotting for nitrotyrosine and actin in the hypothalamus of GRP and ISO rats treated according to the protocol shown in Figure 4A with optical density (OD) protein bands normalized to the actin protein values. Fr(1,40)=236.385 p<0.001; Ftr(1,40)=21.171 p<0.001; Ft(1,40)=17.242 p<0.001; Frxtr(1,40)=21.171 p<0.001; Frxt(1,40)=25.504 p<0.001; Ftrxt(1,40)=21.171 p<0.001; Frxtrxt(1,40)=21.171 p<0.001; **p<0.01; ***p<0.001. (H) Real-time PCR for NOX2 mRNA expression (fold change) in the hypothalamus of GRP and ISO rats treated according to the protocol shown in Figure 4A. Fr(1,40)=305.401 p<0.001; Ftr(1,40)=15.951 p<0.001; Ft(1,40)=10.586 p<0.01; Frxtr(1,40)=15.951 p<0.001; Frxt(1,40)=10.586 p<0.01; Ftrxt(1,40)=15.951 p<0.001; Frxtrxt(1,40)=15.951 p<0.001; *p<0.05; **p<0.01; ***p<0.001.
Apocynin treatment was able to stop the progression of the increase of the hypothalamic 8OhdG levels and nitrotyrosine protein expression observed from week 4 to week 7 of the social isolation period (Fig. 5F, G). Three weeks of apocynin treatment also decreased the NOX2 mRNA expression levels compared to the nontreated isolated rats (Fig. 5H).
Ncf1 polymorphism prevents neuroendocrine and behavioral alterations induced by social isolation
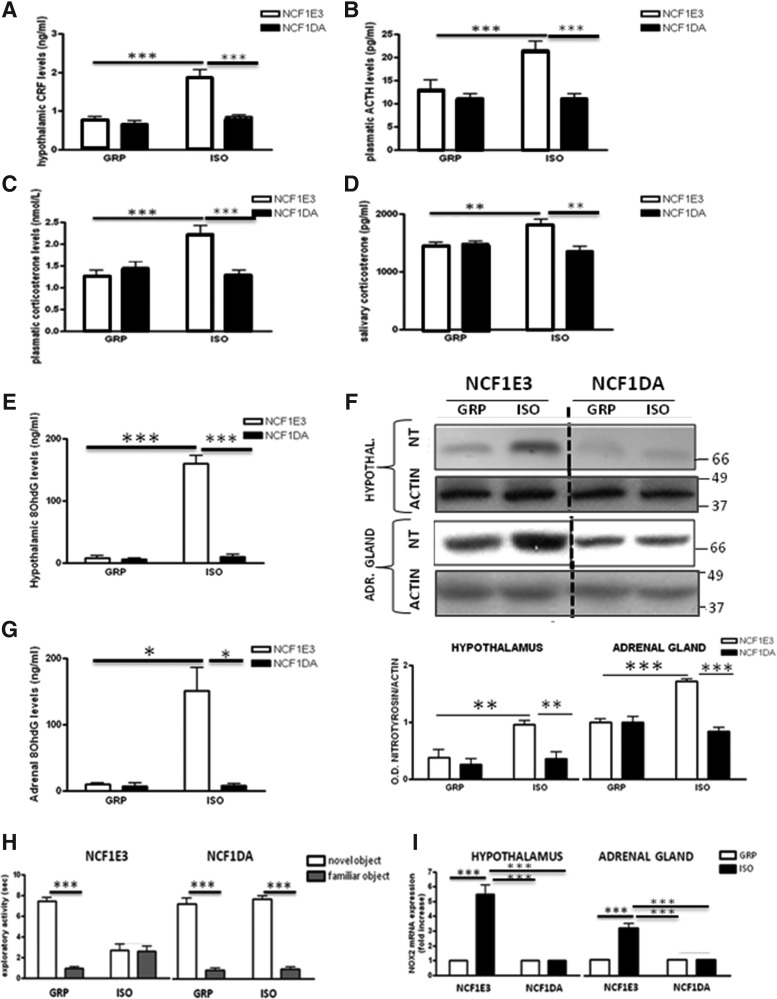
No basal differences in the hypothalamic CRF, plasmatic amount of ACTH, and plasmatic and salivary levels of corticosterone were observed between the Wistar strain and the DA strain (data not shown). Under control conditions, we did not detect any alteration in the markers of the HPA-axis between DA.Ncf1E3 and DA.Ncf1DA rats (Fig. 6A–D). As described above for Wistar rats, 7 weeks of social isolation led to increased hypothalamic levels of CRF, plasmatic amount of ACTH, and plasmatic and salivary levels of corticosterone in the DA.Ncf1E3 strain. In contrast, social isolation had no impact on these biomarkers in DA.Ncf1DA rats (Fig. 6A–D).
FIG. 6.
