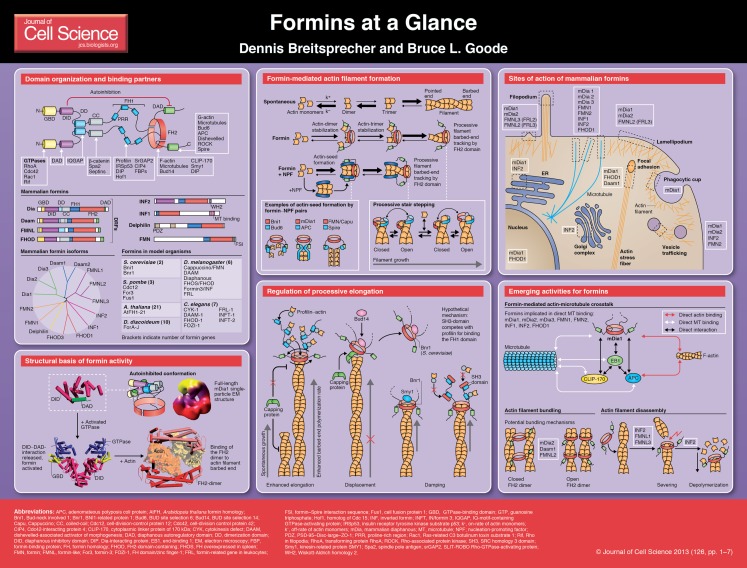
Formins are conserved actin polymerization machines that have instrumental roles in controlling rearrangements of the actin cytoskeleton and have recently been shown to directly regulate microtubule dynamics. Here, and on the accompanying poster, we aim to organize a rapidly expanding body of literature on this diverse protein family, summarizing the common properties that apply to most formins, and highlighting recent advances in understanding formin structure, mechanism, activity and regulation at the molecular and cellular levels.
Formins were first identified in flies, mice and yeast as genes that, when mutated, cause severe defects in cytokinesis, polarity, and cell and tissue morphogenesis (Mass et al., 1990; Jackson-Grusby et al., 1992; Castrillon and Wasserman, 1994; Kohno et al., 1996). Subsequent studies in budding yeast Saccharomyces cerevisiae revealed that formins directly nucleate the assembly of actin filaments, and that these activities are essential in vivo for the assembly of actin cables that direct polarized cell growth (Evangelista et al., 2002; Pruyne et al., 2002; Sagot et al., 2002a; Sagot et al., 2002b). Since then, members of this large protein family (encoded by 15 genes in mammals, two in S. cerevisiae, three in Saccharomyces pombe, and six in Drosophila melangastor) have been demonstrated to have crucial roles in an increasingly wide range of cytoskeleton-based processes (as illustrated in the Poster).
Cellular functions of formins
In yeast, formins have an essential role in the assembly of cytokinetic rings and cables that direct intracellular transport (Chang, 1999; Feierbach and Chang, 2001; Evangelista et al., 2002; Dong et al., 2003; Cheung and Wu, 2004; Ingouff et al., 2005; Cheung et al., 2010). In animal cells, formins are required for the assembly of filopodia (Pellegrin and Mellor, 2005; Schirenbeck et al., 2005; Yang et al., 2007; Block et al., 2008; Matusek et al., 2008; Harris et al., 2010), lamellipodia (Yang et al., 2007; Sarmiento et al., 2008; Block et al., 2012), stress fibers (Ishizaki et al., 2001; Satoh and Tominaga, 2001; Gasteier et al., 2003; Peng et al., 2003; Hotulainen and Lappalainen, 2006; Sato et al., 2006; Takeya et al., 2008), cytoplasmic actin networks used for long-range vesicle transport (Leader et al., 2002; Azoury et al., 2008; Li et al., 2008; Pfender et al., 2011; Schuh, 2011), cytokinetic actin rings (Severson et al., 2002; Watanabe et al., 2008) and phagocytic cups (Brandt et al., 2007). Formins also have essential roles in physiological processes ranging from cell motility in the immune system (Yayoshi-Yamamoto et al., 2000; Eisenmann et al., 2007; Shi et al., 2009), to gastrulation and neural tube closure (Habas et al., 2001; Sato et al., 2006; Lai et al., 2008), heart morphogenesis (Iskratsch et al., 2010; Li et al., 2011), kidney morphogenesis (Brown et al., 2010; Boyer et al., 2011a; Boyer et al., 2011b), and dendritic spine formation in neurons. Some of these functions depend on the actin nucleation and elongation activities of formins (Bartolini et al., 2008; Andrés-Delgado et al., 2010; Madrid et al., 2010; Andrés-Delgado et al., 2012; Ramabhadran et al., 2012; Stastna et al., 2012), which have been demonstrated by investigating point mutations that impair in these activities (Xu et al., 2004; Lu et al., 2007; Ramabhadran et al., 2012). However, for many of the other in vivo functions of formins, it has not yet been determined which of their activities are required. Moreover, some formins exhibit activities beyond actin assembly, e.g. actin bundling, severing, depolymerization and microtubule (MT) binding (see below). Thus, an important future challenge is to develop new mutants as tools that are capable of separately disrupting each formin activity.
Localization and activation of formins
Formins are recruited and activated at different sites in cells, where they perform their diverse roles in cytoskeletal reorganization. Of the 15 vertebrate formins, the largest subset are Diaphanous-related formins (DRFs), which have an N-terminal GTPase-binding domain (GBD), an adjacent DID (Diaphanous inhibitory domain) and a C-terminal DAD (Diaphanous autoregulatory domain) (see Poster) (Alberts, 2001; Li and Higgs, 2003; Otomo et al., 2005a; Rose et al., 2005; Otomo et al., 2010). DRFs are autoinhibited through DID–DAD interactions. Recent crystallographic and single-particle electron microscopy studies show that, in the autoinhibited conformation, the N-terminus physically obstructs the ability of the C-terminus to polymerize actin (Nezami et al., 2010; Otomo et al., 2010; Maiti et al., 2012). Binding of active Rho-GTPases to GBD activates the formin by releasing these DID–DAD interactions. However, activation is noticeably incomplete (Li and Higgs, 2003; Maiti et al., 2012), suggesting that other factors are required to fully activate formins.
A variety of Rho-GTPases recruit DRFs to different locations in the cell for localized actin assembly (Evangelista et al., 1997; Watanabe et al., 1997; Ishizaki et al., 2001; Nakano et al., 2002; Tolliday et al., 2002; Pellegrin and Mellor, 2005; Seth et al., 2006; Martin et al., 2007; Block et al., 2012) (see Poster), yet additional factors can also regulate formins. For example, the S. cerevisiae formin Bnr1 and S. pombe formin Cdc12 each harbor at least two separate localization sequences that independently target the formin in vivo (Gao et al., 2010), suggesting that a combination of cues and binding partners control formin recruitment. Indeed, one of the sequences in Bnr1 was shown to interact with a septin-associated kinase that controls Bnr1 function (Buttery et al., 2012). In addition, the S. cerevisiae DRF Bni1 is phosphorylated at its N- and C-termini by Prk1 kinase, which facilitates its release from autoinhibition (Wang et al., 2009). In mammals, the formin protein diaphanous homolog 2 (DIAPH2, hereafter referred to as mDia3) is phosphorylated by Aurora B kinase (Cheng et al., 2011), the formin homology domain proteins 1 and 3 (FHOD1 and FHOD3, respectively) are phosphorylated by cGMP-dependent protein kinase 1 (PRKG1) and casein kinase 2 subunit α (CSNK2A1, also known as CK2) (Hannemann et al., 2008; Iskratsch et al., 2010; Iskratsch et al., 2012), and mDia2 and FHOD1 are activated through phosphorylation by Rho-associated protein kinase (ROCK) (Takeya et al., 2008; Staus et al., 2011). Inverted formin-2 (INF2) and formin-like protein 2 (FMNL2, also known as FRL3) are also farnesylated and myristoylated, respectively, which promotes their membrane targeting (Chhabra et al., 2009; Block et al., 2012). Moreover, mDia1 and mDia2, as well as the plant formins AFH1, formin1 and class II formin, directly bind phospholipid membranes (Cheung et al., 2010; Ramalingam et al., 2010; Gorelik et al., 2011; Martinière et al., 2011; van Gisbergen et al., 2012). These observations show that localization and activation of formins depend on their diverse interactions and that localization and activation, in some cases, serve as convergent inputs from multiple signalling pathways.
