Summary
Many neuronal mRNAs are transported from cell bodies into axons and dendrites. Localized translation of the mRNAs brings autonomy to these processes that can be vast distances from the cell body. For axons, these translational responses have been linked to growth and injury signaling, but there has been little information about local function of individual axonally synthesized proteins. In the present study, we show that axonal injury increases levels of the mRNA encoding neural membrane protein 35 (NMP35) in axons, with a commensurate decrease in the cell body levels of NMP35 mRNA. The 3′ untranslated region (3′UTR) of NMP35 is responsible for this localization into axons. Previous studies have shown that NMP35 protein supports cell survival by inhibiting Fas-ligand-mediated apoptosis; however, these investigations did not distinguish functions of the locally generated NMP35 protein. Using axonally targeted versus cell-body-restricted NMP35 constructs, we show that NMP35 supports axonal growth, and overexpression of an axonally targeted NMP35 mRNA is sufficient to increase axonal outgrowth.
Key words: RNA transport, Axon growth, Axonal protein synthesis, Regeneration
Introduction
Polarized eukaryotic cells transport mRNAs from the nucleus to subcellular domains, providing a precise spatial and temporal control of protein levels (Jung et al., 2012). For rodent neurons, the ends of their processes can be centimeters distance from the cell body. mRNAs are actively transported into these distal processes by microtubule-based transport mechanisms (Donnelly et al., 2010). Although mRNAs and translational machinery were initially detected in the post-synaptic processes of the neuron (i.e. dendrites), it is now clear that the pre-synaptic or axonal processes also have the capacity to synthesize proteins (Jung et al., 2012). This has been particularly evident in growing axons, both for developing and regenerating axons, but there are several publications supporting the notion that mRNAs and ribosomes are present in mature axons of neurons in the peripheral nervous system (PNS) prior to injury (Ben-Yaakov et al., 2012; Hanz et al., 2003; Koenig et al., 2000; Perlson et al., 2005; Yudin et al., 2008; Zelená, 1970). Studies of cutaneous nerve terminals also suggest that translation of mRNAs can be triggered by pain invoking stimuli (Jimenez-Diaz et al., 2008; Melemedjian et al., 2010). In developing axons, translation of new proteins is needed for response to some guidance cues (Jung et al., 2011). However, a study from the Letourneau lab raised questions on this need for localized protein synthesis in developing axons (Roche et al., 2009). Thus, despite that mRNA translation has been demonstrated in axons, questions remain on what the locally generated proteins do in the axon.
Neurons have proven a particularly appealing model to study mRNA localization. Even in cultured neurons, axons can extend millimeters from the cell body making it possible to physically isolate these processes to purity (Willis and Twiss, 2011). RNA profiling studies of axonal processes in cultured neurons have shown increasing complexity, such that hundreds of different mRNAs are now known to be transported into axons (Gumy et al., 2011; Taylor et al., 2009; Zivraj et al., 2010). It is not known if such a complex population of mRNAs is similarly transported into axons in vivo. We have recently used a transgenic approach to show that β-actin mRNA is transported into axons of neurons in the peripheral and central nervous systems (CNS) (Willis et al., 2011). Moreover, localized translation of β-actin and GAP-43 mRNAs support regeneration of PNS axons (Donnelly et al., 2011). It seems likely that protein products of other axonal mRNAs also contribute to regeneration of axons. Consistent with this, mice lacking the β-actin gene apparently showed normal development and regeneration of motor axons (Cheever et al., 2011). This could indicate that β-actin is not needed for regeneration, or that other locally synthesized proteins can compensate for the loss of β-actin. Thus, in depth analyses for regulation and function of other axonal mRNAs are needed.
The axonal transcriptome of cultured dorsal root ganglion (DRG) neuron includes several transmembrane and secreted proteins (Gumy et al., 2011; Willis et al., 2007). Although the classic ultrastructure of rough endoplasmic reticulum (RER) and Golgi apparatus has not been identified in axons, we have previously shown that growing axons have functional equivalents of RER and Golgi apparatus for secretion of locally synthesized proteins (Merianda et al., 2009). However, evidence for membrane insertion or secretion of individual axonally synthesized proteins has been lacking. Here, we have focused on neural membrane protein 35 (NMP35), which we previously identified by cDNA array hybridization as an axonal mRNA in cultured adult DRG neurons (Gumy et al., 2011; Willis et al., 2007). NMP35 is a transmembrane protein that was initially cloned in attempts to identify developmentally regulated genes in peripheral nerve, but as its name implies its expression is limited to neurons (Schweitzer et al., 2002; Schweitzer et al., 1998). NMP35 has also been termed as the Fas apoptosis inhibitor protein 2 (FAIM2) and Lifeguard (LFG) (Beier et al., 2005; Fernández et al., 2007). The designations FAIM2 and LFG stemmed from studies showing that this protein can prevent apoptosis triggered by Fas ligand (FasL) (Beier et al., 2005; Fernández et al., 2007). However, these studies did not take into account the possibility that NMP35 protein may be synthesized locally. In the studies here, we have used RNA targeting constructs to test functions of cell body versus axonally synthesized NMP35 protein.
NMP35′s sequence was also shown to have homology to the glutamate binding subunit of the rat and Drosophila NMDA receptor (GRINA1; previously termed glutamate binding protein and dNMDARA1, respectively) (Schweitzer et al., 2002). Previous immuno-electron microscopy studies showed NMP35 protein to be present in post-synaptic processes, but the protein was also detected in efferent processes of spinal motor neurons (Schweitzer et al., 2002). Here, we show that NMP35 mRNA localizes to axons as well as dendrites of cultured neurons. This mRNA is targeted to PNS axons in vivo, and the 3′ untranslated region (3′UTR) of NMP35 mRNA is both necessary and sufficient for its transport into axons. Axonal localization of NMP35 mRNA increases after axonal injury. Moreover, expression studies indicate that axonal transport of NMP35 mRNA with subsequent localized translation provides a function in axonal growth that is distinct from cell body restricted NMP35 (FAIM2, LFG) previously used for overexpression studies (Beier et al., 2005; Fernández et al., 2007).
Results
Differential localization of membrane protein mRNAs in the axons
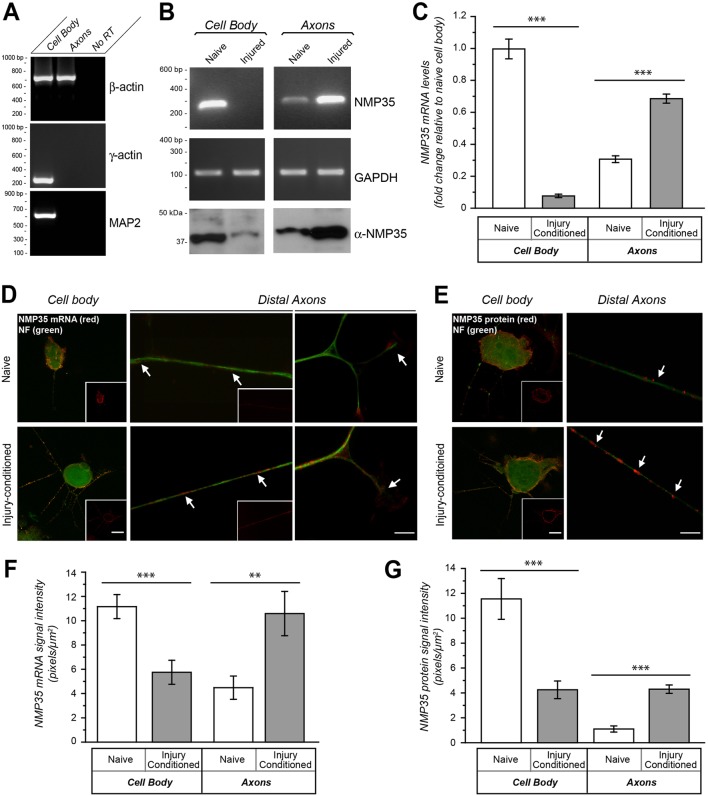
Although initial cloning of NMP35 mRNA focused on identifying Schwann cell gene products (Schweitzer et al., 1998), NMP35 protein expression was shown to be restricted to neurons (Schweitzer et al., 2002). We detected NMP35 as an axonal mRNA using cDNA arrays to profile transcripts from cultures of injury-conditioned adult DRGs (Willis et al., 2007). The transport of NMP35 mRNA into axons of DRG neurons was not significantly affected by neurotrophins, semaphorin, or myelin-associated glycoprotein, stimuli that we previously showed trigger robust changes in transport of other axonal mRNAs (Willis et al., 2007). Thus, we asked if the injury conditioning could affect the axonal levels of NMP35 mRNA. For this, we cultured DRG neurons on porous membranes to isolate axonal processes (Zheng et al., 2001). Reverse transcriptase coupled polymerase chain reaction (RT-PCR) confirmed purity of the axonal preparations showing amplification of β-actin mRNA from the axonal preparations but not the cell body restricted γ-actin and MAP2 mRNAs (Fig. 1A). Both standard and quantitative RT-PCR (RTqPCR) showed increased NMP35 mRNA levels in the axons from the injury-conditioned compared to naïve DRGs (Fig. 1B,C). Although the overall levels of NMP35 mRNA did not appreciably change in these DRG cultures (supplementary material Fig. S1A), the cell body levels of NMP35 mRNA in the injury-conditioned DRG cultures was significantly decreased compared to naïve cultures (Fig. 1B,C). By fluorescence in situ hybridization (FISH), NMP35 mRNA showed granular signals in the axon shaft of the cultured DRG neurons that extended distally into the growth cones (Fig. 1D). Scrambled probes and no probe controls showed minimal background labeling (supplementary material Fig. S1B). Comparing processes of injury-conditioned to naïve DRG neurons, axonal NMP35 mRNA levels showed a significant increase and cell body NMP35 mRNA showed a significant decrease (Fig. 1D,F) consistent with the RT-PCR results above. Furthermore, immunolabeling showed a significant increase in axonal signals and decrease in the cell body signals for NMP35 protein with injury conditioning (Fig. 1E,G).
Fig. 1.
