Summary
Intercellular adhesion molecule-5 (ICAM-5) is a dendrite-specific adhesion molecule, which functions in both the immune and nervous systems. ICAM-5 is the only negative regulator that has been identified for maturation of dendritic spines so far. Shedding of the ICAM-5 ectodomain promotes spine maturation and enhances synaptic activity. However, the mechanism by which ICAM-5 regulates spine development remains poorly understood. In this study, we found that ablation of ICAM5 expression resulted in a significant increase in the formation of synaptic contacts and the frequency of miniature excitatory post-synaptic currents, an indicator of pre-synaptic release probability. Antibodies against ICAM-5 and β1 integrins altered spine maturation. Furthermore, we found that β1 integrins serve as binding partners for ICAM-5. β1 integrins were immunoprecipitated with ICAM-5 from mouse brain and the binding region in ICAM-5 was localized to the two first Ig domains. β1 integrins were juxtaposed to filopodia tips at the early stage of synaptic formation, but as synapses matured, β1 integrins covered the mushroom spines. Loss of β1 integrins from the pre-synaptic sites affected the morphology of the post-synaptic structures. ICAM-5 ectodomain cleavage decreased or increased when the interaction between ICAM-5 and β1 integrins was potentiated or weakened, respectively, using antibodies. These results suggest that the interaction between ICAM-5 and β1 integrins is important in formation of functional synapses.
Key words: ICAM-5, Integrin, Adhesion, Neuron, Spine
Introduction
In the central nervous system (CNS), synapse formation is often referred to as a process in which initial contacts between axonal terminals and dendritic filopodia undergo changes in morphology and molecular content resulting in mature synapses. Besides neurotransmitter receptors and ion channels, a growing body of evidence shows that a multitude of synaptic cell adhesion molecules (CAMs) play important roles in regulating synapse formation. They stabilize the initial synaptic contacts, recruit synaptic structural proteins and trigger intracellular signaling to the actin cytoskeleton that induces synapse formation (Nguyen and Südhof, 1997; Torres et al., 1998; Biederer et al., 2002; Dalva et al., 2007; Ko et al., 2009; Mah et al., 2010; Takahashi et al., 2011).
The intercellular adhesion molecule-5 (ICAM-5, telencephalin) belongs to the immunoglobulin (Ig) superfamily containing nine Ig-domains. It is expressed in the soma, dendritic shafts and dendritic filopodia/spines of excitatory neurons in the telencephalon (Oka et al., 1990; Benson et al., 1998; Gahmberg et al., 2008; Mitsui et al., 2005). Previous studies have revealed both immune and neurodevelopmental functions for this molecule in the CNS (Tian et al., 2009). The interaction of ICAM-5 with the β2 integrin lymphocyte function-associated antigen 1 (LFA-1) has been extensively studied (Mizuno et al., 1997; Tian et al., 1997; Tian et al., 2000a; Tian et al., 2008; Zhang et al., 2008). It mediates the binding of leukocytes to hippocampal neurons and induces spreading of microglia (Mizuno et al., 1999). Furthermore, ICAM-5 induces dendritic outgrowth by homophilic binding (Tian et al., 2000b). ICAM-5-deficient mice exhibit decreased density of filopodia and acceleration of spine maturation (Matsuno et al., 2006), enhanced long-term potentiation (LTP) and altered learning performance (Nakamura et al., 2001). In addition, loss of ICAM-5 in brain accelerates spine maturation on thalamo-recipient cortical neurons (Barkat et al., 2011). N-methyl-D-aspartic acid (NMDA)-induced matrix metalloproteinase-2 and -9 (MMP-2 and -9) activation results in ICAM-5 ectodomain cleavage, which promotes dendritic spine development and affects LTP (Tian et al., 2007; Conant et al., 2010).
Integrins are heterodimeric trans-membrane cell adhesion molecules formed by an α- and a β-chain (Hynes, 2002; Chan and Davis, 2008; Gahmberg et al., 2009). In the CNS, integrins participate in several neuronal events essential for the development and remodeling of the brain, such as neural migration, neurite outgrowth, synapse formation and plasticity, and formation of working memory (Pinkstaff et al., 1998; Cohen et al., 2000; Shi and Ethell, 2006; Gardiner et al., 2007; Webb et al., 2007; Cingolani et al., 2008; Bahr et al., 1997; Chan et al., 2003; Chan et al., 2007; Chan et al., 2010). However, the localization of β1 integrins in neuronal subcellular structures is ambiguous, and no clear correlation with their functional activity has been demonstrated.
In the present study, we found that β1 integrins are expressed on both pre- and post-synaptic structures. We further identified β1 integrins as counter receptors for ICAM-5 and characterized the interaction in detail. The binding involves the two first Ig-domains of ICAM-5. It occurs at the initial stage of synapse formation, which may mediate loose, dynamic contacts between pre- and post-synaptic sites. Blocking or activating of this interaction by antibodies or knockdown β1 integrins in axons leads to altered spine morphology. ICAM-5 ectodomain cleavage is prevented by this interaction. These data expand our knowledge of the physical localization and physiological function of β1 integrins in brain, and provide a possible mechanism by which ICAM-5 regulates the maturation of functional synapses.
Results
ICAM-5 deficiency leads to increased formation of functional synapses
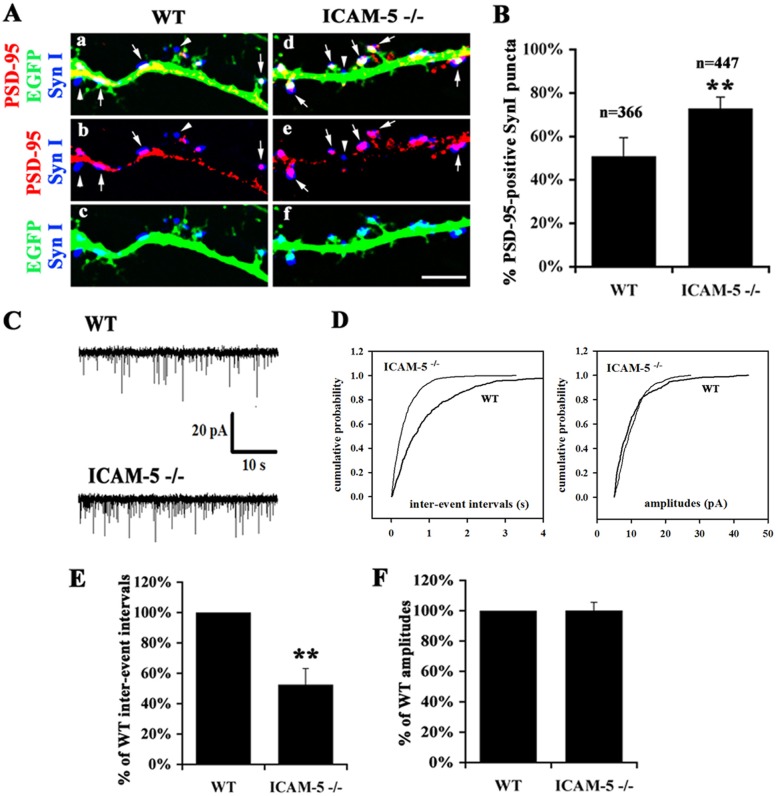
Previous studies showed that in ICAM-5−/− neurons, there was an increase in the size and number of spines and the overlap between synaptophysin and spine heads (Matsuno et al., 2006), indicating that ICAM-5 not only affects spine morphology, but also alters the formation of synaptic contacts. To confirm this, synapsin I, a synaptic vesicle-associated protein present in axon terminals and post-synaptic density (PSD)-95 were used as markers for pre- and post-synapses respectively, and the colocalization of the two markers was used to evaluate the level of the pre- and post-synaptic contacts. At 15 day in vitro (DIV), in ICAM-5−/− neurons, synapsin I puncta accumulated on the spine heads while in WT neurons, they scatter along the dendritic shafts (Fig. 1A). The overlap between synapsin I and PSD-95 was significantly increased in ICAM-5−/− (78%±3%) neurons compared with WT (51%±5%) and the colocalization mainly increased in spine heads (Fig. 1A,B).
Fig. 1.
ICAM-5 inhibits the formation of synaptic contacts and functional synapses. (A) Representitive images of dendrites of cultured hippocampal neurons at 15 DIV from WT (a–c) and ICAM-5−/− (d–f) mice. Colocalization of the PSD-95 (red) and synapsin I (blue) is shown (b and e). (c and f) show the merged images of EGFP-labeled dendrites (green) and synapsin I. Arrows: synapsin I puncta overlapping with PSD-95. Arrowheads: synapsin I puncta closely associated but not overlapping with PSD-95. Scale bar: 5 µm. (B) Diagram showing the quantitative analysis of synapsin I/PSD-95 colocalization. 366 (WT) and 477 (ICAM-5−/−) synapsin I puncta from 3 independent experiments were quantitatively analyzed. Mean±s.d. is shown. **P<0.01. (C) Functional synapses were analyzed by measuring mEPSCs. WT and ICAM-5-deficient mouse hippocampal neurons were cultured until 15–18 DIV and recordings were performed on neurons of the same age (DIV) for both genotypes in each experiment. Shown are representative recordings of spontaneous miniature glutamatergic post-synaptic currents of the neurons. (D) The cumulative probability plots from the recordings presented in C show an increased appearance of shorter inter-event intervals in ICAM-5−/− neurons but no obvious changes in the event amplitudes. (E) Diagram showing the overall effect of ICAM-5-deficiency on inter-event interval and event amplitude. Median inter-event intervals or amplitude of each manipulated cell have been divided by the WT values of the same experimental day (DIV 15–18). The mean time between events was half as long in ICAM-5−/− cells as in WT cells. The amplitude of currents did not change with any manipulation. n = 5 experiments, 60 cells. Mean±s.d. is shown. **P<0.01.
To study whether the increased synaptic contacts in ICAM-5−/− neurons are functionally active, we recorded the inter-event intervals and amplitude of miniature excitatory post-synaptic currents (mEPSCs) in cultured WT and ICAM-5-deficient neurons on 15–18 DIV (60 neurons randomly selected from 5 independent experiments were patched) (Fig. 1C). Compared with WT, ICAM-5−/− neurons exhibited a significant increase in the frequency (decrease in the inter-event intervals) of mEPSCs, however there was no significant effect on the amplitude (Fig. 1C,D). The mean relative inter-event interval was 53±11% shorter in ICAM-5−/− than in WT neurons implying a significant increase in the frequency of mEPSC (Fig. 1E). The corresponding value for event amplitudes in ICAM-5−/− was 100±5% compared to WT (Fig. 1E).
In conclusion, ICAM-5 deficiency increased the probability of successful synaptic events and the lack of change in the amplitude of mEPSCs indicates that the efficacy of the post-synaptic receptors is not changed. These results are not consistent with a post-synaptic mediated mechanism in ICAM5−/− neurons.
