Summary
Dictyostelium discoideum shows chemotaxis towards folic acid (FA) throughout vegetative growth, and towards cAMP during development. We determined the spatiotemporal localization of cytoskeletal and signaling molecules and investigated the FA-mediated responses in a number of signaling mutants to further our understanding of the core regulatory elements that are crucial for cell migration. Proteins enriched in the pseudopods during chemotaxis also relocalize transiently to the plasma membrane during uniform FA stimulation. In contrast, proteins that are absent from the pseudopods during migration redistribute transiently from the PM to the cytosol when cells are globally stimulated with FA. These chemotactic responses to FA were also examined in cells lacking the GTPases Ras C and G. Although Ras and phosphoinositide 3-kinase activity were significantly decreased in Ras G and Ras C/G nulls, these mutants still migrated towards FA, indicating that other pathways must support FA-mediated chemotaxis. We also examined the spatial movements of PTEN in response to uniform FA and cAMP stimulation in phospholipase C (PLC) null cells. The lack of PLC strongly influences the localization of PTEN in response to FA, but not cAMP. In addition, we compared the gradient-sensing behavior of polarized cells migrating towards cAMP to that of unpolarized cells migrating towards FA. The majority of polarized cells make U-turns when the cAMP gradient is switched from the front of the cell to the rear. Conversely, unpolarized cells immediately extend pseudopods towards the new FA source. We also observed that plasma membrane phosphatidylinositol 3,4,5-trisphosphate [PtdIns(3,4,5)P3] levels oscillate in unpolarized cells treated with Latrunculin-A, whereas polarized cells had stable plasma membrane PtdIns(3,4,5)P3 responses toward the chemoattractant gradient source. Results were similar for cells that were starved for 4 hours, with a mixture of polarized and unpolarized cells responding to cAMP. Taken together, these findings suggest that similar components control gradient sensing during FA- and cAMP-mediated motility, but the response of polarized cells is more stable, which ultimately helps maintain their directionality.
Key words: Folate, Signal transduction, Motility, Polarity, Chemotaxis
Introduction
Chemotaxis is the directed migration of cells towards or away from gradients of signaling molecules. This migratory process is implicated in a myriad of physiological activities in single-celled and multicellular species including inflammation, lymphocyte homing, axon guidance, angiogenesis and embryogenesis (Devreotes and Zigmond, 1988; Niggli, 2003; Park et al., 2002; Rickert et al., 2000; Swaney et al., 2010). Improper chemotaxis leads to pathological conditions including infectious and allergic diseases, wound healing, atherosclerosis and tumor metastasis (Lazennec and Richmond, 2010; Libby, 2002; Moore, 2001; Patel and Haynes, 2001). The social amoeba Dictyostelium discoideum is one of the most widely studied of this phenomenon.
Depending upon their physiological state, D. discoideum cells can exhibit chemotaxis towards the chemoattractants folic acid (FA) or cyclic adenosine monophosphate (cAMP) (Devreotes and Zigmond, 1988). Vegetative cells feed on bacteria and other microbes and scavenge for food by sensing and migrating toward FA and other potential chemical signals (Maeda et al., 2009; Pan et al., 1972). When nutrients are limiting, cells enter a cAMP-dependent developmental cycle that culminates in the formation of multicellular fruiting bodies (Bonner, 1971; Bonner, 1978; Katoh et al., 2007; Loomis, 1979). D. discoideum cells are highly chemotactic during these early stages of development and are very polarized, forming a defined front and rear. Altered gene expression in these cells makes them more sensitive to cAMP (Manahan et al., 2004; Williams and Harwood, 2003; Zhang et al., 2007). Both the serpentine cAMP receptor (cAR1) and the heterotrimeric G protein alpha subunit, Gα2, increase in expression as do many other developmentally regulated proteins (Abe and Maeda, 1994; Parent and Devreotes, 1996; Verkerke-Van Wijk et al., 1998). Upon cAMP stimulation, Ras G activates the phosphoinositide 3-kinase 2 (PI3K2), one of five PI3Ks containing a Ras-binding domain in D. discoideum (Funamoto et al., 2002; Janetopoulos et al., 2005; Kae et al., 2004). The marked increase of phosphatidylinositol 3,4-bisphosphate [PtdIns(3,4)P2] and phosphatidylinositol 3,4,5-trisphosphate [PtdIns(3,4,5)P3] at the leading edge of migrating cells can be identified by the plekstrin homology (PH) domain of the cytosolic regulator of adenylyl cyclase (CRAC), a biosensor that measures increased levels of these plasma membrane (PM) phosphoinositides (Dormann et al., 2004; Parent et al., 1998). PKBA [human protein kinase B (PKB) homolog] is also recruited to the PM and binds to PtdIns(3,4,5)P3 through its PH domain and is activated by phosphorylation of its hydrophobic motif and activation loop (Bozulic and Hemmings, 2009; Chung et al., 2001; DiNitto and Lambright, 2006; Feng et al., 2004; Guertin et al., 2006; Kamimura and Devreotes, 2010; Liao et al., 2010; Sarbassov et al., 2005). In a parallel pathway, Ras C activates the TORC2 complex, which in turn triggers PKBA and a second PKB homolog, PKBR1 (Charest et al., 2010; Cai, 2010; Kamimura et al., 2008; Liao et al., 2010). Activated Ras, PI3K2 and PKBA are localized at the leading edge of chemotaxing cells in cAMP gradients and help regulate the actin cytoskeleton (Kamimura et al., 2008; Sasaki and Firtel, 2009). Coronin, dynacortin and LimE are all actin-binding proteins that localize at the leading edge of a migrating cell but interact with actin differently: coronin crosslinks F-actin filaments (de Hostos et al., 1991) and dynacortin bundles F-actin filaments at the leading edge (Girard et al., 2004; Kabacoff et al., 2007; Robinson et al., 2002) while LimE binds and forms a complex with Rac and F-actin and other actin-binding proteins at the cortex of cell projections (Prassler et al., 1998). Conversely, the tumor suppressor and phosphatase and tensin homolog (PTEN) has been shown to be distributed on the lateral and trailing edge of the plasma membrane of the cell (Funamoto et al., 2002; Iijima and Devreotes, 2002). Moreover, cortexillin I stably bundles actin filaments in the rear of the cell (Ren et al., 2009). This reciprocal spatial regulation of PI3K2 and PTEN differentially localizes PtdIns(3,4,5)P3 at the leading edge, which supports F-actin-mediated membrane protrusions at the front of the cell. At the same time, this spatial distribution of the enzymes likely maintains PtdIns(4,5)P2 at the rear, and helps define the back of the cell (Janetopoulos et al., 2004; Ma et al., 2004).