Biochemical activities of formins
Formins are large, dimeric multi-domain proteins with a modular design (see poster). Their signature features are the C-terminal formin homology 1 and 2 domains (FH1 and FH2, respectively) (Chesarone et al., 2010; Schönichen and Geyer, 2010). The only known exception is Dictyostelium ForC, which lacks an FH1 domain (Rivero et al., 2005). Most formins also have C-terminal tail regions that, sometimes, include DAD domains and/or Wiskott-Aldrich syndrome homology region 2 (WH2)-like domains. In comparison, the N-terminal halves of formins are more variable and have main roles in directing their localization.
Most purified formins exhibit the following three activities: (1) nucleation of actin assembly, (2) processive movement on growing barbed ends of actin filaments while protecting them from capping proteins and (3) profilin-dependent acceleration of actin filament elongation. Additional activities in different subsets of formins include MT binding (see below) and actin filament bundling, severing and/or depolymerization (Harris et al., 2004; Michelot et al., 2005; Moseley and Goode, 2005; Chhabra and Higgs, 2006; Harris and Higgs, 2006; Esue et al., 2008; Barkó et al., 2010; Harris et al., 2010; Machaidze et al., 2010; Scott et al., 2011; Skillman et al., 2012). All known interactions of formins with actin and MTs are mediated by the FH1 and FH2 domains and/or the tail regions. The FH1 domain is predicted to be extended and unstructured, and contains multiple proline-rich motifs that recruit complexes that consist of actin monomers and profilin, a small abundant protein that binds the majority of ATP–actin monomers in cells (Chang et al., 1997; Imamura et al., 1997; Sagot et al., 2002b; Kovar et al., 2003; Kovar et al., 2005). The FH2 domain forms a ring-shaped anti-parallel dimer, in which the two halves are held together by interactions of ‘lasso’ and ‘post’ segments, generating a flexibly tethered dimer (Xu et al., 2004). FH2 dimers bind with high affinity to the barbed ends of actin filaments (Pruyne et al., 2002; Kovar et al., 2003; Zigmond, 2004), and each functional half of the dimer contains two different actin-binding sites; one marked by a conserved isoleucine residue (I1431 in Bni1) and the other by a conserved lysine residue (K1601 in Bni1) (Xu et al., 2004; Otomo et al., 2005b; Lu et al., 2007). Mutation of these residues (e.g. in Bni1, mDia1, mDia2, FRL2, Daam1, INF2) strongly reduces actin nucleation and elongation activities of the respective formins and compromises actin assembly in vivo (Shimada et al., 2004; Xu et al., 2004; Lu et al., 2007; Bartolini et al., 2008; Harris et al., 2010; Ramabhadran et al., 2012).
Mechanism of actin nucleation
The precise mechanism by which formins nucleate the assembly of actin filaments is still being worked out. Initially, it was proposed that formins nucleate actin filaments by capturing and stabilizing spontaneously formed actin dimers and trimers (Pring et al., 2003) (see Poster), on the basis that the FH2 domain alone is sufficient for nucleation in vitro but lacks detectable binding affinity for actin monomers. Subsequently, however, it was shown that FH2-domain-mediated nucleation is very inefficient when using profilin-bound actin monomers, the predominant substrate that is available for actin polymerization in cells (Chesarone et al., 2010). More recent studies have shown that the C-terminal tail regions of formins bind actin monomers and enhance nucleation in the presence of profilin, which might explain how formins nucleate actin assembly in vivo (Gould et al., 2011; Heimsath and Higgs, 2012). In addition, the tail regions of formins can interact with other factors that bind actin monomers with high affinity and promote nucleation (see below). Thus, the interplay between formins and nucleation co-factors is a powerful means by which both, tight control and robust stimulation of actin nucleation, can be achieved in vivo (Blanchoin and Michelot, 2012). Moreover, interactions between FH1 and the profilin-actin complex might contribute to nucleation (Paul and Pollard, 2008). Thus, nucleation triggered through formins in the presence of profilin may involve contributions by their FH1 and FH2 domains, and the tail regions.
Although most formins promote actin nucleation and elongation, the strength of these activities can vary drastically. For example, S. pombe Cdc12 is a potent nucleator with a nucleation efficiency of over 50% (every second formin dimer nucleates a filament) (Neidt et al., 2008), whereas other formins, such as Daam1, FMNL3 (also known as FRL2) and FMNL2</emph> have nucleation efficiencies of below 1% (Vaillant et al., 2008; Block et al., 2012). These differences may reflect diverse in vivo requirements, e.g. the need for a slower and more controlled nucleation in some instances, or the dedication of a formin to actin filament elongation rather than nucleation. Indeed, one study has proposed that the primary role of FMNL2 at the leading edge of the cell is to capture free actin filament barbed ends that are nucleated by the Arp2/3 complex and elongate those filaments to drive filopodial and lamellipodial extension (Block et al., 2012). The weaker nucleation by some formins that has been observed in vitro might also reflect their stronger dependence on co-factors or nucleation promoting factors (NPFs) in vivo.
Formin-interacting NPFs are believed to help formins overcome the barrier that profilin constitutes to actin nucleation. Profilin suppresses the self-association of actin into dimers and trimers, and reduces the efficiency of formin nucleation (Neidt et al., 2008; Paul and Pollard, 2008; Scott et al., 2011). However, NPFs effectively compete with profilin for actin monomer binding, and organize mutiple actin monomers in proximity to the formin FH2 domain. Such NPF-formin pairs include Bud6–Bni1, Spire–FMN (Spir and Cappuccino in Drosophila) and adenomateous polyposis coli protein (APC)–mDia1 (Moseley et al., 2004; Quinlan et al., 2007; Webb et al., 2009; Okada et al., 2010; Graziano et al., 2011; Tu et al., 2012). In vivo, Bni1–Bud6 and APC–mDia1 function together to assemble actin cables and pseudocleavage furrows, respectively, and have also been shown to directly interact in vitro to assemble actin in the presence of profilin and/or capping protein (Moseley et al., 2004; Okada et al., 2010; Graziano et al., 2011; Breitsprecher et al., 2012). Spire and FMN co-function in vivo to assemble cytoplasmic actin meshworks (Schumacher et al., 2004; Pfender et al., 2011; Schuh, 2011) but, perplexingly, Spire inhibits rather than enhances the nucleation activity of FMN in vitro (Quinlan et al., 2007; Vizcarra et al., 2011; Zeth et al., 2011), suggesting that additional factors are required to activate collaborative actin assembly through Spire–FMN. More recently, we have begun to address the question of how NPF–formin pairs co-assemble actin filaments by using triple-color total internal reflection fluorescence (TIRF) microscopy at the single-molecule level (Breitsprecher et al., 2012). This study revealed that, during the early phases of nucleation, mDia1 and APC molecules associate, with APC being mainly responsible for recruiting actin monomers. Subsequently, upon actin polymerization, APC and mDia1 separate, with APC remaining at the nucleation site and mDia1 moving processively along the growing barbed end, where it protects the filament from capping protein (Breitsprecher et al., 2012). An important next goal will be to determine whether other NPF–formin pairs use similar or distinct mechanisms.
Mechanisms of actin filament elongation
Once an actin filament is nucleated, the dimeric FH2 domain processively tracks the growing barbed end, which permits the addition of tens of thousands of actin subunits before it finally dissociates. The tracking mechanism is thought to involve transient, alternating contacts of the two halves of the FH2 domain dimer with the two terminal actin subunits of the filament. During these movements, the FH2 dimer is believed to switch between an ‘open state’ that allows actin monomer addition and a capped or ‘closed state’ that does not (see Poster) (Zigmond et al., 2003; Kozlov and Bershadsky, 2004; Moseley et al., 2004; Romero et al., 2004; Xu et al., 2004; Otomo et al., 2005b; Kovar et al., 2006; Vavylonis et al., 2006; Paul and Pollard, 2009a). The fraction of time a formin spends in the open versus the closed state is its ‘gating factor’ (Paul and Pollard, 2009a) and ranges from almost 0 (capped) for S. pombe Cdc12 to nearly 1 (uninhibited elongation) for mDia1 and Caenorhabditis elegans CYK-1 (Kovar et al., 2006; Neidt et al., 2008). Owing to differences in gating factors, different formins slow filament elongation and depolymerization to variable degrees in the absence of profilin (Kovar et al., 2003; Harris et al., 2004; Kovar et al., 2006; Neidt et al., 2008; Neidt et al., 2009; Block et al., 2012).
