Summary
Proteomic studies in unicellular eukaryotes identified a set of centriolar proteins that included proteome of centriole 1 (Poc1). Functional studies in these organisms implicated Poc1 in centriole duplication and length control, as well as ciliogenesis. Using isoform-specific antibodies and RNAi depletion, we have examined the function of the two related human proteins, Poc1A and Poc1B. We find that Poc1A and Poc1B each localize to centrioles and spindle poles, but do so independently and with different dynamics. However, although loss of one or other Poc1 protein does not obviously disrupt mitosis, depletion of both proteins leads to defects in spindle organization with the generation of unequal or monopolar spindles. Our data indicate that, once incorporated, a fraction of Poc1A and Poc1B remains stably associated with parental centrioles, but that depletion prevents incorporation into nascent centrioles. Nascent centrioles lacking both Poc1A and Poc1B exhibit loss of integrity and maturation, and fail to undergo duplication. Thus, when Poc1A and Poc1B are co-depleted, new centrosomes capable of maturation cannot assemble and unequal spindles result. Interestingly, Poc1B, but not Poc1A, is phosphorylated in mitosis, and depletion of Poc1B alone was sufficient to perturb cell proliferation. Hence, Poc1A and Poc1B play redundant, but essential, roles in generation of stable centrioles, but Poc1B may have additional independent functions during cell cycle progression.
Key words: Poc1, Cell cycle, Centriole, Centrosome, Mitosis, Spindle
Introduction
Centrioles are remarkably conserved structures found in most animal cells (Azimzadeh and Marshall, 2010; Kobayashi and Dynlacht, 2011). In post-mitotic differentiated cells, they take the form of basal bodies that subtend the axonemal microtubules found within cilia and flagella, while in dividing cells they form the core of the centrosome, the primary site of microtubule nucleation both in interphase and mitosis. They therefore play essential functions in many aspects of cell biology and multicellular organisms cannot develop properly in their absence (Bettencourt-Dias et al., 2011; Gerdes et al., 2009; Marshall, 2008; Nigg and Raff, 2009).
Centrioles are strikingly beautiful structures, being composed of nine, highly stabilized, microtubule triplets organized as blades into a barrel that is ∼500 nm in length and 200 nm in diameter (Azimzadeh and Marshall, 2010). In G1, animal cells contain two centrioles, an older one called the mother and a younger one called the daughter. These undergo one round of duplication per cell cycle to maintain centriole numbers in dividing cells. Centrioles have a polarity with a proximal end from which new centrioles grow, and a distal end, which, in the case of the mother centriole, has appendages that contribute to both microtubule anchoring and attachment to the plasma membrane during ciliogenesis. The lumen of the centriole also contains a number of internal structures. At the proximal end is a cartwheel-like structure with nine spokes that is thought to dictate the ninefold symmetry of the centriole (Hiraki et al., 2007; Kitagawa et al., 2011; Nakazawa et al., 2007; van Breugel et al., 2011). Additional electron-dense material is detected within the distal half of the centriole lumen although this remains poorly defined. In ciliated cells, the distal end extends towards the base of the cilium through a region called the transition zone, which acts as a gate to control entry of proteins to and from the cilium (Ishikawa and Marshall, 2011).
Understanding the detailed molecular structure of the centriole and how it is assembled has been a major challenge for cell biologists. However, the concerted use of siRNA and mutagenesis screens, combined with ultrastructural electron microscopy studies, has begun to identify the roles of many centriole components (Bettencourt-Dias and Glover, 2007; Loncarek and Khodjakov, 2009; Nigg and Raff, 2009; Nigg and Stearns, 2011; Strnad and Gönczy, 2008). In addition, organelle-specific and comparative proteomics have provided important insights into the composition of centrioles and basal bodies (Andersen et al., 2003; Jakobsen et al., 2011; Keller et al., 2005; Kilburn et al., 2007). Proteomic analyses of basal bodies in Chlamydomonas and Tetrahymena revealed a set of eighteen conserved, but previously uncharacterized, proteins, that were termed Poc, for proteome of centriole (Keller et al., 2005; Kilburn et al., 2007). One such protein, Poc1, has been confirmed as a core centriole/basal body component not only in Chlamydomonas and Tetrahymena, but also the cnidarian Clytia, the insect Drosophila and vertebrates, including Xenopus, zebrafish and humans (Blachon et al., 2009; Fourrage et al., 2010; Hames et al., 2008; Keller et al., 2009; Pearson et al., 2009). Phylogenetic studies suggest the existence of Poc1 proteins in all organisms that contain motile cilia at some point in their life cycle (Woodland and Fry, 2008).
Poc1 proteins have a conserved organization consisting of an N-terminal WD40 domain likely to form a 7-bladed β-propeller, and a C-terminal coiled-coil that includes a highly conserved sequence, known as the Poc1 motif. Separating the WD40 domain and coiled-coil is a less well-conserved spacer sequence. Human cells express two different isoforms of Poc1, termed Poc1A and Poc1B (also previously called Pix2 and Pix1, respectively), encoded by distinct genes. When overexpressed, both Poc1 isoforms localize to centrioles in human cells with truncation studies implicating the WD40 domain in centrosome targeting (Hames et al., 2008; Keller et al., 2009; Pearson et al., 2009). However, it is currently unclear whether the endogenous proteins both localize at the centrosome and whether they have redundant or distinct functions.
Studies of poc1Δ mutants in single-celled eukaryotes indicate a role for Poc1 in basal body stability (Keller et al., 2009; Pearson et al., 2009). Poc1 is also implicated in centriole length control with overexpression in human cells leading to elongated centrioles associated with centrin and γ-tubulin. However, depletion of Poc1 did not lead to shortening of centrioles in human cells, although mutations in Drosophila Poc1 did produce shortened spermatid centrioles (Blachon et al., 2009; Keller et al., 2009). Consistent with a role in centriole organization, EM studies indicate the presence of Poc1 on both the inner luminal walls and proximal ends of centrioles, whilst in ciliated cells, it appears at the transition zone (Hames et al., 2008; Keller et al., 2009; Pearson et al., 2009). Depletion studies suggest that Poc1B, but not Poc1A, is required for ciliogenesis in human cells, whilst Poc1 knockdown in zebrafish causes developmental defects typical of ciliopathies (Pearson et al., 2009). Microinjection of Poc1 antibodies in human cells also interferes with cell division (Hames et al., 2008), Together then, Poc1 proteins appear to be important for centriole assembly and/or stability, as well as ciliogenesis and cell division.
Here, using isoform-specific antibodies and RNAi depletion, we have characterized the properties and functions of the individual human Poc1 isoforms. We find that, while both proteins are stably incorporated into centrioles, their association with the centrosome is independent and exhibits distinct dynamics. Moreover, we observed that Poc1B, but not Poc1A, is phosphorylated in mitosis, and depletion of Poc1B alone was sufficient to perturb cell proliferation. In contrast, depletion of both proteins, but not each one individually, led to a failure of centriole biogenesis and defects in mitotic spindle formation. Hence, human Poc1 proteins have potentially both redundant and distinct functions in centriole integrity and cell cycle progression.