PtdIns(3,4,5)P3 levels are also regulated by the amount of PtdIns(4,5)P2 available as substrate for PI3Ks (Kortholt et al., 2007). Phospholipase C (PLC) cleaves PtdIns(4,5)P2 into inositol trisphosphate (InsP3) and diacylglycerol (DAG) and has been implicated in delocalizing PTEN from the PM. Cells lacking PLC have been shown to have reduced levels of PtdIns(3,4,5)P3 at the leading edge of migrating cells and demonstrate little change in response to a uniform stimulus. However, overexpression of PLC results in an excessive accumulation of PtdIns(3,4,5)P3 and defects in chemotaxis (Kortholt et al., 2007).
The cAMP-mediated chemotaxis signaling pathways are well characterized in D. discoideum. However, the signaling pathways underlying folic acid-based migration are not as well understood. While a receptor (receptors) has not yet been identified, a G alpha subunit, Gα4, was found to be specific for FA-mediated responses. Receptors and heterotrimeric G proteins specific for cAMP have been known for many years (Chen et al., 1996; Dormann et al., 2001; Janetopoulos et al., 2001; Kim et al., 1998; Kortholt and van Haastert, 2008; Kumagai et al., 1991; Milne et al., 1997; Prabhu and Eichinger, 2006; Pupillo et al., 1989; Sonnemann et al., 1998). There is only one functional β-subunit and γ-subunit in D. discoideum; cells lacking the β-subunit do not chemotax or respond to a uniform stimulus of either cAMP or FA (Hadwiger, 2007; Kumagai et al., 1989; Wu et al., 1995) (C.J. and G.A.W., unpublished data). Previous studies have examined the effects of FA treatment alone or with cAMP on the production of cAMP by cells (Devreotes, 1983). While the PKBA/PKBR1 activation and substrate phosphorylation have been studied in response to either FA or cAMP with several targets having been identified (Kamimura et al., 2008; Liao et al., 2010), the spatial and temporal dynamics of signaling and cytoskeletal molecules in response to uniform FA stimulation and in gradients are not well characterized. Recently, it has been shown that the signaling proteins PI3K, TORC2, PLA and sGC are not essential for Ras activation to FA gradients or to steep gradients of cAMP. These proteins, however, provide a memory of direction and persistence that increases the sensitivity 150-fold for chemotaxis toward shallow gradients of cAMP (Kortholt et al., 2011).
The morphological characteristics of vegetatively-grown D. discoideum cells chemotaxing to FA are typically quite distinct in comparison to starved cells undergoing chemotaxis to cAMP. Although vegetative cells are amoeboid-shaped and unpolarized, they are quite capable of migrating directionally in a FA gradient (Bernstein et al., 1981; de Wit and Rinke de Wit, 1986; Devreotes, 1983; Hadwiger and Srinivasan, 1999; Jowhar et al., 2010; Kesbeke et al., 1990; Kortholt et al., 2011; Maeda and Firtel, 1997; Pan et al., 1972; van Haastert et al., 1982). On the other hand, cells that have been starved undergo developmental changes that result in a distinct polarized morphology. While the leading edge can sometimes extend more than one pseudopod, these cells have a well-defined front and back, typically lacking lateral pseudopods as they migrate towards a cAMP source (Andrew and Insall, 2007; Chubb et al., 2002; Devreotes and Janetopoulos, 2003; Insall and Andrew, 2007; Van Haastert and Bosgraaf, 2009; van Haastert and Postma, 2007). Regardless of cell shape, the underlying sensing mechanism regulating directional motility may be functioning in a similar manner to well-fed cells. By eliminating the role of polarity and phenotypes due to developmental delays in cell migration, we can better elucidate the core regulators of the gradient-sensing mechanism. Furthermore, understanding the interactions between the cAMP and FA pathways should provide insight into the regulation of both chemotactic pathways as the only currently known difference between cAMP- and FA-mediated chemotaxis is the Gα-subunits. We speculate that these pathways share the majority of the signaling components downstream of the heterotrimeric G proteins.