NMP35 mRNA and protein are enriched in axons of injury-conditioned neurons in culture. Dissociated DRG cultures prepared from naïve and injured rats at 7 days following sciatic nerve crush were used for fractionation of cell bodies and axons (A–C) or for FISH and immunofluorescence (D–G) after 24 hours in vitro. (A–C) By RT-PCR, β-actin was detected only in the axonal processes, but γ-actin and MAP2 mRNAs were restricted to the cell body samples showing purity of the axonal preparations (A). By both standard (B) and quantitative RT-PCR (C) NMP35 mRNA levels shift from cell body to axon predominance in the neurons from the injured animals. Amplification of GAPDH shows equivalent levels of total RNA in the standard RT-PCR; 12S rRNA was used for normalization of the RTqPCR. (D,E) By FISH (D) and immunofluorescence (E), both NMP35 mRNA and protein appear more abundant in the axons (arrows) and reduced in cell bodies of DRG neurons cultured from injury-conditioned compared with naïve animals. The cell body images in D and E show XYZ maximum projections constructed from 10 optical planes taken at 0.5 µm intervals; the inset panels show only the NMP35 mRNA or protein signals. (F,G) Quantification of FISH (F) and immunofluorescence (G) signals for axons and cell bodies from matched exposure images as in D and E are shown. All image pairs of naïve and injury-conditioned neurons are matched for exposure, gain, offset and post-processing (n≥15 for cell body and n≥20 for axons from at least three separate experiments; **P≤0.01, ***P≤0.001 by Student's t-test). Values in C, F and G are means±s.e.m. Scale bars: 10 µm for main panels, 50 μm for insets.
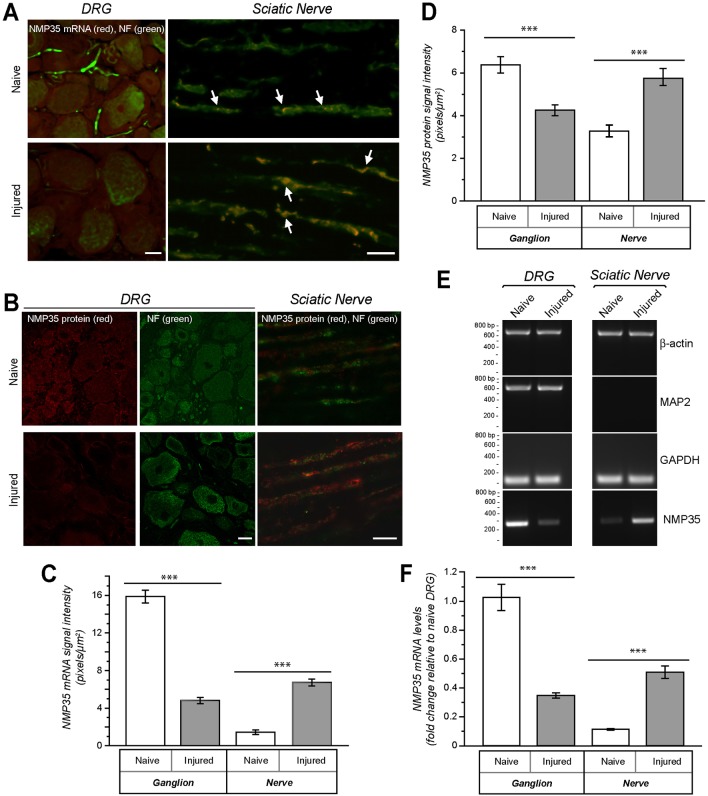
Since NMP35 mRNA increased in axons of injury-conditioned neurons in vitro, we asked if a similar increase in axonal NMP35 mRNA and protein might be seen in vivo after axonal injury. Confocal imaging of DRG and sciatic nerve sections that were processed for RNA-FISH showed clear cell body and axonal signals for NMP35 mRNA (Fig. 2A,C). NMP35 mRNA and protein appeared overall more abundant in the injured nerves and less abundant in the DRG compared with signals in uninjured tissues at seven days after nerve crush (Fig. 2B,D). Since NMP35 mRNA was only seen in the cell bodies and axons by these FISH analyses, we used RT-PCR to compare levels of NMP35 mRNA in nerves at seven days after a unilateral sciatic nerve crush. Axons in the crushed sciatic nerves showed fourfold more NMP35 mRNA than those in the uninjured nerves (Fig. 2E,F). Moreover, NMP35 mRNA levels were threefold lower in L4-5 DRGs ipsilateral to the injury compared to contralateral uninjured ganglia (Fig. 2E,F). Taken together, these data indicate that axotomy by nerve crush injury triggers a shift in NMP35 mRNA localization, with increased levels of NMP35 mRNA and protein in injured or regenerating versus uninjured axons.
Fig. 2.
Axotomy increases axonal localization of NMP35 mRNA in vivo. NMP35 mRNA and protein levels were examined in naïve versus crush-injured sciatic nerves at 7 days after injury. The nerve was sampled just proximal to the crush injury with a corresponding level of naïve nerve sampled; L4-5 DRGs are illustrated. (A) Representative exposure matched confocal image pairs of naïve versus injured nerve for FISH/immunofluorescence show increased signal for NMP35 mRNA in axons of the injured sciatic nerve (arrows) and a decrease in NMP35 mRNA signal in the neuronal cell bodies of L4-5 DRGs compared with uninjured nerve and DRG. (B) Representative exposure-matched confocal image pairs as in A show a similar increased signal for NMP35 protein in the sciatic nerve and decreased signal for NMP35 protein in the L4-5 DRG neuronal cell bodies at 7 days after injury compared with uninjured nerve and DRG. (C) Quantification of NMP35 mRNA intensity that overlapped with neurofilament heavy (NF H) protein signal in naïve and injured sciatic nerve and DRG sections is shown. (D) Quantification of NMP35 protein intensity overlapping with NF H signal in uninjured and injured sciatic nerve versus DRG is shown. (E,F) RNA was purified from sciatic nerve and L4-5 DRGs, both naïve and at 7 days post crush injury, and used for RT-PCR to gain a more quantitative estimate of changes in NMP35 mRNA levels. Standard RT-PCR amplification of β-actin and GAPDH mRNAs shows relative equivalent loading between samples; absence of MAP2 mRNA in the sciatic nerve is consistent with the axonal nature of the neuronal processes in the sciatic nerve (E). NMP35 mRNA appears to increase in the nerve and decrease in the DRG following crush injury. RTqPCR similarly shows an increase in NMP35 mRNA in sciatic nerve and a decrease in DRG (F). 12S rRNA was used to normalize RTqPCR data (n≥20 from at least three separate experiments; ***P≤0.001 by Student's t-test for indicated data pairs). Values in C, D and F are means±s.e.m. Scale bars: 5 µm for DRG images; 10 µm for sciatic nerve images.
NMP35 protein has been shown to concentrate in post-synaptic terminals of mature, neurons (Schweitzer et al., 2002). The DRG neurons used here only extend Tau-positive, MAP2-negative axonal processes in culture, and both the centrally and distally projecting processes of these sensory neurons show axonal features in vivo (Zheng et al., 2001). Thus, we asked if NMP35 mRNA also localizes into axonal processes of fully polarized CNS neurons. By FISH analyses, NMP35 mRNA is seen in both the dendritic and axonal processes of cultured cortical neurons (supplementary material Fig. S2), indicating that NMP35 mRNA can localize into both pre-synaptic and post-synaptic compartments consistent with previous analyses of its protein localization (Schweitzer et al., 2002).
NMP35 mRNA's 3′UTR drives axonal localization in DRG neurons
Given the potential shift in transport of NMP35 mRNA with axonal injury, we asked how this mRNA is localized into axons. UTRs, particularly the 3′UTR, are frequently sites for localization elements (Andreassi and Riccio, 2009). Thus, we asked if NMP35 mRNA's 3′UTR is sufficient for localizing a heterologous mRNA into axons of cultured DRG neurons. For this, we generated a diffusion-limited myristoylated green fluorescent protein (GFPmyr) reporter (Aakalu et al., 2001) carrying the 3′UTR of NMP35 mRNA (GFPmyr3′NMP35). The myristoylation element in GFP coding sequence decreases its diffusion compared to non-modified GFP such that fluorescence recovery after photobleaching (FRAP) can be used to test for localized translation events by comparing recovery with and without active protein synthesis (Vuppalanchi et al., 2010; Yudin et al., 2008). Without a localizing UTR, the GFPmyr mRNA is restricted to the neuronal cell body (Aakalu et al., 2001; Akten et al., 2011; Ben-Yaakov et al., 2012; Perry et al., 2012; Vuppalanchi et al., 2010; Willis et al., 2007; Yudin et al., 2008).
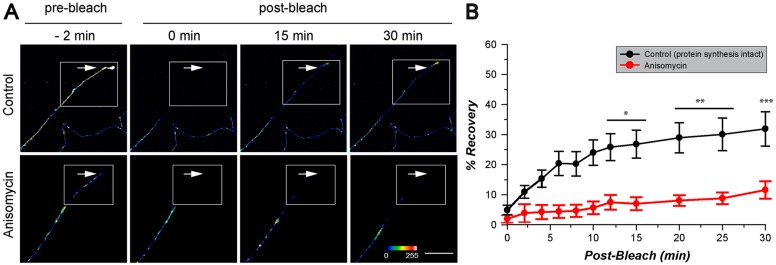
DRG neurons transfected with GFPmyr3′NMP35 showed robust GFP fluorescence in foci along the axon shaft and in the terminal axon/growth cone (Fig. 3A; supplementary material Movies 1 and 2), suggesting that the localization of the endogenous NMP35 mRNA that we had seen by FISH analyses can be driven by the 3′UTR of NMP35 mRNA. Upon photobleaching, the distal axon showed recovery of GFP fluorescence that began to reach statistical significance at 12 min post-bleach (Fig. 3B). This kinetics of recovery is consistent with our previous studies where increase in fluorescence after photobleaching in axons at ≥750 µm from cell body, as used here, occurs before new GFP protein could be transported from the neuronal cell body (Akten et al., 2011; Vuppalanchi et al., 2010; Willis et al., 2007; Yudin et al., 2008). To test if protein synthesis contributed to this recovery, DRG cultures were pre-treated with translation inhibitor anisomycin for 30 min prior to photobleaching. There was no increase in the axonal GFP fluorescence above post-bleach levels in the presence of anisomycin (Fig. 3A,B), indicating that the recovery seen above is protein synthesis dependent. Furthermore, FISH showed that axonal GFP mRNA was only detected if the 3′UTR of NMP35 was included in the reporter constructs (see Fig. 6A below). These data indicate that the 3′UTR of NMP35 mRNA is sufficient for mRNA localization into the axons of cultured DRG neurons.
Fig. 3.