Results
Poc1A and Poc1B are stable components of human centrosomes
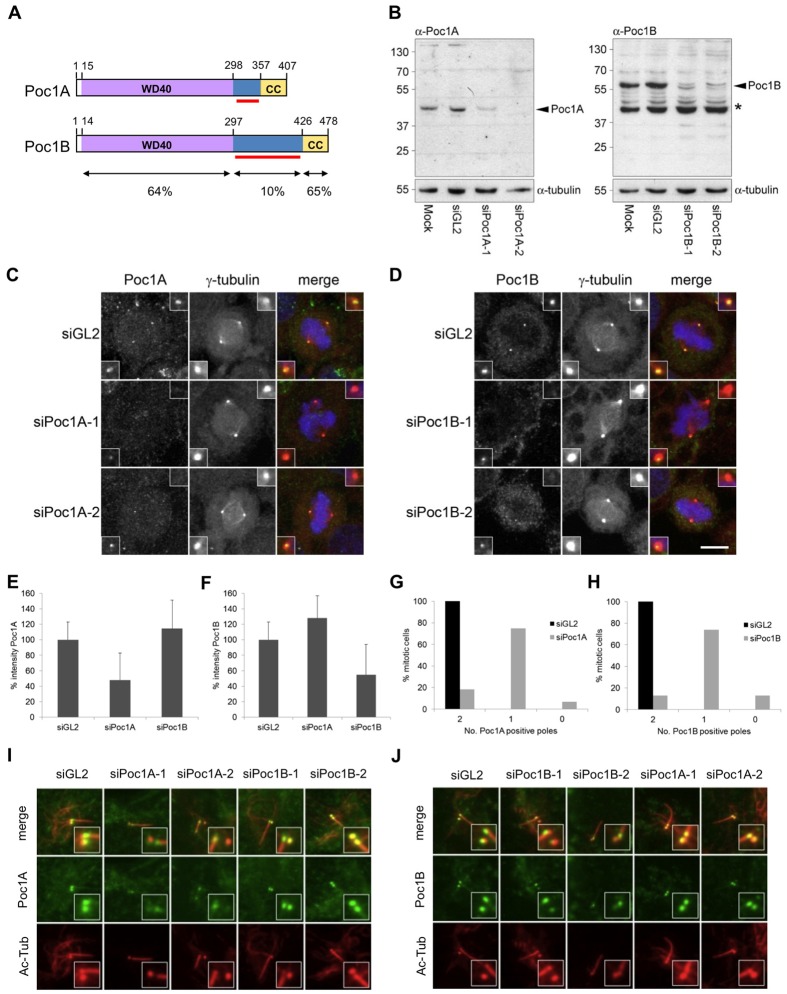
To study the behaviour of the distinct human Poc1A and Poc1B proteins, we first generated isoform-specific antibodies using bacterially expressed fragments of the non-conserved spacer regions that lie between the WD40 and coiled-coil as antigens (Fig. 1A). Western blotting, combined with siRNA-mediated depletion with two different oligonucleotides against each isoform, indicated that these antibodies were not only capable of detecting the endogenous proteins but were specific for the appropriate version of Poc1 (Fig. 1B; supplementary material Fig. S1A). Immunofluorescence microscopy of mitotic cells confirmed localization of both Poc1A and Poc1B at spindle poles as shown by co-localization with γ-tubulin (Fig. 1C,D). Again, this staining pattern was significantly diminished upon depletion with either of the two different siRNAs, whereas there was no significant impact on the localization of the non-depleted isoform (Fig. 1E,F; supplementary material Fig. S1B,C). Importantly, though, we found that loss of Poc1 staining at the two spindle poles upon depletion was not equivalent with one pole usually retaining a detectable amount of Poc1 protein (Fig. 1G,H). However, competition with purified Poc1 proteins led to complete loss of Poc1 staining at spindle poles attesting to the specificity of the antibodies (supplementary material Fig. S1D,E).
Fig. 1.
Poc1A and Poc1B are stable components of centrioles. (A) Schematic representation of Poc1A and Poc1B protein domain organization. WD40, WD40-repeat domain; CC, coiled-coil domain. The red bars show the spacer regions used to generate isoform-specific Poc1 antibodies. Amino-acid positions and the percentage of amino-acid identity of the relative domains are indicated. (B) Western blot characterization of purified Poc1A and Poc1B antibodies in HeLa cell extracts that were either mock-treated or treated with control (siGL2), Poc1A (siPoc1A-1, siPoc1A-2) or Poc1B (siPoc1B-1, siPoc1B-2) siRNAs. Arrowheads indicate Poc1 proteins; the asterisk indicates a non-specific protein detected. α-tubulin served as a loading control. Molecular masses (kDa) are indicated on the left. (C,D) HeLa cells transfected with siRNAs against luciferase (siGL2) or two different siRNAs against Poc1A (C) or Poc1B (D) were fixed after 72 hours. Cells were stained for γ-tubulin (red) and Poc1A (green, C) or Poc1B (green, D). Scale bar: 10 µm. (E,F) The mean intensity of Poc1A (E) and Poc1B (F) staining of mitotic spindle poles was quantified in cells that had been mock-depleted or depleted with Poc1A or Poc1B siRNAs, as indicated. (G,H) The percentage of cells with 0, 1 or 2 Poc1A-positive (A) or Poc1B-positive (B) spindle poles after 72 hours RNAi treatment with GL2 or Poc1A or Poc1B siRNAs is shown (n = 94 for siGL2, n = 60 for siPoc1A, n = 100 for siPoc1B). (I,J) hTERT-RPE1 cells treated with siRNAs against GL2 or two different siRNAs against Poc1A or Poc1B for 32 hours were subsequently serum starved for 40 hours, fixed and stained with Poc1A (I) or Poc1B (J) (green) and acetylated-tubulin (Ac-Tub; red) antibodies.
We hypothesized that a stable fraction of Poc1A and Poc1B that was already incorporated into centrosomes was maintained during depletion, whereas incorporation into new centrosomes did not take place. To test this hypothesis, hTERT-RPE1 cells were depleted with the two different siRNAs for each isoform before serum starvation to induce ciliogenesis. This revealed that Poc1A and Poc1B remain on the basal body that subtends the axonemal microtubules associated with the cilium, i.e. the mother centriole, but are absent from the other (Fig. 1I,J). In support of the notion that Poc1 proteins can be stably incorporated into centrosomes, short-term siRNA-mediated depletion was not able to remove Poc1 proteins from spindle poles in cells that had previously expressed recombinant GFP–Poc1A or GFP–Poc1B (supplementary material Fig. S2). We therefore conclude that a fraction of Poc1A and Poc1B proteins are stably incorporated into centrioles and that depletion only prevents their incorporation into nascent centrioles.
Poc1A and Poc1B localize to centrosomes independently
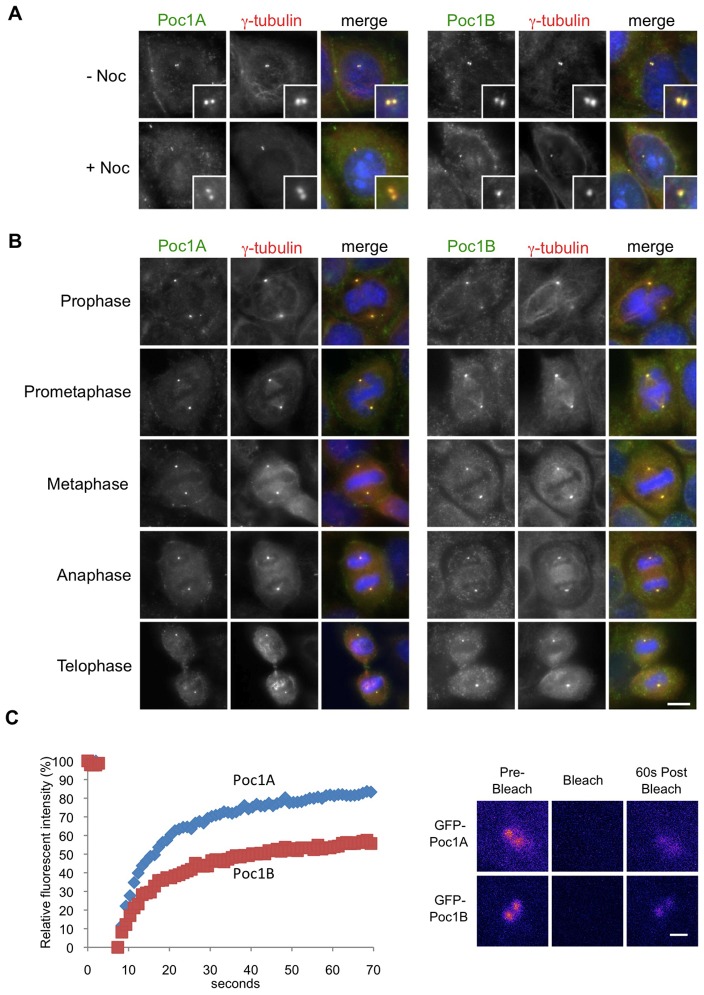
Consistent with data using recombinant proteins (Hames et al., 2008; Keller et al., 2009; Keller et al., 2005; Pearson et al., 2009), we found that endogenous Poc1A and Poc1B also localized to interphase centrosomes and that this was independent of the presence of intact microtubules (Fig. 2A). Analysis through the different phases of mitosis revealed that Poc1A and Poc1B localized to spindle poles from prophase through to telophase, while Poc1B also exhibited a weak association with spindle fibres (Fig. 2B). A previous proteomic study had raised the possibility that Poc1A and Poc1B were present in the same complexes and that their localization to the centrosome may therefore be interdependent (Hutchins et al., 2010). To test this we examined whether the proteins were capable of interacting with each other in cells. By transiently expressing GFP-tagged Poc1 proteins in a cell line that stably expressed TAP-tagged Poc1A, we found that recombinant Poc1 proteins can associate in the same complexes in cells (supplementary material Fig. S3).
Fig. 2.
Poc1A and Poc1B localize independently to centrosomes. (A) HeLa cells were either untreated (− Noc) or incubated with 500 µg/ml nocodazole (+ Noc) for 4 hours to depolymerize microtubules before immunostaining. Methanol-fixed cells were labelled with Poc1A or Poc1B (green) and γ-tubulin (red) antibodies. Merge panels include DNA staining with Hoechst 33258 (blue). Magnified views of centrosomes are shown in the insets. (B) Mitotic HeLa cells at the stages indicated were stained with Poc1A or Poc1B (green) and γ-tubulin (red) antibodies. Merge panels include DNA staining with Hoechst 33258 (blue). Scale bar: 10 µm. (C) HeLa cells were transiently transfected with EGFP-Poc1A (blue diamonds) or EGFP-Poc1B (red squares), and FRAP analysis was performed on an asynchronous population of cells using a confocal microscope. Recovery curves are shown on the left and representative pseudo-coloured images from pre- and post-bleached live samples are shown on the right. Scale bar: 1 µm.