In this study, we have characterized the localization of cytoskeletal and signaling proteins of wild-type and signaling mutants during random motility in response to uniform stimulation and while in a concentration gradient of the chemoattractant FA. We demonstrate that there are many cytoskeletal components that display a reciprocal temporal and spatial localization as has been previously been described for PI3K2 and PTEN. Several of these components have motifs that suggest their localizations are controlled by the PM products of these two enzymes in response to chemoattractants. We also characterize the responses of unpolarized and polarized cells to rapid gradient switching and analyze their gradient-sensing responses in the absence of an actin cytoskeleton. We conclude that the underlying mechanism regulating directionality for both FA- and cAMP-mediated chemotaxis, as well as polarized and unpolarized cells, are likely the same. However, unpolarized cells have an added regulatory component that makes them more difficult to steer. We also find that several signaling molecules that have been shown to be crucial for cAMP-mediated chemotaxis are dispensable for FA-guided motility. While the inability to chemotax to cAMP and form fruiting bodies make an excellent screen for identifying chemotaxis mutants, future studies implicating molecules in gradient sensing and directed migration should also incorporate FA-mediated signaling to ensure that the defects are not a result of incomplete polarization and developmental delay.
Results
Membrane redistribution of signaling and cytoskeletal markers in response to a uniform folic acid stimulus
The spatiotemporal localization of cytoskeletal and signaling molecules have been extensively studied in developed D. discoideum cells exposed to cAMP gradients and during uniform stimulation (Funamoto et al., 2002; Iijima and Devreotes, 2002; Janetopoulos and Firtel, 2008; Kortholt and van Haastert, 2008; Rericha and Parent, 2008; Swaney et al., 2010). Many of these molecules are also regulated during random motility and cytokinesis (Janetopoulos et al., 2005; Sasaki et al., 2007). Ras and PI3K activity localize at the leading edge of a migrating cell and to the poles of cells during cytokinesis while other molecules such as PTEN and myosin II redistribute to the posterior end during chemotaxis and localize to the furrow during cytokinesis (Janetopoulos and Devreotes, 2006; Sasaki et al., 2007). A pattern that has not been well appreciated is that the signaling molecules that redistribute to the PM in response to a uniform stimulus of cAMP also localize to the leading edge of a migratory cell and to the poles of cells undergoing cytokinesis. On the other hand, signaling components such as PTEN, which dissociate from the membrane when globally stimulated, localize to the rear and furrow of migrating and dividing cells, respectively (Iijima and Devreotes, 2002; Janetopoulos et al., 2005).
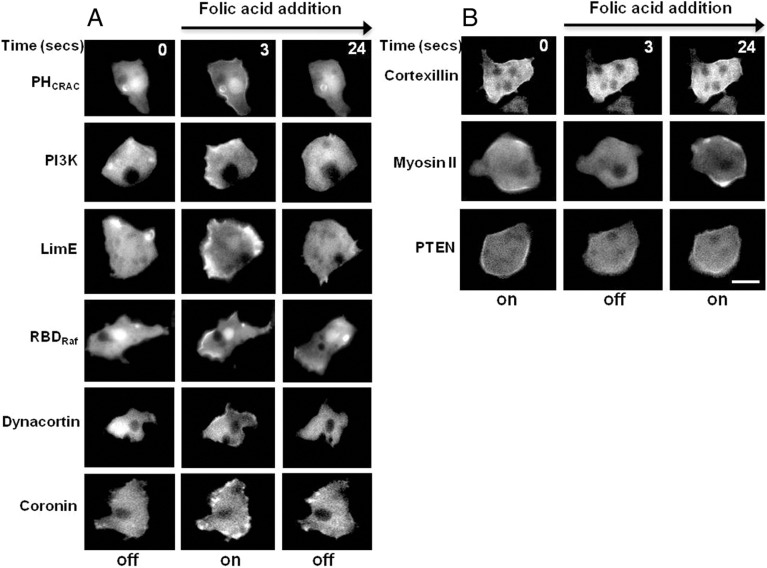
We surmised that a similar localization pattern for signaling and cytoskeletal proteins would be observed in D. discoideum cells when stimulated globally with the chemoattractant FA. To test this, vegetative cells were given a uniform stimulus of FA. PHCrac–GFP, RBDRaf–GFP, PI3K–GFP, LimE–RFP, GFP–dynacortin and coronin–GFP all rapidly translocated to the PM after addition of FA to these unpolarized cells (Fig. 1). On the other hand, PTEN–GFP, myosin II–GFP and GFP–cortexillin-1 all dissociated from the PM (Fig. 1). Interestingly, the two actin bundling proteins dynacortin and cortexillin-1 had opposing responses to FA (similar results not shown were found with uniform cAMP stimulus in developed cells).
Fig. 1.
Membrane redistribution of signaling and cytoskeletal proteins by uniform folic acid stimulation. Fluorescent images of the indicated GFP markers during uniform folic acid stimulation (100 µM). (A) PHCrac–GFP, PI3K2–GFP, LimE–RFP, RBDRaf–GFP, GFP–dynacortin and coronin–GFP translocated to the plasma membrane upon FA addition (middle column). The cells adapt and the molecules return to the cytosol (right column). (B) GFP–cortexillin-1, myosin-II–GFP, and PTEN–GFP proteins are on the plasma membrane prior to stimulus and re-localize to the cytosol. Time between frames designated in seconds. Scale bar: 10 µm.