3′UTR of NMP35 mRNA is sufficient for axonal mRNA localization in vitro. DRGs were transfected with diffusion-limited GFPmyr plus the 3′UTR of NMP35 mRNA to test for axonal-localizing ability of this non-translated region. (A) Representative images from FRAP sequences are shown for indicated pre-bleach and post-bleach intervals for control (top row) and 80 µM anisomycin-treated cultures (bottom row). GFP fluorescence is displayed as a spectrum as indicated; the boxed area represents the region of interest (ROI) for the terminal axon (arrow indicates terminal axon) that was subjected to bleaching. Recovery of GFP signals in the ROI only occurs when protein synthesis is intact. Scale bar: 25 µm. (B) Quantification of fluorescence in the ROI over the indicated time period is shown for n≥7 neurons analyzed over at least three separate transfection experiments. Average fluorescence is shown as a percentage of pre-bleach signals within each image sequence; error bars represent s.e.m. for each experiment (*P≤0.05, **P≤0.01 and ***P≤0.001 by one-way ANOVA compared with t = 0 min).
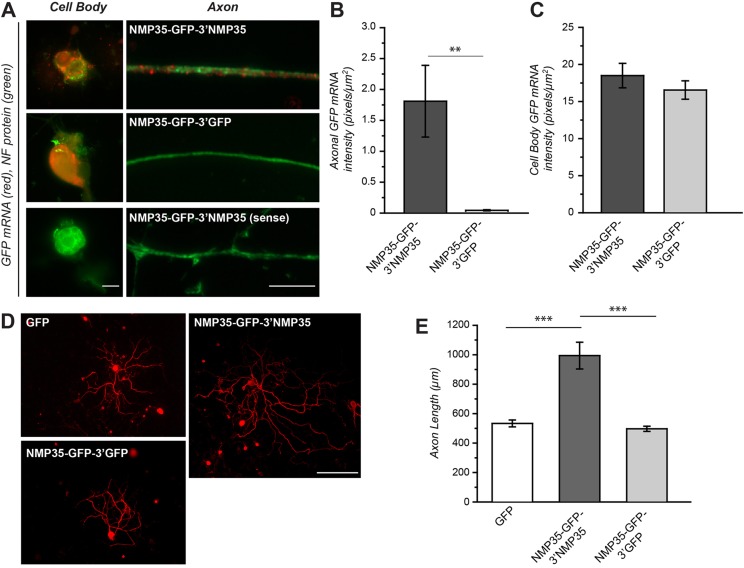
Fig. 6.
Axonal targeting of NMP35 mRNA is sufficient to increase axonal outgrowth. To determine whether targeting NMP35-GFP fusion protein for axonal synthesis might contribute to growth, we transduced cultured DRG neurons with the LV preparations used in Fig. 4 to overexpress axonally targeted versus cell-body-restricted NMP35 mRNA (NMP35-GFP-3′NMP35 and NMP35-GFP-3′GFP, respectively). (A–C) NMP35-GFP-3′NMP35- and NMP35-GFP-3′GFP-transduced cultures were processed for FISH for GFP mRNA. Representative exposure-matched images of cell bodies and axon shafts are shown in A. Here, GFP mRNA is only seen in axons of NMP35-GFP-3′NMP35-transduced cultures. Quantification of axonal and neuronal cell body GFP mRNA signals for multiple cultures is shown in B and C, respectively. There is no axonal GFP mRNA signal above background in the NMP35-GFP-3′GFP-transduced cultures, whereas cell body signals are not significantly different between the NMP35-GFP-3′GFP- and NMP35-GFP-3′NMP35-transduced cultures (**P≤0.01 by Student's t-test). (D) Representative montage images of neurons expressing GFP, NMP35-GFP-3′NMP35 and NMP35-GFP-3′GFP for 72 hours and then stained for neurofilament heavy (NF H) are shown. (E) Quantification of axon length of neurons transduced with GFP, NMP35-GFP-3′NMP35 or NMP35-GFP-3′GFP is shown (n≥25 neurons in at least three separate transduction experiments; **P≤0.01; ***P≤0.001 by Student's t-test). Scale bars: 10 µm (A); 200 µm (D).
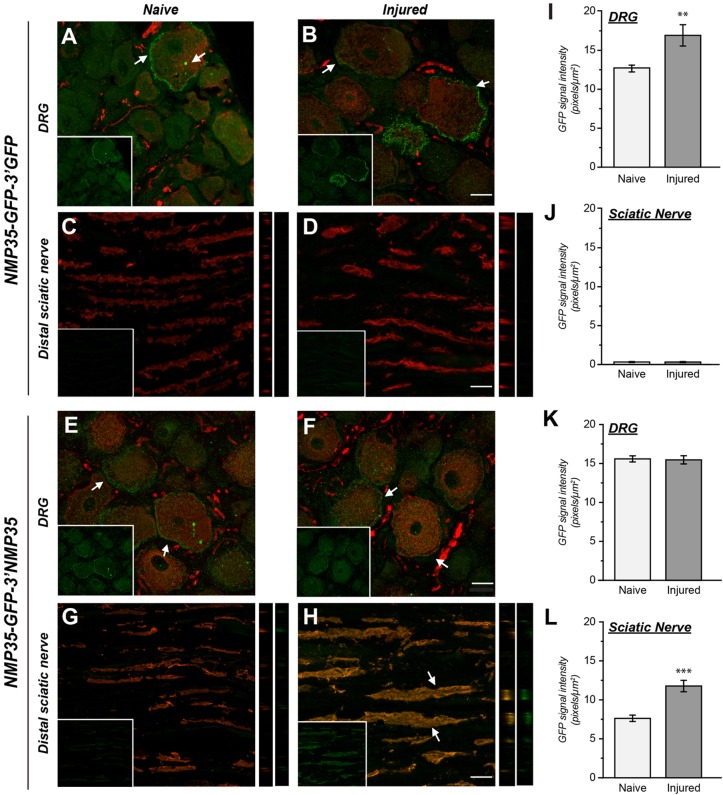
Since the 3′UTR of NMP35 mRNA could localize mRNA into axons of cultured neurons, we asked if it might also be sufficient for axonal localization in vivo. For this, we constructed NMP35-AcGFP fusion protein/reporters with the 3′UTR of NMP35 or 3′UTR of Aequorea coerulescens GFP (AcGFP); we refer to the monomeric AcGFP as simply ‘GFP’ throughout the remainder of this text. These constructs were used to generate lentivirus (LV) for in vivo expression (LV-NMP35-GFP-3′NMP35 and LV-NMP35-GFP-3′GFP, respectively). To test for axonal mRNA translation in vivo, LV-NMP35-GFP-3′NMP35 and LV-NMP35-GFP-3′GFP were used to transduce the L4-5 DRGs in adult rats. Ten days after injecting LV preparations, animals were subjected to unilateral sciatic nerve crush injury at mid-thigh level, ∼4.5 cm from the site of LV injection. GFP signals were then assessed at the site of injection, in the DRG, and in the distal axon proximal to the crush site (or comparable level in the uninjured nerve). Tissue sections were evaluated by immunolabeling for GFP and neuronal markers using confocal microscopy. At the injection site, GFP signals were seen in Schwann cells and in axons for both LV preparations (supplementary material Fig. S3). GFP signals were clearly visible in the naïve and injured DRG for LV-NMP35-GFP-3′GFP and LV-NMP35-GFP-3′NMP35 transduced animals, indicating in vivo transduction of these neurons by LV (Fig. 4A–H). In examining the distal nerve just proximal to the crush site, GFP signals were only seen in the LV-NMP35-GFP-3′NMP35 transduced animals (Fig. 4G,H). Despite the enhanced detection afforded by anti-GFP immunolabeling, we saw no GFP signals in the nerves of the LV-NMP35-GFP-3′GFP transduced animals (Fig. 4C,D). The absence of any GFP signals in the nerves of the LV-NMP35-GFP-3′GFP transduced animals and the distance separating the injection and injury sites (>4 cm) argues that the axonal GFP signals seen when 3′UTR of NMP35 mRNA was included in the LV construct are the result of localized protein synthesis rather than transport or diffusion of NMP35-GFP protein from the cell body into the axonal compartment. Taken together, these data indicate that the 3′UTR of NMP35 transcript is both necessary and sufficient for axonal localization in sensory neurons both in vitro and in vivo.
Fig. 4.
3′UTR of NMP35 mRNA is needed for localization into sensory sciatic nerve. To determine whether the 3′UTR of NMP35 mRNA is needed for axonal localization in vivo, L4-5 DRGs were transduced with LV that expressed an NMP35-GFP fusion protein carrying the 3′UTR of NMP35 or GFP mRNAs (NMP-GFP-3′NMP35 and NMP-GFP-3′GFP, respectively). (A–H) Representative exposure-matched confocal images for GFP signals (green) and neurofilament heavy (NF H) (red) are shown for sections of L4-5 DRGs and sciatic nerves for naïve and 7 day crush-injured nerves and DRGs are shown as indicated. Large panels show merged images and inset panels show only the GFP channel. The right-hand strips for C, D, G and H show YZ images of merged GFP and NF signals for the first strip, and GFP signals only for the second strip. GFP signals are seen along the periphery of DRG cell bodies (arrows) for both NMP35-GFP-3′GFP- (A,B) and NMP35-GFP-3′NMP35- (E,F) transduced animals. This suggests membrane localization of NMP35-GFP protein. Sections of distal sciatic nerve show GFP signals only in the NMP35-GFP-3′NMP35 transduced animals (G,H), with a clear increase in GFP signals in the injured nerve (arrows). The nerves in the NMP35-GFP-3′NMP35-transduced animals are also suggestive of focal NMP35-GFP signals along the periphery of the axon. This is also seen in YZ projections of image stacks that show intra-axonal NMP35-GFP and signals enriched along the periphery of the axoplasm (image strips in G and H; see also teased nerve preparations in supplementary material Fig. S4). (I–L), GFP signal intensity that overlapped with NF H signals was quantified over multiple animals and is shown for DRG and sciatic nerves for the NMP35-GFP-3′GFP- (I,J) and NMP35-GFP-3′NMP35- (K,L) transduced animals. These data confirm the absence of sciatic nerve NMP35-GFP signals in the NMP35-GFP-3′GFP-transduced animals (J) and an increase in sciatic nerve NMP35-GFP signals with nerve crush injury in the NMP35-GFP-3′NMP35-transduced animals (L) (n≥25 from at least three separate transduced experiments; **P≤0.01 and ***P≤0.001 by Student's t-test). Values in I–L are means±s.e.m. Scale bars: 10 μm.