The Ncf1 mutation protects from the neuroendocrine alterations induced by social isolation. (A) Hypothalamic levels of CRF (ng/ml) in Ncf1E3and Ncf1DA GRP and ISO for 7W (n=6 for each group in each time point). Two-way ANOVA followed by Tukey post hoc test. r=rearing, g=genotype Fr(1,20)=26.355 p<0.001; Fg(1,20)=19.878 p<0.001; Ftxg(1,20)=12.526 p=0.002; ***p<0.001. (B) Plasmatic levels of ACTH (pg/ml) in Ncf1E3and Ncf1DA GRP and ISO for 7W (n=6 for each group in each time point). Fr(1,20)=26.355 p<0.001; Fg(1,20)=19.878 p<0.001; Ftxg(1,20)=12.526 p=0.002; ***p<0.001. (C) Plasmatic levels of corticosterone (nM) in Ncf1E3and Ncf1DA GRP and ISO for 7W. Fr(1,20)=7.101 p=0.015; Fg(1,20)=7.101 p=0.015; Ftxg(1,20)=15.517 p<0.001; ***p<0.001. (D) Salivary levels of corticosterone (pg/ml) in Ncf1E3and Ncf1DA GRP and ISO for 7W. Fr(1,20)=2.139 p=0.159; Fg(1,20)=7.911 p=0.011; Fgxr(1,20)=10.646 p=0.004; **p<0.01. (E) Hypothalamic 8OhdG levels (ng/ml) assessed by ELISA in in Ncf1E3and Ncf1DA GRP and ISO for 7W. Fr(1,20)=126.664 p<0.001; Fg(1,20)=120.259 p<0.001; Fgxr(1,20)=114.072 p<0.001; ***p<0.001. (F) Representative images of western blotting for nitrotyrosine and actin in the hypothalamus and the adrenal glands of Ncf1E3and Ncf1DA GRP and ISO for 7W with optical density (OD) protein bands normalized to the actin protein values. Fr(1,20)=16.960 p<0.001; Fg(1,20)=15.486 p<0.001; Fgxr(1,20)=7.044 p<0.05; **p<0.01. For adrenal gland, Fr(1,20)=24.020 p<0.001; Fg(1,20)=47.078 p<0.001; Fgxr(1,20)=59.314 p<0.001; ***p<0.001. (G) Adrenal 8OhdG levels (ng/ml) assessed by ELISA in Ncf1E3and Ncf1DA GRP and ISO for 7W. Fr(1,20)=26.355 p<0.001; Fg(1,20)=19.878 p<0.001; Fgxr(1,20)=12.526 p<0.01; *p<0.05. (H) Exploratory activity (sec) of the familiar and novel object in Ncf1E3and Ncf1DA GRP and ISO for 7W. Student's t-test, ***p<0.001 novel object versus familiar object within Ncf1E3 GRP, Ncf1DA GRP, and Ncf1DA ISO. (I) Real-time PCR for NOX2 mRNA expression (fold change) in the hypothalamus and the adrenal glands of Ncf1E3and Ncf1DA c GRP and ISO for 7W. Fr(1,20)=51.718 p<0.001; Fg(1,20)=50.964 p<0.001; Fgxr(1,20)=50.216 p<0.001; **p<0.01. For adrenal gland, Fr(1,20)=45.882 p<0.001; Fg(1,20)=45.885 p<0.001; Fgxr(1,20)=45.789 p<0.001; ***p<0.001.
Ncf1 loss-of-function mutation also prevented the increase in the hypothalamic and adrenal 8OhdG levels (Fig. 6E–G) and nitrotyrosine protein expression (Fig. 6F) induced by 7 weeks of social isolation.
Exploratory activity of isolated DA.Ncf1E3 was strongly affected by 7 weeks of social isolation, while we did not detect the same negative impact of the social isolation on the exploratory activity of isolated DA.Ncf1DA (Fig. 6H).
Seven weeks of social isolation induced a significant increase in the NOX2 mRNA expression in the hypothalamus and adrenal glands of DA.Ncf1E3 rats, whereas this increase did not occur in DA.Ncf1DA rats (Fig. 6I).
Increased HPA-axis functioning was observed in both DA.Ncf1E3- and DA.Ncf1DA-restrained rats (Supplementary Table. S1). Thus, the Ncf1 polymorphism did not protect from the neuroendocrine alterations induced by acute stress.
Discussion
In this study, we investigated the time dependency of the HPA-axis alterations induced by psychosocial stress and its causal relationship with increased oxidative stress. We demonstrate that social isolation leads to an early elevation of NOX2 expression and oxidative stress in the hypothalamus, followed by increase of CRF levels, plasmatic amount of ACTH, and an altered exploratory behavior. Elevations in the plasmatic and salivary levels of corticosterone, together with increased oxidative stress and NOX2 mRNA expression in the adrenal gland, occur at later time points. A treatment with the antioxidant/NOX inhibitor apocynin stops the progression of HPA-axis dysfunctions induced by social isolation, whereas a loss-of-function polymorphism in the NOX2 subunit p47phox completely prevents neuroendocrine and behavioral alterations, as well as the increase of NOX2 expression in the hypothalamus and adrenal glands induced by social isolation.
To our knowledge, this is the first study showing a time course of the alterations of the HPA-axis and their relation to oxidative stress in the rat model of social isolation. Physiologically, both in humans and in rodents, the HPA-axis is not fully mature at birth. Indeed, there are several developmental changes occurring from childhood until late adolescence/early adultness in both basal HPA activity and cortisol release (16, 26, 62). Referring specifically to the rat life, the three considered time points (2, 4, and 7 weeks after weaning) correspond to this critical period for the development of the neuroendocrine system going from childhood (2 weeks after weaning) toward adolescence (4 weeks after weaning) and early adultness (7 weeks after weaning) (43, 54). Therefore, the responses observed at weeks 2 and 4 after weaning could be considered as an early response, because they involve the first stages of the development of the neuroendocrine system, while the responses observed at week 7 after the weaning could be considered as the late response, because it occurs in the last phase of this critical period of the HPA-axis development. We demonstrate that elevation in oxidative stress (NOX2 mRNA expression and biomarkers of oxidative stress) is an early pathological event occurring in the hypothalamus, preceding the increase of CRF and plasmatic levels of ACTH. This finding is of crucial importance, given the central role of the hypothalamus in the stress response (50) and in the development of stress-related mental disorders (3). Thus, early neuropathological alterations of the hypothalamus have been shown in rodent models of chronic stress (7, 63) and in patients with mental disorders (19, 34). In this context, the early increase of oxidative stress and NOX2 expression shown in our study might represent a triggering event of the stress response and HPA-axis activation.