Reciprocal regulation of signaling and cytoskeletal markers during folic-acid-mediated chemotaxis
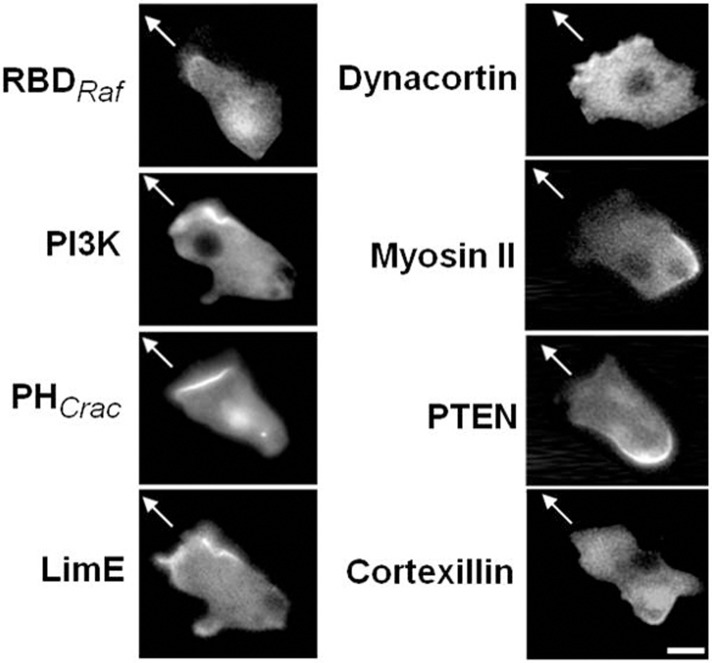
Cells display a polarized distribution of Ras and PI3K activity at the leading edge and PTEN at the trailing edge during cAMP-mediated chemotaxis (Funamoto et al., 2002; Iijima and Devreotes, 2002; Ma et al., 2004). Given that uniform responses to FA mirrored those seen with cAMP, we examined the distribution of a number of signaling and cytoskeletal markers when cells were migrating in a FA gradient. While these cells were mostly unpolarized and extended pseudopods in all directions, the localization of RBDRaf–GFP, PI3K2–GFP, PHCrac–GFP, LimE–RFP and GFP–dynacortin was confined to pseudopodial projections while GFP–cortexillin-1, myosin II–GFP and PTEN–GFP was reciprocally regulated and absent from the periphery of pseudopods (Fig. 2). In some cases, cells can develop a somewhat polarized leading edge, but this is atypical and the cells tend to meander as they make their way up the FA gradient.
Fig. 2.
Localization of signaling proteins during exposure to a folic acid gradient. Representative images of indicated fluorescently tagged proteins migrating towards 10 µM FA in a micropipette. The arrow indicates the position of the micropipette. RBDRaf–GFP, PI3K–GFP, PHCrac–GFP, LimE–RFP and dynacortin–GFP localized in pseudopods projecting in the direction of the micropipette. Myosin II–GFP, PTEN–GFP and GFP–cortexillin-1 delocalized from the pseudopods during migration towards FA. Scale bar: ∼5 µm.
Ras and PI3K activity are significantly reduced in Ras G and Ras C/G null cells
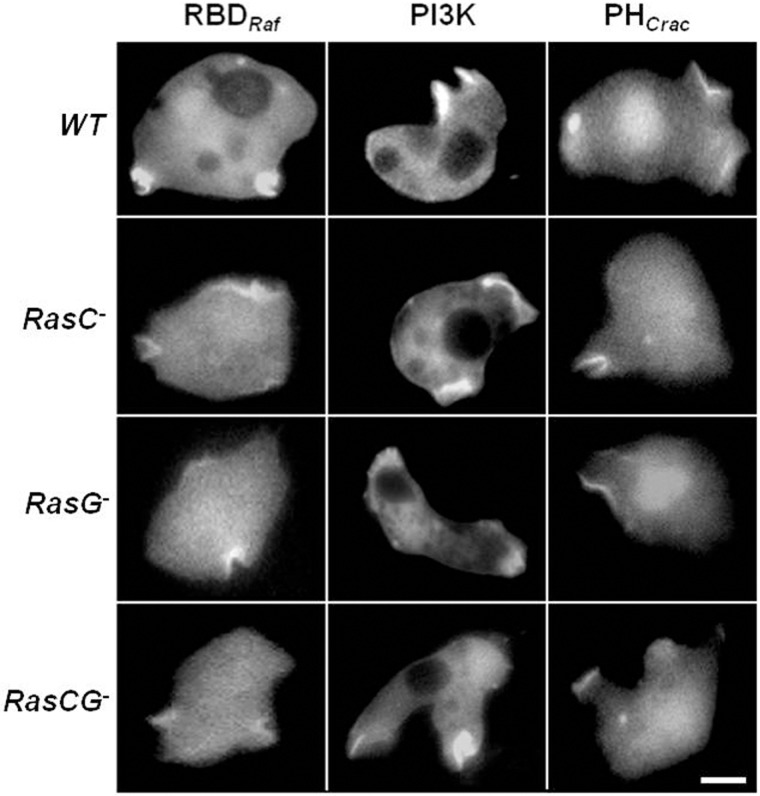
There are four major signaling pathways that have been implicated in cAMP-mediated chemotaxis: PI3K, TORC2, sGC and PLA2. Among these, PI3K and TORC2 pathways are the most extensively studied. The small G proteins Ras G and C regulate PI3K and TORC2, respectively (Kamimura et al., 2008; Kortholt and van Haastert, 2008). It has been reported that Ras C and Ras G null cells are capable of chemotaxis, however, Ras C/G double nulls are completely blind in a cAMP gradient and together are required for directed migration (Bolourani et al., 2006). Ras G is thought to regulate the production of PtdIns(3,4,5)P3 by activating PI3K at the leading edge during cAMP-mediated chemotaxis (Kamimura et al., 2008; Kortholt et al., 2010; Charest et al., 2010). In these studies, cAR1 expression was significantly reduced and delayed during early development in Ras G null cells and undetectable in Ras C/G double null cells (Bolourani et al., 2006). To sidestep this developmental defect, we decided to test PI3K activity and RBD activity in wild-type (WT) cells and in the various Ras mutant lines during random