The FRAP experiments above did not allow us to distinguish changes in localized synthesis of NMP35 in axons of injury-conditioned versus naïve neurons, since the transfected constructs required at least 2 days for optimal expression. Over this incubation period, the naïve neurons begin to transition from an arborizing growth to an elongating axonal growth is similar to the injury-conditioned phenotype (Smith and Skene, 1997). These in vivo experiments brought an opportunity to use GFP protein fluorescence as a surrogate for comparing NMP35 3′UTR-dependent effects on axonal GFP mRNA levels in injured and naïve neurons. Quantification of the axonal GFP signals confirmed a significant increase in GFP intensity in the LV-NMP35-GFP-3′NMP35 transduced animals after injury compared to the uninjured animals (Fig. 4G–H). There was no axonal GFP signal detected above background in nerves from the LV-NMP35-GFP-3′GFP transduced animals (Fig. 4C–D). In contrast to the endogenous NMP35 mRNA, we saw no decrease in GFP levels in the DRG after injury in the LV-NMP35-GFP-3′NMP35 transduced animals but there was an increase in DRG GFP levels in the LV-NMP35-GFP-3′GFP transduced animals (Fig. 4I–K). This may be attributed to overexpression of the NMP35-GFP fusion protein compared to the endogenous NMP35 mRNA.
The NMP35-GFP fusion protein used in these latter studies also allowed us to assess potential for localization of NMP35 protein in axons. In the DRGs of the LV transduced animals, NMP35-GFP signals were concentrated along the periphery of the neurons for both LV preparations, suggestive of membrane localization of the fusion protein (Fig. 4). Projection of confocal image stacks of distal nerve into YZ planes also showed enhanced signals along the periphery of several axons in the LV-NMP35-GFP-3′NMP35 transduced preparations (Fig. 4G,H, inset panels). Finally, teased nerve preparations showed GFP signals that were enriched along the periphery of individual axons in the LV-NMP35-GFP-3′NMP35 transduced animals (supplementary material Fig. S4C,D). The LV-NMP35-GFP-3′GFP transduced animals again showed no detectable GFP signals in these teased nerve preparations (supplementary material Fig. S4A,B). Taken together, these data support the conclusion that the locally translated NMP35, which is targeted to axons through its 3′UTR, is inserted into the axoplasmic membrane.
Although the above data are consistent with an increase in transport of NMP35 mRNA into axons after injury, a shift in the stability of the transcript would also account for the increased levels of NMP35 mRNA and protein in the axons of injured DRG neurons. Since severed DRG axons rapidly undergo Wallerian degeneration even in culture (Zheng et al., 2001), we could not test survival of NMP35 mRNA directly in the isolated axons. Thus, we used cyclosporin A (CsA) to delay the degeneration of severed axons. Barrientos et al. (Barrientos et al., 2011) showed that inhibition of the mitochondrial membrane permeability transition pore with CsA extends the survival of severed axons (Barrientos et al., 2011). Using RNA isolated from CsA-treated axons of naïve and injury conditioned DRG cultures as a template for RT-PCR, the endogenous NMP35 and GAPDH mRNAs were easily detected up to 6 hours after severing (supplementary material Fig. S5). In contrast to the axonal GAPDH mRNA that did not change with injury conditioning, the overall levels of NMP35 mRNA were more abundant in the axons of the injury conditioned versus naïve neurons at every time point. However, there was no difference in stability of the NMP35 mRNA when the injury conditioned and naïve RNA levels at 4 and 6 hour samples were normalized to the 2 hour time point (supplementary material Fig. S5B). These data suggest that a change in stability of NMP35 mRNA does not explain the increase in axonal NMP35 mRNA levels after injury.
Axonally synthesized NMP35 mRNA increases axon outgrowth
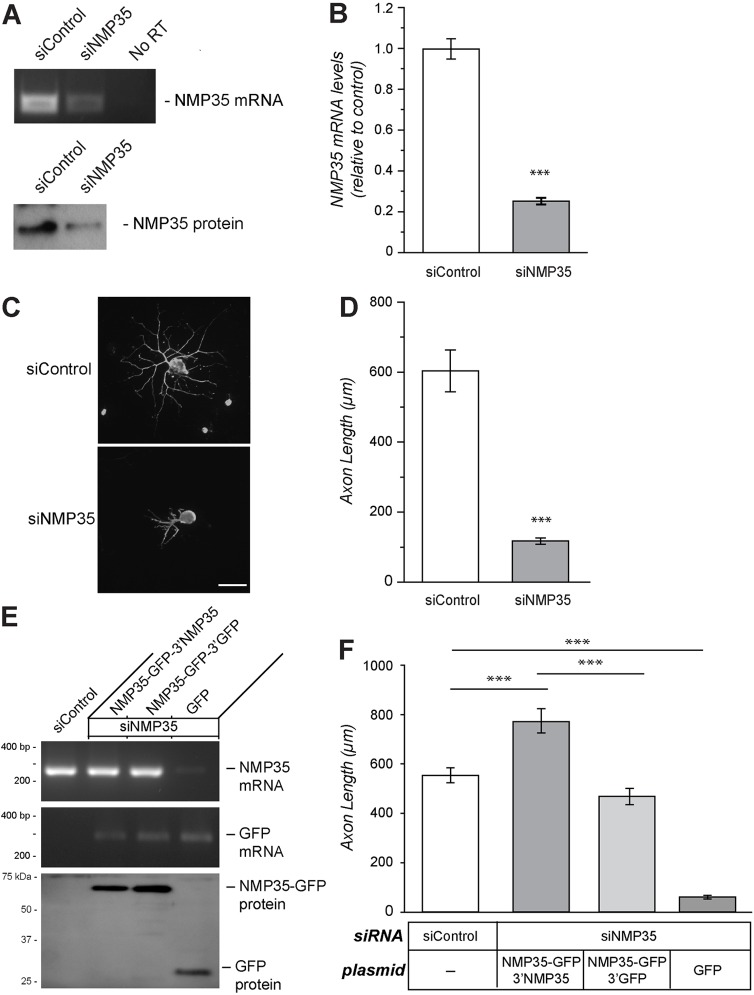
Our experiments suggest that transport of NMP35 mRNA into axons is regulated by injury and/or axonal growth, with the axonally generated NMP35 protein localizing to the axoplasmic membrane. This led us to ask if the locally synthesized NMP35 protein contributes to growth of axons. For this, we used small interfering RNAs (siRNAs) to deplete adult DRG cultures of NMP35 mRNA overall. Transfection with a siRNA pool to the NMP35 sequence depleted both the transcript and protein by ∼75% compared to cultures transfected with non-targeting siControl (Fig. 5A,B). Non-targeting siRNAs (siControl) had no effect on NMP35 mRNA levels compared with non-transfected cultures (data not shown). Depletion of NMP35 mRNA significantly decreased axonal outgrowth in the DRG cultures (Fig. 5C,D). This suggests that NMP35 contributes to axonal outgrowth, but did not distinguish effects of NMP35 synthesis overall from axonally synthesized NMP35. To address this question, we performed rescue experiments using siRNA-resistant NMP35 constructs that were cell body restricted versus axonally targeted. We separately tested each siRNA of the siRNA pool used above (data not shown). From this, the target sequence of a single siRNA oligonucleotide that gave maximal NMP35 mRNA depletion was used to generate siRNA-resistant NMP35-GFP constructs. By RT-PCR, siNMP35 significantly depleted NMP35 mRNA when the neurons were co-transfected with GFP, but co-transfection with siRNA-resistant NMP35-GFP constructs resulted in NMP35 mRNA levels comparable to siControl transfected neurons (Fig. 5E). Immunoblotting confirmed expression of NMP35-GFP fusion proteins and GFP at near equivalent levels (Fig. 5E). Depletion of NMP35 did not appear to affect cell survival since cell numbers for these co-transfections were statistically the same (data not shown). Consistent with the results above, siNMP35 transfection resulted in a significant depletion of axonal outgrowth compared to siControl-transfected neurons, and co-transfection with GFP alone did not rescue this growth deficit (Fig. 5F). Co-transfection of NMP35-GFP, either the cell body restricted or axonally localizing form, rescued the axonal growth deficit with siNMP35; however, axons were significantly longer when the axonally localizing NMP35-GFP-3′NMP35 was used (Fig. 5F).
Fig. 5.
Depletion of NMP35 mRNAs in the DRG neurons attenuates axonal outgrowth. (A) DRG cultures transfected with siRNA targeting NMP35 (siNMP35) mRNA or a non-targeting control (siControl) show reduced NMP35 mRNA and protein at 72 hours post-transfection by RT-PCR and immunoblotting, respectively. (B) RTqPCR: siNMP35 reduced endogenous NMP35 mRNA levels by ∼80%. (C,D) Depletion of NMP35 mRNA from DRG cultures decreases axonal growth. Representative neurofilament heavy (NF H)-stained images are shown in C. Quantification of average axon length for neurons transfected with siNMP35 versus siControl after 72 hours in vitro is shown in D. Scale bar: 100 µm. (E,F) To test for potential off-target effects of the siNMP35, neurons were co-transfected with cell-body-restricted (NMP35-GFP-3′GFP) versus axonally targeted (NMP35-GFP-3′NMP35) constructs; transfection with GFP was used as a control. (E) Representative RT-PCR and immunoblotting for NMP35 mRNA and NMP35-GFP mRNA and proteins. Upon co-transfection with siNMP35 and NMP35-GFP constructs, NMP35 mRNA levels were rescued to near-endogenous levels. GFP mRNA levels were relatively equivalent for the two NMP35-GFP and the GFP transfections. Immunoblotting confirmed expression of NMP35-GFP protein. (F) Analysis of axonal growth in these co-transfection experiments showed the expected reduction in axon length comparing siNMP35+GFP and siControl transfections. Both of the NMP35-GFP constructs rescued axonal outgrowth deficit seen with siNMP35, with the axonally targeting NMP35-GFP-3′NMP35 increasing axonal growth above the siControl-transfected cultures. siNMP35+GFP showed significant reduction in axonal outgrowth compared with siControl-, NMP35-GFP-3′NMP35- and NMP35-GFP-3′GFP-transfected neurons (n≥25 from at least three separate transfection experiments; ***P≤0.001 by Student's t-test for B, D and F). Values in B, D and F are means±s.e.m.
These siRNA studies suggested that simply increasing levels of NMP35 mRNA in axons might be sufficient to support increased axonal outgrowth. To directly test this possibility, we used the LV preparations to overexpress cell body restricted versus axonally localizing NMP35-GFP mRNA in DRG cultures without altering endogenous NMP35 levels. FISH for GFP mRNA confirmed axonal localization of the NMP35-GFP-3′NMP35 mRNA, but not NMP35-GFP-3′GFP, in the LV-transduced cultures (Fig. 6A,B). Cell bodies of the transduced neurons showed no significant differences in GFP mRNA with the different LV preparations (Fig. 6A,C). Axons of neurons transduced with the LV-NMP35-GFP-3′NMP35 were significantly longer than control neurons transduced with LV-NMP35-GFP-3′GFP (Fig. 6D,E). The average axon lengths with expression of the cell body restricted NMP35-GFP-3′GFP were not significantly different than cultures transduced with LV-GFP (Fig. 6D,E). Thus, consistent with the siRNA rescue experiments above, increasing axonal levels of NMP35, but not just simply the overall expression of NMP35, is sufficient for increased axonal outgrowth.