Antibodies against ICAM-5 or β1 integrins affect spine morphology
Conant and co-workers reported an interaction between ICAM-5 and β1 integrins (Conant et al., 2011). They did not, however, study the localization of β1 integrins nor did they address the physiological relevance of the interaction. In this study, we used antibodies, which bind to the ectodomains of ICAM-5 or β1 integrins, to mimic the effect of loss-of- or gain-of-function.
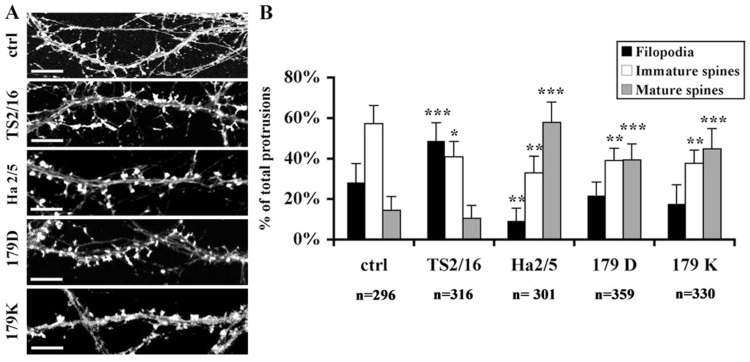
Mouse hippocampal neurons were treated at 11 DIV with antibodies against ICAM-5 or β1 integrins. The properties of the antibodies used in the study are summarized in Table 1. The cells were fixed at 15 DIV and immunostained for F-actin. As shown in Fig. 2, untreated neurons exhibited 57%±9% immature spines (including thin spines and stubby spines), 28%±9% filopodia and 14%±7% mature spines. When cells were treated with the ICAM-5 antibodies 179D and 179K, the ratio of immature spines decreased and that of mature spines significantly increased. Ha2/5, an adhesion-blocking antibody against β1 integrins, showed a similar effect on spine maturation as ICAM-5 antibodies. Interestingly, TS2/16 (Fig. 2A,B, TS2/16), an activating antibody against β1 integrins, which increased the ratio of filopodia and decreased that of the immature spines, showed an opposite effect to the adhesion-blocking antibodies. TS2/16 is usually described as an antibody reacting with human β1 integrins, but we saw effects on mouse neurons. To confirm that this antibody also cross-reacts with mouse β1 integrins, we treated mouse N2A neuroblastoma cells with TS2/16. In the presence of 1 mM MnCl2, the reactivity of 9EG7, an antibody recognizing activated mouse β1 integrins (Bazzoni et al., 1995), was significantly increased in N2A cells (supplementary material Fig. S2). Therefore, TS2/16 also activates mouse β1 integrins.
Table 1.
Antibodies used in functional studies
| Antibodies | Properties | Epitopes |
| TS2/16 | Activating | Human integrin β1 chain 207-218a |
| 9EG7 | Activating | Mouse integrin β1 chain 495-602a |
| Ha2/5 | Blocking | Mouse integrin β1 chain ectodomain |
| M2253z | Blocking | Human integrin β1 chain ectodomain |
| 179 D | Blocking | Mouse and human ICAM-5 1st Ig-like domain |
| 179 K | Blocking | Mouse and human ICAM-5 2nd Ig-like domain |
| 179 H | Blocking | Mouse and human ICAM-5 D2-3 Ig-like domain |
| 246 H | Blocking | Mouse and human ICAM-5 2nd Ig-like domain |
Properties refer to the capability of these antibodies in increasing or decreasing the ligand binding function of their immunogens
Expressed as the sequences in the primary structure
Fig. 2.
Altered ICAM-5/β1 integrin interaction results in abnormal spine formation. (A) Hippocampal neurons were left untreated (ctrl) or treated with antibodies from 11 to 15 DIV and then stained for F-actin. Compared with the untreated condition, the balance of dendritic protrusions was shifted towards more mature spines by blocking Ha2/5, 179D and 179K, yet towards filopodia by activating TS2/16. Scale bar: 5 µm. (B) Quantitative analysis of the ratio of different types of protrusions. The number of analyzed protrusions is listed under the columns. Mean ± s.d. of 3 independent experiments is shown. *P<0.01; **P<0.005; ***P<0.001.
The similar function of ICAM-5 and β1 integrins suggest that the two molecules regulate the morphological change of spines by an interaction between them. Therefore it was important to characterize this interaction in detail.
ICAM-5 binds to β1 integrins directly through the ectodomain
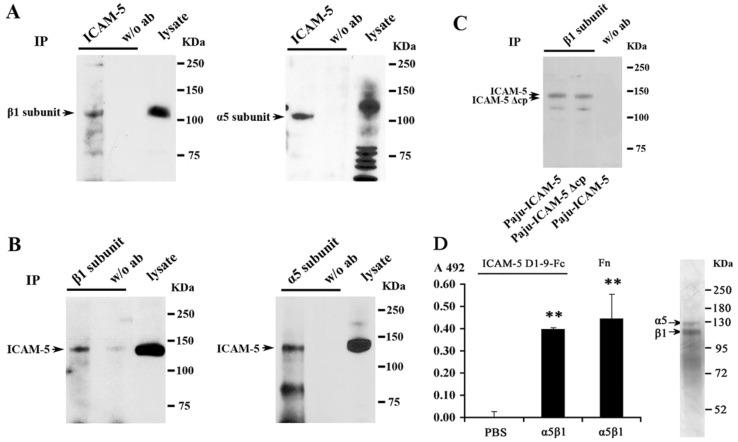
To study ICAM-5 interactions, ICAM-5 was immunoprecipitated from mouse forebrain homogenates. The immunoprecipitation was confirmed by western blotting with an ICAM-5 antibody (not shown). The integrin β1 subunit (Fig. 3A, left panel) and the α5 subunit (Fig. 3A, right panel) were detected in ICAM-5 precipitates. Reciprocally, ICAM-5 was also detected from the precipitates with the β1 (Fig. 3B, left panel) and α5 subunits (Fig. 3B, right panel), but not from the control precipitates. The α3 and α8 integrin subunits were also examined, but they showed very low binding to ICAM-5 (not shown). To determine whether the ectodomain or the cytoplasmic tail of ICAM-5 is more important in the interaction with β1 integrins, Paju cells were transfected with full length ICAM-5 (Paju-ICAM-5), or ICAM-5 lacking the cytoplasmic tail (Paju-ICAM-5 Δcp). The expression level of β1 integrins was similar in the two transfectants (not shown). The β1 integrin subunit was immunoprecipitated from the cell lysates of both Paju transfectants. ICAM-5 was detected in both precipitates by an antibody recognizing the ectodomain of ICAM-5 without any significant change in band intensity (Fig. 3C). Although a role for the transmembrane segment cannot be excluded from these experiments, the results indicate that most probably the ICAM-5 ectodomain interacts with β1 integrins.
Fig. 3.
ICAM-5 binds to β1 integrins. (A,B) Adult mouse brain homogenates were used for immunoprecipitation with antibodies against ICAM-5 (A, left and right), the integrin β1 chain (B, left) and α5 chain (B, right) respectively. Integrin β1 (A, left), α5 chains (A, right) and ICAM-5 (B, left and right) were detected by western blotting from the precipitates as well as the lysates (indicated by arrowheads). In the control precipitates, no antibody was used. The apparently higher molecular weight of the α5 subunit in lysate (A) is likely due to sample overloading. (C) Lysates from transfected Paju-ICAM-5 and Paju-ICAM-5 Δcp cells were precipitated with an antibody recognizing the integrin β1 chain. Full length ICAM-5 and cytoplasmic tail truncated ICAM-5 (ICAM-5 Δcp) were detected by western blotting with an antibody against the ectodomain of ICAM-5. (D) Binding of purified ICAM-5 D1–9-Fc to immobilized α5β1 integrin. Fibronectin was used as a positive control in binding to α5β1 integrin. Mean±s.d. of 3 independent experiments is shown. **P<0.01. The right panel shows SDS-PAGE of the integrin purification.
A direct interaction between ICAM-5 and β1 integrins was confirmed by using purified ICAM-5 D1–9-Fc and α5β1 integrin (Fig. 3D).
Binding of ICAM-5 to β1 integrins occurs in trans
To address whether the interaction between ICAM-5 and β1 integrins occurs in a trans manner, we performed cell adhesion assays.
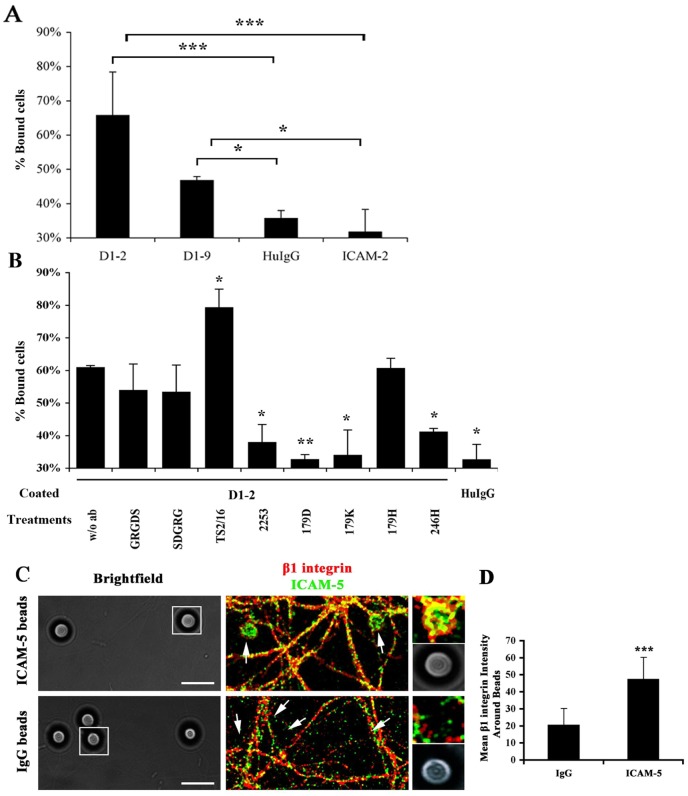
We used Paju-Neo cells, which do not express ICAM-5, making it a tool of choice to avoid homophilic binding between ICAM-5 and study its interaction with β1 integrins. β1 integrin expression in Paju-Neo cells was examined by flow cytometry (not shown). Cells were seeded onto the immobilized ICAM-5-Fc proteins D1–2 and D1–9 for 30 min and the unbound cells were washed away. As shown in Fig. 4A, compared with negative controls (human IgG and ICAM-2-Fc), both ICAM-5-Fc proteins increased cell adhesion. D1–2 exhibited strong binding with 66% bound cells. ICAM-5 D1–9 showed less binding, with 47% bound cells. A previous study has shown that ICAM-5 D1–2 is a more efficient binder to LFA-1 than ICAM-5 D1–9 (Tian et al., 2000a).
Fig. 4.