migration and in response to FA. Previous work has shown that Ras, PI3K and PTEN are regulated in cells lacking heterotrimeric G-protein signaling and without the presence of an external stimulus (Sasaki et al., 2007). Activated Ras was monitored using RBDRaf in the absence of chemoattractants during random motility in both WT and Ras mutant backgrounds (Fig. 3). RBD–GFPRaf localized strongly at the pseudopods and macropinosomes in WT cells. There was a gradual decrease in RBD–GFPRaf localization in cells lacking Ras C, Ras G and Ras C/G double nulls, respectively. To determine the corresponding activity of PI3K, we visualized both recruitment of PI3K2–GFP to pseudopods and the levels of PtdIns(3,4,5)P3 using PHCrac–GFP. Previous studies have shown that recruitment of PI3K2 to the PM is sufficient for activity (Huang et al., 2003). The fluorescence intensity of the PI3K markers mirrored that of RBDRaf (Fig. 3).
Fig. 3.
Random motility of WT and Ras mutants. The vegetative cells expressing RBDRaf–GFP, PI3K–GFP and PHCrac–GFP were plated in the Na/KPO4 buffer. The fluorescence images were captured in the absence of chemoattractant. All three markers localized to the pseudopods and macropinosomes. Scale bar: ∼5 µm.
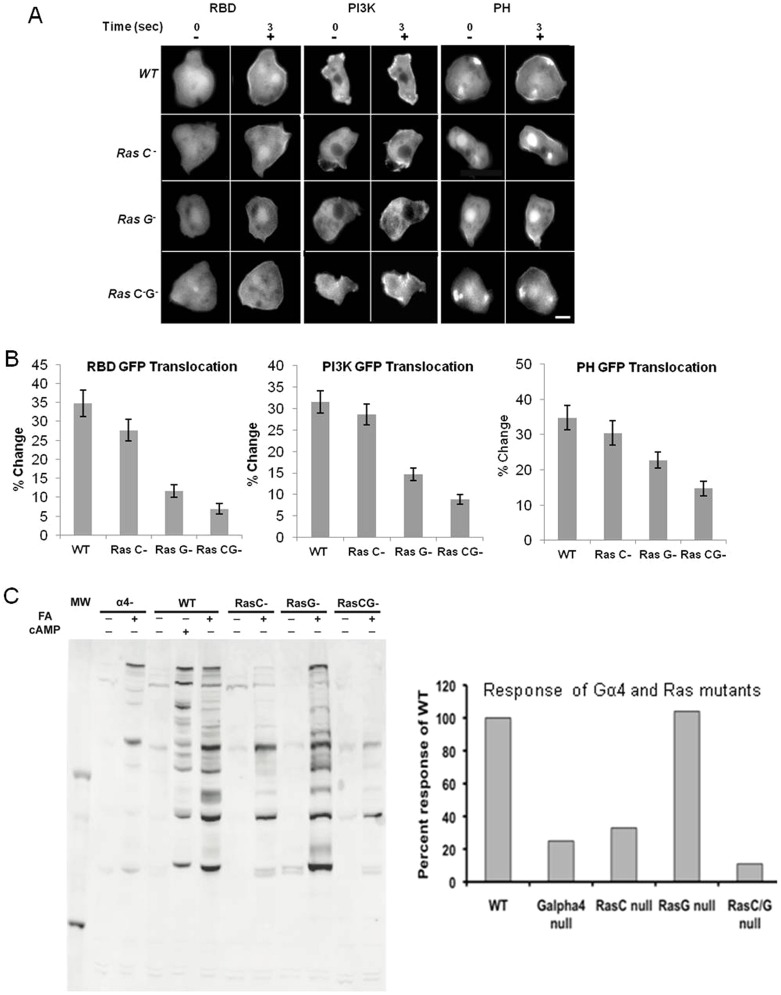
To examine the downstream signaling responses of cells in the absence of Ras, we stimulated cells with a uniform stimulus of FA and measured the translocation of RBDRaf–GFP, PI3K–GFP and PHCrac–GFP from the cytosol to the PM. These responses were significantly reduced in the Ras G and Ras C/G double null cells (Fig. 4A,B). Next, we analyzed the regulation of PKB substrate phosphorylation in WT and mutant cell lines in response to FA. Cells lacking the Gα4 subunit showed a very weak, but substantial response after uniform addition of FA (Fig. 4C). Numerous PKB substrates were phosphorylated in WT cells stimulated with FA or cAMP, with many similar targets. Several identified protein substrates including Talin B (280 kDa) Ras GefN (180 kDa), Ras GefS (110 kDa), PI4P5K domain containing protein (110 kDa), RhoGap GacQ (65 kDa), PHAPS (86 kDa) and SHAPS (53 kDa) had increases in phosphorylation (Kamimura et al., 2008; Liao et al., 2010). As a negative control, PKB substrate phosphorylation was observed in PKBA/PKBR1 double nulls in response to uniform FA stimulation. As expected, the PKBA/PKBR1 double nulls showed no measurable PKB substrate phosphorylation (supplementary material Fig. S1). The relative quantification of the western blot (Fig. 4C, bar chart) demonstrated that Gα4 and Ras C were the primary positive regulators of PKB substrate phosphorylation in response to FA. Ras G seems to play only a minor role, if any, in the regulation of PKB substrate phosphorylation in response to FA.