Discussion
Several studies in different neuronal preparations have now shown that axons of cultured neurons contain hundreds of mRNAs (Gumy et al., 2011; Taylor et al., 2009; Willis et al., 2007; Zivraj et al., 2010). We previously showed that transport of mRNAs into axons cultured from the adult rats used here can be regulated by stimulation with axonal growth-promoting and growth-inhibiting cues; however, transport of several mRNAs into DRG axons did not respond to neurotrophins, semaphorin, or myelin-associated glycoprotein (Willis et al., 2007). This suggested that either the appropriate stimulus was not tested or these mRNAs are constitutively transported into axons. Some of these transcripts, such as Importin β1, RanBP1 and Stat3α mRNAs, are resident in mature PNS axons with their local translation in axons being triggered by injury (Ben-Yaakov et al., 2012; Hanz et al., 2003; Yudin et al., 2008). Here, we show that NMP35 mRNA is also regulated by injury. However, our data suggest that transport of NMP35 mRNA into axons rather than its translation within axons is injury-dependent. Axonal levels of NMP35 mRNA are increased after axonal injury with no change in its overall expression of the transcript. Moreover, the locally generated NMP35 protein enhances axonal growth. Thus, this shift in NMP35 mRNA levels from cell body predominant in naïve neurons to axon predominant in axotomized neurons suggests that injury triggers an inherent change in the neuron's ability to localize this mRNA.
Transport of mRNAs from the cell body into axons is an active process, driven by specific RNA elements that serve as binding sites for RNA binding proteins (RBP). These structural elements have proven difficult to predict based on primary sequence alone, but have most frequently been documented within the 3′UTRs of localized mRNAs (Andreassi and Riccio, 2009). Consistent with this, NMP35 mRNA's 3′UTR shows no clear homology to known localization elements. The localizing 3′UTRs of calreticulin and grp78/BiP mRNAs show striking interspecies sequence identity among vertebrates (Ben-Yaakov et al., 2012; Kislauskis et al., 1994; Vuppalanchi et al., 2010). In contrast, the 3′UTR of rat NMP35 mRNA shows 83% identity with mouse, less than 75% identity with primate, and essentially no identity with other available vertebrate NMP35 3′UTR sequences. Nonetheless, the rat NMP35 mRNA's 3′UTR was sufficient for localizing GFP reporter mRNA in cultured neurons and was necessary for localizing an NMP35-GFP fusion protein mRNA into adult peripheral axons in vivo. Similar to NMP35 mRNA, CGRP mRNA shifts from cell body to axons in injured DRG neurons (Toth et al., 2009) and sensorin mRNA shifts from cell body to neurites in Aplysia sensory neurons upon synapse formation (Andreassi et al., 2010; Lyles et al., 2006; Natera-Naranjo et al., 2010). The overall levels of NMP35 mRNA do not change with injury, only the ratio of axonal to cell body mRNA increases comparing compared injured and naive DRG neurons. With CGRP showing similar regulation (Toth et al., 2009), it is appealing to hypothesize the existence of a cohort of axonal mRNAs that share RBPs for enhanced targeting after axotomy. Future studies will be needed to determine the RBP(s) that bind to NMP35's 3′UTR for localizing this transcript, but this shift in axonal levels of NMP35 mRNA without an apparent change in overall expression of the transcript likely reflect either an increase in availability or activity of this RBP after axotomy. Such a shift in availability could be secondary to decreased levels of other mRNAs as we have recently shown for GAP-43 and β-actin mRNAs competing for limited quantities of ZBP1 (Donnelly et al., 2011). However, in contrast to ZBP1, overexpression of the axonally localizing NMP35-GFP-3′NMP35 resulted in increased axonal growth suggesting that the level of the RBP(s) needed for axonal localization of NMP35 mRNA is not limiting.
Functions of locally synthesized proteins have largely been extrapolated from what is known of the overall functions of these proteins, but the locally generated proteins have been found to have distinct functions in some circumstances. For example, the microfilament protein β-actin allows for directional migration rather than overall motility of migrating fibroblasts when it is locally generated (Shestakova et al., 2001). Axonal translation of Importin β1 and RanBP1 is used to generate an Importin α/β heterodimer for transporting axonal signaling proteins to the cell body, with introduction of these proteins providing a precise temporal indicator of axon injury (Ben-Yaakov et al., 2012; Hanz et al., 2003; Perry et al., 2012; Yudin et al., 2008). Although NMP35 mRNA was initially cloned from developing sciatic nerve (Schweitzer et al., 1998) and NMP35 protein concentrates at synapses in post-mitotic neurons (Schweitzer et al., 2002), the possibility for localization of NMP35 mRNA was not considered in these early expression studies. Our data linking increased axonal levels of NMP35 mRNA to increased axonal growth likely accounts for the initial cloning of NMP35 mRNA from sciatic nerve.
NMP35 was independently isolated by other groups as FAIM2 or LFG and has been functionally implicated in cell survival (Beier et al., 2005; Fernández et al., 2007). Depletion of FAIM2/LFG from neurons increases sensitivity to Fas-mediated apoptosis and developmental cell death, while overexpression of FAIM2/LFG was protective from effects of FasL-mediated death of neurons (Beier et al., 2005; Fernández et al., 2007; Hurtado de Mendoza et al., 2011). Although no role in neurite or axonal growth was detected for the overexpressed FAIM2/LFG proteins, these constructs did not contain the 3′UTR that we have shown localizes NMP35 mRNA into neuronal processes based on primers and ESTs used for their cloning. Consequently, the overexpression studies used by Beier et al. (Beier et al., 2005) and Fernández et al. (Fernández et al., 2007) did not examine the effects of the locally synthesized protein that we show here plays a role in axonal growth. Thus, we have uncovered a previously unrecognized function of NMP35 in promoting axonal growth that is distinct for the locally synthesized NMP35.
A similarly named but unrelated mRNA, FAIM (GenBank accession number NM_080895), encodes a protein that also protects neurons and other cell types from FasL-induced apoptosis (Segura et al., 2007; Sole et al., 2004). Interestingly, an alternatively spliced form of FAIM mRNA generates a shorter protein that lacks 22 N-terminal amino acids (FAIMs) (Zhong et al., 2001). Similar to the axonally localizing NMP35 studied here, overexpression of FAIMs increases neurite outgrowth (Sole et al., 2004). However, it should be emphasized that NMP35 and FAIMs show no sequence homology at the RNA or protein level and the alternatively spliced FAIMs alters the 5′ end of the transcript. Furthermore, unlike NMP35/FAIM2, both long and short forms of FAIM are cytoplasmic proteins without any predicted transmembrane domains (Segura et al., 2007; Sole et al., 2004).
In our hands, we do not see increased cell death with depletion of NMP35 from the DRG cultures under standard culture conditions; thus, it is not clear if the role in axonal growth is linked to NMP35's inhibition of Fas signaling. There have been studies showing that Fas activation can support neurite growth. FasL was shown to increase neurite branching in hippocampal cultures, neurite outgrowth from DRG explants, and axonal regeneration after sciatic nerve crush (Desbarats et al., 2003; Zuliani et al., 2006). These studies could argue that NMP35 has some function beyond Fas inhibition, since NMP35 is known to inhibit Fas signaling and our data indicate that NMP35 supports rather than attenuates axon growth. However, Fas activation through FasL was recently shown to increase secretion of NGF in Schwann cells (Mimouni-Rongy et al., 2011). Thus, the data from Desbarats et al. (Desbarats et al., 2003) on FasL supporting nerve regeneration may be a secondary effect of FasL on the Schwann cells. Further studies will be needed to determine molecular mechanisms that NMP35 utilizes to support growth of axons. Nonetheless, our work clearly shows axonal targeting of an mRNA encoding a transmembrane protein can be used to modulate axonal growth from adult neurons. Moreover, these studies point to a previously unrecognized mechanism that neurons can use to modify axonal transport of mRNAs beyond the extracellular stimuli-induced transport and competition for RBPs that we have previously published (Donnelly et al., 2011; Vuppalanchi et al., 2010; Willis and Twiss, 2010). For NMP35 mRNA transport, the axonal injury must either increase the levels or activity of the RBP(s) needed for targeting the transcript for transport from the cell body into distal axons.
Materials and Methods
Animal care and surgery
All animal experiments were conducted under Institutional Animal Care and Use Committee (IACUC)-approved protocols at Alfred I duPont Hospital for Children and Drexel University. For nerve injury, animals were subjected to a conditioning sciatic nerve crush at mid-thigh level as previously described (Twiss et al., 2000).
Cell culture
For DRG culture, L4-6 ganglia were isolated from adult male Sprague Dawley rats and dissociated using collagenase (500 U/ml; Sigma, St. Louis, MO) and trypsin-EDTA (0.05%; Cellgro, Manassas, VA) (Twiss et al., 2000). Cells were washed with DMEM/F12 and then resuspended in DMEM/F12 medium containing N1 supplement (Sigma), 10% horse serum (Hyclone, Salt Lake City), and 10 µM cytosine arabinoside (Sigma). Cells were cultured on poly-L-lysine (Sigma)+laminin (Millipore, Billerica, MA)-coated surfaces at 37°C with 5% CO2. For isolation of axons, dissociated cells were plated at moderate density on polyethylene-tetrathalate (PET) membrane (8 µm pores; BD Falcon) inserts (Zheng et al., 2001); for FISH analyses, immunofluorescencea and neurite outgrowth assays, dissociated cells were plated at low density on coated glass coverslips.
DRG cultures were transfected immediately after dissociation using the AMAXA Nucleofector apparatus (Lonza, Allendale, NJ). For this, dissociated ganglia were pelleted at 100 g for 5 min and resuspended in transfection solution from the Rat Neuron Nucleofector kit (Lonza). A 3–5 µg portion of each plasmid DNA was transfected using the G-013 program. Cells were resuspended in culture media and plated; medium was replaced 4 and 24 hours later. For in vivo transduction with LV, an MOI of 100 was added to the dissociated DRGs 3–4 hours after plating cells; media was replaced the next day; LV was titered as described previously (Vuppalanchi et al., 2010).