In trans binding between ICAM-5 and β1 integrins. (A) Purified ICAM-5-Fc proteins were pre-coated on 96-well microtiter plates. Human IgG and ICAM-2-Fc fusion proteins were used as negative controls. The ICAM-5-Fc proteins increased the binding of Paju-Neo cells, with D1–2-Fc being most efficient. (B) Effect of antibodies and peptides on Paju cell adhesion to ICAM-5 D1–2-Fc. The ICAM-5 abs 179D, 179K and 246 H and the β1 integrin adhesion blocking antibody 2253 were effective in inhibiting cell adhesion, while the β1 integrin activating antibody TS2/16 increased cell adhesion. (C) ICAM-5-Fc coated beads recruit β1 integrins in cultured neurons. 13 DIV neurons incubated for 24 h with ICAM-5 or human IgG-coated beads were fixed and stained for ICAM-5 (green) and β1 integrins (red). The DIC images were presented for each corresponding fluorescent image. Arrows indicate the location of beads. Inset: higher magnification images of the selected area. ICAM-5 coated beads efficiently recruited β1 integrins. Scale bar: 10 µm. The mean fluorescent intensity within the beads area was quantitated (D). >200 beads from 3 independent experiments were analyzed for each treatment. Mean ± s.d. is shown. *P<0.05, **P<0.01, ***P<0.001.
These results suggest that ICAM-5 promoted cell adhesion through interactions with counter-receptor(s) on the surface of Paju-Neo cells. To further prove that β1 integrins are responsible for the interaction, we employed antibodies against β1 integrins or ICAM-5 (the properties of the antibodies used in the study are summarized in Table 1), and an arginine-glycine-aspartate (RGD) peptide, which blocks interactions between integrin β1 subunit and several of its binding partners (Fig. 4B). Compared with the binding to D1–2 without antibody treatment (Fig. 4B, first bar), the β1 integrin adhesion blocking antibody 2253 inhibited the binding significantly. The ICAM-5 antibodies 179D, 179K and 246 H recognizing the first two Ig-like domains in ICAM-5 were also effective blockers of the binding. In contrast, 179H, which recognizes domains D2–3, showed a minimal blocking effect. Both the GRGDS and the SDGRG peptides slightly decreased the binding, but the decrease was not statistically significant, which is in accordance with the fact that ICAM-5 lacks of the RGD sequence. Importantly, TS2/16, a β1 integrin activating antibody, increased the adhesion significantly. We conclude that β1 integrins take part in ICAM-5-mediated cell adhesion via binding to ICAM-5 in trans.
Moreover, the interaction between ICAM-5 and β1 integrins in cultured neurons was further confirmed by a bead recruitment assay. We used ICAM-5-Fc-coated beads to mimic the endogenous ICAM-5 and studied their binding to β1 integrins. ICAM-5 D1–9-Fc or human IgG coated beads were incubated with cultured hippocampal neurons for 24 h. Cells were fixed and immunostained for ICAM-5 and β1 integrins. ICAM-5 coated beads efficiently recruited β1 integrins and the two proteins colocalized on the surface of the beads (Fig. 4C,D).
β1 integrins are expressed in both pre- and post-synapses
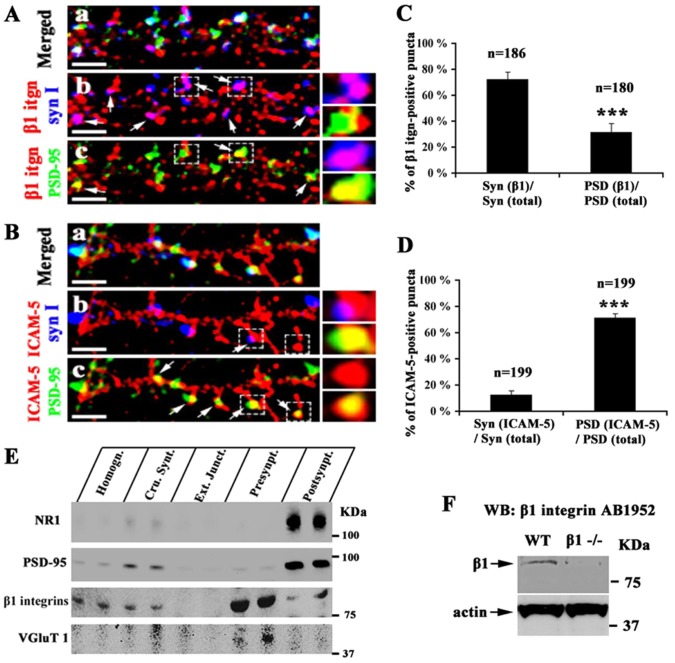
The discovery of the trans interaction between ICAM-5 and β1 integrins, and the fact that ICAM-5 is a post-synaptic protein in neurons (Benson et al., 1998; Yoshihara and Mori, 1994) led us to study whether β1 integrins are located at the pre-synaptic sites. Immunofluorescent staining of cultured hippocampal neurons was performed with a rabbit monoclonal antibody (mAb) against β1 integrins. Synapsin I and PSD-95 were used as pre- and post-synaptic markers, respectively. The majority of synapsin I immunoreactive puncta (72%±6%) colocalized with β1 integrins (Fig. 5Ab; Fig. 5C). PSD-95 positive puncta partially but less abundantly colocalized with β1 integrins (32%±7%) (Fig. 5Ac; Fig. 5C). In comparison, the colocalization of ICAM-5/synapsin I and ICAM-5/PSD-95 is also shown. ICAM-5 colocalized with PSD-95 along the dendritic shaft and spine heads (Fig. 5Bc) and synapsin I puncta apposed to ICAM-5-labeled dendrites and spine heads (Fig. 5Bb). 16%±3% synapsin I vs 71%±3% PSD-95 puncta colocalized with ICAM-5 (Fig. 5D). Notably, some β1 integrin puncta neither colocalized with synapsin I nor PSD-95 (Fig. 5Ab), which indicates the existence of non-synaptic β1 integrins.
Fig. 5.
Expression of β1 integrins on synapses. Mouse hippocampal neurons were fixed at 15 DIV and triple stained for PSD-95 (green), β1 integrins (red) and synapsin I (blue) (A) or PSD-95 (green), ICAM-5 (red) and synapsin I (blue) (B). (A) Colocalization of β1 integrins with synapsin I (b), or with PSD-95 puncta (c). β1 integrins colocalize with synapsin I at a higher level than with PSD-95. In comparison, ICAM-5 colocalizes with PSD-95 along the dendritic shaft and protrusions (c), but not with synapsin I (b). Arrows indicate the area where two proteins colocalized. Scale bar: 5 µm. Small windows: higher magnification view of the area marked by dashed frames. (C,D) The colocalization of β1 integrin/synapsin I, β1 integrin/PSD95, ICAM-5/synapsin I and ICAM-5/PSD-95 was quantitated. The number of puncta analyzed is shown above the columns. Mean ± s.d. from 3 independent experiments is shown. ***, P<0.001. (E) Mouse forebrains were used to isolate pre- and post-synaptic fractions. 30 µg protein from each following fractions was applied in duplicate to SDS-PAGE followed by western blotting: homogenate, crude synaptosome, external junction fraction, pre-synaptic fraction, and post-synaptic fraction. β1 integrins were found on both pre- and post-synaptic fractions but enriched in the pre-synaptic fraction. (F) 50 µg brain homogenates from WT and β1 integrin conditional knockout mice were applied to SDS-PAGE followed by western blotting. The β1 integrin antibody recognized a single band at 88 kDa, which was present in WT but almost absent in β1 integrin−/− mouse brain homogenates. This shows that the antibody is specific.
Pre- and post-synaptic fractionation is an often used method to study the distribution of synaptic proteins. Vesicular glutamate transporter 1(VGLUT1), NMDA receptor subunit 1 (NR1) and PSD-95 were used as the markers for the fractionation. Synaptosomes were hence fractionated into an external junction, a pre-synaptic and a post-synaptic fraction. The external junction fraction constitutes plasma and vesicle membranes, the pre-synaptic fraction mainly contains the pre-synaptic active zones, and the post-synaptic fraction is composed of PSDs. As expected, VGLUT1 was enriched in the pre-synaptic fraction while NR1 and PSD-95 were enriched in the post-synaptic fraction. β1 integrins were found in both pre- and post-synaptic fractions but were enriched in the pre-synaptic fraction. Absence of β1 integrins in the external junction fraction is possibly due to their cytoplasmic interactions with cytoskeletal proteins, which form a tight anchorage to the active zones and PSDs (Fig. 5E).
The specificity of β1 integrin antibodies for the studies above was tested using excitatory neuron-specific β1 integrin knockout mouse brain homogenates by western blotting. AB1952 recognized a single band at 88 kDa in WT but the intensity of a corresponding band in β1 integrin−/− brain was significantly lower (Fig. 5F). The same band was also recognized by other β1 integrin antibodies (sc-6622, Santa Cruz and Ab 52971, Abcam) (not shown). The band may represent a proteolytic fragment of the β1 chain.
These results clearly demonstrate that β1 integrins are expressed at both pre- and post-synaptic sites.
Development-related expression of β1 integrins at excitatory synapses
Dendritic protrusions are classified into filopodia, thin and long protrusions arising from the dendritic shafts, and spines, which are relatively shorter and usually carry an enlarged head on their tips (Ethell and Pasquale, 2005). Filopodia are usually considered to be precursors of spines, and nascent synapses are initially formed between axonal terminals and the dendritic filopodia. The filopodia-to-spine transition accompanies synapse maturation and stabilization (Ziv and Smith, 1996).
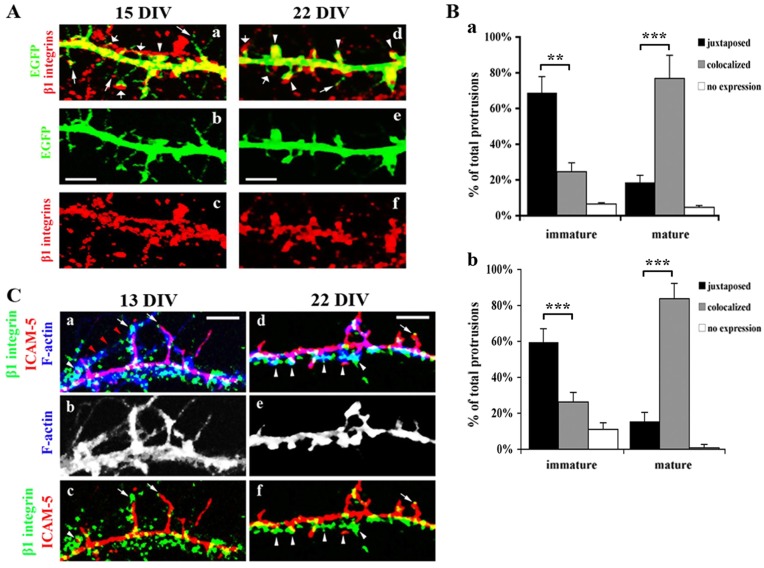
Previous studies have shown that ICAM-5 is enriched in filopodia and immature spines, but becomes excluded from mature spines (Matsuno et al., 2006; Tian et al., 2007). To further clarify the distinctive role of β1 integrins at different synaptic developmental stages, we studied β1 integrin expression in different types of dendritic protrusions. To visualize the fine structure of dendritic protrusions, neurons were transfected with Enhanced Green Fluorescent Protein (EGFP) and immunostained for β1 integrins on 15 and 22 DIV after fixation. The colocalization of β1 integrins and different subtypes of protrusions was studied. At 15 DIV, in some filopodia, β1 integrins were weakly expressed at their tips (Fig. 6Aa and Fig. 6Ad, narrow arrows), but increased in immature (Fig. 6Aa and Fig. 6Ad, wide arrows) and mature spines (Fig. 6Aa and Fig. 6Ad, arrowheads). Interestingly, β1 integrins were more often found juxtaposed to the heads of immature spines rather than colocalized with them (Fig. 6Ba, 66%±7% vs 23%±5%). However, most of mature spines (Fig. 6B, 78%±8%) had β1 integrins overlapping with the enlarged spine heads. At 22 DIV, even though the proportion of mature spines greatly increased compared with 15 DIV, the correlation of β1 integrin reactivity with subtypes of spines remains similar (Fig. 6Bb).