Fig. 4.
Ras and PI3K activity are significantly reduced in Ras G and Ras C/G null cells. (A) Fluorescent images of wild-type and Ras mutants expressing RBDRaf –GFP, PI3K–GFP and PHCrac–GFP. Cells were stimulated with 100 µM FA (+), and the indicated GFP-tagged proteins translocated to the plasma membrane. Scale bar: ∼5 µm. (B) The percentage change of membrane to cytosol ratio is significantly decreased in Ras G and Ras C/G null cells (P<0.05). Error bars show the standard error of mean. (C) Western blot band intensity (left) was quantified using ImageJ and plotted (right) as the percentage change of Ras mutants over the wild type.
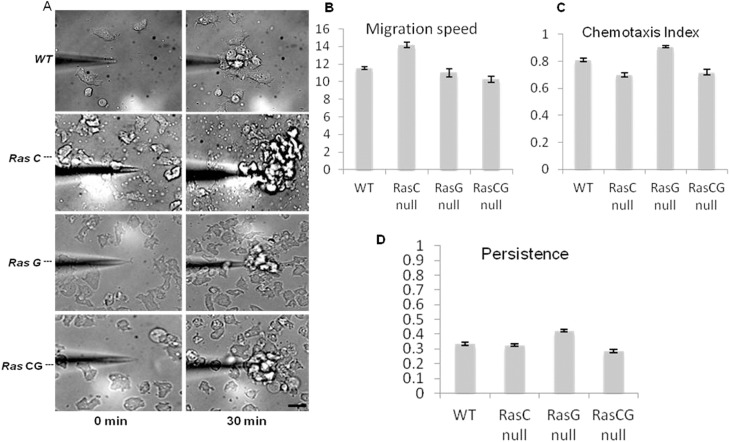
Ras C and G are not required for folic acid chemotaxis
Given the strong defects in cAMP-mediated chemotaxis in Ras C/G nulls (Bolourani et al., 2006) and the decrease in Ras and PI3K activity shown in Fig. 4, we hypothesized that the Ras C, G and C/G double nulls might also have strong defects in FA chemotaxis. Surprisingly, we found that Ras C, G and C/G double nulls were able to migrate directionally quite robustly and with no significant difference between WT and Ras mutants in migration speed, chemotactic index or persistence (Fig. 5). We re-examined the Ras C/G double nulls and found that when starved and pulsed with cAMP, they can polarize and migrated well in gradients of cAMP (supplementary material Fig. S2A). Interestingly, we also tested the ability of the double nulls lacking PKBA and PKBR1 to chemotax. These mutants chemotaxed well to FA (supplementary material Fig. S2B) and also migrated to cAMP, as previously reported (Meili et al., 1999), but did not polarize well (data not shown).
Fig. 5.
Chemotaxis and quantification of WT, Ras C, Ras G and Ras C/G null cells during migration to FA. (A) Frames of wild-type and Ras mutants at time 0 and after 30 minutes in an FA gradient. Cells migrate up the concentration gradient and towards the micropipette. Scale bar: ∼20 µm. (B–D) Quantification from three independent experiments of (B) migration speed, (C) chemotaxis index, and (D) persistence. Migration speed chemotaxis index and persistence of wild-type, Ras C, Ras G and Ras C/G null cells are not significantly different (P<0.05).