Another novelty of the present work is the finding that the social isolation-induced increase of NOX2 expression and oxidative stress in the adrenal gland (the peripheral component of the HPA-axis) is a late event. This might lead ultimately to an excessive release of corticosterone in the plasma (associated also with elevations in salivary corticosterone) and consequent worsening of an altered behavior. As well as the hypothalamus, the adrenal glands are also considered a key anatomical and physiological player in the response to psychosocial stress, given its crucial pathological role in rodent models of chronic stress (57, 64) and in patients with psychiatric disorders (17, 18, 38). However, to the best of our knowledge, no studies exist investigating specifically the temporal contribution of the increase of oxidative stress in the adrenal glands to the neuropathological response induced by chronic psychosocial stress. In this context, a possible, although still speculative, outline of the temporal events linking the increase of NOX2-derived oxidative stress to the HPA-axis dysfunctions might be as follows: chronic psychosocial stress might induce an early increase of NOX2 expression and oxidative stress in the hypothalamus, leading first to increased release of the central biomarkers of the HPA-axis functioning (CRF and ACTH) and an altered exploratory behavior. The long-lasting elevation in NOX2 expression and oxidative stress in the hypothalamus, together with the appearance of elevations of NOX2 expression and oxidative stress in the adrenal gland (at later time points), might determine the alterations in the peripheral biomarkers of the HPA-axis (plasmatic and salivary corticosterone) and, consequently, worsening of the altered behavior. This outline is supported by recent evidence showing that increase in brain oxidative stress and/or reduced antioxidant defense has been found in the early phases of stress-induced mental disorders (39, 42, 48), and that both cerebral and peripheral long-lasting oxidative stress is one of the main contributors to the worsening of the psychosocial stress-induced altered behavior in rodents (53, 56) and psychotic symptoms in humans (15, 35).
Mechanisms leading to NOX2 upregulation in neurons are not yet understood. In the ketamine mouse model of psychosis, neuronal production of interleukin-6 is necessary for the activation of NADPH oxidase in brain (12). Thus, it could be speculated that psychosocial stress leads to early activation of neuroinflammatory in the hypothalamus, mediated by an increase of interleukin production and activation of microglial cells. Indeed, activated microglia after many different types of stimuli have been associated with NOX2 expression (28, 66). Release of mineralocorticoids, such as aldosterone, represents an important pathophysiological response to the activation the of HPA-axis, induced by psychosocial stress, and it is thought to be associated to increased oxidative stress in the adrenal glands (32, 49). Impairment in the aldosterone production rates has been found in depressive manic-depressive psychosis (29). Moreover, major depression has been found to occur in patients with elevated plasma concentrations of both cortisol and aldosterone after heart failure [reviewed in (25, 31)]. Treatment with spironolactone, an aldosterone antagonist, has been shown to attenuate this depression (1, 4). Thus, a role for aldosterone increase after elevation of oxidative stress induced by psychosocial stress could not be excluded from the above-described scenario.
In this study, we demonstrate the involvement of NOX2-derived oxidative stress in the development of neuroendocrine alterations and altered behavior by using a pharmacological approach. Although apocynin cannot be considered a specific NOX2 inhibitor rather than an antioxidant compound, its neuroprotective properties in neurodegenerative diseases (40, 60, 61, 67) and its role in the prevention of cell death after stroke have been recently shown (20). Apocynin effects have also been previously tested in rodent models of psychosis. Indeed, its prophylactic effect in the loss of phenotype of GABAergic neurons in the ketamine mouse model of psychosis (11, 12) and in the development of neuropathological and behavioral alterations in the rat model of social isolation (53) has been reported. In the present study, we show a therapeutic effect of apocynin. Thus, administration of this compound (started when neuroendocrine alterations and altered behaviour begin to be detectable) stops the progression of the HPA-axis dysfunctions induced by social isolation. This finding might open innovative therapeutic insights for the treatment of the early phases of human psychosis. Treatment with apocynin also prevents the increase of NOX2 expression, induced by social isolation. This phenomenon could be explained by the fact that apocynin has not a direct effect on NOX2 enzyme transcription (6, 58). Thus, the block of NOX2 activation by apocynin might result in a decrease of NOX2 expression at mRNA levels, as a consequence of a homeostatic circuit.
The data presented here provide an important genetic proof of principle of NOX2-derived oxidative stress involvement in the alterations of the HPA-axis and behavior induced by social isolation. Given the difficulties to obtain knockout rats, existence of rats with a loss-of-function mutation in the Ncf1 gene, coding for the p47phox subunit for NOX2, represents an important tool to verify the role of NOX2-derived oxidative stress in the development of neuroendocrine alterations induced by psychosocial stress. The exchange of a single amino acid (threonine to methionine at position 153) in the Ncf1 allele leads to a lower oxidative burst (30, 46). The Ncf1 locus is highly polymorphic in wild rats and the fully functional E3 allele (with threonine at position 153) has dominant effects. More than half of the wild rat population (Rattus norvegicus trapped in Sweden and Germany) are homozygous for the Ncf1DA alleles, having a low ROS response (46, 47). The data shown in Figure 5 of this article have been obtained by using inbred DA rats, polymorphic only with respect to the Ncf1 locus. Thus, importantly, the total prevention of the development of neuroendocrine alterations induced by chronic social stress is due to a single-nucleotide polymorphism. Interestingly, the Ncf1 polymorphism does not confer protection from the increased release of the central and peripheral biomarkers of the HPA-axis functioning induced by acute stress. Indeed, the neuroendocrine response of rats with low oxidative burst to 60 min of restraint stress does not differ from the response of rats with high oxidative burst to the same acute stress. How these results could be explained in the context of stress response in wild nature? It is possible that exposure to acute stress in nature leads to an immediate increase of oxidative stress and activation of the HPA-axis to assure the fight-or-fly response (51). On the other hand, the effects of the Ncf1 polymorphism might represent a form of adaptation to long-lasting stress, needing more time to be evident and, therefore, not detectable in the immediate response to an acute stress. Thus, the Ncf1 polymorphism might be a crucial new candidate in the understanding of the mechanisms leading to the positive adaptation and to the ability to maintain or regain mental health (despite experiencing severe adversity), also known as resilience (27).
In conclusion, we provide evidence for a key role of early NOX2-derived oxidative stress in the development of neuroendocrine alterations induced by psychosocial stress. Our results suggest that NOX2 might represent a crucial molecular link between HPA-axis dysfunctions and mental disorders. Therefore, targeting NOX2 enzyme might provide innovative approaches for the cure of psychiatric diseases.