However, as noted above, RNAi experiments revealed that depletion of Poc1A did not prevent centrosome localization of Poc1B and vice versa. We therefore performed fluorescence recovery after photobleaching (FRAP) experiments with HeLa cells transfected with GFP-tagged Poc1A or Poc1B to assess their turnover rates at centrosomes (Fig. 2C). Interestingly, we found that the two Poc1 proteins exhibited distinct dynamics with 81.8% of Poc1A exchanging at the centrosome with a t1/2 of 7.8 seconds, whereas only 55.1% of Poc1B exchanged within a t1/2 of 8.7 seconds. These dynamics were independent of the presence or absence of the other isoform, as depletion of Poc1A did not substantially alter the dynamics of Poc1B, and nor vice versa (Table 1). Note that a region of interest (ROI) of 4 µm2 was chosen that allowed for the mobile nature of the centrosomes and recovery was measured in the entire ROI. Hence, this region will contain some cytoplasmic recovery and as a result the % mobile fraction is likely to be an overestimate. Together, then, these data support the conclusion that at least 20% of Poc1A and 45% of Poc1B are stably incorporated into the centrosome and would therefore be resistant to depletion.
Table 1.
Summary of FRAP data on Poc1A and Poc1B in asynchronous HeLa cells transiently expressing GFP–Poc1A or GFP–Poc1B
| GFP–Poc1A | GFP–Poc1B | GFP–Poc1A (siPoc1B) | GFP–Poc1B (siPoc1A) | |
| % Mobile fraction | 81±8 | 55±12 | 76±10 | 56±11 |
| Half-life | 7.8±2.8 | 8.7±2.9 | 5.8±1.7 | 10.4±3.6 |
| Number of cells | 12 | 14 | 15 | 10 |
A 4×4 µm region of interest encompassing the centrosomes was bleached and the percentage mobile fraction within a 60 second period, and half-life (t1/2, seconds) were determined using easyFRAP software.
Poc1B undergoes mitotic specific phosphorylation
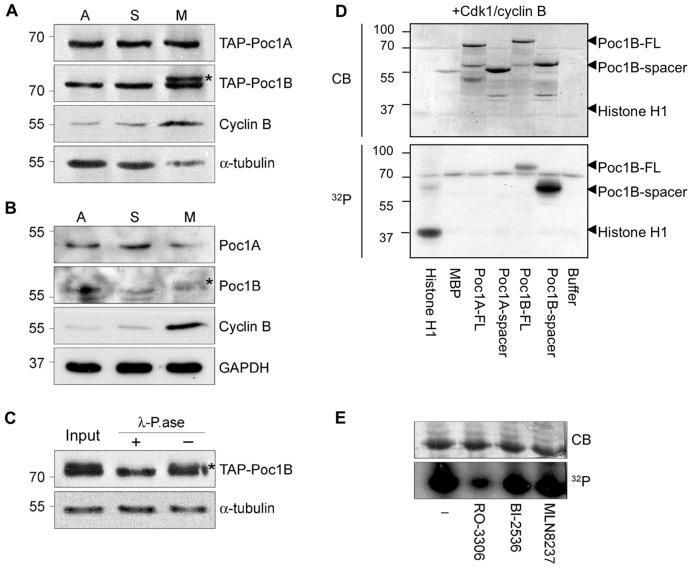
During analysis of recombinant Poc1 proteins by western blot, a doublet was sometimes observed for Poc1B. To examine whether this might reflect a cell cycle-dependent regulation, we first analysed the migration of recombinant TAP–Poc1 proteins in extracts taken from asynchronous or synchronized cells. A retarded gel mobility was apparent for TAP–Poc1B, but not TAP–Poc1A, in extracts prepared from M-phase-, but not S-phase-, arrested cells (Fig. 3A). A similar mitotic-specific modification could be detected, albeit more weakly, with endogenous Poc1B, but not Poc1A (Fig. 3B). Incubation of TAP–Poc1B isolated from mitotic extracts with λ-phosphatase led to loss of the gel-shift indicating that Poc1B undergoes phosphorylation during mitotic arrest (Fig. 3C). Confirmation that this is a mitotic-specific event, rather than a consequence of microtubule depolymerisation, came from showing that cells treated with nocodazole during an S-phase arrest do not exhibit phosphorylation, whilst cells arrested in mitosis with a Plk1 inhibitor rather than nocodazole show phosphorylation (supplementary material Fig. S4A).
Fig. 3.
Poc1B is phosphorylated in mitosis. (A) Extracts from asynchronous (A), hydroxyurea-arrested (S) and nocodazole-arrested (M) HEK293 cells stably expressing TAP–Poc1A or TAP–Poc1B were separated on phos-tag gels and western blotted with the appropriate isoform-specific Poc1 antibody and antibodies against cyclin B and α-tubulin. (B) Extracts from HeLa cells prepared as in A were western blotted with isoform-specific Poc1 antibodies or antibodies against cyclin B or GAPDH. The Poc1B gel-shift in M-phase cells is indicated with an asterisk. (C) Extracts from nocodazole-arrested HEK293 cells stably expressing TAP–Poc1B were either untreated (Input) or incubated in phosphatase buffer with (+) or without (−) λ-phosphatase for 30 min at 30°C, before western blotting with Poc1B and α-tubulin antibodies. (D) Histone H1, maltose-binding protein (MBP) and full-length (FL) or spacer fragments of MBP-tagged Poc1A or Poc1B were used as substrates in kinase assays with Cdk1/cyclin B for 30 min at 30°C. Samples were analysed by SDS-PAGE, Coomassie Blue staining (CB) and autoradiography (32P). In A–D, molecular masses (in kDa) are indicated on the left. (E) The His-Poc1B-spacer was incubated in soluble mitotic HeLa cell extracts with [γ-32P]ATP in the absence (−) or presence of small molecule inhibitors of Cdk1 (RO-3306), Plk1 (BI-2536) and Aurora A (MLN8237) before analysis as in D.
To determine which kinase(s) may be responsible for Poc1B phosphorylation, we incubated MBP-tagged Poc1B with a number of mitotic or centrosome-associated kinases. Whilst Plk1, Plk4, Aurora A and Nek2 did not phosphorylate Poc1B in vitro, Cdk1/cyclin B strongly phosphorylated this protein (Fig. 3D; supplementary material Fig. S4B). Indeed, Cdk1/cyclin B phosphorylated the full-length and spacer region of Poc1B but not Poc1A (Fig. 3D). We also found that a tagged version of the Poc1B spacer region could be phosphorylated in an extract prepared from mitotically arrested cells and that this phosphorylation was blocked by a chemical inhibitor of Cdk1, but not Plk1 or Aurora A (Fig. 3E). Hence, we conclude that Poc1B undergoes phosphorylation in mitotic human cells that may be mediated by Cdk1 within the non-conserved spacer region of the protein.
Poc1B is required for cell proliferation
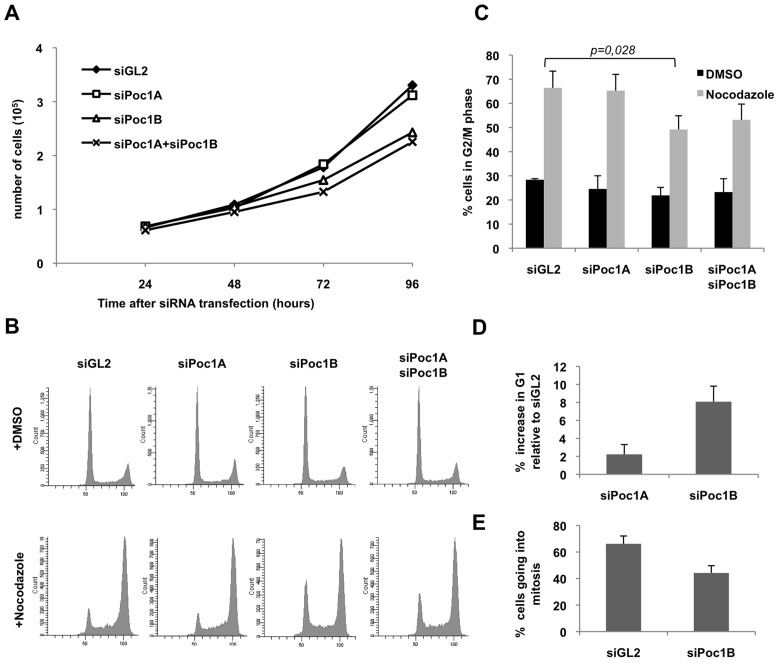
To explore whether human cells might have different requirements for the two Poc1 proteins, we used the two different siRNAs to deplete each isoform, individually or in combination, in HeLa cells. Analysing cell numbers at increasing times after depletion revealed a consistent reduction in proliferation of cells treated with siRNAs against Poc1B, but not Poc1A, as compared to control depletions (Fig. 4A; supplementary material Fig. S5A). Combined depletion of Poc1A and Poc1B led to a similar reduction in proliferation as depletion of Poc1B alone. Analysis of annexin V staining revealed no significant increase in apoptotic cells following Poc1B depletion compared to controls indicating that reduced proliferation was not a result of programmed cell death (data not shown). We therefore tested whether cells were still cycling by analysing DNA content by flow cytometry after incubating cells with nocodazole. Strikingly, this revealed that depletion of Poc1B, but not Poc1A, reduced nocodazole-induced accumulation of G2/M cells pointing to an interphase delay (Fig. 4B,C). Indeed, flow cytometry analysis revealed an increase in the fraction of G1 cells in response to Poc1B depletion even in the absence of nocodazole (Fig. 4B,D). A similar increase in the percentage of cells in G1 and failure to accumulate a G2/M peak in the presence of nocodazole was found upon depletion of Poc1B from the non-transformed cell line, hTERT-RPE1 (supplementary material Fig. S5B–D). By time-lapse imaging we also observed a reduction in the number of Poc1B-depleted cells entering mitosis in a 24 hour period compared to controls (Fig. 4E). Together, these data demonstrate that loss of Poc1B, but not Poc1A, leads to reduced proliferation and delay of cells in G1.