Fig. 6.
β1 integrins localize opposite to early spines and colocalize with mature spines. (A) Rat hippocampal neurons were transfected with EGFP 24 h before fixation (15 DIV or 22 DIV) and immunostained for β1 integrins. The colocalization of the β1 integrins with EGFP-labeled dendritic protrusions was studied. β1 integrins were weakly expressed at the tip of filopodia (narrow arrows), juxtaposed to immature spines (wide arrows), and colocalized with the head of mushroom spines (arrowheads). Scale bar: 10 µm. (B) Quantitative analysis of the correlation of the β1 integrin expression with different spines was performed using 355 immature spines and 304 mature spines from 3 independent experiments. Data from 15 (a) and 22 (b) DIV cultures are shown, respectively. Colocalized: >50% areas of spine heads overlapping with β1 integrin staining; juxtaposed: integrin β1 expression associated with the tip of filopodia or the spines from the counter cell and <50% areas of spine heads overlapping with β1 integrin staining; no expression: β1 integrin staining not seen in the protrusions. Mean±s.d. is shown. **P<0.01, ***P<0.001. (C) Representative images of rat hippocampal neurons at 13 and 22 DIV immunostained for β1 integrins (green), ICAM-5 (red) and F-actin (blue). Arrows indicate filopodia or immature spines, in which β1 integrins were opposite to ICAM-5 on the tips of the protrusions. Arrowheads indicate mature spines, which were mostly covered by β1 integrins but with minimal overlap with ICAM-5. Note that in panel a, red arrowheads show a fragment of an axon, which was making contacts with filopodia. Scale bar: 5 µm.
The distribution of the two proteins during synapse maturation was also examined. Cultured hippocampal neurons were used to study the colocalization of ICAM-5 and β1 integrins in different dendritic protrusions. On 13 DIV (Fig. 6Ca–c), dendrites contained abundant filopodia and ICAM-5 was enriched in them. β1 integrins were scattered around dendritic shafts and were apposed to some filopodia (Fig. 6Ca, arrows). A fragment of an axon (ICAM-5 negative), weakly seen but indicated by red arrowheads (Fig. 6Ca), made contacts with filopodia, in which two ICAM-5-stained filopodia are associated with β1 integrins. A small population of mushroom spines were also observed at this stage, in which ICAM-5 did not colocalize with β1 integrins (Fig. 6Ca, arrowheads). On 22 DIV (Fig. 6Cd–f), dendrites contained more spines than filopodia (Fig. 6Ce, F-actin). ICAM-5 was well colocalized with F-actin in filopodia and immature spines, but was partially excluded from the mushroom spines. In filopodia and immature spines, β1 integrins were opposite to ICAM-5 at the tip of filopodia or immature spines (Fig. 6Cd and Fig. 6Cf, arrows). In mature mushroom spines, β1 integrins covered most of the spine heads. However, ICAM-5 was found on one side of the spine, and minimally overlapped with β1 integrins (Fig. 6Cd and Fig. 6Cf, arrowheads).
These results indicate that at the early stage of synapse formation, β1 integrins are more often expressed in the pre-synaptic structures, making contacts with the tips of filopodia and immature spines from the counter neuron. It is difficult to determine the localization of β1 integrins after mature spines formed. The enhanced overlap of β1 integrins with mature spine heads may result from increased expression of post-synaptic β1 integrins or a shortened distance between pre- and post-synaptic membranes at the late stage of synapse formation.
ICAM-5 binding to β1 integrins affects its ectodomain shedding
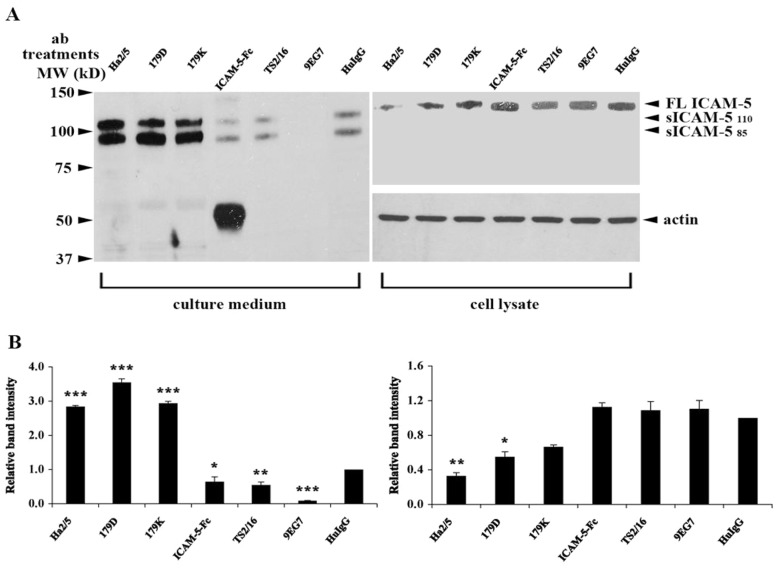
The ectodomains of ICAM-5 seem important in delaying synapse maturation as MMP-dependent cleavage of ICAM-5 promotes spines maturation and synaptic activity (Tian et al., 2007; Conant et al., 2010; Conant et al., 2011). Moreover, MMP-9 enzymatic activity has been shown to increase the surface trafficking of NR1 through a β1 integrin-dependent pathway (Michaluk et al., 2009; Wang et al., 2008). We found that the interaction of ICAM-5 with β1 integrins affects spine structures. Therefore it was tempting to study the putative role of ICAM-5/β1 integrin interaction in ICAM-5 ectodomain cleavage. Mouse hippocampal neurons were pre-treated with antibodies against the β1 integrins (TS2/16, 9EG7 and Ha2/5) and ICAM-5 (179D and 179K) and purified ICAM-5 D1–2-Fc protein for 3 days, and changed into HBSS/Ca++ medium. After 16 h incubation, cell lysates and culture media were collected. The levels of sICAM-5 released into the culture media (Fig. 7A, left panel) and full-length ICAM-5 present in cell lysates (Fig. 7A, right panel) were measured by western blotting. Two sICAM-5 fragments of 85 and 110 kDa were detected in the culture media as previously reported (Tian et al., 2007). Our cell adhesion assays showed that TS2/16 enhanced the interaction between ICAM-5 and β1 integrins, while 179D and 179K inhibited this interaction (Fig. 3B,C). Interestingly, we found that these antibodies also affected ICAM-5 cleavage. The adhesion blocking antibodies Ha2/5, 179D and 179K increased the level of soluble sICAM-5. In contrast, the β1 integrin-activating antibodies TS2/16 and 9EG7 effectively reduced the level of sICAM-5. Notably, ICAM-5 D1–2-Fc treatment also led to increased sICAM-5 levels released into the culture media (Fig. 7).
Fig. 7.
Antibody treatments against ICAM-5 and β1 integrins affect ICAM-5 ectodomain cleavage. (A) Hippocampal neurons were left untreated or treated with 20 µg/ml antibodies between days 11 to 14. After changing the cell culture medium to HBSS with 1.8 mM CaCl2 for 16 h, cell lysates and the conditioned media was collected separately, and ICAM-5 was detected by an antibody against the ICAM-5 cytoplasmic tail (ICAM-5cp, cell lysate) or ectodomains (1000J, culture medium). Soluble ICAM-5 85- and 110-kDa fragments were released to the culture media. Antibodies Ha2/5, 179D and 179K caused an increased level of sICAM-5. Purified ICAM-5 D1–2-Fc protein and the β1 integrin-activating antibodies TS2/16 and 9EG7 inhibited the release of sICAM-5. Actin in cell lysates was used to quantitate the amount of cellular material from which the culture media were collected. (B) Intensity of the released (85-kDa fragment) and membrane-bound ICAM-5 was quantified by ImageJ. Mean ± s.d. of 3 independent experiments is shown. *P<0.05; **P<0.01; ***P<0.001.
Knockdown of β1 integrins in axons affects synapse formation
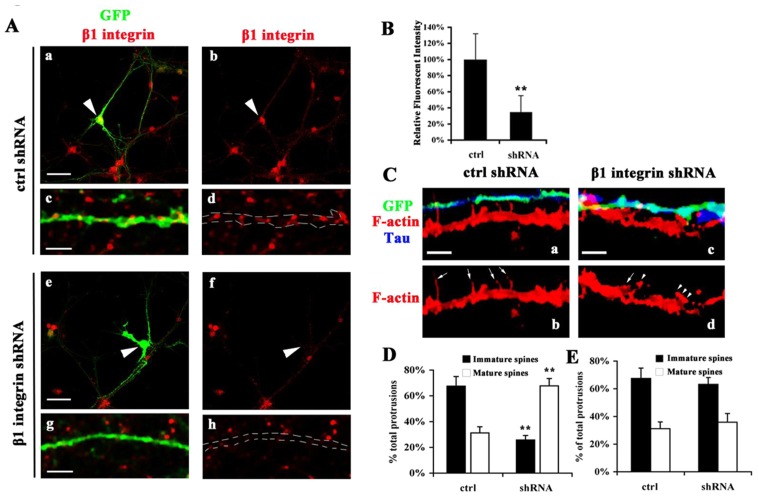
Interfering with the ICAM-5/β1 integrin interaction by antibody treatments resulted in altered spine morphology (Fig. 2). However, because β1 integrins are present in both pre- and post-synaptic structures, the effects may also come from the cis interaction of ICAM-5/β1 integrins. To eliminate this possibility, we studied the loss-of-function of pre-synaptic β1 integrins in synapse formation. Ten DIV hippocampal neuron cultures were transfected with small hairpin RNA (shRNA) for β1 integrins or control plasmids and the expression levels of β1 integrins were examined by immunofluorescent staining. A prominent decrease of β1 integrin expression was found in the soma (Fig. 8A, indicated by arrowheads) and neurites (Fig. 8A, indicated by dash lines) of shRNA transfected neurons. The mean fluorescent intensity of soma was quantitated and β1 integrin expression was found to be downregulated by ∼70% by shRNA in transfected cells (Fig. 8B). To study the interaction of pre- and post-synaptic structures, neurons were stained with axon marker Tau (Fig. 8C, blue) and F-actin (Fig. 8C, red), which visualizes the morphology of spines. The neurites labeled with GFP and Tau are axons, which originate from β1 integrin knocked-down neurons (Fig. 8C, cyan). Since shRNA downregulated β1 integrins in axons as well as dendrites, in this study, we only focused on those dendrites which were not transfected. At 12 DIV, in the control cultures, dendrites exhibit 63% of immature (including filopodia, thin spines and stubby spines) and 35% of mature (mushroom) spines among all the protrusions and there is no significant difference between the dendrites with or without contact with GFP labeled axons (Fig. 8C–E). In β1 integrin shRNA transfected cultures, dendrites, when in contact with transfected axons, carry an increased number of mature spines (61%) and a decreased number of immature spines (37%) (Fig. 8C,D); these dendrites, which failed to make contact with shRNA transfected axons, have a similar proportion of filopodia and spines with the control neurons (Fig. 8E).