Materials and Methods
Animals
An equal number of adult male and female Wistar rats (Harlan, S. Pietro al Natisone), DA (with the ROS-low responder Ncf1DA allele) and DA.Pia4 (with the ROS high-responder Ncf1E3 allele) (Medical Inflammation Research animal house, Karolinska Institute, Stockholm) rats were used to obtain litters. All animals were housed at a constant room temperature (22°C°±3°C) and a relative humidity (55%±5%) under a 12-h light/dark cycle (lights on from 7:00 AM to 7:00 PM). Food and water were freely available. All efforts were made to minimize the number of animals used and their suffering in conformity with the ethics guidelines and national and international laws (DL Number 116, G. U, suppl. 40, 18 February 1992, Circolare Number 8, GU, July 14, 1994; EEC Council Directive 86/609, OJ L 358, December 1, 2012, 1987; Guide for the Care and Use of Laboratory Animals, U.S. National Research Council, 1996).
Genotyping of animals
The 3 DA/E3 single nucleotide polymorphisms (snps) in Ncf1 were genotyped by the high-resolution melt curve analysis. First, DNA was purified from tail tips using a DNAeasy DNA isolation kit from Qiagen. The primers for the HRM single nucleotide polymorphism (snp) typing were designed using primer express software, and the sequences were as follows:
Ncf1exon4_R GGTGGCTACTCACTGGCTGT;
Ncf1exon4_F CCGAGTACTTCAACAGCCTCA;
Ncf1exon 6_F TGGCCCGATAGGTCTGAAG;
Ncf1exon 6_R CCCTGCTGTGTCCATTCA;
Ncf1exon 11_F ACACGGCGGACGTCAGTT;
Ncf1exon 11_R ACCCAGCTCGGACCTCATC.
HRM curve analysis was performed in a BIORAD C1000 thermal cycler immediately after a 40-cycle amplification using Sso-fast evagreen cybermix from Biorad and the annealing temperature 60 degrees. To determine the allelic melting condition for differentiation between the DA and E3 alleles of the three snps, the real-time polymerase chain reaction (PCR) products were subjected to the ramping of 0.1°C/s between 83°C and 94°C. All specimens were tested in triplicate, and their melting profiles analyzed using Biorad HRM melt software. Control homozygous DA, homozygous E3, and the DA/E3 heterozygous were used as standards for the melting-temperature curve classification. For the standards, each allelic-variant melt curve was set as a genotype, and the samples were classified as either DA or E3 according to their melt curve.
All Wistar rats used in this study were genotyped, and all of them had the Ncf1E3 allele, thus with a higher ROS response (data not shown).
Social isolation protocol
The social isolation procedure (37) was performed on male rats. At weaning (postnatal day 21), pups were separated from their mothers and reared either as isolated rats (ISO; one rat per cage) or in social groups [control rats (reared in group), GRP; three to four rats per cage]. To avoid a litter effect, each litter contributed only one subject to the GRP and one subject to the ISO. All animals were reared in Plexiglas cages (ISO: 48.0×27.0×20.0 cm; GRP: 59.0×38.5×20.0 cm). Animals exposed to 2 weeks of social isolation were 5 weeks old; animals exposed to 4 weeks of social isolation were 7 weeks old; and animals exposed to 7 weeks of social isolation were 10 weeks old. Animals were disturbed only for cleaning purposes, which consisted of changing the cage once a week for ISO and twice a week for GRP. Both ISO and GRP rats were housed in the same room, so that ISO rats maintained a visual, auditory, and olfactory contact with the other animals.
Blindness of the study
Researchers performing behavioral, histological, and biochemical analysis were blind with respect to the rearing and treatment conditions. Indeed, it was not possible to deduce from the labeling whether an animal was isolated or not. The social isolation procedure was performed in a dedicated part of the animal facility, not accessible to the investigators during the entire period of the social isolation protocol. The blinding of the data was maintained until the analysis was terminated.
Apocynin treatment
Apocynin treatment (5 mg/kg/day in drinking water) was applied to the control and isolated animals, as previously described (7, 40), from week 4 to week 7 of social isolation.
Acute restraint stress
Rats were transported to the experimental room in their home cages and allowed to adapt to this environment for at least 30 min. Animals were then submitted to a 60-min restraint period in a plastic cylindrical restraining tube (diameter 6.5 cm and length 15 cm). After restraint, the animals returned to their cages.
Measurement and quantification of markers of the HPA-axis
CRF
Rats were killed by decapitation, and the brain was immediately removed for hypothalamus dissection. Tissues were frozen and stored at −80°C until the analysis was performed. Hypothalamic CRF concentrations (ng/ml) were determined using a commercial ELISA kit (CRF Rat/Mouse Sensitive ELISA Kit; Biovendor).
Plasmatic ACTH and corticosterone
Blood was collected from the trunk of killed rats into heparinized tubes and then centrifuged at 10,000 g for 20 min at 4°C. The supernatants were removed and frozen at −80°C until the analysis was performed. Plasmatic ACTH concentrations (pg/ml) were determined using a commercial ELISA kit (ACTH Rat/Mouse Ultrasensitive lumELISA; Demeditec Diagnostics). Plasmatic corticosterone levels (nM) were determined using a commercial ELISA kit (Corticosterone ELISA kit; Abnova, DBA Italia S.r.l, and Corticosterone EIA kit; Alexis Biochemical, Vinci-Biochem)
Salivary corticosterone
Salivary samples were collected in plastic tubes after intraperitoneal injection of pilocarpin (5 mg/kg). The absence of blood contamination was checked using a salivary blood contamination kit (Salimetrics Europe Ltd.). Samples were stored at −80°C until they were assayed after a proper dilution (1:16) with an assay buffer.
The salivary corticosterone (pg/ml) levels were determined using a commercial ELISA kit (Corticosterone ELISA kit; Abnova, DBA Italia S.r.l, and Corticosterone EIA kit; Alexis Biochemical, Vinci-Biochem).