Fig. 4.
Depletion of Poc1B proteins leads to loss of proliferation. (A) Growth curves of HeLa cells transfected with siRNA oligonucleotides against luciferase as control (siGL2), Poc1A, Poc1B or Poc1A+Poc1B; data are means from two separate experiments. (B) HeLa cells treated with siRNAs for 55 hrs were treated with 0.5 µg/ml nocodazole or DMSO for 17 hours, fixed, and analysed by flow cytometry. (C) Histogram of the percentage of cells in G2/M phase; data are means ± s.d. from three separate experiments including that shown in B. The P-value indicates the significance of difference in the percentage of cells in G2/M in the nocodazole-treated siGL2 versus siPoc1B samples. (D) HeLa cells were depleted with siRNAs against GL2, Poc1A or Poc1B and analysed by flow cytometry after 72 hours. The percentage increase in G1 phase relative to siGL2 is indicated; Data are means ± s.d. from three separate experiments. (E) HeLa cells stably expressing GFP–α-tubulin were treated with siRNAs against GL2 or Poc1B for 48 hours and the percentage of cells entering mitosis in the subsequent 24 hour period was determined by time-lapse imaging. n = 113 for siGL2 and 100 for siPoc1B.
Co-depletion of Poc1A and Poc1B interferes with mitotic spindle formation
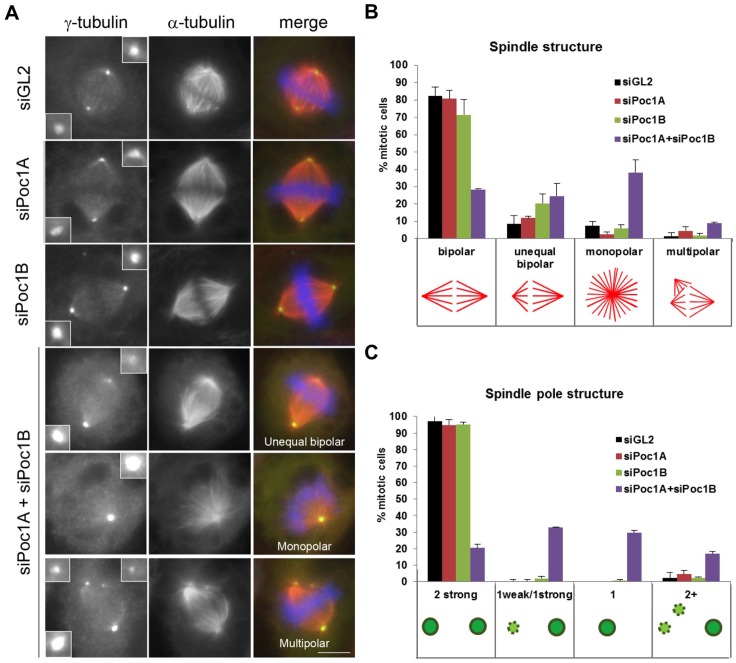
We next investigated the consequences of Poc1 loss on mitotic progression. Again, Poc1A and Poc1B were depleted either individually or in combination from HeLa cells for 72 hours before analysis of spindle structure in mitotic cells. Importantly, depletion of Poc1A or Poc1B alone showed no significant mitotic defects. In contrast, the combined depletion of Poc1A and Poc1B led to frequent aberrations in spindle organization (Fig. 5A,B). Three major categories of spindle defect were observed: unequal bipolar spindles in which one half-spindle was shorter than the other, monopolar spindles and multipolar spindles. The most prominent phenotype was the existence of monopolar spindles, which accounted for 38.1% of spindles in the Poc1A/Poc1B co-depleted cells versus 7.4% in controls. However, unequal bipolar spindles were also common (24.6% in the co-depletion versus 8.8% in controls). Multipolar spindles were found in 8.9% of the mitotic cells co-depleted of both Poc1A and Poc1B, compared to 1.4% in the controls. Staining of spindle poles with antibodies against γ-tubulin revealed that the spindle defects correlated with abnormalities in spindle pole organization. The monopolar spindles in Poc1-depleted cells presented with only one detectable pole instead of two. Similarly, the unequal bipolar spindles had one strongly stained pole and one much weaker staining pole that was present on the shorter half spindle (Fig. 5A,C). Multipolar spindles had one strong pole and one pole that appeared fragmented. These observations indicate that in the majority of cells co-depleted for both Poc1A and Poc1B centrosome maturation or integrity had failed at one pole but not the other.
Fig. 5.
Co-depletion of Poc1A and Poc1B leads to mitotic spindle defects. (A) HeLa cells transfected with siRNAs against GL2, Poc1A and/or Poc1B were fixed after 72 hours. Cells were stained with γ-tubulin (green) and α-tubulin (red) antibodies. Merge panels include DNA staining with Hoechst 33258 (blue). Scale bar: 6 µm. (B) The percentage of cells with the spindle structures indicated. (C) The percentage of mitotic cells with γ-tubulin-positive centrosomes of the appearances indicated. Data in B and C are means ± s.d. from two separate experiments; n = 215 for siGL2, n = 218 for siPoc1A, n = 210 for siPoc1B and n = 235 for siPoc1A+siPoc1B. Error bars in B and C represent s.d.
Co-depletion of Poc1A and Poc1B leads to failure of centriole biogenesis
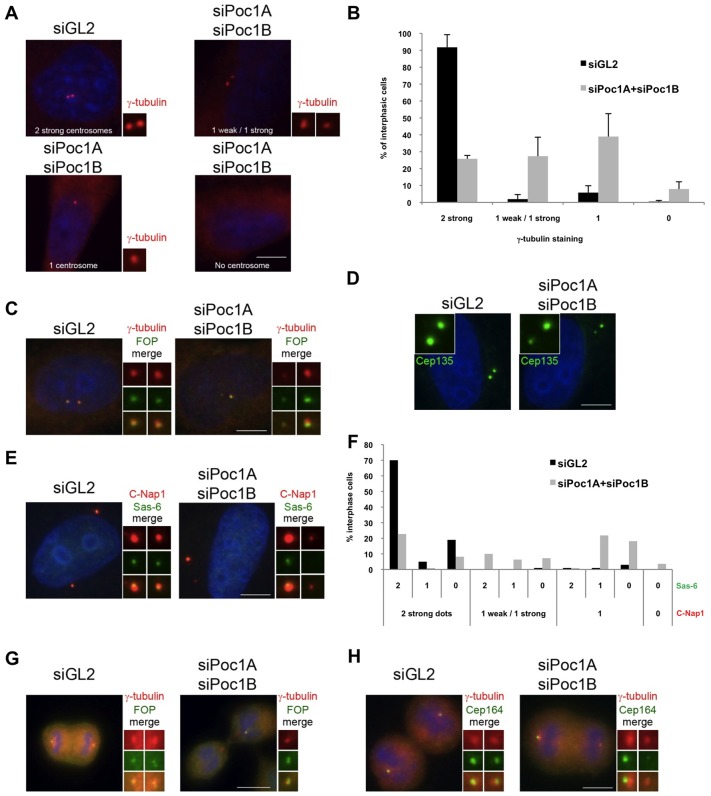
It was notable that in cells depleted of Poc1A alone or Poc1B alone, there was no substantial loss of γ-tubulin from the centrosome that was devoid of Poc1. Strikingly, though, we found that whereas two strong γ-tubulin signals were detected in 90% of control depleted cells, cells co-depleted for both Poc1A and Poc1B exhibited substantial loss of γ-tubulin from one centrosome. 27% of co-depleted cells had two detectable γ-tubulin dots but had one that was at least 2-fold weaker than the other, and 39% of cells had only one detectable γ-tubulin dot (Fig. 6A,B). A similar loss was observed for the centrosomal marker, FOP (Yan et al., 2006) (Fig. 6C). This could be explained by a defect in centrosome maturation specifically leading to loss of PCM recruitment. However, staining with antibodies against markers of centriole proximal ends, notably Cep135, Sas-6 and C-Nap1 (Fry et al., 1998; Kleylein-Sohn et al., 2007; Leidel et al., 2005), revealed similar losses of staining from one centrosome, such that either only one dot was detected or one strong and one weak dot (Fig. 6D–F). Hence, the defect was not simply in recruitment of PCM. Indeed, the frequent detection of one strong and one weak dot with these centriole markers provides persuasive evidence that while co-depletion of Poc1A and Poc1B does not block the initiation of centriole duplication, it does interfere with assembly of nascent centrioles.