Fig. 8.
Pre-synaptic β1 integrin knockdown alters synapse formation. 10 DIV cultured hippocampal neurons were transfected with GIPZ β1 integrin-shRNA or control plasmids and cultured for another 48 h before fixation. (A) Cells were stained for β1 integrins (red). Arrowheads indicate the soma of transfected cells. Dash lines indicate fragments of GFP labeled neurites. a and e, Scale bar: 20 µm (a,e) 5 µm (c,g). (B) Mean fluorescent intensity in somas was quantitated. Mean ± s. d. of 3 independent experiments is shown. **P<0.01. (C) Transfected axons were labeled with GFP and Tau (blue) and fine structures of dendrites were visualized by F-actin (red). Dendrites, which make contacts with β1 integrin shRNA transfected axons, exhibit an increased number of spines. Arrows indicate filopodia and immature spines. Arrowheads indicate mature spines. (D) Quantification from dendrites in contact with GFP labeled axons. (E) Quantification from dendrites not in contact with GFP labeled axons. ∼300 µm of dendritic fragments randomly picked from 10 untransfected neurons were analyzed for each group. The number of filopodia and spines was quantitated. Mean±s. d. from 3 independent experiments is shown. **P<0.005.
Discussion
ICAM-5 was first described as a telencephalon-specific molecule (telencephalin) with high homology to the previously described ICAM-molecules (Gahmberg, 1997; Yoshihara and Mori, 1994). In the immune system, the receptor of ICAM-5 is the β2 integrin LFA-1 expressed on peripheral blood leukocytes and microglia (Tian et al., 1997; Mizuno et al., 1999; Tian et al., 2000a; Zhang et al., 2008; Ransohoff and Cardona, 2010). In the CNS, the roles of ICAM-5 in stimulating dendrite outgrowth, delaying spine maturation and increasing LTP have been extensively studied (Tian et al., 2000b; Nyman-Huttunen et al., 2006; Matsuno et al., 2006; Nakamura et al., 2001). Upon stimulation of NMDA receptors and MMP activation, ICAM-5 ectodomain cleavage is promoted, which induces spine maturation (Tian et al., 2007). Furthermore, the addictive drug methamphetamine stimulates ICAM-5 cleavage, and this cleavage was blocked with MMP inhibitors (Conant et al., 2010). These findings highlight important roles of ICAM-5 in the CNS. Finding and characterizing the counter receptor for ICAM-5 in the brain would significantly advance our understanding of the neuronal functions of ICAM-5.
In this study, we have characterized the interaction between ICAM-5 and β1 integrins in detail. Importantly, we found that by regulating the ectodomain cleavage of ICAM-5, β1 integrins modulate spine morphology and synapse maturation.
The interaction was first observed by coimmunoprecipitation. According to previous studies, α3, α5 and α8 integrins are present in synaptic regions and play distinctive roles in synapse formation and plasticity. We found that the α5β1 integrin is the predominant binding partner of ICAM-5 in brain, whereas binding of α3β1 and α8β1 integrins was not clearly observed (not shown). The binding between ICAM-5 and β1 integrins is direct and occurs through the extracellular domains of the two proteins.
The cell adhesion results show that ICAM-5 binds to β1 integrins through its ectodomain and the ICAM-5-D1–2 domains form the most important binding region. The lower binding capability of the longer polypeptide D1–9 probably results from intra-molecular interactions that partially mask the binding site of ICAM-5 to β1 integrins. A similar finding was observed for its binding to LFA-1 (Tian et al., 2000a). Several extracellular matrix (ECM) proteins are well-known ligands for β1 integrins (Hynes, 2002) and many studies have shown a role of the interaction between ECM proteins and β1 integrins in synapse differentiation. Although RGD-containing peptides block the interactions between β1 integrins and some proteins, such as fibronectin (FN) (Ruoslahti and Pierschbacher, 1986), the peptides did not inhibit the binding of β1 integrin-mediated cell adhesion to ICAM-5-Fc proteins. In fact, ICAM-5 does not contain an RGD-sequence. β1 integrins do not contain an I-domain found in the LFA-1 integrin known to bind ICAM-5 (Zhang et al., 2008). Therefore, the binding site in β1 integrins must differ from that of LFA-1.
As synaptic adhesion molecules, β1 integrins are often considered to be located at the post-synaptic site, even though Hellwig and co-workers earlier showed a pre-synaptic localization of β1 integrins by electron microscopy (EM) (Hellwig et al., 2011). Our studies using fluorescent immunostaining and synaptosome fractionation show that, besides being present on dendrites, β1 integrins are also found in pre-synaptic structures. A similar expression pattern of β1 integrins was also shown by immune-EM (supplementary material Fig. S3). It is possible that the pre- and post-synaptic β1 integrins play different roles during development. In nascent synapses, β1 integrins appear to be more predominant on the pre-synaptic sites, suggesting that they may serve as counter-receptors for ICAM-5. Taking into account the expression pattern of ICAM-5, we speculate that the in trans interaction of ICAM-5 and β1 integrins is more important during early stages of synapse formation. In fact, soluble ICAM-5-coated beads failed to induce pre-synaptic protein clustering (supplementary material Fig. S1) even though they recruited β1 integrins efficiently, suggesting that the ICAM-5/β1 integrin interaction is irrelevant for synaptic protein clustering. They may form a loose and dynamic contact between pre- and post-synaptic membranes and the interaction is likely to be released upon to further signaling.
ICAM-5 is known as a negative regulator of spine maturation. When we used antibodies recognizing the first and second Ig-like domain of ICAM-5, the neurons exhibited an increased number of spines and a higher ratio of mature/immature spines, which indicates enhanced spine maturation. Interestingly, a blocking antibody against β1 integrins showed a similar effect on spine morphology. These phenomena resemble the phenotype of ICAM-5 knockout neurons. In contrast, neurons treated with an activating antibody against β1 integrins showed little effect on mature spines but exhibited a significant increase in the filopodia/immature spines ratio. This indicates a block in the filopodia-to-immature spine transition, but less effect on mature spines. This observation is similar to the phenotype of ICAM-5-overexpressing neurons. Therefore, an important function of the interaction of ICAM-5/β1 integrins is to promote filopodia elongation or to maintain the morphology of filopodia-like spines before they develop into mature spines. Although we cannot exclude the possibility that the antibodies interfere with the binding of β1 integrins to other ligands, the effects are at least partially due to the ICAM-5/β1 integrin interaction.
Downregulating β1 integrin expression by shRNA in axons resulted in a decreased number of filopodia and an increased number of spines in dendrites making contact. This indicates that the pre-synaptic β1 integrins are important in the filopodia-to-spine transition of the post-synaptic structures. Thus the effects of antibodies on spine morphology mainly come from the modulation of the trans interaction. These and other findings in the present manuscript suggest that the functional phenotype in ICAM-5−/− neurons that show higher frequency but no change in amplitude, is not derived from a post-synaptic but rather pre-synaptic mechanism.
The regulation of ICAM-5/β1 integrins on spine morphology likely occurs through fine-tuning of ICAM-5 ectodomain cleavage. At the early stage of synapse formation, β1 integrins likely start to interact with ICAM-5 when the initial contact forms between the axonal terminal and the filopodia tip. Possibly, upon binding to β1 integrins, the ICAM-5 ectodomain is partially covered, which makes the proteolytic sites less accessible to MMPs. Thus, the interaction of ICAM-5 and β1 integrins seems to provide a protecting mechanism affecting ICAM-5 ectodomain cleavage early during spine maturation.
ICAM-5 ectodomain cleavage was efficiently inhibited by activating β1 integrins, whereas the cleavage was enhanced by blocking antibodies. Importantly, ICAM-5 mAbs also showed similar effects. Interestingly, sICAM-5 treatment also inhibited ICAM-5 ectodomain cleavage. In fact, when treated with ICAM-5-Fc proteins, cultured hippocampal neurons exhibited an increased number and length of filopodia but an unchanged number of spines (Tian et al., 2007), which resembles the phenotype of β1 integrin-activating antibody-treated neurons. Added sICAM-5 may compete out the binding of β1 integrins to the endogenous ICAM-5. It has earlier been shown that soluble ICAM-1 and ICAM-2 promote β2 integrin-dependent T lymphocyte adhesion (Kotovuori et al., 1999). It is possible that sICAM-5 binding also activates β1 integrins and increases their binding affinity to the post-synaptic ICAM-5. ICAM-5 also shows homophilic binding (Tian et al., 2000b), and this binding may protect the ectodomain of ICAM-5 from being cleaved. The exact mechanism how sICAM-5 works remains to be elucidated.
In conclusion, we show that β1 integrins are also pre-synaptic and that they regulate synapse formation through the interaction with ICAM-5. This interaction, which inhibits the filopodia-to-spine transition, is most important at the early stage of synapse formation.
Materials and Methods
Reagents and antibodies
Human IgG, poly-L-lysine, GRGDS and SDGRG peptides were obtained from Sigma-Aldrich (St. Louis, MO). The PEF-BOS-ICAM-5 construct was made as described (Tian et al., 1997).
The polyclonal antibody (pAb) anti-ICAM-5cp against the mouse ICAM-5 cytoplasmic tail was a gift from Dr Y. Yoshihara (Brain Science Institute/Institute of Physical and Chemical Research, Wako City, Japan). The pAb 1000J and mAbs 127E, 179D, 179K, 246H, 246A and 179H against ICAM-5 ectodomains were gifts from P. Kilgannon (ICOS Corporation, Seattle, WA). The β1 integrin activating antibody TS2/16 was a gift from Dr T. A. Springer (Harvard Medical School, MA). The rabbit pAb against human FN was a gift from Dr Antti Vaheri (University of Helsinki). The rabbit anti-α5 integrin pAb (sc-10729), the goat anti-β1 integrin pAb (sc-6622), and the rabbit anti-β1 integrin pAb (sc-8978) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The rabbit anti-β1 integrin pAb (AB1952), the mouse anti-human β1 integrin adhesion blocking mAb 2253, the rabbit anti-α5 integrin pAb and the guinea pig anti-VGLUT1 pAb were purchased from Millipore (Billerica, MA). The hamster β1 integrin adhesion blocking mAb Ha2/5, which cross-reacts with mouse and rat β1 integrins (Michaluk et al., 2009), and the mouse anti-NR1 mAb were purchased from BD Biosciences (San Jose, CA). The rabbit anti-β1 integrin mAb (9EG7) and the PSD-95 mAb were obtained from Abcam (Cambridge, UK). Horseradish peroxidase (HRP)-conjugated anti-mouse, rabbit, goat and human IgGs were obtained from GE Healthcare Life Sciences (Uppsala, Sweden). Alexa488-, Cy3-, and Cy5-conjungated mouse, and rabbit IgGs, TRITC-conjugated phalloidin and Zenon labeling kit for rabbit IgG were all obtained from Invitrogen (Carlsbad, CA). Cy5-conjugated synapsin I mAb was obtained from Synaptic Systems (Göttingen, Germany).