In the presence of profilin, however, the FH1 domains recruit profilin–actin complexes and accelerate the addition of actin monomers to the FH2-capped barbed end of up to tenfold over the rate of elongation at free barbed ends (Kovar et al., 2003; Romero et al., 2004; Kovar et al., 2006; Paul and Pollard, 2008; Paul and Pollard, 2009b; Vidali et al., 2009) (see Poster). How actin subunits are delivered from the FH1 to the FH2 domains is not fully understood, but might involve direct interactions between FH2– and FH1–profilin–actin (Ezezika et al., 2009; Neidt et al., 2009). Furthermore, the profilin-binding sites in the FH1 domain are arranged in a specific order, with low-affinity sites nearest to the FH2 domain and high-affinity sites more distal from it (Courtemanche and Pollard, 2012), possibly to optimize actin transfer to the barbed end. Importantly, it has been shown that formin processivity depends neither on profilin nor on the hydrolysis of ATP on newly added actin subunits (Kovar et al., 2006; Michelot et al., 2007; Paul and Pollard, 2009b; Ramalingam et al., 2010; Mizuno et al., 2011; Breitsprecher et al., 2012). Thus, the release of free energy, which accompanies the addition of actin subunits at the barbed end of the filament seems to be sufficient to drive formin FH2 processive movement.
Owing to the helical pitch of the actin filament, each step taken by an untethered formin on the barbed end is accompanied by a rotation of 14 degrees. At in vivo rates of actin polymerization, this would cause a formin to ‘spin’ at over 1000 rpm, which may be difficult to achieve for membrane-bound, immobilized formins. This problem has been referred to as the ‘rotation paradox’, and was initially addressed using single-filament TIRF microscopy and passively immobilized formins, which led to the conclusion that formins do not faithfully rotate during filament elongation, suggesting that they, instead, slip around the barbed end to release torsional stress (Shemesh et al., 2005). However, this model has recently been challenged in a different study, which employed single-molecule fluorescence polarization and in which helical rotations of filaments were observed that are elongated by tightly immobilized formins (Mizuno et al., 2011). Thus, further experimental studies are needed to determine whether formins are capable of either rotating or slipping depending on the forces or constraints that are applied.
Regulation of formin processivity
In vitro, formins remain processively attached to growing ends of filaments for minutes without dissociating, and produce actin polymers that are over 50 µm long (Kovar et al., 2006; Neidt et al., 2008; Breitsprecher et al., 2012). These properties suggest that, in vivo, there is a need for regulatory mechanisms to limit the duration and/or rate of formin processivity because actin filaments in cells are typically no longer than 1 µm (Pollard and Borisy, 2003). To date, two such mechanisms have been described for S. cerevisiae Bnr1: (1) Bud14 binds to its FH2 domain, which catalyzes Bnr1 displacement from filament ends (Chesarone et al., 2009); (2) the myosin-passenger protein Smy1, which also binds to the Bnr1 FH2 domain, interferes with actin filament elongation, slowing it down to half the normal speed (Chesarone-Cataldo et al., 2011) (see Poster). In vivo, both Bud14 and Smy1 are required to prevent the overgrowth of actin cables; loss of both proteins at the same time results in even more-severe defects of cable organization and cell growth. In addition, capping protein itself might competitively displace formins from filament ends, as suggested by its genetic interactions with Bud14 in S. cerevisiae (Chesarone et al., 2009) and its antagonistic interactions with mDia1 in mammalian cells (Bartolini et al., 2012). Another potential group of formin regulators are FH1-domain ligands, for example the Slit–Robo GTPase-activating proteins (srGAPs) (Mason et al., 2011), which might interfere with the binding of profiling–actin complexes to slow or arrest elongation (see Poster). Moreover, the Arp2/3-activating WAVE complex has been shown to directly inhibit mDia2, possibly as part of a regulatory circuit that balances the activity of the WAVE–Arp2/3 complex in lamellipodia formation with that of mDia2 in extending filopodia (Beli et al., 2008).
Regulation of microtubule dynamics
In vivo observations have long suggested that formins also regulate MT organization and dynamics (Kikyo et al., 1999; Lee et al., 1999; Chang, 2000; Ishizaki et al., 2001; Kato et al., 2001; Leader et al., 2002; Deeks et al., 2010; Li et al., 2010). Expression of constitutively active mDia constructs results in co-alignment of MTs and actin fibers (Ishizaki et al., 2001), promotes MT targeting to the cell periphery (Yamana et al., 2006; Goulimari et al., 2008; Zaoui et al., 2010) and stabilizes MTs (Palazzo et al., 2001; Wen et al., 2004; Eng et al., 2006; Bartolini et al., 2008). mDia mutants that fail to polymerize actin still bind to and stabilize MTs in vitro and in vivo (Bartolini et al., 2008). Furthermore, mDia3 mediates MT attachment to kinetochores independent of actin polymerization (Yasuda et al., 2004; Cheng et al., 2011), and INF2 promotes MT stabilization to reorient centrosomes (Andrés-Delgado et al., 2012). Furthermore, inverted formin 1 (INF1) colocalizes with MTs and promotes their bundling and acetylation in vivo, consistent with a role in stabilizing MT arrays (Young et al., 2008). More recently, it was shown that MT acetylation in cells is induced upon overexpression of each of ten different mammalian formins (Thurston et al., 2012). Thus, formins appear to stabilize MTs both through their direct binding (Bartolini et al., 2008; Gaillard et al., 2011) and/or by altering the post-translational state of MTs (Wen et al., 2004; Bartolini et al., 2008; Thurston et al., 2012).
Biochemical studies have begun to define the molecular interactions of the formins mDia1, mDia2 and INF2 with MTs (Bartolini et al., 2008; Gaillard et al., 2011). These studies have revealed surprising differences in how formin domains interact with MTs – as well as differences in their binding affinities and stoichiometries – suggesting that these formins have distinct roles in regulating MTs. In addition, these studies have shown that the presence of MTs inhibits the actin assembly activities of mDia1 and mDia2 but not of INF2 (Gaillard et al., 2011), suggesting that some formins can coordinate their effects on MTs and actin filaments. This view is supported by the interaction of formins with the MT plus-end tracking proteins (+TIPs) APC and EB1, and the cytoplasmic linker protein 170 (CLIP-170; tea1p in yeast) (Feierbach et al., 2004; Wen et al., 2004; Bartolini et al., 2008; Lewkowicz et al., 2008; Cheng et al., 2011), thereby representing an area ripe for future investigation at both biochemical and cellular levels.
Conclusions and perspectives
Ten years of genetic and biochemical characterization has demonstrated that formins in different species directly catalyze actin filament nucleation and elongation, but that the strength of their activities vary greatly, as do their in vivo binding partners, localization and regulation. Furthermore, many formins have additional activities (e.g. actin filament depolymerization, severing and bundling, and MT stabilization and bundling) that are still not well understood and deserve more attention (see Poster). For these reasons, it is crucial to characterize the properties of each formin individually before drawing conclusions about its cellular roles. Moreover, it is important to consider formin activities in the context of their ligands, which can transform their activities in a variety of ways.
Supplementary Material
Acknowledgments
We thank J. Eskin, B. Graziano, R. Jaiswal, A. Rodal and C. Ydenberg for critical reading.