Measurement and quantification of markers of oxidative stress
Hypothalamic and adrenal 8OHdG (ng/ml) were determined using a commercial ELISA kit (Highly Sensitive 8-OHdG Check Elisa; JaiCa). The hypothalamic and adrenal nitrotyrosine protein expression levels were determined by Western blotting, as previously described (Schiavone et al.), using a rabbit polyclonal anti-nitrotyrosine antibody (1:100; Millipore) and α-actin (1:4000; Sigma-Aldrich). The antibody we used to detect nitrotyrosine protein revealed one major band at ∼67 KDa. Other very weak bands are present in the background of the blot. The optical density measurements refer only to the major band of the blot.
Optical densities of the bands were measured using ImageJ software (http://rsb.info.nih.gov/ij/) and normalized with α-actin.
Novel-object recognition test
Rats were exposed to two habituation sessions (intersession interval: 24 h) where they were allowed 5 min to explore the apparatus. Twenty-four h after the last habituation, two 3-min trials separated by a 1-min intertrial interval were carried out. In the first trial, rats were exposed to two identical objects (white glasses or light bulbs). During the second trial, rats were exposed to one familiar object and to a new, differently shaped object. Each object was placed at an equidistant position between the center and the wall of the arena. At the beginning of each trial, the rats were placed in the center of the arena with their heads oriented in the opposite direction to the objects. Exploration of the objects was defined as sniffing or touching the object with the nose. Turning around or sitting on the object was not considered as exploration. Exploratory activity was expressed as time (sec) of exploration. Objects and arena were carefully cleaned between each session to avoid confounding olfactory stimuli.
Immunohistochemistry
Immunohistochemical analyses were performed in the hypothalamus and in the adrenal glands as previously described (53, 56), using monoclonal and polyclonal antibodies against 8OhdG (1:10; JaICA), nitrotyrosine (1:100; Millipore), Neun (1:2000; Chemicon), Iba-1 (1:500; Wako), and GFAP (1: 2000; Millipore).
Quantifications of immunohistochemistry have been performed using Metamorph software (Molecular Devices) and expressed as the number of positive-stained cells per area analyzed. Concerning the 8OHdG and nitrotyrosine immunostaining experiments in the hypothalamus, the mean of the total number of cells per field was around 800. No differences were detected in the total number of cells per field among experimental groups (Supplementary Fig. S3A, B). The magnification used for the quantification was 20×, and the number of fields studied for the sample was four. No differences were found in the total cell numbers among analyzed fields per sample (data not shown). Concerning 8OHdG and nitrotyrosine immunostaining experiments in the adrenal glands, the mean of the total number of cells per field was around 600. No differences were detected in the total number of cells per field among experimental groups (data not shown). The magnification used for the quantification was 20×, and the number of fields studied for sample was two. No differences were found in the total cell numbers among the analyzed fields per sample (data not shown).
Confocal microscopy
An LSM 510 Meta confocal laser scanner mounted on an Axio Imager Z1 microscope (Carl Zeiss) was used for confocal microscopy as previously described (55). Negative controls consisting of the tissue incubated without primary antibodies were performed for each experiment (data not shown).
Real-time quantitative PCR
Total RNA from the hypothalamus and the adrenal glands was isolated using an RNeasy mini kit (Qiagen) according to the instructions of the manufacturer. The residual genomic DNA was removed using an RNase-Free DNase set (Qiagen). Total RNA (1 μg) was reverse transcribed using the superscript II kit according to the instructions of the manufacturer (Invitrogen). Real-time quantitative PCRs were performed using Power SYBR Green PCR master mix (Applied Biosystems) and a Chromo 4TM Real-Time system (Bio-Rad). Quantification was performed at a threshold detection line (Ct value). The Ct value for the target genes (NOX2) was normalized with the relative levels of Rps9 (ribosomal protein S9) and Tbp (TATA-box-binding protein) mRNAs used as housekeeping genes. Triplicates were performed for each condition. Results are expressed as fold increase. The sequence of the primers used has been previously described (56).
Statistical analysis
All statistical analyses were performed using SigmaStat® 3.1 and GraphPad® 5.0 for Windows. The statistical tests are indicated in the figure legends. For all tests, a p-value<0.05 was considered statistically significant. Results are expressed as means±standard error.
As comparative results were obtained for controls at 2, 4, and 7 weeks, data of these three experimental groups have been plotted and showed as a single group (GRP) to avoid redundancy in the graphs. Statistical analysis has been performed both on single or plotted data. In both cases, the same statistical significances have been obtained.
Supplementary Material
Abbreviations Used
- 8OhdG
8-hydroxy-2-deoxyguanosine
- ACTH
adrenocorticotropic hormone
- APCN
apocynin
- CNS
central nervous system
- CRF
corticotropin-releasing factor
- GABA
γ-aminobutyric acid
- GRP
control rats (reared in group)
- HPA-axis
hypothalamic-pituitary-adrenal axis
- ISO
isolated rats
- NCF1
neutrophil cytosolic factor 1
- NOX2
NADPH oxidase 2
- PCR
polymerase chain reaction
- ROS
reactive oxygen species
Acknowledgments
The authors were supported by the Swiss National Foundation (Grant No. 3200A0-103725), the Italian PRIN to LT for 2009 from MIUR, the Swedish Medical Research Council, the European Union Grants MASTERSWITCH (HEALTH-F2-2008-223404), and EURATRANS (HEALTH-F4-2010-241504).
Author Disclosure Statement
The authors declare no biomedical financial interests or potential conflicts of interest relevant to the subject matter of this work. KHK is the funding member of GenKyoTex, which develops NOX inhibitors.