Animals
ICAM-5 knockout mice were generated by gene targeting (Nakamura et al., 2001). Excitatory neuron-specific β1-integrin knockout mice were generated by crossing floxed β1-integrin and α-calcium/calmodulin-dependent protein kinase II-cAMP response element Cre (CaMKII-Cre) mice (Chan et al., 2006). The genetic background of all animals was normalized by backcrossing at least six generations with C57Bl/6 and maintained as homozygous lines. All experiments were approved by and performed according to the guidelines of the ethic committees for animal research at the University of Helsinki and Scripps Florida.
Cell lines
The stable neuronal cell lines, Paju-Neo, Paju-ICAM-5 and Paju-ICAM-5Δcp, were made as described earlier (Nyman-Huttunen et al., 2006). Cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) (Invitrogen), 1% penicillin-streptomycin, and 1% L-glutamine (Lonza Group Ltd, Switzerland), in the presence of 0.5 mg/ml G418 (Sigma-Aldrich). COS-1 cells were from ATCC and maintained as described above without G418.
Mouse brain homogenization and coimmunoprecipitation
Two adult mouse forebrains were homogenized in 10 volumes of ice-cold homogenization buffer (1% Triton X-100 in phosphate buffered saline (PBS), 1×protease inhibitor cocktail (Roche Diagnostics GmbH, Germany), 1×phosphatase inhibitor (Roche)), and centrifuged at 100,000 g for 1 h. The supernatant was precleared with 1 ml 50% Protein G Sepharose (Invitrogen) at 4°C for 1 h, and divided into 1 ml aliquots, which were left untreated or incubated with 2 µg/ml antibodies against ICAM-5 (246A, ICOS, WA), β1 (sc-6622, Santa Cruz) and α5 (sc-10729, Santa Cruz) subunits respectively, overnight at 4°C. 30 µl Protein G Sepharose was added and the samples were incubated with rotation for an additional hour at 4°C. The precipitates were washed with ice-cold homogenization buffer three times and resuspended in 25 µl 2×SDS sample loading buffer.
Recombinant protein purification
ICAM-5 ectodomain cDNAs coding for ICAM-5 Ig domains 1–2 (D1–2) and 1–9 (D1–9) were prepared as described earlier (Tian et al., 1997). COS-1 cells were transiently transfected with recombinant ICAM-5 cDNAs by using Fugene 6 transfection reagent (Roche), and recombinant ICAM-5 ectodomains were purified from the cell culture media by Protein A sepharose CL 4B (Invitrogen). Purified proteins were examined by SDS-PAGE and their concentrations determined using the BCA assay (Pierce, Rockford, IL).
Purification of α5β1 integrin
The α5β1 integrin was purified from human placenta by affinity chromatography using a wheat germ agglutinin agarose column and an affinity matrix of Sepharose coupled with the 110 kDa thermolysin fragment of FN (Argraves and Tran, 1994; Forsberg et al., 1994).
Enzyme-linked immunoassay (ELISA)
One µg of purified α5β1 integrin was attached to flat-bottom 96-well microtiter plates (Nunc, Denmark) in 25 mM Tris, pH 8.0, 150 mM NaCl, 1 mM CaCl2 and 1 mM MgCl2 by incubation overnight at 4°C. The wells were blocked with 1% milk powder for 1–2 h at room temperature (RT). Ten µg ICAM-5 D1–9-Fc or FN were then incubated on the plates at 1 µg/100 µl/well for 1 h at RT. The bound proteins were detected by HRP-conjugated anti-human IgG (for ICAM-5 D1–9-Fc) or FN rabbit pAb followed by HRP-conjugated anti-rabbit IgG (for FN). After washing, the plates were incubated in 100 µl/well 0.1 M phosphate-citrate, pH 5.0 containing 0.8 mg/ml OPD tablet (Dako, Finland), at 37°C for 30 min. The reaction was stopped by adding 100 µl 1 M H2SO4 and the absorbance at 492 nm was measured by spectrometry.
Cell adhesion assays
100 µl of purified recombinant ICAM-5-Fc, ICAM-2-Fc and human IgG (8.8 µM) were pre-coated on 96-well microtiter plates (Nunc) overnight at 4°C. After washing with PBS, the wells were blocked with 1% Bovine Serum Albumin (BSA)/PBS for 2 h at RT. Paju cells were detached, washed, and resuspended in DMEM containing 1% BSA, 2 mM MnCl2, 20 mM HEPES pH 7.4, at 106 cells/ml. 6×104 cells were added per well and incubated for 30 min at RT. For blocking experiments, ICAM-5-Fc protein-coated wells were treated with 100 µg/ml antibodies against ICAM-5: 179D, 179K, 246H and 246K. Meanwhile, the cells were treated with 100 µg/ml RGD-containing peptides or β1 integrin antibodies for 30 min at RT before being added to 96-well microtiter plates. Non-adherent cells were removed by gentle washing with PBS. After washing, the bound cells were lysed in 100 µl/well phosphatase substrate-containing lysis buffer (1% Triton X-100, 50 mM sodium acetate, pH 5.0, 3 mg/ml P-nitrophenyl phosphate), and incubated at 37°C for 30 min. The reaction was stopped by adding 50 µl/well 1M NaOH. The absorbance at 405 nm was measured by spectrometry. The percentage of bound cells was calculated as:
Western blotting
Samples were separated using 4–12% SDS-PAGE (Invitrogen) and transferred to nitrocellulose membranes (Whatman GmbH, Germany). After blocking, membranes were incubated with primary antibodies followed by incubation with HRP-conjugated secondary antibodies (Invitrogen). After washings with TBS and 0.05% Tween 20, labeled bands were visualized with an ECL kit (Pierce). Band intensity was quantified by ImageJ (NIH).
Isolation of pre- and post-synaptic fractions
The method was adapted from Bouvier et al., 2008 with modification. The whole procedure was carried out at 0–4°C unless otherwise stated. Forebrains from four male adult C57Bl/6 mice were homogenized in ice-cold sucrose/HEPES buffer (0.32 M sucrose, 10 mM HEPES, pH 7.4) containing protease inhibitors and centrifuged at 1000 g for 10 min. The supernatant was centrifuged at 17,500 g for 30 min. The pellet (P1, crude synaptosome) was resuspended in sucrose/HEPES buffer and layered on top of a discontinuous sucrose gradient (2.6 M, 1.2 M, 0.8 M). After ultra-centrifugation at 110,000 g for 2 h, the fraction between the 0.8 M/1.2 M sucrose interfaces, which contains the purified synaptosomal fraction was collected, and treated with 50 mM CaCl2, 1% Triton X-100, 20 mM Tris, pH 6.0 for 30 min. After centrifugation at 40,000 g for 30 min, the supernatant (S2, external junction), which mainly contains plasma and vesicle constituents, was saved. The pellet, constituting the pre-synaptic active zone and PSD proteins, was resuspended in 1% Triton X-100, 20 mM Tris, pH 8.0 and incubated for 30 min. The increase of pH from 6 to 8 released pre-synaptic active zones (S3) from PSDs (P3). Proteins in S2 and S3 were precipitated with acetone at −20°C overnight and all pellets were solubilized in 5% SDS.
The protein concentrations of the fractions were determined by the BCA assay. 30 µg protein/sample was applied in duplicates to SDS-PAGE followed by western blotting.
Primary hippocampal neuron cultures
Primary cultures of hippocampal neurons were prepared from C57Bl/6 mouse fetuses at gestational day 18 as previously described (Nyman-Huttunen et al., 2006). Dissociated neurons were counted and seeded on poly-L-lysine coated cell culture surfaces at the concentrations of 6×104/well for 24-well plates and 106/well for 6-well plates. All hippocampal neurons were cultured in glial cell-conditioned Neurobasal medium (Invitrogen) containing 2% B27 and 1% L-glutamine. One third of the culture media were refreshed every 3–4 days.
Immunofluorescence microscopy
Hippocampal neurons were transfected with the pEGFP-N1 plasmid by Lipofectamine 2000 (Invitrogen) 24 h before fixation and cultured until 15–22 DIV. Neurons were fixed with 4% paraformaldehyde (PFA) in PBS and permeabilized with 0.2% saponin. After blocking with 5% BSA/saponin/PBS, the cells were incubated with primary antibodies for 2 h at RT or overnight at 4°C, followed by incubation with the secondary antibodies for 1 h at RT. For ICAM-5/β1 integrin co-staining (Fig. 6C), ICAM-5-cp pAb was labeled with Alexa488-conjugated rabbit IgG by Zenon antibody labeling kit (Invitrogen) before being mixed with β1 integrin pAb. Images were obtained with a confocal laser-scanning microscope under 63×magnification (TSC SP2 AOBS, HCX OIL APO 63×objective/1.4-0.6; Leica) using a charge-coupled device camera (Leica) and the LCSLite software.
Cell stimulation
Primary hippocampal neurons were cultured in 6-well plates at 106 cells/well. The β1 integrin adhesion blocking antibody Ha2/5 and activating antibody TS2/16, and ICAM-5 antibodies 179D and 179K, were added to the culture medium respectively at 20 µg/ml on 11 DIV. On 14 DIV, cell culture medium was replaced with 1 ml Hank's Buffered Salt Solution (HBSS) containing 1.8 mM CaCl2. After 16 h, 1-ml aliquots of the conditioned medium were concentrated 20-fold by vivaspin centrifugal concentrators (Sartorius Ltd), and the cells were stripped with Laemmli sample buffer. 10% (v/v) lysates and the culture media were applied to SDS-PAGE.
Bead recruitment assay
4.84 µm diameter polystyrene beads (Bangs Laboratories) were washed with PBS and mixed with ligand proteins at 40 µg/ml overnight at 4°C. The beads were then blocked with 0.1% BSA for 1 h at RT, washed three times with PBS and resuspended in Neurobasal medium. 1–1.5×105 beads/coverslip were added to neuron cultures on 12–13 DIV and incubated for 24 h before fixation.