For culturing cortical neurons, cortices were dissected from P1-4 rat pups, incubated in papain for 30 min, and then dissociated by trituration in Hibernate E media (Brainbits, Springfield, IL). After gentle centrifugation, dissociated cortices were plated on poly-L-lysine-coated glass coverslips in Neurobasal medium with 10% fetal bovine serum, B27 supplement, 2 mM glutamine (Invitrogen, Grand Island, NY), and penicillin/streptomycin (Cellgro) at 37°C and 5% CO2 (Vuppalanchi et al., 2010).
Isolation of axons
DRG neurons cultured on PET membranes for 16–20 hours were used for axon isolation. For this, the upper membrane surface containing cell bodies and non-neuronal cells was scraped using a cotton-tipped applicator as previously described (Willis and Twiss, 2010). For analysis of cell body, the axonal processes were scraped from the upper membrane surface. RNA was then isolated from the cellular elements remaining on the membrane as outlined below. These isolated axonal preparations were tested for purity by RT-PCR for γ-actin, β-actin and MAP2 mRNAs (Merianda et al., 2009; Willis et al., 2005).
DNA and viral constructs
All PCR products used for cloning were sequence verified as individual clones. RNA from adult rat DRGs was used as template for reverse transcription using iScript (BioRad, Hercules, CA); Pfu DNA polymerase (Stratagene, La Jolla, CA) was used for PCR amplifications.
Diffusion limited GFP reporter constructs (GFPmyr) (Aakalu et al., 2001) were used to test for axonal localizing ability of NMP35 RNA sequences. 3′UTR of NMP35 (GenBank accession NM_144756) with terminal Not1 and Apa1 restriction sites was PCR-amplified with the following primers: sense, 5′-GCGGCCGCAACCGGGAATGAGGAGCCCTCC-3′ and anti-sense, 5′-GGGCCCGACTGAGGGGACATGGCTGGAACTTG-3′. PCR products were cloned into pTOPO 2.1 (Invitrogen), and then sub-cloned into Not1 and Apa1 sites of the GFPmyr expression vector replacing the 3′UTR of αCAMKII (Aakalu et al., 2001).
For expression of NMP35 coding sequence, constructs were generated to encode an NMP35-GFP fusion protein using pAcGFP1-N3 (Clontech, Mountain View, CA). For this the complete 5′UTR through coding sequence (i.e. next to last codon) was using the following primers, constructed with EcoR1 and BamH1 restriction sites for cloning: sense, 5′-GAATTCGAGACGCAGGCAGGCTGCGGTGA-3′ and anti-sense, 5′-GGATCCAGCTTTTTGGCACCAACCGGGAA-3′. This generated a plasmid encoding an mRNA with NMP35-GFP fusion protein and 3′UTR of GFP (pNMP35-GFP-3′GFP). The GFP 3′UTR was then replaced with NMP35's 3′UTR to generate pNMP35-GFP-3′NMP35. NMP35′s 3′UTR was generated as above, but with terminal Not1 sites for cloning downstream of GFP.
For generating LV, we first used a QuikChange XL Site-Directed Mutagenesis kit to mutate the polyadenylation signals in the NMP35 and GFP 3′UTRs (Vuppalanchi et al., 2010). After sequence verification, the cDNA cassettes containing the CMV promoter, 5′UTR, NMP35-GFP fusion protein coding sequence were subcloned into the pENTR shuttle vector (Invitrogen). NMP35-GFP sequence was then recombined into a Gateway-compatible derivative of the pCDH-CMV–MCS1-Ef1α-copGFP (SBI System Biosci., Mountain View, CA) as described previously (Vuppalanchi et al., 2010). LV was generated as described previously (Blesch, 2004). Serial dilutions were used to determine MOI of these LV preparations in DRG cultures.
To generate siRNA resistant NMP35-GFP constructs, four nucleotides in the siRNA-targeted regions (CCGTATTCTTTGCAACATA) were mutated as above using the following primers: sense, 5′-GGGCATCCTATGCCGTG*TTCTTC*GCAACG*TACCTGACTCTGGCTT-3′ and anti-sense, 5′-AAGCCAGAGTCAGGTAC*GTTGCG*AAGAAC*ACGGCATAGGATGCCC-3′ (* indicates mutated nucleotides).
RNA isolation and analyses
Total RNA was extracted from the fractionated DRG cultures using the RNAqueous Micro kit (Ambion, Austin, TX) as per the manufacturer's protocol. For tissue preparations, RNA was isolated using the RNAqueous kit (Ambion) per the manufacturer's protocol. Isolated RNA was quantified using the VersaFluor fluorimeter (BioRad) with a RiboGreen assay (Invitrogen) (Willis et al., 2005). Tissue samples were normalized for RNA content. For the axonal preparations, flow-through from the affinity-based RNA isolation was used to measure the protein content of the isolates by fluorimetry using the NanoOrange reagent (Invitrogen). Axonal RNA preparations were then normalized for protein content to control for axon yield (Merianda et al., 2009; Willis et al., 2005; Willis et al., 2007). Normalized RNA samples (60 ng each) were used for reverse transcription (RT) using the iScript RT kit (BioRad).
Standard PCR was performed using HotStarTaq Mastermix (Qiagen, Valencia, CA). RNA isolated from adult rat brain was used as a positive control for RT-PCR. Negative control consisted of PCR using RT reactions processed without the addition of enzyme (‘no RT’). Cycling parameters for standard PCR were: 15 min ‘hotstart’ at 95°C and then 45 sec at 95°C, 45 sec at 58°C, and 3 min at 72°C for 30 cycles.
Reverse transcribed pure axonal RNA samples were further processed for quantitative PCR (qPCR) using 2× SsoFast Evagreen Supermix on a CFX 384 Touch qPCR instrument (Biorad). For the qPCR on axonal samples, we used signals for the 12S mitochondrial rRNA to normalize RNA content and RT efficiency between samples by differential cycle threshold (ΔCt) calculations (Willis and Twiss, 2010). Primers for rat γ-actin, β-actin, and MAP2 mRNAs and 12S mitochondrial rRNA have been published (Willis et al., 2005). Other primers used were as follows: glyceraldehyde-3-phosphate dehydrogenase (GAPDH; GenBank accession number NM_017008) – sense, 5′-GGAGAAACCTGCCAAGTATG-3′ and antisense, 5′-AGACAACCTGGTCCTCAGTG 3′; NMP35 – sense, 5′-ACCTGACTCTGGCTTGCTGT-3′ and antisense, 5′-CGAGGAGGAGTCCACTGAAG-3′.
Axonal mRNA decay analyses
Naïve and injury-conditioned DRGs were cultured on PET membranes for 16 hours and then treated with 20 µM Cyclosporin A (CsA; LC Laboratories). After 20 mins, cell bodies and non-neuronal cells were removed as described above; severed axons were incubated at 37°C, 5% CO2 for up to 6 hr. Axonal RNA was then isolated and processed for RT-PCR as described above. The qPCR analyses were performed in quadruplicate over at least 3 separate experiments.
In situ hybridization and immunofluorescence analyses
Digoxigenin-labeled oligonucleotide or cRNA probes were used for FISH. Oligonucleotide probes were used to detect endogenous mRNAs as previously described (Vuppalanchi et al., 2010). Antisense oligonucleotides to nucleotides 842–891 and 980–1029 of rat NMP35 (GenBank accession NM_144756) were designed using Oligo6 software (Molecular Biology Insights, Cascade, CO). BLAST analyses showed no homology to rat mRNAs deposited into GenBank. These were synthesized with 5′-amino C6 modifier C6 at four thymidines per oligonucleotide, and then labeled with digoxigenin succinamide ester (Roche, Indianapolis, IN) per manufacturer's protocol. Digoxigenin-labeled, scrambled probes were used for specificity control. cRNA probes were used to detect GFP reporter mRNA. These were generated by in vitro transcription from linearized pcDNA3-eGFP (Addgene, Plasmid 13031) with SP6 or T7 RNA polymerases and digoxigenin-labeled nucleotide mixture (Roche). Oligonucleotide probes were used at 1.66 ng/µl for cultured neurons and 50 ng/µl for tissue sections (Vuppalanchi et al., 2010). cRNA probes were used at 5 ng/µl probes. After hybridization and washing, samples were processed for immunofluorescence as previously described (Vuppalanchi et al., 2010). The following primary antibodies were used: chicken anti-neurofilament heavy (NF H) (1:1000; Millipore), mouse anti-digoxigenin (1:200; Jackson ImmunoResearch, West Grove, PA), and Cy3-conjugated mouse anti-digoxigenin (1:200; Jackson ImmunoResearch). After vigorous washing, secondary antibodies were applied for 2 hours. The following secondary antibodies were used: FITC-conjugated donkey anti-chicken (1:200; Jackson ImmunoResearch) or AMCA-conjugated anti-chicken (1:200; Jackson ImmunoResearch) and Cy3-conjugated anti-mouse (1:200; Jackson ImmunoResearch). After series of washes, samples were mounted with Prolong Gold Antifade (Invitrogen).
Immunofluorescence was performed as previously published (Merianda et al., 2009), with the exception of either 4% paraformaldehyde or cold methanol fixed samples were used for cultures (methanol fixation was used to visualize NMP35 protein in cultured neurons). Tissue sections were equilibrated in PBS and then incubated in 20 mM Glycine followed by 0.25 M NaBH4 for 30 min to quench autofluorescence (Vuppalanchi et al., 2010). The antibody to rat NMP35 was generated in chicken using a synthetic NMP35 peptide (SYEEATSGEGLKAGAF; Swissport NO. AAC32463) by GeneTel Labs (Madison, WI); this antibody was used at 1:200 dilutions. Mouse anti-neurofilament antibody cocktail (1:400; Sigma) was used to detect axons; GFP antibody (1:200, Abcam) to enhance GFP signals. Secondary antibodies were FITC or Texas red conjugated donkey anti-chicken, -rabbit or -mouse antibodies (1:300; Jackson ImmunoResearch). After vigorous washes, samples were mounted as above. Cultured neurons were imaged with Leica DMRXA2 or Zeiss Axioplan epifluorescent microscopes fitted with an ORCA-ER charge-coupled device (CCD) camera (Hamamatsu, Bridgewater, NJ). All images were matched for acquisition parameters and post-processing. Tissue sections were imaged using Leica TCS/SP2 or Zeiss LSM700 laser scanning confocal microscope.