References
- 1.Adams K. Pathophysiologic role of the renin-angiotensin-aldosterone and sympathetic nervous systems in heart failure. Am J Health Syst Pharm. 2004;61:S4–S13. doi: 10.1093/ajhp/61.suppl_2.S4. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed Z. Shaw G. Sharma VP. Yang C. McGowan E. Dickson DW. Actin-binding proteins coronin-1a and IBA-1 are effective microglial markers for immunohistochemistry. J Histochem Cytochem. 2007;55:687–700. doi: 10.1369/jhc.6A7156.2007. [DOI] [PubMed] [Google Scholar]
- 3.Ahs F. Furmark T. Michelgard A. Langstrom B. Appel L. Wolf OT. Kirschbaum C. Fredrikson M. Hypothalamic blood flow correlates positively with stress-induced cortisol levels in subjects with social anxiety disorder. Psychosom Med. 2006;68:859–862. doi: 10.1097/01.psy.0000242120.91030.d8. [DOI] [PubMed] [Google Scholar]
- 4.Albert NM. Yancy CW. Liang L. Zhao X. Hernandez AF. Peterson ED. Cannon CP. Fonarow GC. Use of aldosterone antagonists in heart failure. JAMA. 2009;302:1658–1665. doi: 10.1001/jama.2009.1493. [DOI] [PubMed] [Google Scholar]
- 5.Albrecht P. Lewerenz J. Dittmer S. Noack R. Maher P. Methner A. Mechanisms of oxidative glutamate toxicity: the glutamate/cystine antiporter system xc- as a neuroprotective drug target. CNS Neurol Disord Drug Targets. 2010;9:973–982. doi: 10.2174/187152710791292567. [DOI] [PubMed] [Google Scholar]
- 6.Aldieri E. Riganti C. Polimeni M. Gazzano E. Lussiana C. Campia I. Ghigo D. Classical inhibitors of NOX NAD(P)H oxidases are not specific. Curr Drug Metab. 2008;9:686–696. doi: 10.2174/138920008786049285. [DOI] [PubMed] [Google Scholar]
- 7.Alexa HV. Early life stress, the development of aggression and neuroendocrine and neurobiological correlates: what can we learn from animal models? Front Neuroendocrinol. 2009;30:497–518. doi: 10.1016/j.yfrne.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Altamura AC. Boin F. Maes M. HPA axis and cytokines dysregulation in schizophrenia: potential implications for the antipsychotic treatment. Eur Neuropsychopharmacol. 1999;10:1–4. doi: 10.1016/s0924-977x(99)00017-6. [DOI] [PubMed] [Google Scholar]
- 9.Asaba K. Iwasaki Y. Yoshida M. Asai M. Oiso Y. Murohara T. Hashimoto K. Attenuation by reactive oxygen species of glucocorticoid suppression on proopiomelanocortin gene expression in pituitary corticotroph cells. Endocrinology. 2004;145:39–42. doi: 10.1210/en.2003-0375. [DOI] [PubMed] [Google Scholar]
- 10.Bedard K. Krause K. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 11.Behrens M. Ali S. Dao D. Lucero J. Shekhtman G. Quick K. Dugan L. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318:1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- 12.Behrens M. Ali S. Dugan L. Interleukin-6 mediates the increase in NADPH-oxidase in the ketamine model of schizophrenia. J Neurosci. 2008;28:13957–13966. doi: 10.1523/JNEUROSCI.4457-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belvederi Murri M. Pariante CM. Dazzan P. Hepgul N. Papadopoulos AS. Zunszain P. Di Forti M. Murray RM. Mondelli V. Hypothalamic-pituitary-adrenal axis and clinical symptoms in first-episode psychosis. Psychoneuroendocrinology. 2012;37:629–644. doi: 10.1016/j.psyneuen.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Breen AP. Murphy JA. Reactions of oxyl radicals with DNA. Free Radic Biol Med. 1995;18:1033–1077. doi: 10.1016/0891-5849(94)00209-3. [DOI] [PubMed] [Google Scholar]
- 15.Bulut M. Savas HA. Altindag A. Virit O. Dalkilic A. Beneficial effects of N-acetylcysteine in treatment resistant schizophrenia. World J Biol Psychiatry. 2009;10:626–628. doi: 10.1080/15622970903144004. [DOI] [PubMed] [Google Scholar]
- 16.Carlson M. Earls F. Psychological and neuroendocrinological sequelae of early social deprivation in institutionalized children in Romania. Ann N Y Acad Sci. 1997;807:419–428. doi: 10.1111/j.1749-6632.1997.tb51936.x. [DOI] [PubMed] [Google Scholar]
- 17.Checkley S. The neuroendocrinology of depression and chronic stress. Br Med Bull. 1996;52:597–617. doi: 10.1093/oxfordjournals.bmb.a011570. [DOI] [PubMed] [Google Scholar]
- 18.Cohen SI. Cushing's syndrome: a psychiatric study of 29 patients. Br J Psychiatry. 1980;136:120–124. doi: 10.1192/bjp.136.2.120. [DOI] [PubMed] [Google Scholar]
- 19.Collip D. Nicolson NA. Lardinois M. Lataster T. van Os J. Myin-Germeys I. Daily cortisol, stress reactivity and psychotic experiences in individuals at above average genetic risk for psychosis. Psychol Med. 2011;41:2305–2315. doi: 10.1017/S0033291711000602. [DOI] [PubMed] [Google Scholar]
- 20.Connell BJ. Saleh TM. Co-administration of apocynin with lipoic acid enhances neuroprotection in a rat model of ischemia/reperfusion. Neurosci Lett. 2012;507:43–46. doi: 10.1016/j.neulet.2011.11.047. [DOI] [PubMed] [Google Scholar]
- 21.Corcoran CM. Smith C. McLaughlin D. Auther A. Malaspina D. Cornblatt B. HPA axis function and symptoms in adolescents at clinical high risk for schizophrenia. Schizophr Res. 2012;135:170–174. doi: 10.1016/j.schres.2011.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eng LF. Ghirnikar RS. GFAP and Astrogliosis. Brain Pathol. 1994;4:229–237. doi: 10.1111/j.1750-3639.1994.tb00838.x. [DOI] [PubMed] [Google Scholar]