Patch clamp recordings, data acquisition and analysis
mEPSCs were recorded from cultured mouse hippocampal neurons (15–18 DIV) in a whole-cell voltage-clamp configuration at RT. The composition of the extracellular solution was: 124 mM NaCl, 3 mM KCl, 2 mM CaCl2, 1.1 mM NaH2PO4, 1.3 mM MgSO4, 20 mM HEPES, and 10 mM D-glucose, pH 7.4. Neurons were in the extracellular solution maximally for 2 h. To eliminate synchronized action potential-induced spontaneous post-synaptic currents, all experiments were performed in the presence of 1 µM tetrodotoxin (TTX). mEPSCs of glutamatergic origin were isolated by extracellular application of 10 µM bicuculline methiodide. Miniature spontaneous events were completely and reversibly blocked by NBQX and AP5 (data not shown). Patch pipettes were fabricated from borosilicate glass (GC-150F; Harvard Apparatus); and their resistance, when filled with intracellular solution containing 18 mM KCl, 111 mM potassium gluconate, 0.5 mM CaCl2, 5 mM BAPTA, 2 mM Mg-ATP, 10 mM HEPES, 10 mM glucose, and 2 mM NaOH, at pH 7.3, ranged from 6 to 8 MΩ. Membrane potential was clamped at −60 mV. Only cells with access resistance that did not exceed 20 MΩ were accepted for analysis. Access- and cell membrane resistance were regularly monitored by 10-mV hyperpolarizing voltage pulses 100 ms long, and recordings were terminated if any of these resistances changed by 10% or more.
A patch-clamp amplifier (EPC 9; HEKA) was used for voltage clamp and data acquisition. Data were acquired at sampling rate of 2 kHz and then band-pass filtered (1 Hz–1 kHz). Synaptic events were detected and analyzed using Mini Analysis software (version 6; Synaptosoft Inc.). After automatic screening (amplitude threshold set to 5 pA), events were approved manually according to their onset and decay. Because glutamatergic synaptic activity strongly depends on the age of neurons, the effect of ICAM-5 deficiency was assessed from recordings obtained on the same DIV. Only recordings that contained at least 200 synaptic events were accepted for analysis. The inter-event interval and event amplitude for each individual cell were characterized by their median values. The mean of median inter-event intervals and event amplitudes for each experimental paradigm was normalized to corresponding mean control values obtained on the same experimental day.
Lentivirus-based β1 integrin knockdown
Mouse GIPZ lentiviral shRNAmir plasmids for β1 integrin subunit (Clones V2LMM_39157, V2LMM_188403 and V3LMM_429934) and a control GIPZ empty plasmid were purchased from Open Biosystems/ThermoFisher. The level of β1 integrin knockdown was accessed by transfecting N2A cells and the most effective shRNA plasmids were selected for transfecting neurons (supplementary material Fig. S4). Ten DIV cultured hippocampal neurons were transfected with β1 integrin-shRNA or control plasmids and cultured for an additional 48 h before fixation.
β1 integrin activation by TS2/16 in N2A cells
N2A cells were grown in DMEM with 10% FBS, 1% penicillin-streptomycin and 1% L-glutamine for 24 h and changed into HBSS without or with 1 mM MnCl2. 10 µg/ml mIgG or β1 integrin-activating antibody TS2/16 were added into cells and incubated for 30 min at 37°C. Cells were then fixed with 4% PFA and immunostained with β1 integrin monoclonal antibody 9EG7. This antibody has been previously found to recognize the ligand-bound conformation of β1 integrins (Bazzoni et al., 1995).
Electron microscopy
12–16 weeks old male C57 Bl/6 mice were anaesthetized and perfused with PBS and 0.1 M phosphate buffer, pH 7.4, followed by 4% PFA. Hippocampi were dissected into 1 mm3 cubes and further fixed in 4% PFA at 4°C for 4 days. To improve the plasticity of tissue blocks, tissue blocks were infused with 1.8 M sucrose and 20% (w/v) polyvinylpyrrolidone and frozen in liquid nitrogen (Tokuyasu, 1989). Frozen blocks were trimmed and cut into 60-nm-thick sections at −120°C. After being blocked with 1% fish skin gelatin (FSG), 1% FBS in 50 mM NH4Cl/PBS, pH 7.4, sections were incubated with anti β1 integrin pAb at 5 µg/ml (AB1952, Millipore), followed by protein A-conjugated 10 nm gold particles (University of Utrecht, The Netherlands). Excessive washing was performed after each antibody incubation. To increase the contrast, sections were incubated with 2% neutral uranyl acetate for 10 min at RT, followed by incubation with 1.8% methyl cellulose/0.4% uranyl acetate for 15 min on ice. Images were taken with an electron microscope (Tecnai 12, FEI Company, Holland) running at 80 kV under 11,000×magnification. The labeling of β1 integrins in active zones was quantified from ∼150 synapses which were randomly imaged from four grids. The active zone was divided horizontally into four equal sections along the pre-synaptic membrane. The two sections in the middle composed the ‘central’ sub-region, while the two sections on both sides of the active zone were named as the ‘peripheral’ sub-region. The number of gold particles was quantified and presented as the percentage of β1 integrin labeling on each sub-region: % β1 integrin labeling on the sub-region = number of gold particles (sub-region)/number of gold particles (whole active zone)×100%.
Quantitative analysis for immunofluorescence
Quantification was performed on more than 10 neurons, which were randomly selected from three independent experiments. Segments of dendrites less than 100 µm apart from the somas were used for quantification. For colocalization analysis, region of interest (ROI) was selected manually in a random manner. Only those puncta which have ≥50% area fallen in the ROIs were counted as ‘colocalized’. Analysis was performed with the same criteria in all experiments and the genotypes or treatments were unknown to the analyzer.
For spine analysis, dendritic protrusions were categorized by the following criteria: mushroom spine: length <3 µm and with an enlarged head; thin spine: length >3 µm and with an enlarged head; filopodium: length = 3–10 µm, without an enlarged head. To quantify the correlation of β1 integrins with the EGFP-labeled spines, >50% areas of spine heads overlapping with β1 integrin staining was defined as ‘colocalized’, while <50% was ‘juxtaposed’. Images were processed with Photoshop and ImageJ.
Statistical analysis
Mann–Whitney U tests were used to measure the significance of inter-group differences between datasets.
Supplementary Material
Acknowledgments
We thank Dr Yoshihiro Yoshihara for providing the anti-ICAM-5cp pAb; Dr Patrick Kilgannon for rat ICAM-5 mAbs and the pAb 1000J; Dr Timothy A. Springer for the β1 integrin activating antibody TS2/16; Dr Antti Vaheri for the rabbit anti-fibronectin pAb; Juha Kuja-Panula for help with Lentiviral shRNA; Leena Kuoppasalmi, Seija Lehto, Outi Nikkilä, Erja Huttu and Maria Aatonen for technical assistance; and Yvonne Heinilä and Leea Sokura for secretarial help.
Footnotes
Funding
This study was supported by the Academy of Finland, the Sigrid Jusélius Foundation, the Magnus Ehrnrooth Foundation, the Finnish Medical Association, Wilhelm och Else Stockmanns stiftelse and the Liv och Hälsa Foundation. Additional support was from the National Institutes of Health [grant number MH060420 to R.L.D.]. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.106674/-/DC1
References
- Argraves W. S., Tran H. (1994). Purification of α5β1 integrin by ligand affinity chromotography. J. Tissu. Cult. Meth. 16, 243–247 10.1007/BF01540660 [DOI] [Google Scholar]
- Bahr B. A., Staubli U., Xiao P., Chun D., Ji Z. X., Esteban E. T., Lynch G. (1997). Arg-Gly-Asp-Ser-selective adhesion and the stabilization of long-term potentiation: pharmacological studies and the characterization of a candidate matrix receptor. J. Neurosci. 17, 1320–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkat T. R., Polley D. B., Hensch T. K. (2011). A critical period for auditory thalamocortical connectivity. Nat. Neurosci. 14, 1189–1194 10.1038/nn.2882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzoni G., Shih D-T., Buck C. A., Hemler M. E. (1995). Monoclonal antibody 9EG7 defines a novel β1 integrin epitope induced by soluble ligand and manganese, but inhibited by calcium. J. Biol. Chem. 270, 25570–25577 10.1074/jbc.270.43.25570 [DOI] [PubMed] [Google Scholar]
- Benson D. L., Yoshihara Y., Mori K. (1998). Polarized distribution and cell type-specific localization of telencephalin, an intercellular adhesion molecule. J. Neurosci. Res. 52, 43–53 [DOI] [PubMed] [Google Scholar]
- Biederer T., Sara Y., Mozhayeva M., Atasoy D., Liu X., Kavalali E. T., Südhof T. C. (2002). SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science 297, 1525–1531 10.1126/science.1072356 [DOI] [PubMed] [Google Scholar]
- Bouvier D., Corera A. T., Tremblay M. E., Riad M., Chagnon M., Murai K. K., Pasquale E. B., Fon E. A., Doucet G. (2008). Pre-synaptic and post-synaptic localization of EphA4 and EphB2 in adult mouse forebrain. J. Neurochem. 106, 682–695 10.1111/j.1471-4159.2008.05416.x [DOI] [PubMed] [Google Scholar]
- Chan C. S., Davis R. L.2008). Integrins and cadherins – Extracellular matrix in memory formation. Learning and Memory: A Comprehensive Reference. Byrne721–740 Oxford, UK: Elsevier [Google Scholar]