Protein isolation, electrophoresis and immunoblotting
DRG cultures were lysed for 20 min at 4°C in RIPA buffer supplemented with protease inhibitor cocktail (Sigma). Lysates were cleared of debris by centrifugation at 16,000 g for 15 min at 4°C and then normalized for protein content using Bradford assay (BioRad). Normalized lysates were precipitated overnight with acetone at −80°C, and then resuspended and denatured in Laemmli sample buffer. Samples were resolved by standard SDS-PAGE and then electrophoretically transferred to PVDF membranes (Millipore). Membranes were rinsed in Tris-buffered saline with 0.1% Tween 20 (TBST) and then blocked in 5% nonfat dry milk. Blots were incubated in the following antibodies overnight at 4°C: rabbit anti-LFG (1:500; Pro Sci, Poway, CA) or rabbit anti-GFP (1:2000; Abcam, Cambridge, MA). Blots were rinsed several times in TBST and then incubated with HRP-conjugated anti-rabbit IgG (1:5000; Millipore) in the blocking buffer for 1 h at room temperature. Blots were washed for 30 min in TBST and developed with ECLplus (GE Healthcare, Piscataway, NJ).
Fluorescence recovery after photobleaching
FRAP was used to test for axonal translation of GFPmyr reporter mRNAs with NMP35 3′UTR as described (Vuppalanchi et al., 2010). For this, DRG cultures that had been transfected with GFPmyr3′NMP35 were initially evaluated for GFP expression by epifluorescence microscopy. 40× oil immersion objective (0.7 NA) on an inverted Leica TCS/SP2 confocal microscope with an environmental chamber maintained at 37°C was used with pinhole was set at 4 AU. For baseline fluorescence intensity, neurons were imaged every 30 sec over 2 min with 488 nm laser line set at 15% power before bleaching. For photobleaching, ≥150 µm2 ROI comprising the terminal 70–120 µm of distal axon was exposed to 100% power of the 488 nm laser line for 40 frames at 1.6 sec intervals. Recovery was then monitored every 30 sec over 30 min using 15% power of 488 nm laser line and GFP emission was collected over band filter of 498–530 nm with PMT energy, offset, and gain matched for all collection sets. To assess role of protein synthesis in this recovery, cultures were pre-treated with 80 µM anisomycin for 30 min prior to photobleaching. ImageJ was used to calculate average pixels/µm2 in the ROIs of the raw confocal images, which was then normalized to baseline intensity to calculate percent recovery. Mean for this normalized intensity for each post-bleach interval was calculated.
siRNA-based depletion
A pool of four synthetic siRNAs targeting rat NMP35 mRNA was designed using Dharmacon's siDESIGN Center (www.dharmacon.com/sigenome/default.aspx). For siRNA transfection, dissociated DRGs were cultured and then transfected with 200 nM siRNA after 24 hours using DharmaFECT3 transfection reagent in serum free medium per manufacturer's protocol (Dharmacon, Chicago, IL). Transfection efficiency for siRNAs was monitored by co-transfection with a stable, fluorescent, non-targeting control siRNA with RISC-free modification (siGLO, Dharmacon). To test for non-specific effects of siRNA transfection, cultures were transfected with non-targeting siCONTROL (Dharmacon). Efficiency of siRNA-based depletion was quantitated by RTqPCR for NMP35 mRNAs at 72 hours after transfection as outlined above.
In vivo expression of NMP35-GFP
For testing localization of NMP35-GFP in vivo, L4-5 DRGs were transduced with LV preparations as outlined above. 40 MOI (10 µl) of each LV preparation was injected into the L4-5 nerve roots adjacent to the DRG. 10 days later, animals were subjected to sciatic nerve crush injury at mid-thigh as previously described (Twiss et al., 2000). The L4-5 DRGs, nerve roots (injection site) and sciatic nerve (0.5 cm proximal to 0.5 cm distal to the crush site) were dissected fixed in 4% paraformaldehyde and processed for cryosectioning. Cryostat sections were immunolabeled as described above with anti-neurofilament (1∶750; Millipore) and anti-GFP (1∶200; Abcam) antibodies. Teased nerve preparations were generated from a segment of fixed sciatic nerve that was equilibrated in PBS. A 30-ga needle was used to strip away the epineurium and teased apart the nerve into individual fibers. Teased nerves were air dried on Superfrostplus glass slides (Fisher, Pittsburgh, PA), hydrated in PBS, and then processed for immunostaining. Teased nerves were mounted under coverslips with Prolong Gold Antifade and analyzed by confocal microscopy. Mean for GFP protein intensity (pixels/µm2) in DRG, distal and proximal nerves (both naïve and injured) was calculated using Image J.
Axon outgrowth assays
Axonal length was assessed after 72 hours in culture by immunostaining with neuronal markers as above. Length of longest axon of randomly captured images was measured by a blinded observer using Image J as described (Donnelly et al., 2011). At least three separate culture preparations were analyzed for each condition.
Fluorescence intensity studies
Intensity was calculated from digital images that were matched for exposure time, gain, offset, and post-processing parameters. mRNA and protein signals in cell body and axons was analyzed for intensity for n≥15 for cell body and n≥20 for axons from at least 3 separate experiments. Neurofilament signals were used to trace neuronal cell body and axons. Image J was then used to calculate the average pixels/µm2 in these areas as recently described (Vuppalanchi et al., 2012).
Statistical analyses
GraphPad Prism 4 software package (La Jolla, CA) was used for statistical analyses. Student's t-test was used to compare two means of independent groups. These included fluorescent intensities from FISH and immunofluorescence images calculated using Image J. One-way ANOVA with Bonferroni post-hoc test was used to test for significance the FRAP studies where more independent groups were compared as previously published (Akten et al., 2011; Ben-Yaakov et al., 2012; Perry et al., 2012; Vuppalanchi et al., 2010; Vuppalanchi et al., 2012; Yudin et al., 2008).
Supplementary Material
Footnotes
Funding
The initial studies in this work were funded by the Paralyzed Veterans of America Spinal Cord Research Foundation [grant number 2520 to T.T.M.]. Additional grant funding came from the National Institutes of Health [grant number R01-NS041596 to J.L.T.]; the Miriam and Sheldon G. Adelson Medical Research Foundation and the International Foundation for Research in Paraplegia [grant number P116 to J.L.T.]. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.107268/-/DC1
References
- Aakalu G., Smith W. B., Nguyen N., Jiang C., Schuman E. M. (2001). Dynamic visualization of local protein synthesis in hippocampal neurons. Neuron 30, 489–502 10.1016/S0896-6273(01)00295-1 [DOI] [PubMed] [Google Scholar]
- Akten B., Kye M. J., Hao T., Wertz M. H., Singh S., Nie D., Huang J., Merianda T. T., Twiss J. L., Beattie C. E.et al. (2011). Interaction of survival of motor neuron (SMN) and HuD proteins with mRNA cpg15 rescues motor neuron axonal deficits. Proc. Natl. Acad. Sci. USA 108, 10337–10342 10.1073/pnas.1104928108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreassi C., Riccio A. (2009). To localize or not to localize: mRNA fate is in 3′UTR ends. Trends Cell Biol. 19, 465–474 10.1016/j.tcb.2009.06.001 [DOI] [PubMed] [Google Scholar]
- Andreassi C., Zimmermann C., Mitter R., Fusco S., De Vita S., Saiardi A., Riccio A. (2010). An NGF-responsive element targets myo-inositol monophosphatase-1 mRNA to sympathetic neuron axons. Nat. Neurosci. 13, 291–301 10.1038/nn.2486 [DOI] [PubMed] [Google Scholar]
- Barrientos S. A., Martinez N. W., Yoo S., Jara J. S., Zamorano S., Hetz C., Twiss J. L., Alvarez J., Court F. A. (2011). Axonal degeneration is mediated by the mitochondrial permeability transition pore. J. Neurosci. 31, 966–978 10.1523/JNEUROSCI.4065-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier C. P., Wischhusen J., Gleichmann M., Gerhardt E., Pekanovic A., Krueger A., Taylor V., Suter U., Krammer P. H., Endres M.et al. (2005). FasL (CD95L/APO-1L) resistance of neurons mediated by phosphatidylinositol 3-kinase-Akt/protein kinase B-dependent expression of lifeguard/neuronal membrane protein 35. J. Neurosci. 25, 6765–6774 10.1523/JNEUROSCI.1700-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben–Yaakov K., Dagan S. Y., Segal–Ruder Y., Shalem O., Vuppalanchi D., Willis D. E., Yudin D., Rishal I., Rother F., Bader M.et al. (2012). Axonal transcription factors signal retrogradely in lesioned peripheral nerve. EMBO J. 31, 1350–1363 10.1038/emboj.2011.494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blesch A. (2004). Lentiviral and MLV based retroviral vectors for ex vivo and in vivo gene transfer. Methods 33, 164–172 10.1016/j.ymeth.2003.11.005 [DOI] [PubMed] [Google Scholar]
- Cheever T. R., Olson E. A., Ervasti J. M. (2011). Axonal regeneration and neuronal function are preserved in motor neurons lacking ß-actin in vivo. PLoS ONE 6, e17768 10.1371/journal.pone.0017768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbarats J., Birge R. B., Mimouni–Rongy M., Weinstein D. E., Palerme J. S., Newell M. K. (2003). Fas engagement induces neurite growth through ERK activation and p35 upregulation. Nat. Cell Biol. 5, 118–125 10.1038/ncb916 [DOI] [PubMed] [Google Scholar]
- Donnelly C. J., Fainzilber M., Twiss J. L. (2010). Subcellular communication through RNA transport and localized protein synthesis. Traffic 11, 1498–1505 10.1111/j.1600-0854.2010.01118.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly C. J., Willis D. E., Xu M., Tep C., Jiang C., Yoo S., Schanen N. C., Kirn–Safran C. B., van Minnen J., English A.et al. (2011). Limited availability of ZBP1 restricts axonal mRNA localization and nerve regeneration capacity. EMBO J. 30, 4665–4677 10.1038/emboj.2011.347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández M., Segura M. F., Solé C., Colino A., Comella J. X., Ceña V. (2007). Lifeguard/neuronal membrane protein 35 regulates Fas ligand-mediated apoptosis in neurons via microdomain recruitment. J. Neurochem. 103, 190–203 [DOI] [PubMed] [Google Scholar]
- Gumy L. F., Yeo G. S., Tung Y. C., Zivraj K. H., Willis D., Coppola G., Lam B. Y., Twiss J. L., Holt C. E., Fawcett J. W. (2011). Transcriptome analysis of embryonic and adult sensory axons reveals changes in mRNA repertoire localization. RNA 17, 85–98 10.1261/rna.2386111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanz S., Perlson E., Willis D., Zheng J. Q., Massarwa R., Huerta J. J., Koltzenburg M., Kohler M., van–Minnen J., Twiss J. L.et al. (2003). Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron 40, 1095–1104 10.1016/S0896-6273(03)00770-0 [DOI] [PubMed] [Google Scholar]