Fig. 6.
Co-depletion of Poc1A and Poc1B leads to loss of centrosome proteins. HeLa cells were transfected with siRNAs against luciferase (siGL2) or Poc1A+Poc1B, as indicated, and fixed after 72 hours. Cells were stained with γ-tubulin antibodies alone (A, red), γ-tubulin (red) and FOP antibodies (green; C,G), Cep135 antibodies (green) (D), C-Nap1 (red) and Sas-6 (green; E), γ-tubulin (red) and Cep164 (green; H). (B) The percentage of interphase cells with 2 strong, 1 weak and 1 strong, 1 alone or 0 γ-tubulin-stained spots. Data are means ± s.d. from two independent experiments (n = 207 for siGL2 and n = 222 for siPoc1A+siPoc1B). (F) The percentage of interphase cells with 2 strong, 1 weak and 1 strong, 1 alone or 0 C-Nap1-positive centrosomes were scored with respect to the number of Sas-6 dots seen in the same cells. Data are from a single experiment (n = 100 for siGL2 and n = 110 for siPoc1A+siPoc1B). Merged images include DNA staining with Hoechst 33258 (blue). Magnified views of centrosomes, when visible, are shown. Scale bars: 6 µm (A,C,D,E,H); 11 µm (G).
Interestingly, 8% of Poc1A and Poc1B co-depleted interphase cells had no detectable centrosome staining at all with γ-tubulin or FOP antibodies. This is consistent with the observation of co-depleted cells undergoing cytokinesis, with one daughter cell having one detectable centrosome and the other with none (Fig. 6G). Indeed, during mitosis, the daughter centriole matures into a mother and acquires distal end appendages that can be detected with antibodies against Cep164 (Graser et al., 2007). Staining for Cep164 revealed that in control-depleted telophase cells both emerging daughter cells were positive for Cep164 and contain mother centrioles, whereas co-depletion of Poc1A and Poc1B led to only one daughter cell being positive for Cep164 (Fig. 6H). This result confirms the time-dependent loss of mother centrioles during division of cells lacking both Poc1A and Poc1B.
Co-depletion of Poc1A and Poc1B leads to loss of centriole integrity and failure of daughter centriole maturation
As Poc1 proteins can associate with microtubules and localize to the inner centriole walls, we wished to determine whether the loss of centriole proteins upon Poc1 depletion reflects a loss of integrity of the centriolar microtubules. For this purpose, we analysed depleted cells using antibodies against acetylated tubulin that directly stain centriole microtubules, as well as antibodies against the distal end centriole marker, centrin-2. Following control depletions, >95% of interphase cells had two strong acetylated-tubulin dots (Fig. 7A). Of these, approximately one-third had two centrin-2 dots (i.e. were unduplicated and in G1), whilst two-thirds had four centrin-2 dots (i.e. were in the process of duplicating and in S or G2). This indicates that in these cells only the microtubules of the parental centrioles, and not the new procentrioles, are substantially modified by acetylation. Following Poc1 co-depletion, 41.6% of cells had only one detectable acetylated-tubulin dot, suggesting the complete absence of a daughter centriole. Of these, 43% contained only one centriole as detected with centrin-2 antibodies, i.e. were unduplicated in G1, whilst 57% had two centrioles, i.e. were duplicated in S or G2 (Fig. 7B). We also found co-depleted cells that had one strong and one weak acetylated-tubulin signal, i.e. they retained some remnant of a daughter centriole, but in these >90% had less than four centrin-2 dots indicating a failure of duplication from at least one centriole. Hence, co-depletion of Poc1A and Poc1B clearly interferes with generation of centrioles with stabilized microtubules.
Fig. 7.
Co-depletion of Poc1 proteins leads to loss of centriole integrity. (A–D) HeLa cells transfected with siRNAs against luciferase (siGL2) or Poc1A+Poc1B were fixed after 72 hours. Cells were stained with acetylated-tubulin (red) and centrin-2 (green) antibodies. Interphase cells are shown in A and mitotic cells in C. (B) The percentage of interphase cells with 2 strong, 1 weak and 1 strong or only 1 acetylated-tubulin-positive centrosomes is indicated with respect to the number of Centrin-2 dots. Data are means ± s.d. from two separate experiments (n = 157 for siGL2 and n = 92 for siPoc1A+siPoc1B). (D) The percentage of mitotic cells with 2 centrin dots at each pole (2/2), 2 centrin dots at one pole and one at the other (2/1), 2 centrin dots at one pole and none at the other (2/0), 1 centrin dot at each pole (1/1) or 1 centrin dot at one pole and none at the other (1/0). Data are from a single experiment (n = 176 for siGL2 and n = 179 for siPoc1A+siPoc1B). (E) HeLa cells transfected with siRNAs against luciferase (siGL2) or Poc1A+Poc1B for 72 hours were stained with antibodies against γ-tubulin (red) and centrobin (green). DNA was stained with Hoechst 33258 (blue). (F) A schematic model showing the consequences of depleting Poc1 protein (green) on centriole stability and duplication, together with the predicted localization of centrobin under these conditions (pink). Upon siRNA depletion, Poc1 remains stably associated with the parental centrioles but is not incorporated into the nascent procentrioles, preventing their stabilization. When this cell enters mitosis, it will produce daughter cells that have a stable mother centriole but an unstable daughter centriole. When this cell then enters S-phase, the stable mother centriole will initiate duplication but produce another unstable centriole, which is incapable of duplication. When this cell arrives at the next mitosis, the cell contains one spindle pole (pole no. 1) containing the old mother centriole and an unstable procentriole, and one pole (pole no. 2) containing a single unstable daughter centriole or no centriole at all. Such a cell may generate an unequal bipolar or monopolar spindle.
In line with our interphase data, when mitotic cells were examined, almost all control depleted cells contained two centrin-2 dots and one acetylated-tubulin dot at each pole. However, this was not the case in Poc1A and Poc1B co-depleted cells. Of these, ∼30% had two centrin-2 dots at one pole but none at the other, whilst 20% had two dots at one pole and one at the other (Fig. 7C,D). Importantly, in the co-depleted cells, the acetylated-tubulin antibodies only stained the pole containing a pair of centrin dots, and did not stain the pole with a single centrin dot. This strongly supports the hypothesis that cells lacking Poc1A and Poc1B undergo one round of duplication, but the new centrioles formed do not mature and cannot initiate a second round of duplication in the subsequent cycle.
In order to confirm the presence of an immature centriole in cells co-depleted for Poc1A and Poc1B, we stained cells with an antibody against centrobin, a marker of procentrioles that is lost once centrioles initiate duplication in S-phase (Zou et al., 2005). In control depleted mitotic cells, both spindle poles stained positively for γ-tubulin and each of these was associated with a strong centrobin dot, comprising the new pro-centriole within each pole (Fig. 7E). Consistent with our previous data, though, the majority of mitotic cells co-depleted for Poc1A and Poc1B had only one pole that is positive for γ-tubulin. However, they frequently possessed two separated centrobin dots, only one of which was associated with the γ-tubulin-positive pole (Fig. 7E). Hence, we propose that following the combined depletion of Poc1A and Poc1B, cells retain one mature centriole that can undergo duplication and recruit γ-tubulin, but acquire one immature centriole that fails to duplicate or recruit γ-tubulin.
Discussion
Here, using isoform-specific antibodies combined with siRNA-mediated depletion, we have explored the function of the two isoforms of the centrosomal Poc1 protein expressed in human cells. Intriguingly, our data suggest that Poc1A and Poc1B act redundantly in centriole organization, but that Poc1B has additional, non-redundant functions in cell cycle progression. These conclusions are based on the fact that depletion of Poc1B, but not Poc1A, interfered with cell proliferation, whereas depletion of both proteins, but not either protein alone, caused a loss of centriole integrity, which in turn led to defects in mitotic spindle formation.