Footnotes
Funding
This work was supported by the Deutsche Forschungsgemeinschaft [grant number BR 4116/1-1] to D.B. and the National Institutes of Health [grant number GM083137] to B.G. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.107250/-/DC1
References
- Alberts A. S. (2001). Identification of a carboxyl-terminal diaphanous-related formin homology protein autoregulatory domain. J. Biol. Chem. 276, 2824–2830 10.1074/jbc.M006205200 [DOI] [PubMed] [Google Scholar]
- Andrés–Delgado L., Antón O. M., Madrid R., Byrne J. A., Alonso M. A. (2010). Formin INF2 regulates MAL-mediated transport of Lck to the plasma membrane of human T lymphocytes. Blood 116, 5919–5929 10.1182/blood-2010-08-300665 [DOI] [PubMed] [Google Scholar]
- Andrés–Delgado L., Antón O. M., Bartolini F., Ruiz–Sáenz A., Correas I., Gundersen G. G., Alonso M. A. (2012). INF2 promotes the formation of detyrosinated microtubules necessary for centrosome reorientation in T cells. J. Cell Biol. 198, 1025–1037 10.1083/jcb.201202137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azoury J., Lee K. W., Georget V., Rassinier P., Leader B., Verlhac M. H. (2008). Spindle positioning in mouse oocytes relies on a dynamic meshwork of actin filaments. Curr. Biol. 18, 1514–1519 10.1016/j.cub.2008.08.044 [DOI] [PubMed] [Google Scholar]
- Barkó S., Bugyi B., Carlier M. F., Gombos R., Matusek T., Mihály J., Nyitrai M. (2010). Characterization of the biochemical properties and biological function of the formin homology domains of Drosophila DAAM. J. Biol. Chem. 285, 13154–13169 10.1074/jbc.M109.093914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolini F., Moseley J. B., Schmoranzer J., Cassimeris L., Goode B. L., Gundersen G. G. (2008). The formin mDia2 stabilizes microtubules independently of its actin nucleation activity. J. Cell Biol. 181, 523–536 10.1083/jcb.200709029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolini F., Ramalingam N., Gundersen G. G. (2012). Actin-capping protein promotes microtubule stability by antagonizing the actin activity of mDia1. Mol. Biol. Cell 23, 4032–4040 10.1091/mbc.E12-05-0338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beli P., Mascheroni D., Xu D., Innocenti M. (2008). WAVE and Arp2/3 jointly inhibit filopodium formation by entering into a complex with mDia2. Nat. Cell Biol. 10, 849–857 10.1038/ncb1745 [DOI] [PubMed] [Google Scholar]
- Blanchoin L., Michelot A. (2012). Actin cytoskeleton: a team effort during actin assembly. Curr. Biol. 22, R643–R645 10.1016/j.cub.2012.07.026 [DOI] [PubMed] [Google Scholar]
- Block J., Stradal T. E., Hänisch J., Geffers R., Köstler S. A., Urban E., Small J. V., Rottner K., Faix J. (2008). Filopodia formation induced by active mDia2/Drf3. J. Microsc. 231, 506–517 10.1111/j.1365-2818.2008.02063.x [DOI] [PubMed] [Google Scholar]
- Block J., Breitsprecher D., Kühn S., Winterhoff M., Kage F., Geffers R., Duwe P., Rohn J. L., Baum B., Brakebusch C.et al. (2012). FMNL2 drives actin-based protrusion and migration downstream of Cdc42. Curr. Biol. 22, 1005–1012 10.1016/j.cub.2012.03.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer O., Benoit G., Gribouval O., Nevo F., Tête M. J., Dantal J., Gilbert–Dussardier B., Touchard G., Karras A., Presne C.et al. (2011a). Mutations in INF2 are a major cause of autosomal dominant focal segmental glomerulosclerosis. J. Am. Soc. Nephrol. 22, 239–245 10.1681/ASN.2010050518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer O., Nevo F., Plaisier E., Funalot B., Gribouval O., Benoit G., Cong E. H., Arrondel C., Tête M. J., Montjean R.et al. (2011b). INF2 mutations in Charcot-Marie-Tooth disease with glomerulopathy. N. Engl. J. Med. 365, 2377–2388 10.1056/NEJMoa1109122 [DOI] [PubMed] [Google Scholar]
- Brandt D. T., Marion S., Griffiths G., Watanabe T., Kaibuchi K., Grosse R. (2007). Dia1 and IQGAP1 interact in cell migration and phagocytic cup formation. J. Cell Biol. 178, 193–200 10.1083/jcb.200612071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitsprecher D., Jaiswal R., Bombardier J. P., Gould C. J., Gelles J., Goode B. L. (2012). Rocket launcher mechanism of collaborative actin assembly defined by single-molecule imaging. Science 336, 1164–1168 10.1126/science.1218062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. J., Schlöndorff J. S., Becker D. J., Tsukaguchi H., Tonna S. J., Uscinski A. L., Higgs H. N., Henderson J. M., Pollak M. R. (2010). Mutations in the formin gene INF2 cause focal segmental glomerulosclerosis. Nat. Genet. 42, 72–76 10.1038/ng.505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttery S. M., Kono K., Stokasimov E., Pellman D. (2012). Regulation of the formin Bnr1 by septins and a MARK/Par1-family septin-associated kinase. Mol. Biol. Cell. 23, 4041–4053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrillon D. H., Wasserman S. A. (1994). Diaphanous is required for cytokinesis in Drosophila and shares domains of similarity with the products of the limb deformity gene. Development 120, 3367–3377 [DOI] [PubMed] [Google Scholar]
- Chang F. (1999). Movement of a cytokinesis factor cdc12p to the site of cell division. Curr. Biol. 9, 849–852 10.1016/S0960-9822(99)80372-8 [DOI] [PubMed] [Google Scholar]
- Chang F. (2000). Microtubule and actin-dependent movement of the formin cdc12p in fission yeast. Microsc. Res. Tech. 49, 161–167 [DOI] [PubMed] [Google Scholar]
- Chang F., Drubin D., Nurse P. (1997). cdc12p, a protein required for cytokinesis in fission yeast, is a component of the cell division ring and interacts with profilin. J. Cell Biol. 137, 169–182 10.1083/jcb.137.1.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L., Zhang J., Ahmad S., Rozier L., Yu H., Deng H., Mao Y. (2011). Aurora B regulates formin mDia3 in achieving metaphase chromosome alignment. Dev. Cell 20, 342–352 10.1016/j.devcel.2011.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesarone M., Gould C. J., Moseley J. B., Goode B. L. (2009). Displacement of formins from growing barbed ends by bud14 is critical for actin cable architecture and function. Dev. Cell 16, 292–302 10.1016/j.devcel.2008.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesarone M. A., DuPage A. G., Goode B. L. (2010). Unleashing formins to remodel the actin and microtubule cytoskeletons. Nat. Rev. Mol. Cell Biol. 11, 62–74 10.1038/nrm2816 [DOI] [PubMed] [Google Scholar]
- Chesarone–Cataldo M., Guérin C., Yu J. H., Wedlich–Soldner R., Blanchoin L., Goode B. L. (2011). The myosin passenger protein Smy1 controls actin cable structure and dynamics by acting as a formin damper. Dev. Cell 21, 217–230 10.1016/j.devcel.2011.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung A. Y., Wu H. M. (2004). Overexpression of an Arabidopsis formin stimulates supernumerary actin cable formation from pollen tube cell membrane. Plant Cell 16, 257–269 10.1105/tpc.016550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung A. Y., Niroomand S., Zou Y., Wu H. M. (2010). A transmembrane formin nucleates subapical actin assembly and controls tip-focused growth in pollen tubes. Proc. Natl. Acad. Sci. USA 107, 16390–16395 10.1073/pnas.1008527107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhabra E. S., Higgs H. N. (2006). INF2 Is a WASP homology 2 motif-containing formin that severs actin filaments and accelerates both polymerization and depolymerization. J. Biol. Chem. 281, 26754–26767 10.1074/jbc.M604666200 [DOI] [PubMed] [Google Scholar]
- Chhabra E. S., Ramabhadran V., Gerber S. A., Higgs H. N. (2009). INF2 is an endoplasmic reticulum-associated formin protein. J. Cell Sci. 122, 1430–1440 10.1242/jcs.040691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtemanche N., Pollard T. D. (2012). Determinants of Formin Homology 1 (FH1) domain function in actin filament elongation by formins. J. Biol. Chem. 287, 7812–7820 10.1074/jbc.M111.322958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks M. J., Fendrych M., Smertenko A., Bell K. S., Oparka K., Cvrcková F., Zársky V., Hussey P. J. (2010). The plant formin AtFH4 interacts with both actin and microtubules, and contains a newly identified microtubule-binding domain. J. Cell Sci. 123, 1209–1215 10.1242/jcs.065557 [DOI] [PubMed] [Google Scholar]