- Hurtado de Mendoza T., Perez–Garcia C. G., Kroll T. T., Hoong N. H., O'Leary D. D., Verma I. M. (2011). Antiapoptotic protein Lifeguard is required for survival and maintenance of Purkinje and granular cells. Proc. Natl. Acad. Sci. USA 108, 17189–17194 10.1073/pnas.1114226108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez–Diaz L., Geranton S. M., Passmore G. M., Leith J. L., Fisher A. S., Berliocchi L., Sivasubramaniam A. K., Sheasby A., Lumb B. M., Hunt S. P. (2008). Local translation in primary afferent fibers regulates nociception. PLoS ONE 3, e1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H., O'Hare C. M., Holt C. E. (2011). Translational regulation in growth cones. Curr. Opin. Genet. Dev. 21, 458–464 10.1016/j.gde.2011.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H., Yoon B. C., Holt C. E. (2012). Axonal mRNA localization and local protein synthesis in nervous system assembly, maintenance and repair. Nat. Rev. Neurosci. 13, 308–324 10.1038/nrn3274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kislauskis E. H., Zhu X., Singer R. H. (1994). Sequences responsible for intracellular localization of beta-actin messenger RNA also affect cell phenotype. J. Cell Biol. 127, 441–451 10.1083/jcb.127.2.441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig E., Martin R., Titmus M., Sotelo–Silveira J. R. (2000). Cryptic peripheral ribosomal domains distributed intermittently along mammalian myelinated axons. J. Neurosci. 20, 8390–8400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyles V., Zhao Y., Martin K. C. (2006). Synapse formation and mRNA localization in cultured Aplysia neurons. Neuron 49, 349–356 10.1016/j.neuron.2005.12.029 [DOI] [PubMed] [Google Scholar]
- Melemedjian O. K., Asiedu M. N., Tillu D. V., Peebles K. A., Yan J., Ertz N., Dussor G. O., Price T. J. (2010). IL-6- and NGF-induced rapid control of protein synthesis and nociceptive plasticity via convergent signaling to the eIF4F complex. J. Neurosci. 30, 15113–15123 10.1523/JNEUROSCI.3947-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merianda T. T., Lin A. C., Lam J. S., Vuppalanchi D., Willis D. E., Karin N., Holt C. E., Twiss J. L. (2009). A functional equivalent of endoplasmic reticulum and Golgi in axons for secretion of locally synthesized proteins. Mol. Cell. Neurosci. 40, 128–142 10.1016/j.mcn.2008.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimouni–Rongy M., White J. H., Weinstein D. E., Desbarats J., Almazan G. (2011). Fas ligand acts as a counter-receptor in Schwann cells and induces the secretion of bioactive nerve growth factor. J. Neuroimmunol. 230, 17–25 10.1016/j.jneuroim.2010.08.010 [DOI] [PubMed] [Google Scholar]
- Natera–Naranjo O., Aschrafi A., Gioio A. E., Kaplan B. B. (2010). Identification and quantitative analyses of microRNAs located in the distal axons of sympathetic neurons. RNA 16, 1516–1529 10.1261/rna.1833310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlson E., Hanz S., Ben–Yaakov K., Segal–Ruder Y., Seger R., Fainzilber M. (2005). Vimentin-dependent spatial translocation of an activated MAP kinase in injured nerve. Neuron 45, 715–726 10.1016/j.neuron.2005.01.023 [DOI] [PubMed] [Google Scholar]
- Perry R. B-T., Doron–Mandel E., Iavnilovitch E., Rishal I., Dagan S. Y., Tsoory M., Coppola G., McDonald M. K., Gomes C., Geschwind D. H.et al. (2012). Subcellular knockout of importin β1 perturbs axonal retrograde signaling. Neuron 75, 294–305 10.1016/j.neuron.2012.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche F. K., Marsick B. M., Letourneau P. C. (2009). Protein synthesis in distal axons is not required for growth cone responses to guidance cues. J. Neurosci. 29, 638–652 10.1523/JNEUROSCI.3845-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer B., Taylor V., Welcher A. A., McClelland M., Suter U. (1998). Neural membrane protein 35 (NMP35): a novel member of a gene family which is highly expressed in the adult nervous system. Mol. Cell. Neurosci. 11, 260–273 10.1006/mcne.1998.0697 [DOI] [PubMed] [Google Scholar]
- Schweitzer B., Suter U., Taylor V. (2002). Neural membrane protein 35/Lifeguard is localized at postsynaptic sites and in dendrites. Brain Res. Mol. Brain Res. 107, 47–56 10.1016/S0169-328X(02)00445-X [DOI] [PubMed] [Google Scholar]
- Segura M. F., Sole C., Pascual M., Moubarak R. S., Perez–Garcia M. J., Gozzelino R., Iglesias V., Badiola N., Bayascas J. R., Llecha N.et al. (2007). The long form of Fas apoptotic inhibitory molecule is expressed specifically in neurons and protects them against death receptor-triggered apoptosis. J. Neurosci. 27, 11228–11241 10.1523/JNEUROSCI.3462-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shestakova E. A., Singer R. H., Condeelis J. (2001). The physiological significance of beta -actin mRNA localization in determining cell polarity and directional motility. Proc. Natl. Acad. Sci. USA 98, 7045–7050 10.1073/pnas.121146098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. S., Skene J. H. (1997). A transcription-dependent switch controls competence of adult neurons for distinct modes of axon growth. J. Neurosci. 17, 646–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sole C., Dolcet X., Segura M. F., Gutierrez H., Diaz–Meco M. T., Gozzelino R., Sanchis D., Bayascas J. R., Gallego C., Moscat J.et al. (2004). The death receptor antagonist FAIM promotes neurite outgrowth by a mechanism that depends on ERK and NF-kapp B signaling. J. Cell Biol. 167, 479–492 10.1083/jcb.200403093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. M., Berchtold N. C., Perreau V. M., Tu C. H., Li Jeon N., Cotman C. W. (2009). Axonal mRNA in uninjured and regenerating cortical mammalian axons. J. Neurosci. 29, 4697–4707 10.1523/JNEUROSCI.6130-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth C. C., Willis D., Twiss J. L., Walsh S., Martinez J. A., Liu W. Q., Midha R., Zochodne D. W. (2009). Locally synthesized calcitonin gene-related Peptide has a critical role in peripheral nerve regeneration. J. Neuropathol. Exp. Neurol. 68, 326–337 10.1097/NEN.0b013e31819ac71b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twiss J. L., Smith D. S., Chang B., Shooter E. M. (2000). Translational control of ribosomal protein L4 mRNA is required for rapid neurite regeneration. Neurobiol. Dis. 7, 416–428 10.1006/nbdi.2000.0293 [DOI] [PubMed] [Google Scholar]
- Vuppalanchi D., Coleman J., Yoo S., Merianda T. T., Yadhati A. G., Hossain J., Blesch A., Willis D. E., Twiss J. L. (2010). Conserved 3′-untranslated region sequences direct subcellular localization of chaperone protein mRNAs in neurons. J. Biol. Chem. 285, 18025–18038 10.1074/jbc.M109.061333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuppalanchi D., Merianda T. T., Donnelly C., Pacheco A., Williams G., Yoo S., Ratan R. R., Willis D. E., Twiss J. L. (2012). Lysophosphatidic acid differentially regulates axonal mRNA translation through 5′UTR elements. Mol. Cell. Neurosci. 50, 136–146 10.1016/j.mcn.2012.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis D. E., Twiss J. L. (2010). Regulation of protein levels in subcellular domains through mRNA transport and localized translation. Mol. Cell. Proteomics 9, 952–962 10.1074/mcp.R900005-MCP200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis D. E., Twiss J. L. (2011). Profiling axonal mRNA transport. Methods Mol. Biol. 714, 335–352 10.1007/978-1-61779-005-8_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis D., Li K. W., Zheng J. Q., Chang J. H., Smit A. B., Kelly T., Merianda T. T., Sylvester J., van Minnen J., Twiss J. L. (2005). Differential transport and local translation of cytoskeletal, injury-response, and neurodegeneration protein mRNAs in axons. J. Neurosci. 25, 778–791 10.1523/JNEUROSCI.4235-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis D. E., van Niekerk E. A., Sasaki Y., Mesngon M., Merianda T. T., Williams G. G., Kendall M., Smith D. S., Bassell G. J., Twiss J. L. (2007). Extracellular stimuli specifically regulate localized levels of individual neuronal mRNAs. J. Cell Biol. 178, 965–980 10.1083/jcb.200703209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis D. E., Xu M., Donnelly C. J., Tep C., Kendall M., Erenstheyn M., English A. W., Schanen N. C., Kirn–Safran C. B., Yoon S. O.et al. (2011). Axonal Localization of transgene mRNA in mature PNS and CNS neurons. J. Neurosci. 31, 14481–14487 10.1523/JNEUROSCI.2950-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudin D., Hanz S., Yoo S., Iavnilovitch E., Willis D., Gradus T., Vuppalanchi D., Segal–Ruder Y., Ben–Yaakov K., Hieda M.et al. (2008). Localized regulation of axonal RanGTPase controls retrograde injury signaling in peripheral nerve. Neuron 59, 241–252 10.1016/j.neuron.2008.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelená J. (1970). Ribosome-like particles in myelinated axons of the rat. Brain Res. 24, 359–363 10.1016/0006-8993(70)90120-4 [DOI] [PubMed] [Google Scholar]
- Zheng J-Q., Kelly T. K., Chang B., Ryazantsev S., Rajasekaran A. K., Martin K. C., Twiss J. L. (2001). A functional role for intra-axonal protein synthesis during axonal regeneration from adult sensory neurons. J. Neurosci. 21, 9291–9303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X., Schneider T J., Cabral D. S., Donohoe T. J., Rothstein T. L. (2001). An alternatively spliced long form of Fas apoptosis inhibitory molecule (FAIM) with tissue-specific expression in the brain. Mol. Immunol. 38, 65–72 10.1016/S0161-5890(01)00035-9 [DOI] [PubMed] [Google Scholar]
- Zivraj K. H., Tung Y. C., Piper M., Gumy L., Fawcett J. W., Yeo G. S., Holt C. E. (2010). Subcellular profiling reveals distinct and developmentally regulated repertoire of growth cone mRNAs. J. Neurosci. 30, 15464–15478 10.1523/JNEUROSCI.1800-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuliani C., Kleber S., Klussmann S., Wenger T., Kenzelmann M., Schreglmann N., Martinez A., del Rio J. A., Soriano E., Vodrazka P.et al. (2006). Control of neuronal branching by the death receptor CD95 (Fas/Apo-1). Cell Death Differ. 13, 31–40 10.1038/sj.cdd.4401720 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.