Firstly, our data confirm that both human Poc1 proteins are core components of the centrosome throughout the cell cycle. Although this is the first time that the localization of each isoform at the endogenous level has been studied, this result was not a surprise as expression of recombinant proteins had shown that Poc1A and Poc1B could associate with the centrosome (Hames et al., 2008; Keller et al., 2009; Keller et al., 2005; Pearson et al., 2009). We found that, despite the ability of recombinant proteins to dimerise, Poc1 proteins were not dependent on each other for their localization to the centrosome. Moreover, FRAP-based experiments indicate that they have distinct dynamics at the centrosome, with a higher fraction of Poc1A protein undergoing rapid exchange than Poc1B. Interestingly, a stable and dynamic population of Poc1 was also identified in Tetrahymena, which only expresses one Poc1 protein (Pearson et al., 2009). However, it is worth noting that a fraction of both Poc1A and Poc1B was stably incorporated, explaining why a signal for both proteins remains after depletion and potentially why depletion of each protein alone is not sufficient to destabilize centrioles.
Functional studies in Tetrahymena first suggested a role for Poc1 in basal body stability (Pearson et al., 2009). Deletion of the POC1 gene led to structural defects in basal bodies that interfered with ciliogenesis and eventually led to cell death. Specifically, although the cartwheel structure was intact, there were defects in luminal and microtubule structures at the proximal ends of basal bodies arguing strongly that Poc1 is required to correctly assemble a basal body. Our data provide persuasive evidence that this role is conserved in human cells, but that the presence of either Poc1A or Poc1B may be sufficient to fulfil this function. Analysis of mitotic cells revealed that depletion of either protein alone had no obvious consequence, whereas their co-depletion led to clear defects in spindle organization. The predominant phenotypes were unequal bipolar spindles and monopolar spindles, and in both cases, this correlated with the presence of one normal pole and one pole that either had much reduced γ-tubulin or none at all. Further analysis of centriole markers led us to conclude that combined loss of Poc1A and Poc1B leads not simply to loss of centrosome maturation but a more profound loss of centriole integrity. The occasional observation of multipolar spindles with one apparently fragmented pole supports the notion that Poc1 proteins contribute to centriole stability.
Depletion of Poc1A or Poc1B led to their absence specifically from one of the two centrosomes. This is most easily explained by the maintenance of a stable population of Poc1 proteins in the pre-existing centrioles, but no incorporation of Poc1 into new centrioles. We therefore hypothesize that when Poc1 proteins are co-depleted, centrosomes are initially capable of undergoing centriole duplication, but Poc1 proteins are not available for incorporation into nascent centrioles rendering them unstable. When these cells enter mitosis, a relatively normal bipolar division results. However, each offspring cell now contains only one stable centriole, so when these enter the next S-phase, the stable centriole initiates duplication, but the unstable centriole does not. When this cell enters mitosis, it assembles an unequal bipolar or monopolar spindle due to the presence of only one functional centrosome, or even a multipolar spindle due to a fragmented centriole. And if this cell manages to complete a bipolar division, then it will generate one cell with a stable centriole, but the other cell will have only an unstable centriole or, possibly, none at all (Fig. 7F).
The question arises as to how precisely Poc1 proteins ensure centriole integrity. We have previously shown that Poc1 is a microtubule-binding protein, while immuno-EM studies indicate that a fraction of the protein localizes to the centriole lumen walls (Hames et al., 2008; Pearson et al., 2009). The loss of acetylated tubulin from structures that still stained, albeit weakly, for centrin-2 suggests that immature centrioles are being generated but that the centriolar microtubules are not getting stabilized. Poc1 may therefore be directly binding and stabilizing centriolar microtubules, in a manner similar to that recently shown for centrobin (Gudi et al., 2011). Alternatively, it could be recruiting other microtubule-associated proteins, including those involved in post-translational modifications of tubulin. Finally, it could be that the release of proteins like centrobin is required to generate stable centrioles and this does not happen in the absence of Poc1.
It has been reported that depletion of human Poc1 proteins prevents centrosome duplication (Keller et al., 2009). Whilst we agree that unstable centrioles lose the ability to duplicate, our data best support a model whereby the primary function of Poc1 is to contribute to centriole stability rather than initiation of duplication. We frequently detected one strong and one weak signal with a number of different centriole markers, including Sas-6 and Cep135, which represent proximal end markers, and FOP, which localizes more towards the distal end. This, together with the presence of centrobin at structures that lack γ-tubulin, is indicative of an immature or unstable structure rather than no structure at all. Indeed, the presence of a strong and weak dot of C-Nap1 argues for the presence of a correctly disengaged centriole, suggesting that unstable centrioles can persist through one cell division event. Interestingly, overexpression of GFP–Poc1B promoted formation of abnormally long centrioles (Keller et al., 2009), which is consistent with the notion that Poc1 not only stabilizes, but also perhaps promotes extension of, centrioles.
Depletion of Poc1B, but not Poc1A, also interfered with proliferation of human cells. Whether this is indicative of a hypomorphic response that is potentially related to centriole integrity is not clear. However, it is intriguing that we also observed a potential difference in the mitotic regulation of Poc1A and Poc1B. Poc1B is clearly phosphorylated in mitosis. Whether Poc1A is also phosphorylated in mitosis is not known, but we did not observe a gel mobility shift for Poc1A, as we did for Poc1B, and we did not detect phosphorylation of Poc1A by Cdk1 in vitro. This, together with the fact that Cdk1 phosphorylated the divergent spacer region, supports the notion that these two proteins may be differentially regulated in mitosis. Sites phosphorylated in vitro, either by Cdk1 or in a mitotic extract, were mapped in Poc1B (data not shown). However, mutation of these sites did not lead to loss of the gel-shift in mitotic cells and nor did it perturb centrosome localization, so further studies will be required to understand the function behind this phosphorylation.
Interestingly, depletion of another core centriolar protein, Poc5, reduced proliferation of HeLa cells as a result of interphase delay and cell death (Azimzadeh et al., 2009). Poc5 is incorporated into the distal end of nascent centrioles and its depletion blocked centriole elongation. Like Poc1, Poc5 was only stably incorporated into mature centrioles, such that Poc5-depleted cells also revealed a single spot of Poc5 staining. It is clear then that besides the core set of centriole duplication factors originally identified in C. elegans (Dammermann et al., 2004; Leidel et al., 2005; Pelletier et al., 2006), there are a set of other important proteins that play key roles in centriole biogenesis. The challenge going forward will be to identify how they interact to define and maintain the stable architecture of centrioles and basal bodies.
Materials and Methods
Plasmid constructions
The divergent spacer regions, corresponding to amino acid residues 304–362 for Poc1A and 303–431 for Poc1B, were amplified by PCR from the previously generated GFP-Poc1A and GFP-Poc1B plasmids (Hames et al., 2008), and cloned into the pMAL-c2E vector (New England Biolabs) to produce DNA encoding N-terminal MBP-tagged Poc1 spacer peptides. For the production of TAP–Poc1A, the full-length Poc1A cDNA was cloned into a pCMV-TAP vector (Stratagene). All plasmid constructs were verified by DNA sequencing (PNACL, Leicester).
Antibody generation
For production of Poc1A and Poc1B antibodies, rabbits were immunized with bacterially expressed fragments corresponding to the divergent spacer region of Poc1A (amino acid residues 304–362) or Poc1B (amino acid residues 303–431), fused to an N-terminal MBP tag. For affinity purification, the MBP-tagged Poc1A and Poc1B spacer peptides were coupled to CNBr-activated Sepharose according to the manufacturer’s instructions (Amersham). Antisera were passed over the appropriate columns, and after extensive washing with 10 mM Tris-HCl pH 7.5 followed by 10 mM Tris-HCl pH 7.5, 500 mM NaCl, specific antibodies were eluted with 100 mM glycine pH 2.5 or 100 mM triethanolamine pH 12.5, into tubes containing neutralizing quantities of 1 M Tris-HCl pH 8.0.
Cell culture and transfection
U2OS, HeLa, HEK293, HEK293-TAP–Poc1A and HeLa-GFP–α-tubulin cells were grown in Dulbecco’s modified Eagle’s medium (DMEM). hTERT-RPE1 cells were grown in DMEM/Ham’s F12 (1∶1) supplemented with 0.348% sodium bicarbonate solution. All cells were supplemented with 10% foetal calf serum, 100 IU/ml penicillin, and 100 µg/ml streptomycin (1% Pen/Strep). Cells were grown at 37°C in a 5% CO2 atmosphere. Primary cilia formation was induced by culturing cells in serum-free medium for 40 hours. M-phase-arrested cells were obtained by 16 hours treatment with 500 ng/ml nocodazole and collected by shake off. To prepare S-phase-arrested cells, 1 mM hydroxyurea was added to cells for 16 hours. Synchronization was confirmed by flow cytometry. For proliferation assays, HeLa cells grown on 12-well plate for 24 hours were treated with siRNA oligonucleotides and collected every 24 hours for 4 days. Cells were trypsinised and resuspended in 1 ml of DMEM medium. 100 µl of resuspended cells were added to 10 ml of ISOTON solution and counted using a CASY cells counter (Schärfe System). Transient transfections were performed with Lipofectamine 2000 (Invitrogen) according to manufacturer’s instructions.