- Dong Y., Pruyne D., Bretscher A. (2003). Formin-dependent actin assembly is regulated by distinct modes of Rho signaling in yeast. J. Cell Biol. 161, 1081–1092 10.1083/jcb.200212040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenmann K. M., West R. A., Hildebrand D., Kitchen S. M., Peng J., Sigler R., Zhang J., Siminovitch K. A., Alberts A. S. (2007). T cell responses in mammalian diaphanous-related formin mDia1 knock-out mice. J. Biol. Chem. 282, 25152–25158 10.1074/jbc.M703243200 [DOI] [PubMed] [Google Scholar]
- Eng C. H., Huckaba T. M., Gundersen G. G. (2006). The formin mDia regulates GSK3beta through novel PKCs to promote microtubule stabilization but not MTOC reorientation in migrating fibroblasts. Mol. Biol. Cell 17, 5004–5016 10.1091/mbc.E05-10-0914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esue O., Harris E. S., Higgs H. N., Wirtz D. (2008). The filamentous actin cross-linking/bundling activity of mammalian formins. J. Mol. Biol. 384, 324–334 10.1016/j.jmb.2008.09.043 [DOI] [PubMed] [Google Scholar]
- Evangelista M., Blundell K., Longtine M. S., Chow C. J., Adames N., Pringle J. R., Peter M., Boone C. (1997). Bni1p, a yeast formin linking cdc42p and the actin cytoskeleton during polarized morphogenesis. Science 276, 118–122 10.1126/science.276.5309.118 [DOI] [PubMed] [Google Scholar]
- Evangelista M., Pruyne D., Amberg D. C., Boone C., Bretscher A. (2002). Formins direct Arp2/3-independent actin filament assembly to polarize cell growth in yeast. Nat. Cell Biol. 4, 32–41 10.1038/ncb718 [DOI] [PubMed] [Google Scholar]
- Ezezika O. C., Younger N. S., Lu J., Kaiser D. A., Corbin Z. A., Nolen B. J., Kovar D. R., Pollard T. D. (2009). Incompatibility with formin Cdc12p prevents human profilin from substituting for fission yeast profilin: insights from crystal structures of fission yeast profilin. J. Biol. Chem. 284, 2088–2097 10.1074/jbc.M807073200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feierbach B., Chang F. (2001). Roles of the fission yeast formin for3p in cell polarity, actin cable formation and symmetric cell division. Curr. Biol. 11, 1656–1665 10.1016/S0960-9822(01)00525-5 [DOI] [PubMed] [Google Scholar]
- Feierbach B., Verde F., Chang F. (2004). Regulation of a formin complex by the microtubule plus end protein tea1p. J. Cell Biol. 165, 697–707 10.1083/jcb.200403090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard J., Ramabhadran V., Neumanne E., Gurel P., Blanchoin L., Vantard M., Higgs H. N. (2011). Differential interactions of the formins INF2, mDia1, and mDia2 with microtubules. Mol. Biol. Cell 22, 4575–4587 10.1091/mbc.E11-07-0616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L., Liu W., Bretscher A. (2010). The yeast formin Bnr1p has two localization regions that show spatially and temporally distinct association with septin structures. Mol. Biol. Cell 21, 1253–1262 10.1091/mbc.E09-10-0861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasteier J. E., Madrid R., Krautkrämer E., Schröder S., Muranyi W., Benichou S., Fackler O. T. (2003). Activation of the Rac-binding partner FHOD1 induces actin stress fibers via a ROCK-dependent mechanism. J. Biol. Chem. 278, 38902–38912 10.1074/jbc.M306229200 [DOI] [PubMed] [Google Scholar]
- Gorelik R., Yang C., Kameswaran V., Dominguez R., Svitkina T. (2011). Mechanisms of plasma membrane targeting of formin mDia2 through its amino terminal domains. Mol. Biol. Cell 22, 189–201 10.1091/mbc.E10-03-0256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould C. J., Maiti S., Michelot A., Graziano B. R., Blanchoin L., Goode B. L. (2011). The formin DAD domain plays dual roles in autoinhibition and actin nucleation. Curr. Biol. 21, 384–390 10.1016/j.cub.2011.01.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulimari P., Knieling H., Engel U., Grosse R. (2008). LARG and mDia1 link Galpha12/13 to cell polarity and microtubule dynamics. Mol. Biol. Cell 19, 30–40 10.1091/mbc.E06-11-1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano B. R., DuPage A. G., Michelot A., Breitsprecher D., Moseley J. B., Sagot I., Blanchoin L., Goode B. L. (2011). Mechanism and cellular function of Bud6 as an actin nucleation-promoting factor. Mol. Biol. Cell 22, 4016–4028 10.1091/mbc.E11-05-0404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas R., Kato Y., He X. (2001). Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell 107, 843–854 10.1016/S0092-8674(01)00614-6 [DOI] [PubMed] [Google Scholar]
- Hannemann S., Madrid R., Stastna J., Kitzing T., Gasteier J., Schönichen A., Bouchet J., Jimenez A., Geyer M., Grosse R.et al. (2008). The Diaphanous-related Formin FHOD1 associates with ROCK1 and promotes Src-dependent plasma membrane blebbing. J. Biol. Chem. 283, 27891–27903 10.1074/jbc.M801800200 [DOI] [PubMed] [Google Scholar]
- Harris E. S., Higgs H. N. (2006). Biochemical analysis of mammalian formin effects on actin dynamics. Methods Enzymol. 406, 190–214 10.1016/S0076-6879(06)06015-0 [DOI] [PubMed] [Google Scholar]
- Harris E. S., Li F., Higgs H. N. (2004). The mouse formin, FRLalpha, slows actin filament barbed end elongation, competes with capping protein, accelerates polymerization from monomers, and severs filaments. J. Biol. Chem. 279, 20076–20087 10.1074/jbc.M312718200 [DOI] [PubMed] [Google Scholar]
- Harris E. S., Gauvin T. J., Heimsath E. G., Higgs H. N. (2010). Assembly of filopodia by the formin FRL2 (FMNL3). Cytoskeleton (Hoboken) 67, 755–772 10.1002/cm.20485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimsath E. G., Jr and Higgs H. N. (2012). The C terminus of formin FMNL3 accelerates actin polymerization and contains a WH2 domain-like sequence that binds both monomers and filament barbed ends. J. Biol. Chem. 287, 3087–3098 10.1074/jbc.M111.312207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotulainen P., Lappalainen P. (2006). Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J. Cell Biol. 173, 383–394 10.1083/jcb.200511093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura H., Tanaka K., Hihara T., Umikawa M., Kamei T., Takahashi K., Sasaki T., Takai Y. (1997). Bni1p and Bnr1p: downstream targets of the Rho family small G-proteins which interact with profilin and regulate actin cytoskeleton in Saccharomyces cerevisiae. EMBO J. 16, 2745–2755 10.1093/emboj/16.10.2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingouff M., Fitz Gerald J. N., Guérin C., Robert H., Sørensen M. B., Van Damme D., Geelen D., Blanchoin L., Berger F. (2005). Plant formin AtFH5 is an evolutionarily conserved actin nucleator involved in cytokinesis. Nat. Cell Biol. 7, 374–380 10.1038/ncb1238 [DOI] [PubMed] [Google Scholar]
- Ishizaki T., Morishima Y., Okamoto M., Furuyashiki T., Kato T., Narumiya S. (2001). Coordination of microtubules and the actin cytoskeleton by the Rho effector mDia1. Nat. Cell Biol. 3, 8–14 10.1038/35050598 [DOI] [PubMed] [Google Scholar]
- Iskratsch T., Lange S., Dwyer J., Kho A. L., dos Remedios C., Ehler E. (2010). Formin follows function: a muscle-specific isoform of FHOD3 is regulated by CK2 phosphorylation and promotes myofibril maintenance. J. Cell Biol. 191, 1159–1172 10.1083/jcb.201005060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iskratsch T., Reijntjes S., Dwyer J., Toselli P., Degano I. R., Dominguez I., Ehler E. (2012). Two distinct phosphorylation events govern the function of muscle FHOD3. Cell Mol. Life Sci. [Epub ahead of print] doi. 10.1007/s00018–012–1154–7 10.1007/s00018-012-1154-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson–Grusby L., Kuo A., Leder P. (1992). A variant limb deformity transcript expressed in the embryonic mouse limb defines a novel formin. Genes Dev. 6, 29–37 10.1101/gad.6.1.29 [DOI] [PubMed] [Google Scholar]
- Kato T., Watanabe N., Morishima Y., Fujita A., Ishizaki T., Narumiya S. (2001). Localization of a mammalian homolog of diaphanous, mDia1, to the mitotic spindle in HeLa cells. J. Cell Sci. 114, 775–784 [DOI] [PubMed] [Google Scholar]