- Chan C. S., Weeber E. J., Kurup S., Sweatt J. D., Davis R. L. (2003). Integrin requirement for hippocampal synaptic plasticity and spatial memory. J. Neurosci. 23, 7107–7116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C. S., Weeber E. J., Zong L., Fuchs E., Sweatt J. D., Davis R. L. (2006). β1-integrins are required for hippocampal AMPA receptor-dependent synaptic transmission, synaptic plasticity, and working memory. J. Neurosci. 26, 223–232 10.1101/lm.648607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C. S., Levenson J. M., Mukhopadhyay P. S., Zong L., Bradley A., Sweatt J. D., Davis R. L. (2007). Alpha3-integrins are required for hippocampal long-term potentiation and working memory. Learn. Mem. 14, 606–615 10.1101/lm.648607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C. S., Chen H., Bradley A., Dragatsis I., Rosenmund C., Davis R. L. (2010). α8-integrins are required for hippocampal long-term potentiation but not for hippocampal-dependent learning. Genes Brain Behav. 9, 402–410 10.1111/j.1601-183X.2010.00569.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani L. A., Thalhammer A., Yu L. M., Catalano M., Ramos T., Colicos M. A., Goda Y. (2008). Activity-dependent regulation of synaptic AMPA receptor composition and abundance by beta3 integrins. Neuron 58, 749–762 10.1016/j.neuron.2008.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. W., Hoffstrom B. G., DeSimone D. W.2000). Active zones on motor nerve terminals contain alpha 3beta 1 integrin. J. Neurosci. 204912–4921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conant K., Wang Y., Szklarczyk A., Dudak A., Mattson M. P., Lim S. T. (2010). Matrix metalloproteinase-dependent shedding of intercellular adhesion molecule-5 occurs with long-term potentiation. Neuroscience 166, 508–521 10.1016/j.neuroscience.2009.12.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conant K., Lonskaya I., Szklarczyk A., Krall C., Steiner J., Maguire–Zeiss K., Lim S. T. (2011). Methamphetamine-associated cleavage of the synaptic adhesion molecule intercellular adhesion molecule-5. J. Neurochem. 118, 521–532 10.1111/j.1471-4159.2010.07153.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalva M. B., McClelland A. C., Kayser M. S.2007). Cell adhesion molecules: signalling functions at the synapse. Nat. Rev. Neurosci. 8206–220 10.1038/nrn2075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethell I. M., Pasquale E. B. (2005). Molecular mechanisms of dendritic spine development and remodeling. Prog. Neurobiol. 75, 161–205 10.1016/j.pneurobio.2005.02.003 [DOI] [PubMed] [Google Scholar]
- Forsberg E., Ek B., Engström Å., Johansson S. (1994). Purification and characterization of integrin α9β1. Exp. Cell Res. 213, 183–190 10.1006/excr.1994.1189 [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G. (1997). Leukocyte adhesion: CD11/CD18 integrins and intercellular adhesion molecules. Curr. Opin. Cell Biol. 9, 643–650 10.1016/S0955-0674(97)80117-2 [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Tian L., Ning L., Nyman–Huttunen H. (2008). ICAM-5—a novel two-facetted adhesion molecule in the mammalian brain. Immunol. Lett. 117, 131–135 10.1016/j.imlet.2008.02.004 [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Fagerholm S. C., Nurmi S. M., Chavakis T., Marchesan S., Grönholm M.2009). Regulation of integrin activity and signalling. Biochim. Biophys. Acta 1790431–444 10.1016/j.bbagen.2009.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner N. J., Moffatt S., Fernyhough P., Humphries M. J., Streuli C. H., Tomlinson D. R. (2007). Preconditioning injury-induced neurite outgrowth of adult rat sensory neurons on fibronectin is mediated by mobilisation of axonal alpha5 integrin. Mol. Cell. Neurosci. 35, 249–260 10.1016/j.mcn.2007.02.020 [DOI] [PubMed] [Google Scholar]
- Hellwig S., Hack I., Kowalski J., Brunne B., Jarowyj J., Unger A., Bock H. H., Junghans D., Frotscher M. (2011). Role for Reelin in neurotransmitter release. J. Neurosci. 31, 2352–2360 10.1523/JNEUROSCI.3984-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. (2002). Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 10.1016/S0092-8674(02)00971-6 [DOI] [PubMed] [Google Scholar]
- Ko J., Fuccillo M. V., Malenka R. C., Südhof T. C. (2009). LRRTM2 functions as a neurexin ligand in promoting excitatory synapse formation. Neuron 64, 791–798 10.1016/j.neuron.2009.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotovuori A., Pessa–Morikawa T., Kotovuori P., Nortamo P., Gahmberg C. G. (1999). ICAM-2 and a peptide from its binding domain are efficient activators of leukocyte adhesion and integrin affinity. J. Immunol. 162, 6613–6620 [PubMed] [Google Scholar]
- Mah W., Ko J., Nam J., Han K., Chung W. S., Kim E. (2010). Selected SALM (synaptic adhesion-like molecule) family proteins regulate synapse formation. J. Neurosci. 30, 5559–5568 10.1523/JNEUROSCI.4839-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno H., Okabe S., Mishina M., Yanagida T., Mori K., Yoshihara Y. (2006). Telencephalin slows spine maturation. J. Neurosci. 26, 1776–1786 10.1523/JNEUROSCI.2651-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaluk P., Mikasova L., Groc L., Frischknecht R., Choquet D., Kaczmarek L. (2009). Matrix metalloproteinase-9 controls NMDA receptor surface diffusion through integrin beta1 signaling. J. Neurosci. 29, 6007–6012 10.1523/JNEUROSCI.5346-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsui S., Saito M., Hayashi K., Mori K., Yoshihara Y. (2005). A novel phenylalanine-based targeting signal directs telencephalin to neuronal dendrites. J. Neurosci. 25, 1122–1131 10.1523/JNEUROSCI.3853-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T., Yoshihara Y., Inazawa J., Kagamiyama H., Mori K. (1997). cDNA cloning and chromosomal localization of the human telencephalin and its distinctive interaction with lymphocyte function-associated antigen-1. J. Biol. Chem. 272, 1156–1163 10.1074/jbc.272.2.1156 [DOI] [PubMed] [Google Scholar]
- Mizuno T., Yoshihara Y., Kagamiyama H., Ohsawa K., Imai Y., Kohsaka S., Mori K. (1999). Neuronal adhesion molecule telencephalin induces rapid cell spreading of microglia. Brain Res. 849, 58–66 10.1016/S0006-8993(99)01984-8 [DOI] [PubMed] [Google Scholar]
- Nakamura K., Manabe T., Watanabe M., Mamiya T., Ichikawa R., Kiyama Y., Sanbo M., Yagi T., Inoue Y., Nabeshima T.et al. (2001). Enhancement of hippocampal LTP, reference memory and sensorimotor gating in mutant mice lacking a telencephalon-specific cell adhesion molecule. Eur. J. Neurosci. 13, 179–189 10.1046/j.0953-816X.2000.01366.x [DOI] [PubMed] [Google Scholar]
- Nguyen T., Südhof T. C. (1997). Binding properties of neuroligin 1 and neurexin 1beta reveal function as heterophilic cell adhesion molecules. J. Biol. Chem. 272, 26032–26039 10.1074/jbc.272.41.26032 [DOI] [PubMed] [Google Scholar]
- Nyman–Huttunen H., Tian L., Ning L., Gahmberg C. G. (2006). alpha-Actinin-dependent cytoskeletal anchorage is important for ICAM-5-mediated neuritic outgrowth. J. Cell Sci. 119, 3057–3066 10.1242/jcs.03045 [DOI] [PubMed] [Google Scholar]
- Oka S., Mori K., Watanabe Y. (1990). Mammalian telencephalic neurons express a segment-specific membrane glycoprotein, telencephalin. Neuroscience 35, 93–103 10.1016/0306-4522(90)90124-M [DOI] [PubMed] [Google Scholar]
- Pinkstaff J. K., Lynch G., Gall C. M.1998). Localization and seizure-regulation of integrin β 1 mRNA in adult rat brain. Brain Res. Mol. Brain Res. 55265–276 10.1016/S0169-328X(98)00007-2 [DOI] [PubMed] [Google Scholar]
- Ransohoff R. M., Cardona A. E. (2010). The myeloid cells of the central nervous system parenchyma. Nature 468, 253–262 10.1038/nature09615 [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. (1986). Arg-Gly-Asp: a versatile cell recognition signal. Cell 44, 517–518 10.1016/0092-8674(86)90259-X [DOI] [PubMed] [Google Scholar]
- Shi Y., Ethell I. M. (2006). Integrins control dendritic spine plasticity in hippocampal neurons through NMDA receptor and Ca2+/calmodulin-dependent protein kinase II-mediated actin reorganization. J. Neurosci. 26, 1813–1822 10.1523/JNEUROSCI.4091-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Arstikaitis P., Prasad T., Bartlett T. E., Wang Y. T., Murphy T. H., Craig A. M.2011). post-synaptic TrkC and presynaptic PTPσ function as a bidirectional excitatory synaptic organizing complex. Neuron 69287–303 10.1016/j.neuron.2010.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L., Yoshihara Y., Mizuno T., Mori K., Gahmberg C. G. (1997). The neuronal glycoprotein telencephalin is a cellular ligand for the CD11a/CD18 leukocyte integrin. J. Immunol. 158, 928–936 [PubMed] [Google Scholar]
- Tian L., Kilgannon P., Yoshihara Y., Mori K., Gallatin W. M., Carpén O., Gahmberg C. G. (2000a). Binding of T lymphocytes to hippocampal neurons through ICAM-5 (telencephalin) and characterization of its interaction with the leukocyte integrin CD11a/CD18. Eur. J. Immunol. 30, 810–818 [DOI] [PubMed] [Google Scholar]
- Tian L., Nyman H., Kilgannon P., Yoshihara Y., Mori K., Andersson L. C., Kaukinen S., Rauvala H., Gallatin W. M., Gahmberg C. G. (2000b). Intercellular adhesion molecule-5 induces dendritic outgrowth by homophilic adhesion. J. Cell Biol. 150, 243–252 10.1083/jcb.150.1.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L., Stefanidakis M., Ning L., Van Lint P., Nyman–Huttunen H., Libert C., Itohara S., Mishina M., Rauvala H., Gahmberg C. G. (2007). Activation of NMDA receptors promotes dendritic spine development through MMP-mediated ICAM-5 cleavage. J. Cell Biol. 178, 687–700 10.1083/jcb.200612097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L., Lappalainen J., Autero M., Hänninen S., Rauvala H., Gahmberg C. G.2008). Shedded neuronal ICAM-5 suppresses T-cell activation. Blood 1113615–3625 10.1182/blood-2007-09-111179 [DOI] [PubMed] [Google Scholar]
- Tian L., Rauvala H., Gahmberg C. G.2009). Neuronal regulation of immune responses in the central nervous system. Trends Immunol. 3091–99 10.1016/j.it.2008.11.002 [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. T. (1989). Use of poly(vinylpyrolidone) and poly(vinykalcohol) for cryo-ultramicrotomy. Histochem. J. 21, 163–171 [DOI] [PubMed] [Google Scholar]
- Torres R., Firestein B. L., Dong H., Staudinger J., Olson E. N., Huganir R. L., Bredt D. S., Gale N. W., Yancopoulos G. D. (1998). PDZ proteins bind, cluster, and synaptically colocalize with Eph receptors and their ephrin ligands. Neuron 21, 1453–1463 10.1016/S0896-6273(00)80663-7 [DOI] [PubMed] [Google Scholar]
- Wang X. B., Bozdagi O., Nikitczuk J. S., Zhai Z. W., Zhou Q., Huntley G. W. (2008). Extracellular proteolysis by matrix metalloproteinase-9 drives dendritic spine enlargement and long-term potentiation coordinately. Proc. Natl. Acad. Sci. USA 105, 19520–19525 10.1073/pnas.0807248105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb D. J., Zhang H., Majumdar D., Horwitz A. F. (2007). alpha5 integrin signaling regulates the formation of spines and synapses in hippocampal neurons. J. Biol. Chem. 282, 6929–6935 10.1074/jbc.M610981200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara Y., Mori K. (1994). Telencephalin: a neuronal area code molecule? Neurosci. Res. 21, 119–124 10.1016/0168-0102(94)90153-8 [DOI] [PubMed] [Google Scholar]
- Zhang H., Casasnovas J. M., Jin M., Liu J. H., Gahmberg C. G., Springer T. A., Wang J. H. (2008). An unusual allosteric mobility of the C-terminal helix of a high-affinity alphaL integrin I domain variant bound to ICAM-5. Mol. Cell 31, 432–437 10.1016/j.molcel.2008.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv N. E., Smith S. J. (1996). Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron 17, 91–102 10.1016/S0896-6273(00)80283-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.