RNAi
siRNA oligonucleotides specific to Poc1A (Hs_WDR51A_2, Hs_WDR51A_6, Hs_WDR51A_4) or Poc1B (Hs_WDR51B_4, Hs_WDR51B_2, Hs_TUWD12_3) or negative control luciferase GL2 were obtained from Qiagen (Crawley, UK) and transfected into cells at 50 nM using HiPerfect transfection reagent (Qiagen). 48 or 72 hours after transfection, cells were either fixed for immunocytochemistry or prepared for western blot or flow cytometry analysis.
Fixed- and live-cell microscopy
Immunofluorescence microscopy was carried out as previously described (Hames et al., 2008). Cells grown on acid-etched glass coverslips were fixed and permeabilised using ice-cold methanol. For visualization of centrioles with Sas-6, C-Nap1, centrin-2, acetylated tubulin and centrobin antibodies, cells grown on coverslips were left on ice for half an hour followed by treatment with an extraction buffer (80 mM Pipes pH 6.8, 1 mM MgCl2, 1 mM EGTA, 0.5% Triton X-100) prior to fixation with ice-cold methanol at −20°C. Primary antibodies used were raised against Poc1A (2.7 µg/ml), Poc1B (3 µg/ml), γ-tubulin (0.15 µg/ml; Sigma), α-tubulin (0.3 µg/ml; Sigma), acetylated tubulin (0.5 µg/ml; Sigma), centrin-2 (1 µg/ml; Santa Cruz), Sas-6 (2 µg/ml; Santa Cruz), FOP (1:500; gift from E. Nigg), Cep164 (1:1000; gift from E. Nigg), Cep135 (1 µg/ml; Abcam), C-Nap1 (0.75 µg/ml; (Fry et al., 1998) and centrobin (1:100, gift from K. Rhee). Secondary antibodies used were Alexa-Fluor-488- and Alexa-Fluor-594-conjugated goat anti-rabbit and goat anti-mouse IgGs (1 µg/ml; Sigma). Cells were imaged using a TE300 inverted microscope (Nikon, UK) or a Leica SP5 laser scanning confocal microscope. FRAP analysis was performed on the Leica SP5 laser scanning confocal microscope and analysed using easyFRAP software (Rapsomaniki et al., 2012). For time-lapse imaging, HeLa-GFP–α-tubulin cells grown in 6-well plate for 24 hours were treated were siRNA oligonucleotides. Images were captured once every 10 mins over a 24 hour period from 48 to 72 hours after RNAi treatment using an Olympus inverted IX81 motorized microscope equipped with a 40× objective and Cell∧R software. Phase contrast and GFP images were captured for three positions in each condition. Cells were maintained at 37°C in the presence of 5% CO2 in a thermo-controlled chamber during the time of the experiment.
Flow cytometry
To determine cell cycle distributions, cell populations were harvested as appropriate, pelleted by centrifugation and washed in 1× PBS before being resuspended in 1 ml 70% ice-cold ethanol. Cells were maintained in ethanol at 4°C for a minimum of 30 min before being stained with propidium iodide. Briefly, cells were washed twice in 1× PBS to remove all traces of ethanol, and resuspended in 1× PBS supplemented with 100 µg/ml RNase A and 50 µg/ml propidium iodide. Cells were stained in the dark at 4°C for a minimum of 4 hours. Cells were then analysed via flow cytometry, using a FACSCanto™ II analyser and FACSDiva™ software (BD Biosciences).
Preparation of cell extracts, SDS-PAGE and western blotting
Preparation of cell extracts, SDS-PAGE and western blotting were performed as previously described (Hames et al., 2008). Phos-Tag gel were prepared as described (Kinoshita et al., 2006). For western blotting, primary antibodies were used against Poc1A (2.7 µg/ml), Poc1B (3 µg/ml), α-tubulin (0.3 µg/ml; Sigma), cyclin B (1∶1000; Cell Signaling Technology), GAPDH (0.2 µg/ml; Sigma), Protein A (2 µg/ml; Sigma) and GFP (0.1 µg/ml; Abcam). Secondary antibodies were anti-rabbit or anti-mouse horseradish peroxidase (HRP)-labelled IgGs (1∶2000; Sigma).
Immunoprecipitations
For co-immunoprecipitation experiments, HEK293 cells stably expressing TAP–Poc1A were transiently transfected with GFP-tagged Poc1A or Poc1B or GFP alone. After 24 hours, soluble extracts were incubated with pre-washed IgG-Sepharose 6 Fast Flow resin (GE Healthcare) at a ratio of 5 µl beads/mg extract for 2 hours. Tubes were then centrifuged to remove the unbound supernatant and the IgG beads washed in lysis buffer. Protein complexes were eluted by boiling in 3× Laemmli buffer and analysed by SDS-PAGE and western blotting.
Kinase assays
In vitro kinase assays were carried out using purified recombinant proteins, expressed in E. coli, as substrates. Briefly, 1–5 µg of the appropriate substrate was incubated with 1 µCi of [γ-32P]ATP in kinase buffer (50 mM Hepes.KOH pH 7.4; 5 mM MnCl2; 5 mM β-glycerophosphate; 5 mM NaF; 4 µM ATP; 1 mM DTT) and 5 µg of the appropriate purified kinase for 30 min at 30°C. Reactions were stopped by addition of 25 µl of sample buffer and analysed by SDS-PAGE and autoradiography.
Mitotic phosphorylation assays
Mitotic phosphorylation assays were adapted from Hutchins et al. (Hutchins et al., 2004). Briefly, M-phase-arrested HeLa cells prepared by nocodazole treatment were washed and collected in EBS buffer (80 mM β-glycerophosphate; 20 mM EGTA; 15 mM MgCl2; 100 mM sucrose; 1 mM DTT and 1 mM PMSF). One volume of EBS buffer was added to the cell pellet, and after 30 min on ice, cells were freeze-thawed three times and centrifuged at 16,000 g for 30 min. The supernatant was collected and centrifuged again at 16,000 g for 15 min. The resulting mitotic extracts were quantified by BCA assay, aliquoted and stored in liquid nitrogen. Upon thawing, extracts were supplemented with an ATP-regenerating system consisting of 10 mM creatine phosphate, 40 µg/ml creatine kinase, and 1 mM ATP. Mitotic phosphorylation assays were carried out on 0.5–1 µg of purified recombinant protein by incubation in 50 mM Tris-HCl pH 7.5, 100 µM [γ-32P]ATP, 10 mM MgCl2, 1 µM okadaic acid plus 10 µg of mitotic HeLa cell extract, for 30 min at 30°C. Reactions were stopped by addition of 25 µl 3× Laemmli buffer and analysed by SDS-PAGE and autoradiography.
Supplementary Material
Acknowledgments
We are very grateful to all members of our laboratory for useful discussion. We also thank Sun Xiao Ming and Marion MacFarlane (Leicester) for help with proliferation assays, Erich Nigg (Basel) for FOP and Cep164 antibodies and Kunsoo Rhee (Seoul) for centrobin antibody.