- Kikyo M., Tanaka K., Kamei T., Ozaki K., Fujiwara T., Inoue E., Takita Y., Ohya Y., Takai Y. (1999). An FH domain-containing Bnr1p is a multifunctional protein interacting with a variety of cytoskeletal proteins in Saccharomyces cerevisiae. Oncogene 18, 7046–7054 10.1038/sj.onc.1203184 [DOI] [PubMed] [Google Scholar]
- Kohno H., Tanaka K., Mino A., Umikawa M., Imamura H., Fujiwara T., Fujita Y., Hotta K., Qadota H., Watanabe T.et al. (1996). Bni1p implicated in cytoskeletal control is a putative target of Rho1p small GTP binding protein in Saccharomyces cerevisiae. EMBO J. 15, 6060–6068 [PMC free article] [PubMed] [Google Scholar]
- Kovar D. R., Kuhn J. R., Tichy A. L., Pollard T. D. (2003). The fission yeast cytokinesis formin Cdc12p is a barbed end actin filament capping protein gated by profilin. J. Cell Biol. 161, 875–887 10.1083/jcb.200211078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovar D. R., Wu J. Q., Pollard T. D. (2005). Profilin-mediated competition between capping protein and formin Cdc12p during cytokinesis in fission yeast. Mol. Biol. Cell 16, 2313–2324 10.1091/mbc.E04-09-0781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovar D. R., Harris E. S., Mahaffy R., Higgs H. N., Pollard T. D. (2006). Control of the assembly of ATP- and ADP-actin by formins and profilin. Cell 124, 423–435 10.1016/j.cell.2005.11.038 [DOI] [PubMed] [Google Scholar]
- Kozlov M. M., Bershadsky A. D. (2004). Processive capping by formin suggests a force-driven mechanism of actin polymerization. J. Cell Biol. 167, 1011–1017 10.1083/jcb.200410017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai S. L., Chan T. H., Lin M. J., Huang W. P., Lou S. W., Lee S. J. (2008). Diaphanous-related formin 2 and profilin I are required for gastrulation cell movements. PLoS ONE 3, e3439 10.1371/journal.pone.0003439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leader B., Lim H., Carabatsos M. J., Harrington A., Ecsedy J., Pellman D., Maas R., Leder P. (2002). Formin-2, polyploidy, hypofertility and positioning of the meiotic spindle in mouse oocytes. Nat. Cell Biol. 4, 921–928 10.1038/ncb880 [DOI] [PubMed] [Google Scholar]
- Lee L., Klee S. K., Evangelista M., Boone C., Pellman D. (1999). Control of mitotic spindle position by the Saccharomyces cerevisiae formin Bni1p. J. Cell Biol. 144, 947–961 10.1083/jcb.144.5.947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewkowicz E., Herit F., Le Clainche C., Bourdoncle P., Perez F., Niedergang F. (2008). The microtubule-binding protein CLIP-170 coordinates mDia1 and actin reorganization during CR3-mediated phagocytosis. J. Cell Biol. 183, 1287–1298 10.1083/jcb.200807023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Higgs H. N. (2003). The mouse Formin mDia1 is a potent actin nucleation factor regulated by autoinhibition. Curr. Biol. 13, 1335–1340 10.1016/S0960-9822(03)00540-2 [DOI] [PubMed] [Google Scholar]
- Li H., Guo F., Rubinstein B., Li R. (2008). Actin-driven chromosomal motility leads to symmetry breaking in mammalian meiotic oocytes. Nat. Cell Biol. 10, 1301–1308 10.1038/ncb1788 [DOI] [PubMed] [Google Scholar]
- Li Y., Shen Y., Cai C., Zhong C., Zhu L., Yuan M., Ren H. (2010). The type II Arabidopsis formin14 interacts with microtubules and microfilaments to regulate cell division. Plant Cell 22, 2710–2726 10.1105/tpc.110.075507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Hallett M. A., Zhu W., Rubart M., Liu Y., Yang Z., Chen H., Haneline L. S., Chan R. J., Schwartz R. J.et al. (2011). Dishevelled-associated activator of morphogenesis 1 (Daam1) is required for heart morphogenesis. Development 138, 303–315 10.1242/dev.055566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Meng W., Poy F., Maiti S., Goode B. L., Eck M. J. (2007). Structure of the FH2 domain of Daam1: implications for formin regulation of actin assembly. J. Mol. Biol. 369, 1258–1269 10.1016/j.jmb.2007.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas R. L., Zeller R., Woychik R. P., Vogt T. F., Leder P. (1990). Disruption of formin-encoding transcripts in two mutant limb deformity alleles. Nature 346, 853–855 10.1038/346853a0 [DOI] [PubMed] [Google Scholar]
- Machaidze G., Sokoll A., Shimada A., Lustig A., Mazur A., Wittinghofer A., Aebi U., Mannherz H. G. (2010). Actin filament bundling and different nucleating effects of mouse Diaphanous-related formin FH2 domains on actin/ADF and actin/cofilin complexes. J. Mol. Biol. 403, 529–545 10.1016/j.jmb.2010.09.017 [DOI] [PubMed] [Google Scholar]
- Madrid R., Aranda J. F., Rodríguez–Fraticelli A. E., Ventimiglia L., Andrés–Delgado L., Shehata M., Fanayan S., Shahheydari H., Gómez S., Jiménez A.et al. (2010). The formin INF2 regulates basolateral-to-apical transcytosis and lumen formation in association with Cdc42 and MAL2. Dev. Cell 18, 814–827 10.1016/j.devcel.2010.04.001 [DOI] [PubMed] [Google Scholar]
- Maiti S., Michelot A., Gould C., Blanchoin L., Sokolova O., Goode B. L. (2012). Structure and activity of full-length formin mDia1. Cytoskeleton (Hoboken) 69, 393–405 10.1002/cm.21033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. G., Rincón S. A., Basu R., Pérez P., Chang F. (2007). Regulation of the formin for3p by cdc42p and bud6p. Mol. Biol. Cell 18, 4155–4167 10.1091/mbc.E07-02-0094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinière A., Gayral P., Hawes C., Runions J. (2011). Building bridges: formin1 of Arabidopsis forms a connection between the cell wall and the actin cytoskeleton. Plant J. 66, 354–365 10.1111/j.1365-313X.2011.04497.x [DOI] [PubMed] [Google Scholar]
- Mason F. M., Heimsath E. G., Higgs H. N., Soderling S. H. (2011). Bi-modal regulation of a formin by srGAP2. J. Biol. Chem. 286, 6577–6586 10.1074/jbc.M110.190397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matusek T., Gombos R., Szécsényi A., Sánchez–Soriano N., Czibula A., Pataki C., Gedai A., Prokop A., Raskó I., Mihály J. (2008). Formin proteins of the DAAM subfamily play a role during axon growth. J. Neurosci. 28, 13310–13319 10.1523/JNEUROSCI.2727-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelot A., Guérin C., Huang S., Ingouff M., Richard S., Rodiuc N., Staiger C. J., Blanchoin L. (2005). The formin homology 1 domain modulates the actin nucleation and bundling activity of Arabidopsis FORMIN1. Plant Cell 17, 2296–2313 10.1105/tpc.105.030908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelot A., Berro J., Guérin C., Boujemaa–Paterski R., Staiger C. J., Martiel J. L., Blanchoin L. (2007). Actin-filament stochastic dynamics mediated by ADF/cofilin. Curr. Biol. 17, 825–833 10.1016/j.cub.2007.04.037 [DOI] [PubMed] [Google Scholar]
- Mizuno H., Higashida C., Yuan Y., Ishizaki T., Narumiya S., Watanabe N. (2011). Rotational movement of the formin mDia1 along the double helical strand of an actin filament. Science 331, 80–83 10.1126/science.1197692 [DOI] [PubMed] [Google Scholar]
- Moseley J. B., Goode B. L. (2005). Differential activities and regulation of Saccharomyces cerevisiae formin proteins Bni1 and Bnr1 by Bud6. J. Biol. Chem. 280, 28023–28033 10.1074/jbc.M503094200 [DOI] [PubMed] [Google Scholar]
- Moseley J. B., Sagot I., Manning A. L., Xu Y., Eck M. J., Pellman D., Goode B. L. (2004). A conserved mechanism for Bni1- and mDia1-induced actin assembly and dual regulation of Bni1 by Bud6 and profilin. Mol. Biol. Cell 15, 896–907 10.1091/mbc.E03-08-0621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano K., Imai J., Arai R., Toh–E A., Matsui Y., Mabuchi I. (2002). The small GTPase Rho3 and the diaphanous/formin For3 function in polarized cell growth in fission yeast. J. Cell Sci. 115, 4629–4639 10.1242/jcs.00150 [DOI] [PubMed] [Google Scholar]
- Neidt E. M., Skau C. T., Kovar D. R. (2008). The cytokinesis formins from the nematode worm and fission yeast differentially mediate actin filament assembly. J. Biol. Chem. 283, 23872–23883 10.1074/jbc.M803734200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidt E. M., Scott B. J., Kovar D. R. (2009). Formin differentially utilizes profilin isoforms to rapidly assemble actin filaments. J. Biol. Chem. 284, 673–684 10.1074/jbc.M804201200 [DOI] [PubMed] [Google Scholar]