PLC can regulate the localization of PTEN–GFP on the plasma membrane
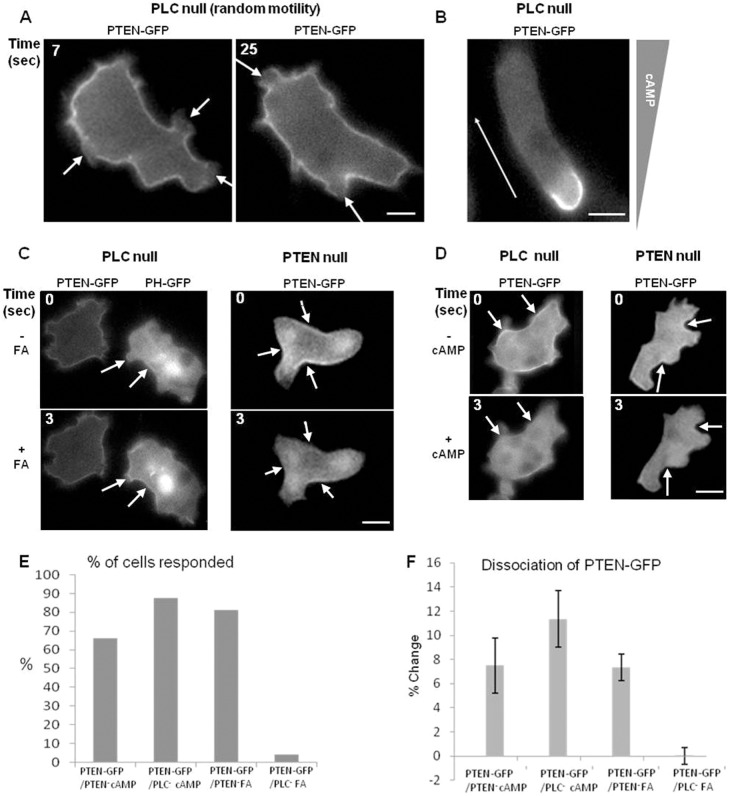
The PM distribution of PtdIns(4,5)P2 may be a major contributor to the spatial and temporal distribution of signaling and cytoskeletal markers described above. Both PTEN and cortexillin-1 have putative PtdIns(4,5)P2-binding motifs (Iijima et al., 2004; Stock et al., 1999). PtdIns(4,5)P2 is the substrate for PI3K for synthesis of PtdIns(3,4,5)P3 and for phospholipase C (PLC) in the production of InsP3 and DAG. The binding of PTEN to PtdIns(4,5)P2 is thought to play a regulatory role in lipid signaling and is necessary for proper PM localization (Iijima et al., 2004). In the absence of chemical gradients, it was observed that vegetative PLC null cells formed random pseudopods and accumulated PtdIns(3,4,5)P3 at the pseudopods during random cell migration (not shown). More strikingly, PTEN was often missing from peripheral pseudopodial projections, suggesting that loss of PLC had little effect on the normal reciprocal regulation of PtdIns(3,4,5)P3 levels and PTEN in random migration (Fig. 6A; supplementary material Movie 1). We also found that these cells polarized after only four hours of starvation and had a sharp localization of PTEN at the rear of the cell during chemotaxis (Fig. 6B). Since this was a surprising result, we elected to test the localization of PTEN–GFP in PLC null cells in response to uniform FA stimulation. While we did sometimes see PTEN relocalize from the PM to the cytosol in response to FA, the response was greatly diminished (Fig. 6C,E,F). This observation is similar to the PTEN–GFP response reported when PLC null cells were stimulated with cAMP (Kortholt et al., 2007). However, in our hands, the PTEN–GFP delocalized in a manner that was indistinguishable from loss of PTEN from the PM in PTEN null cells stimulated with cAMP (Fig. 6D) (Iijima and Devreotes, 2002). We counted the amount of cells showing a response and quantified the percentage of cells that responded to the stimulation event. When uniformly stimulated with cAMP, 66% of the PTEN null cells showed a loss of PTEN–GFP from the PM (total 65 cells), while 87% of the PLC null cells responded (out of 41 cells). There was no significant difference in PTEN–GFP responses to cAMP in these two cell lines (Fig. 6F).
Fig. 6.
Localization of PTEN–GFP in PLC and PTEN null cells. (A) PTEN–GFP delocalizes (arrows) from pseudopods during random motility when expressed in PLC null cells. Shown is a cell at two different time points. (B) Fluorescence image of PTEN–GFP localized to the rear of a polarized PLC null cell during chemotaxis. (C; left panel) PTEN–GFP is distributed on the PM prior and subsequent to folic acid stimulation in PLC null cells. WT cells expressing PHCrac–GFP were used as controls and show a response during uniform folic acid stimulation. (Right panel) PTEN–GFP is localized on the PM prior to FA stimulation and delocalized subsequent to FA stimulation in PTEN null cells. (D) PTEN–GFP is localized on the PM prior to cAMP stimulation and delocalized subsequent to cAMP stimulation in PLC null (left panel) and PTEN null cells (right panel). (E) The number of cells that responded to cAMP and FA in PTEN–GFP/PTEN null and PLC null were manually counted and expressed as a percentage of response. (F) The percentage change of the membrane to cytosol ratio is significantly decreased in PTEN–GFP/PLC null-FA over all other cells (P<0.05). Error bars show the standard error of mean. Scale bars: ∼5 µm.