Footnotes
Funding
This work was supported by the Biotechnology and Biological Sciences Research Council [grant number BB/F010702/1 to A.M.F. and H.R.W.]. A.M.F. also acknowledges funding from The Wellcome Trust [grant number 082828] and Cancer Research UK [grant number C1420/A9363]. We are also grateful for access to facilities within the Centre for Core Biotechnology Services. Deposited in PMC for release after 6 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.111203/-/DC1
References
- Andersen J. S., Wilkinson C. J., Mayor T., Mortensen P., Nigg E. A., Mann M. (2003). Proteomic characterization of the human centrosome by protein correlation profiling. Nature 426, 570–574 10.1038/nature02166 [DOI] [PubMed] [Google Scholar]
- Azimzadeh J., Marshall W. F. (2010). Building the centriole. Curr. Biol. 20, R816–R825 10.1016/j.cub.2010.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azimzadeh J., Hergert P., Delouvée A., Euteneuer U., Formstecher E., Khodjakov A., Bornens M. (2009). hPOC5 is a centrin-binding protein required for assembly of full-length centrioles. J. Cell Biol. 185, 101–114 10.1083/jcb.200808082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettencourt–Dias M., Glover D. M. (2007). Centrosome biogenesis and function: centrosomics brings new understanding. Nat. Rev. Mol. Cell Biol. 8, 451–463 10.1038/nrm2180 [DOI] [PubMed] [Google Scholar]
- Bettencourt–Dias M., Hildebrandt F., Pellman D., Woods G., Godinho S. A. (2011). Centrosomes and cilia in human disease. Trends Genet. 27, 307–315 10.1016/j.tig.2011.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blachon S., Cai X., Roberts K. A., Yang K., Polyanovsky A., Church A., Avidor–Reiss T. (2009). A proximal centriole-like structure is present in Drosophila spermatids and can serve as a model to study centriole duplication. Genetics 182, 133–144 10.1534/genetics.109.101709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammermann A., Müller–Reichert T., Pelletier L., Habermann B., Desai A., Oegema K. (2004). Centriole assembly requires both centriolar and pericentriolar material proteins. Dev. Cell 7, 815–829 10.1016/j.devcel.2004.10.015 [DOI] [PubMed] [Google Scholar]
- Fourrage C., Chevalier S., Houliston E. (2010). A highly conserved Poc1 protein characterized in embryos of the hydrozoan Clytia hemisphaerica: localization and functional studies. PLoS ONE 5, e13994 10.1371/journal.pone.0013994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry A. M., Mayor T., Meraldi P., Stierhof Y. D., Tanaka K., Nigg E. A. (1998). C-Nap1, a novel centrosomal coiled-coil protein and candidate substrate of the cell cycle-regulated protein kinase Nek2. J. Cell Biol. 141, 1563–1574 10.1083/jcb.141.7.1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes J. M., Davis E. E., Katsanis N. (2009). The vertebrate primary cilium in development, homeostasis, and disease. Cell 137, 32–45 10.1016/j.cell.2009.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graser S., Stierhof Y. D., Lavoie S. B., Gassner O. S., Lamla S., Le Clech M., Nigg E. A. (2007). Cep164, a novel centriole appendage protein required for primary cilium formation. J. Cell Biol. 179, 321–330 10.1083/jcb.200707181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudi R., Zou C., Li J., Gao Q. (2011). Centrobin-tubulin interaction is required for centriole elongation and stability. J. Cell Biol. 193, 711–725 10.1083/jcb.201006135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hames R. S., Hames R., Prosser S. L., Euteneuer U., Lopes C. A., Moore W., Woodland H. R., Fry A. M. (2008). Pix1 and Pix2 are novel WD40 microtubule-associated proteins that colocalize with mitochondria in Xenopus germ plasm and centrosomes in human cells. Exp. Cell Res. 314, 574–589 10.1016/j.yexcr.2007.10.019 [DOI] [PubMed] [Google Scholar]
- Hiraki M., Nakazawa Y., Kamiya R., Hirono M. (2007). Bld10p constitutes the cartwheel-spoke tip and stabilizes the 9-fold symmetry of the centriole. Curr. Biol. 17, 1778–1783 10.1016/j.cub.2007.09.021 [DOI] [PubMed] [Google Scholar]
- Hutchins J. R., Moore W. J., Hood F. E., Wilson J. S., Andrews P. D., Swedlow J. R., Clarke P. R. (2004). Phosphorylation regulates the dynamic interaction of RCC1 with chromosomes during mitosis. Curr. Biol. 14, 1099–1104 10.1016/j.cub.2004.05.021 [DOI] [PubMed] [Google Scholar]
- Hutchins J. R., Toyoda Y., Hegemann B., Poser I., Hériché J. K., Sykora M. M., Augsburg M., Hudecz O., Buschhorn B. A., Bulkescher J.et al. (2010). Systematic analysis of human protein complexes identifies chromosome segregation proteins. Science 328, 593–599 10.1126/science.1181348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H., Marshall W. F. (2011). Ciliogenesis: building the cell’s antenna. Nat. Rev. Mol. Cell Biol. 12, 222–234 10.1038/nrm3085 [DOI] [PubMed] [Google Scholar]
- Jakobsen L., Vanselow K., Skogs M., Toyoda Y., Lundberg E., Poser I., Falkenby L. G., Bennetzen M., Westendorf J., Nigg E. A.et al. (2011). Novel asymmetrically localizing components of human centrosomes identified by complementary proteomics methods. EMBO J. 30, 1520–1535 10.1038/emboj.2011.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller L. C., Romijn E. P., Zamora I., Yates J. R., 3rd, Marshall W. F. (2005). Proteomic analysis of isolated chlamydomonas centrioles reveals orthologs of ciliary-disease genes. Curr. Biol. 15, 1090–1098 10.1016/j.cub.2005.05.024 [DOI] [PubMed] [Google Scholar]
- Keller L. C., Geimer S., Romijn E., Yates J., 3rd, Zamora I., Marshall W. F. (2009). Molecular architecture of the centriole proteome: the conserved WD40 domain protein POC1 is required for centriole duplication and length control. Mol. Biol. Cell 20, 1150–1166 10.1091/mbc.E08-06-0619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilburn C. L., Pearson C. G., Romijn E. P., Meehl J. B., Giddings T. H., Jr, Culver B. P., Yates J. R., 3rd, Winey M. (2007). New Tetrahymena basal body protein components identify basal body domain structure. J. Cell Biol. 178, 905–912 10.1083/jcb.200703109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita E., Kinoshita–Kikuta E., Takiyama K., Koike T. (2006). Phosphate-binding tag, a new tool to visualize phosphorylated proteins. Mol. Cell. Proteomics 5, 749–757 10.1074/mcp.T500024-MCP200 [DOI] [PubMed] [Google Scholar]
- Kitagawa D., Vakonakis I., Olieric N., Hilbert M., Keller D., Olieric V., Bortfeld M., Erat M. C., Flückiger I., Gönczy P.et al. (2011). Structural basis of the 9-fold symmetry of centrioles. Cell 144, 364–375 10.1016/j.cell.2011.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleylein–Sohn J., Westendorf J., Le Clech M., Habedanck R., Stierhof Y. D., Nigg E. A. (2007). Plk4-induced centriole biogenesis in human cells. Dev. Cell 13, 190–202 10.1016/j.devcel.2007.07.002 [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Dynlacht B. D. (2011). Regulating the transition from centriole to basal body. J. Cell Biol. 193, 435–444 10.1083/jcb.201101005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidel S., Delattre M., Cerutti L., Baumer K., Gönczy P. (2005). SAS-6 defines a protein family required for centrosome duplication in C. elegans and in human cells. Nat. Cell Biol. 7, 115–125 10.1038/ncb1220 [DOI] [PubMed] [Google Scholar]
- Loncarek J., Khodjakov A. (2009). Ab ovo or de novo? Mechanisms of centriole duplication. Mol. Cells 27, 135–142 10.1007/s10059-009-0017-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall W. F. (2008). The cell biological basis of ciliary disease. J. Cell Biol. 180, 17–21 10.1083/jcb.200710085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa Y., Hiraki M., Kamiya R., Hirono M. (2007). SAS-6 is a cartwheel protein that establishes the 9-fold symmetry of the centriole. Curr. Biol. 17, 2169–2174 10.1016/j.cub.2007.11.046 [DOI] [PubMed] [Google Scholar]
- Nigg E. A., Raff J. W. (2009). Centrioles, centrosomes, and cilia in health and disease. Cell 139, 663–678 10.1016/j.cell.2009.10.036 [DOI] [PubMed] [Google Scholar]
- Nigg E. A., Stearns T. (2011). The centrosome cycle: Centriole biogenesis, duplication and inherent asymmetries. Nat. Cell Biol. 13, 1154–1160 10.1038/ncb2345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson C. G., Osborn D. P., Giddings T. H., Jr, Beales P. L., Winey M. (2009). Basal body stability and ciliogenesis requires the conserved component Poc1. J. Cell Biol. 187, 905–920 10.1083/jcb.200908019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier L., O’Toole E., Schwager A., Hyman A. A., Müller–Reichert T. (2006). Centriole assembly in Caenorhabditis elegans. Nature 444, 619–623 10.1038/nature05318 [DOI] [PubMed] [Google Scholar]
- Rapsomaniki M. A., Kotsantis P., Symeonidou I. E., Giakoumakis N. N., Taraviras S., Lygerou Z. (2012). easyFRAP: an interactive, easy-to-use tool for qualitative and quantitative analysis of FRAP data. Bioinformatics 28, 1800–1801 10.1093/bioinformatics/bts241 [DOI] [PubMed] [Google Scholar]
- Strnad P., Gönczy P. (2008). Mechanisms of procentriole formation. Trends Cell Biol. 18, 389–396 10.1016/j.tcb.2008.06.004 [DOI] [PubMed] [Google Scholar]
- van Breugel M., Hirono M., Andreeva A., Yanagisawa H. A., Yamaguchi S., Nakazawa Y., Morgner N., Petrovich M., Ebong I. O., Robinson C. V.et al. (2011). Structures of SAS-6 suggest its organization in centrioles. Science 331, 1196–1199 10.1126/science.1199325 [DOI] [PubMed] [Google Scholar]
- Woodland H. R., Fry A. M. (2008). Pix proteins and the evolution of centrioles. PLoS ONE 3, e3778 10.1371/journal.pone.0003778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X., Habedanck R., Nigg E. A. (2006). A complex of two centrosomal proteins, CAP350 and FOP, cooperates with EB1 in microtubule anchoring. Mol. Biol. Cell 17, 634–644 10.1091/mbc.E05-08-0810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou C., Li J., Bai Y., Gunning W. T., Wazer D. E., Band V., Gao Q. (2005). Centrobin: a novel daughter centriole-associated protein that is required for centriole duplication. J. Cell Biol. 171, 437–445 10.1083/jcb.200506185 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.