- Nezami A., Poy F., Toms A., Zheng W., Eck M. J. (2010). Crystal structure of a complex between amino and carboxy terminal fragments of mDia1: insights into autoinhibition of diaphanous-related formins. PLoS ONE 5, e12992 10.1371/journal.pone.0012992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K., Bartolini F., Deaconescu A. M., Moseley J. B., Dogic Z., Grigorieff N., Gundersen G. G., Goode B. L. (2010). Adenomatous polyposis coli protein nucleates actin assembly and synergizes with the formin mDia1. J. Cell Biol. 189, 1087–1096 10.1083/jcb.201001016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otomo T., Otomo C., Tomchick D. R., Machius M., Rosen M. K. (2005a). Structural basis of Rho GTPase-mediated activation of the formin mDia1. Mol. Cell 18, 273–281 10.1016/j.molcel.2005.04.002 [DOI] [PubMed] [Google Scholar]
- Otomo T., Tomchick D. R., Otomo C., Panchal S. C., Machius M., Rosen M. K. (2005b). Structural basis of actin filament nucleation and processive capping by a formin homology 2 domain. Nature 433, 488–494 10.1038/nature03251 [DOI] [PubMed] [Google Scholar]
- Otomo T., Tomchick D. R., Otomo C., Machius M., Rosen M. K. (2010). Crystal structure of the Formin mDia1 in autoinhibited conformation. PLoS ONE 5, e12896 10.1371/journal.pone.0012896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzo A. F., Cook T. A., Alberts A. S., Gundersen G. G. (2001). mDia mediates Rho-regulated formation and orientation of stable microtubules. Nat. Cell Biol. 3, 723–729 10.1038/35087035 [DOI] [PubMed] [Google Scholar]
- Paul A. S., Pollard T. D. (2008). The role of the FH1 domain and profilin in formin-mediated actin-filament elongation and nucleation. Curr. Biol. 18, 9–19 10.1016/j.cub.2007.11.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul A. S., Pollard T. D. (2009a). Review of the mechanism of processive actin filament elongation by formins. Cell Motil. Cytoskeleton 66, 606–617 10.1002/cm.20379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul A. S., Pollard T. D. (2009b). Energetic requirements for processive elongation of actin filaments by FH1FH2-formins. J. Biol. Chem. 284, 12533–12540 10.1074/jbc.M808587200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrin S., Mellor H. (2005). The Rho family GTPase Rif induces filopodia through mDia2. Curr. Biol. 15, 129–133 10.1016/j.cub.2005.01.011 [DOI] [PubMed] [Google Scholar]
- Peng J., Wallar B. J., Flanders A., Swiatek P. J., Alberts A. S. (2003). Disruption of the Diaphanous-related formin Drf1 gene encoding mDia1 reveals a role for Drf3 as an effector for Cdc42. Curr. Biol. 13, 534–545 10.1016/S0960-9822(03)00170-2 [DOI] [PubMed] [Google Scholar]
- Pfender S., Kuznetsov V., Pleiser S., Kerkhoff E., Schuh M. (2011). Spire-type actin nucleators cooperate with Formin-2 to drive asymmetric oocyte division. Curr. Biol. 21, 955–960 10.1016/j.cub.2011.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T. D., Borisy G. G. (2003). Cellular motility driven by assembly and disassembly of actin filaments. Cell 112, 453–465 10.1016/S0092-8674(03)00120-X [DOI] [PubMed] [Google Scholar]
- Pring M., Evangelista M., Boone C., Yang C., Zigmond S. H. (2003). Mechanism of formin-induced nucleation of actin filaments. Biochemistry 42, 486–496 10.1021/bi026520j [DOI] [PubMed] [Google Scholar]
- Pruyne D., Evangelista M., Yang C., Bi E., Zigmond S., Bretscher A., Boone C. (2002). Role of formins in actin assembly: nucleation and barbed-end association. Science 297, 612–615 10.1126/science.1072309 [DOI] [PubMed] [Google Scholar]
- Quinlan M. E., Hilgert S., Bedrossian A., Mullins R. D., Kerkhoff E. (2007). Regulatory interactions between two actin nucleators, Spire and Cappuccino. J. Cell Biol. 179, 117–128 10.1083/jcb.200706196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramabhadran V., Gurel P. S., Higgs H. N. (2012). Mutations to the formin homology 2 domain of INF2 protein have unexpected effects on actin polymerization and severing. J. Biol. Chem. 287, 34234–34245 10.1074/jbc.M112.365122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalingam N., Zhao H., Breitsprecher D., Lappalainen P., Faix J., Schleicher M. (2010). Phospholipids regulate localization and activity of mDia1 formin. Eur. J. Cell Biol. 89, 723–732 10.1016/j.ejcb.2010.06.001 [DOI] [PubMed] [Google Scholar]
- Rivero F., Muramoto T., Meyer A. K., Urushihara H., Uyeda T. Q., Kitayama C. (2005). A comparative sequence analysis reveals a common GBD/FH3-FH1-FH2-DAD architecture in formins from Dictyostelium, fungi and metazoa. BMC Genomics 6, 28 10.1186/1471-2164-6-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero S., Le Clainche C., Didry D., Egile C., Pantaloni D., Carlier M. F. (2004). Formin is a processive motor that requires profilin to accelerate actin assembly and associated ATP hydrolysis. Cell 119, 419–429 10.1016/j.cell.2004.09.039 [DOI] [PubMed] [Google Scholar]
- Rose R., Weyand M., Lammers M., Ishizaki T., Ahmadian M. R., Wittinghofer A. (2005). Structural and mechanistic insights into the interaction between Rho and mammalian Dia. Nature 435, 513–518 10.1038/nature03604 [DOI] [PubMed] [Google Scholar]
- Sagot I., Klee S. K., Pellman D. (2002a). Yeast formins regulate cell polarity by controlling the assembly of actin cables. Nat. Cell Biol. 4, 42–50 [DOI] [PubMed] [Google Scholar]
- Sagot I., Rodal A. A., Moseley J., Goode B. L., Pellman D. (2002b). An actin nucleation mechanism mediated by Bni1 and profilin. Nat. Cell Biol. 4, 626–631 [DOI] [PubMed] [Google Scholar]
- Sarmiento C., Wang W., Dovas A., Yamaguchi H., Sidani M., El–Sibai M., Desmarais V., Holman H. A., Kitchen S., Backer J. M.et al. (2008). WASP family members and formin proteins coordinate regulation of cell protrusions in carcinoma cells. J. Cell Biol. 180, 1245–1260 10.1083/jcb.200708123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A., Khadka D. K., Liu W., Bharti R., Runnels L. W., Dawid I. B., Habas R. (2006). Profilin is an effector for Daam1 in non-canonical Wnt signaling and is required for vertebrate gastrulation. Development 133, 4219–4231 10.1242/dev.02590 [DOI] [PubMed] [Google Scholar]
- Satoh S., Tominaga T. (2001). mDia-interacting protein acts downstream of Rho-mDia and modifies Src activation and stress fiber formation. J. Biol. Chem. 276, 39290–39294 10.1074/jbc.M107026200 [DOI] [PubMed] [Google Scholar]
- Schirenbeck A., Bretschneider T., Arasada R., Schleicher M., Faix J. (2005). The Diaphanous-related formin dDia2 is required for the formation and maintenance of filopodia. Nat. Cell Biol. 7, 619–625 10.1038/ncb1266 [DOI] [PubMed] [Google Scholar]
- Schönichen A., Geyer M. (2010). Fifteen formins for an actin filament: a molecular view on the regulation of human formins. Biochim. Biophys. Acta 1803, 152–163 10.1016/j.bbamcr.2010.01.014 [DOI] [PubMed] [Google Scholar]
- Schuh M. (2011). An actin-dependent mechanism for long-range vesicle transport. Nat. Cell Biol. 13, 1431–1436 10.1038/ncb2353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher N., Borawski J. M., Leberfinger C. B., Gessler M., Kerkhoff E. (2004). Overlapping expression pattern of the actin organizers Spir-1 and formin-2 in the developing mouse nervous system and the adult brain. Gene Expr. Patterns 4, 249–255 10.1016/j.modgep.2003.11.006 [DOI] [PubMed] [Google Scholar]
- Scott B. J., Neidt E. M., Kovar D. R. (2011). The functionally distinct fission yeast formins have specific actin-assembly properties. Mol. Biol. Cell 22, 3826–3839 10.1091/mbc.E11-06-0492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth A., Otomo C., Rosen M. K. (2006). Autoinhibition regulates cellular localization and actin assembly activity of the diaphanous-related formins FRLalpha and mDia1. J. Cell Biol. 174, 701–713 10.1083/jcb.200605006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severson A. F., Baillie D. L., Bowerman B. (2002). A Formin Homology protein and a profilin are required for cytokinesis and Arp2/3-independent assembly of cortical microfilaments in C. elegans. Curr. Biol. 12, 2066–2075 10.1016/S0960-9822(02)01355-6 [DOI] [PubMed] [Google Scholar]