- 23.Fone KCF. Porkess MV. Behavioural and neurochemical effects of post-weaning social isolation in rodents—relevance to developmental neuropsychiatric disorders. Neurosci Biobehav Rev. 2008;32:1087–1102. doi: 10.1016/j.neubiorev.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Greti A. HPA axis responsiveness to stress: implications for healthy aging. Exp Gerontol. 2011;46:90–95. doi: 10.1016/j.exger.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grippo AJ. Johnson AK. Stress, depression and cardiovascular dysregulation: A review of neurobiological mechanisms and the integration of research from preclinical disease models. Stress. 2009;12:1–21. doi: 10.1080/10253890802046281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gunnar M. Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27:199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- 27.Herrman H. Stewart D. Diaz-Granados N. Berger E. Jackson B. Yuen T. What is resilience? Can J Psychiatry. 2011;56:258–265. doi: 10.1177/070674371105600504. [DOI] [PubMed] [Google Scholar]
- 28.Hu X. Zhang D. Pang H. Caudle WM. Li Y. Gao H. Liu Y. Qian L. Wilson B. Di Monte DA. Ali SF. Zhang J. Block ML. Hong J-S. Macrophage antigen complex-1 mediates reactive microgliosis and progressive dopaminergic neurodegeneration in the MPTP model of Parkinson's disease. J Immunol. 2008;181:7194–7204. doi: 10.4049/jimmunol.181.10.7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hullin R. Jerram T. Lee M. Levell M. Tyrer S. Renin and aldosterone relationships in manic depressive psychosis. Br J Psychiatry. 1977;131:575–581. doi: 10.1192/bjp.131.6.575. [DOI] [PubMed] [Google Scholar]
- 30.Hultqvist M. Sareila O. Vilhardt F. Norin U. Olsson L. Olofsson P. Hellman U. Holmdahl R. Positioning of a polymorphic quantitative trait nucleotide in the Ncf1 gene controlling oxidative burst response and arthritis severity in rats. Antioxid Redox Signal. 2011;14:2373–2383. doi: 10.1089/ars.2010.3440. [DOI] [PubMed] [Google Scholar]
- 31.Johnson A. Grippo A. Sadness and broken hearts: neurohumoral mechanisms and co-morbidity of ischemic heart disease and psychological depression. J Physiol Pharmacol. 2006;57:5–29. [PubMed] [Google Scholar]
- 32.Kasal D. Schiffrin E. Angiotensin II, aldosterone, and anti-inflammatory lymphocytes: interplay and therapeutic opportunities. Int J Hypertens. 2012;2012:829786. doi: 10.1155/2012/829786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kehne JH. Cain CK. Therapeutic utility of non-peptidic CRF1 receptor antagonists in anxiety, depression, and stress-related disorders: evidence from animal models. Pharmacol Ther. 2010;128:460–487. doi: 10.1016/j.pharmthera.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klomp A. Koolschijn PC. Hulshoff Pol HE. Kahn RS. Van Haren NEM. Hypothalamus and pituitary volume in schizophrenia: a structural MRI study. Int J Neuropsychopharmacol. 2012;15:281–288. doi: 10.1017/S1461145711000794. [DOI] [PubMed] [Google Scholar]
- 35.Krebs M. Leopold K. Hinzpeter A. Schaefer M. Neuroprotective agents in schizophrenia and affective disorders. Expert Opin Pharmacother. 2006;7:837–848. doi: 10.1517/14656566.7.7.837. [DOI] [PubMed] [Google Scholar]
- 36.Kudielka BM. Buske-Kirschbaum A. Hellhammer DH. Kirschbaum C. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: impact of age and gender. Psychoneuroendocrinology. 2004;29:83–98. doi: 10.1016/s0306-4530(02)00146-4. [DOI] [PubMed] [Google Scholar]
- 37.Leng A. Feldon J. Ferger B. Long-term social isolation and medial prefrontal cortex: dopaminergic and cholinergic neurotransmission. Pharmacol Biochem Behavr. 2004;77:371–379. doi: 10.1016/j.pbb.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 38.Lennart W. The relationship between the pineal gland and the pituitary-adrenal axis in health, endocrine and psychiatric conditions. Psychoneuroendocrinology. 1983;8:75–80. doi: 10.1016/0306-4530(83)90042-2. [DOI] [PubMed] [Google Scholar]
- 39.Lieberman JA. Tollefson GD. Charles C. Zipursky R. Sharma T. Kahn RS. Keefe RSE. Green AI. Gur RE. McEvoy J. Perkins D. Hamer RM. Gu H. Tohen M HGDH Study Group. Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch Gen Psychiatry. 2005;62:361–370. doi: 10.1001/archpsyc.62.4.361. [DOI] [PubMed] [Google Scholar]
- 40.Lull M. Levesque S. Surace M. Block M. Chronic apocynin treatment attenuates beta amyloid plaque size and microglial number in hAPP(751)(SL) mice. PLoS One. 2011;6:e20153. doi: 10.1371/journal.pone.0020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meyza KZ. Boguszewski PM. Nikolaev E. Zagrodzka J. Age increases anxiety and reactivity of the fear/anxiety circuit in Lewis rats. Behav Brain Res. 2011;225:192–200. doi: 10.1016/j.bbr.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 42.Mico J. Rojas-Corrales M. Gibert-Rahola J. Parellada M. Moreno D. Fraguas D. Graell M. Gil J. Irazusta J. Castro-Fornieles J. Soutullo C. Arango C. Otero S. Navarro A. Baeza I. Martinez-Cengotitabengoa M. Gonzalez-Pinto A. Reduced antioxidant defense in early onset first-episode psychosis: a case-control study. BMC Psychiatry. 2011;11:26. doi: 10.1186/1471-244X-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morgane PJ. Mokler DJ. Galler JR. Effects of prenatal protein malnutrition on the hippocampal formation. Neurosci Biobehav Rev. 2002;26:471–483. doi: 10.1016/s0149-7634(02)00012-x. [DOI] [PubMed] [Google Scholar]
- 44.Mullen RJ. Buck CR. Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- 45.Myers B. McKlveen J. Herman J. Neural regulation of the stress response: the many faces of feedback. Cell Mol Neurobiol. 2012 doi: 10.1007/s10571-012-9801-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olofsson P. Holmberg J. Tordsson J. Lu S. Akerström B. Holmdahl R. Positional identification of Ncf1 as a gene that regulates arthritis severity in rats. Nat Genet. 2003;33:25–32. doi: 10.1038/ng1058. [DOI] [PubMed] [Google Scholar]
- 47.Olofsson P. Johansson Å. Wedekind D. Klöting I. Klinga-Levan K. Lu S. Holmdahl R. Inconsistent susceptibility to autoimmunity in inbred LEW rats is due to genetic crossbreeding involving segregation of the arthritis-regulating gene Ncf1. Genomics. 2004;83:765–771. doi: 10.1016/j.ygeno.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 48.Owe-Larsson B. Ekdahl K. Edbom T. Ösby U. Karlsson H. Lundberg C. Lundberg M. Increased plasma levels of thioredoxin-1 in patients with first episode psychosis and long-term schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry. 2011;35:1117–1121. doi: 10.1016/j.pnpbp.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 49.Queisser N. Schupp N. Aldosterone, oxidative stress, and NF-κB activation in hypertension-related cardiovascular and renal diseases. Free Radic Biol Med. 2012;53:314–327. doi: 10.1016/j.freeradbiomed.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 50.Roubos EW. Dahmen M. Kozicz Ts. Xu L. Leptin and the hypothalamo-pituitary-adrenal stress axis. Gen Comp Endocrinol. 2012;177:28–36. doi: 10.1016/j.ygcen.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 51.Sapolsky R. Armanini M. Packan D. Sutton S. Plotsky P. Glucocorticoid feedback inhibition of adrenocorticotropic hormone secretagogue release. Relationship to corticosteroid receptor occupancy in various limbic sites. Neuroendocrinology. 1990;51:328–336. doi: 10.1159/000125357. [DOI] [PubMed] [Google Scholar]
- 52.Schen C. When mothers leave their children behind. Harv Rev Psychiatry. 2005;13:233–243. doi: 10.1080/10673220500243380. [DOI] [PubMed] [Google Scholar]
- 53.Schiavone S. Sorce S. Dubois-Dauphin M. Jaquet V. Colaianna M. Zotti M. Cuomo V. Trabace L. Krause K. Involvement of NOX2 in the development of behavioral and pathologic alterations in isolated rats. Biol Psychiatry. 2009;66:384–392. doi: 10.1016/j.biopsych.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 54.Seckl. Prenatal glucocorticoids and long-term programming. Eur J Endocrinol. 2004;151:U49–U62. doi: 10.1530/eje.0.151u049. [DOI] [PubMed] [Google Scholar]
- 55.Sorce S. Krause K-H. NOX enzymes in the central nervous system: from signaling to disease. Antioxid Redox Signal. 2009;11:2481–2504. doi: 10.1089/ars.2009.2578. [DOI] [PubMed] [Google Scholar]
- 56.Sorce S. Schiavone S. Tucci P. Colaianna M. Jaquet V. Cuomo V. Dubois-Dauphin M. Trabace L. Krause K-H. The NADPH oxidase NOX2 controls glutamate release: a novel mechanism involved in psychosis-like ketamine responses. J Neurosci. 2010;30:11317–11325. doi: 10.1523/JNEUROSCI.1491-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stachowiak M. Sebbane R. Stricker EM. Zigmond MJ. Kaplan BB. Effect of chronic cold exposure on tyrosine hydroxylase mRNA in rat adrenal gland. Brain Res. 1985;359:356–359. doi: 10.1016/0006-8993(85)91450-7. [DOI] [PubMed] [Google Scholar]
- 58.Stefanska J. Pawliczak R. Apocynin: molecular aptitudes. Mediators Inflamm. 2008;2008:106507. doi: 10.1155/2008/106507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taliaz D. Loya A. Gersner R. Haramati S. Chen A. Zangen A. Resilience to chronic stress is mediated by hippocampal brain-derived neurotrophic factor. J Neurosci. 2011;31:4475–4483. doi: 10.1523/JNEUROSCI.5725-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tang LL YK. Yang XF. Zheng JS. Apocynin attenuates cerebral infarction after transient focal ischaemia in rats. J Int Med Res. 2007;35:517–522. doi: 10.1177/147323000703500411. [DOI] [PubMed] [Google Scholar]
- 61.Tang XN. Cairns B. Cairns N. Yenari MA. Apocynin improves outcome in experimental stroke with a narrow dose range. Neuroscience. 2008;154:556–562. doi: 10.1016/j.neuroscience.2008.03.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tarullo AR. Gunnar MR. Child maltreatment and the developing HPA axis. Horm Behav. 2006;50:632–639. doi: 10.1016/j.yhbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 63.Ueta Y. Dayanithi G. Fujihara H. Hypothalamic vasopressin response to stress and various physiological stimuli: visualization in transgenic animal models. Horm Behav. 2011;59:221–226. doi: 10.1016/j.yhbeh.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 64.Ulrich-Lai YM. Figueiredo HF. Ostrander MM. Choi DC. Engeland WC. Herman JP. Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. Am J Physiol—Endocrinol Metab. 2006;291:E965–E973. doi: 10.1152/ajpendo.00070.2006. [DOI] [PubMed] [Google Scholar]
- 65.van Winkel R. Stefanis NC. Myin-Germeys I. Psychosocial stress and psychosis. a review of the neurobiological mechanisms and the evidence for gene-stress interaction. Schizophr Bull. 2008;34:1095–1105. doi: 10.1093/schbul/sbn101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vilhardt F. Microglia: phagocyte and glia cell. Int J Biochem Cell Biol. 2005;37:17–21. doi: 10.1016/j.biocel.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 67.Wang Q. Tompkins KD. Simonyi A. Korthuis RJ. Sun AY. Sun GY. Apocynin protects against global cerebral ischemia-reperfusion-induced oxidative stress and injury in the gerbil hippocampus. Brain Res. 2006;1090:182–189. doi: 10.1016/j.brainres.2006.03.060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.