- Shemesh T., Otomo T., Rosen M. K., Bershadsky A. D., Kozlov M. M. (2005). A novel mechanism of actin filament processive capping by formin: solution of the rotation paradox. J. Cell Biol. 170, 889–893 10.1083/jcb.200504156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Zhang J., Mullin M., Dong B., Alberts A. S., Siminovitch K. A. (2009). The mDial formin is required for neutrophil polarization, migration, and activation of the LARG/RhoA/ROCK signaling axis during chemotaxis. J. Immunol. 182, 3837–3845 10.4049/jimmunol.0803838 [DOI] [PubMed] [Google Scholar]
- Shimada A., Nyitrai M., Vetter I. R., Kühlmann D., Bugyi B., Narumiya S., Geeves M. A., Wittinghofer A. (2004). The core FH2 domain of diaphanous-related formins is an elongated actin binding protein that inhibits polymerization. Mol. Cell 13, 511–522 10.1016/S1097-2765(04)00059-0 [DOI] [PubMed] [Google Scholar]
- Skillman K. M., Daher W., Ma C. I., Soldati–Favre D., Sibley L. D. (2012). Toxoplasma gondii profilin acts primarily to sequester G-actin while formins efficiently nucleate actin filament formation in vitro. Biochemistry 51, 2486–2495 10.1021/bi201704y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stastna J., Pan X., Wang H., Kollmannsperger A., Kutscheidt S., Lohmann V., Grosse R., Fackler O. T. (2012). Differing and isoform-specific roles for the formin DIAPH3 in plasma membrane blebbing and filopodia formation. Cell Res. 22, 728–745 10.1038/cr.2011.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staus D. P., Taylor J. M., Mack C. P. (2011). Enhancement of mDia2 activity by Rho-kinase-dependent phosphorylation of the diaphanous autoregulatory domain. Biochem. J. 439, 57–65 10.1042/BJ20101700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya R., Taniguchi K., Narumiya S., Sumimoto H. (2008). The mammalian formin FHOD1 is activated through phosphorylation by ROCK and mediates thrombin-induced stress fibre formation in endothelial cells. EMBO J. 27, 618–628 10.1038/emboj.2008.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston S. F., Kulacz W. A., Shaikh S., Lee J. M., Copeland J. W. (2012). The ability to induce microtubule acetylation is a general feature of formin proteins. PLoS ONE 7, e48041 10.1371/journal.pone.0048041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolliday N., VerPlank L., Li R. (2002). Rho1 directs formin-mediated actin ring assembly during budding yeast cytokinesis. Curr. Biol. 12, 1864–1870 10.1016/S0960-9822(02)01238-1 [DOI] [PubMed] [Google Scholar]
- Tu D., Graziano B. R., Park E., Zheng W., Li Y., Goode B. L., Eck M. J. (2012). Structure of the formin-interaction domain of the actin nucleation-promoting factor Bud6. Proc. Natl. Acad. Sci. USA 109, E3424–E3433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillant D. C., Copeland S. J., Davis C., Thurston S. F., Abdennur N., Copeland J. W. (2008). Interaction of the N- and C-terminal autoregulatory domains of FRL2 does not inhibit FRL2 activity. J. Biol. Chem. 283, 33750–33762 10.1074/jbc.M803156200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gisbergen P. A., Li M., Wu S. Z., Bezanilla M. (2012). Class II formin targeting to the cell cortex by binding PI(3,5)P(2) is essential for polarized growth. J. Cell Biol. 198, 235–250 10.1083/jcb.201112085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavylonis D., Kovar D. R., O'Shaughnessy B., Pollard T. D. (2006). Model of formin-associated actin filament elongation. Mol. Cell 21, 455–466 10.1016/j.molcel.2006.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidali L., van Gisbergen P. A., Guérin C., Franco P., Li M., Burkart G. M., Augustine R. C., Blanchoin L., Bezanilla M. (2009). Rapid formin-mediated actin-filament elongation is essential for polarized plant cell growth. Proc. Natl. Acad. Sci. USA 106, 13341–13346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizcarra C. L., Kreutz B., Rodal A. A., Toms A. V., Lu J., Zheng W., Quinlan M. E., Eck M. J. (2011). Structure and function of the interacting domains of Spire and Fmn-family formins. Proc. Natl. Acad. Sci. USA 108, 11884–11889 10.1073/pnas.1105703108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Neo S. P., Cai M. (2009). Regulation of the yeast formin Bni1p by the actin-regulating kinase Prk1p. Traffic 10, 528–535 10.1111/j.1600-0854.2009.00893.x [DOI] [PubMed] [Google Scholar]
- Watanabe N., Madaule P., Reid T., Ishizaki T., Watanabe G., Kakizuka A., Saito Y., Nakao K., Jockusch B. M., Narumiya S. (1997). p140mDia, a mammalian homolog of Drosophila diaphanous, is a target protein for Rho small GTPase and is a ligand for profilin. EMBO J. 16, 3044–3056 10.1093/emboj/16.11.3044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Ando Y., Yasuda S., Hosoya H., Watanabe N., Ishizaki T., Narumiya S. (2008). mDia2 induces the actin scaffold for the contractile ring and stabilizes its position during cytokinesis in NIH 3T3 cells. Mol. Biol. Cell 19, 2328–2338 10.1091/mbc.E07-10-1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb R. L., Zhou M. N., McCartney B. M. (2009). A novel role for an APC2-Diaphanous complex in regulating actin organization in Drosophila. Development 136, 1283–1293 10.1242/dev.026963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y., Eng C. H., Schmoranzer J., Cabrera–Poch N., Morris E. J., Chen M., Wallar B. J., Alberts A. S., Gundersen G. G. (2004). EB1 and APC bind to mDia to stabilize microtubules downstream of Rho and promote cell migration. Nat. Cell Biol. 6, 820–830 10.1038/ncb1160 [DOI] [PubMed] [Google Scholar]
- Xu Y., Moseley J. B., Sagot I., Poy F., Pellman D., Goode B. L., Eck M. J. (2004). Crystal structures of a Formin Homology-2 domain reveal a tethered dimer architecture. Cell 116, 711–723 10.1016/S0092-8674(04)00210-7 [DOI] [PubMed] [Google Scholar]
- Yamana N., Arakawa Y., Nishino T., Kurokawa K., Tanji M., Itoh R. E., Monypenny J., Ishizaki T., Bito H., Nozaki K.et al. (2006). The Rho-mDia1 pathway regulates cell polarity and focal adhesion turnover in migrating cells through mobilizing Apc and c-Src. Mol. Cell. Biol. 26, 6844–6858 10.1128/MCB.00283-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Czech L., Gerboth S., Kojima S., Scita G., Svitkina T. (2007). Novel roles of formin mDia2 in lamellipodia and filopodia formation in motile cells. PLoS Biol. 5, e317 10.1371/journal.pbio.0050317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda S., Oceguera–Yanez F., Kato T., Okamoto M., Yonemura S., Terada Y., Ishizaki T., Narumiya S. (2004). Cdc42 and mDia3 regulate microtubule attachment to kinetochores. Nature 428, 767–771 10.1038/nature02452 [DOI] [PubMed] [Google Scholar]
- Yayoshi–Yamamoto S., Taniuchi I., Watanabe T. (2000). FRL, a novel formin-related protein, binds to Rac and regulates cell motility and survival of macrophages. Mol. Cell. Biol. 20, 6872–6881 10.1128/MCB.20.18.6872-6881.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young K. G., Thurston S. F., Copeland S., Smallwood C., Copeland J. W. (2008). INF1 is a novel microtubule-associated formin. Mol. Biol. Cell 19, 5168–5180 10.1091/mbc.E08-05-0469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaoui K., Benseddik K., Daou P., Salaün D., Badache A. (2010). ErbB2 receptor controls microtubule capture by recruiting ACF7 to the plasma membrane of migrating cells. Proc. Natl. Acad. Sci. USA 107, 18517–18522 10.1073/pnas.1000975107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeth K., Pechlivanis M., Samol A., Pleiser S., Vonrhein C., Kerkhoff E. (2011). Molecular basis of actin nucleation factor cooperativity: crystal structure of the Spir-1 kinase non-catalytic C-lobe domain (KIND)•formin-2 formin SPIR interaction motif (FSI) complex. J. Biol. Chem. 286, 30732–30739 10.1074/jbc.M111.257782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond S. H. (2004). Formin-induced nucleation of actin filaments. Curr. Opin. Cell Biol. 16, 99–105 10.1016/j.ceb.2003.10.019 [DOI] [PubMed] [Google Scholar]
- Zigmond S. H., Evangelista M., Boone C., Yang C., Dar A. C., Sicheri F., Forkey J., Pring M. (2003). Formin leaky cap allows elongation in the presence of tight capping proteins. Curr. Biol. 13, 1820–1823 10.1016/j.cub.2003.09.057 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.