Directional changes by U turns and cell switching
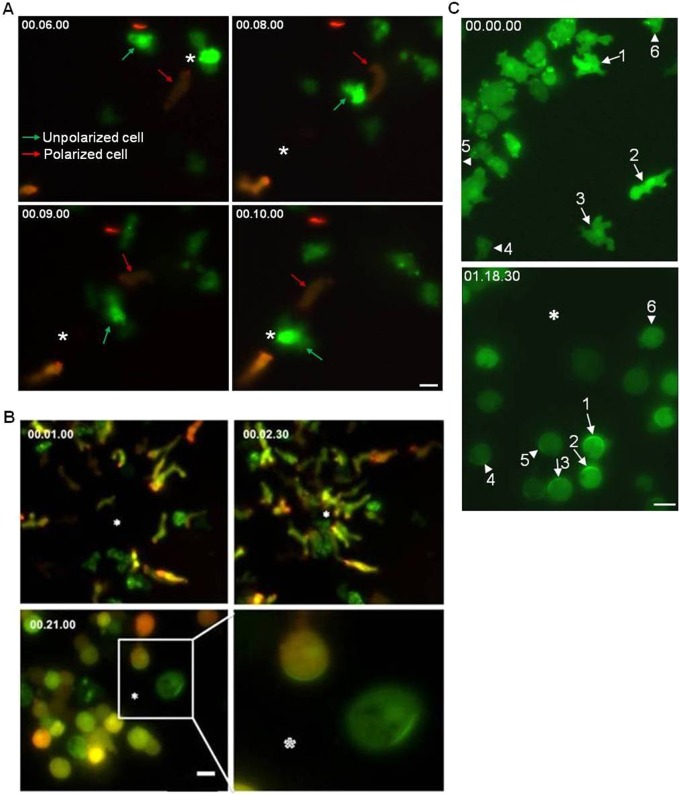
Vegetative cells are capable of net cell translocation in multiple directions by extending ‘random’ pseudopods. Cells that are migrating during aggregation to form a fruiting body, however, are highly polarized and generally have a leading edge. Typically, polarized cells responding to chemotaxis appear to migrate faster in cAMP gradients than unpolarized cells in FA gradients (Jowhar et al., 2010). We tested the ability of polarized and unpolarized cells to change directions in response to an 180° gradient reversal. For this experiment, we mixed highly polarized cells expressing PHCrac–GFP and LimE–RFP (red arrows in Fig. 7A) that were starved and polarized, with unpolarized cells expressing PHCrac–GFP alone (green arrows in Fig. 7A) grown on a bacterial lawn. A micropipette was filled with a mixture of both FA and cAMP. Polarized cells that responded to cAMP typically maintained their polarity before and after the gradient was moved to a location 180° behind the cell. The polarized cell sensed the change in gradient and made a slow U-shaped turn and then proceeded to migrate to the new source position (Fig. 7A; supplementary material Movie 2). This is in stark contrast to unpolarized cells that extend random pseudopods in a biased manner toward the source of FA. When the gradient was moved to the back of unpolarized cells, these cells quickly sensed the change in gradient and extended pseudopods in the new direction (Fig. 7A; supplementary material Movie 2). We also observed that partially polarized cells responding to cAMP were capable of reversing their polarity and extending lateral pseudopods and moved in the new direction (data not shown). Unpolarized cells in general are capable of changing directions more quickly than polarized cells.
Fig. 7.
Spatial and directional sensing during FA- and cAMP-mediated chemotaxis. (A) Unpolarized cells switch directions whereas polarized cells do U-turns when gradients are reversed. Polarized cells expressing PHCrac–GFP and LimE–RFP appear orange, and one such cell is indicated by a red arrow. Vegetative cells expressing only PHCrac–GFP are green and one unpolarized cell is indicated by the green arrow. These cells were imaged while exposed to a gradient formed by a micropipette containing both 10 µM cAMP and 100 µM FA. The micropipette (indicated by the asterisk) was moved from the front to the rear of the cells after 8 seconds at the first position. Note how quickly the cell indicated with green arrow moves towards the micropipette after it is moved to a new position while the cell indicated with the red arrow performs a U-turn. Polarized cells maintain front and rear polarity during migration and perform U-turns. Vegetative PHCrac–GFP cells extend pseudopods from the ‘rear’ of the cell and migrate towards the FA source (see also supplementary material Movie 2). Scale bar: ∼5 µm. (B) PHCrac–GFP localizes to random pseudopods in unpolarized cells in response to a folic acid gradient and to the front of polarized cells in response to a cAMP gradient. Top panels depicts polarized cells expressing PHCrac–GFP and LimE–RFP mixed with unpolarized cells expressing PHCrac–GFP alone, all migrating toward a micropipette loaded with 10 µM cAMP and 100 µM FA. Bottom left panel shows both cell types responding to the mixed chemoattractant source after 10 µM Latrunculin-A treatment. Bottom right panel is a cropped and enlarged image of the area outlined with a white rectangle in bottom left panel. The PHCrac–GFP crescent is in the direction of the gradient source in the cell responding to cAMP and is randomly distributed in the cell that was responding to FA. The white asterisk represents the location of the mixed chemoattractant-loaded micropipette tip. Scale bar: 10 µm. (C) Unpolarized cells do not form stable PHCrac–GFP crescents toward a gradient of cAMP. WT cells expressing PHCrac–GFP were starved for 4 hours and tested for cAMP responses. All of the cells responded to temporal cAMP stimulus (supplementary material Fig. S3) and migrated towards the micropipette (supplementary material Movie 3). The top panel (a frame from supplementary material Movie 3) shows semi-polarized cells (arrows numbered 1–3) and unpolarized cells (arrowheads labeled 4–6). The bottom panel (frame from supplementary material Movie 4) shows the same cells after Latrunculin-A treatment at steady state in a 10 µM cAMP gradient. Semi-polarized cells (1–3) make stable PHCrac–GFP crescents towards the micropipette, whereas unpolarized cells (4–6) make oscillatory PHCrac–GFP crescents when immobilized (see supplementary material Movie 4). An asterisk indicates the position of the pipette. Scale bar: ∼25 µm.
Directional sensing can be visualized in Latrunculin-A-treated polarized cells, but not in vegetative cells
Treatment of migrating D. discoideum cells expressing PHCrac–GFP with the actin polymerization inhibitor Latrunculin-A first demonstrated the ability of eukaryotic cells to do spatial sensing (Parent and Devreotes, 1999). Since unpolarized cells appear to bias random pseudopods toward the gradient source, we hypothesized that the unpolarized cells might have trouble forming a stable PHCrac–GFP crescent toward a gradient source as has been shown for polarized cells. This was tested by mixing unpolarized cells expressing PHCrac–GFP with polarized cells expressing LimE–RFP and PHCrac–GFP. Both unpolarized cells chemotaxing to FA and polarized cells migrating towards cAMP rapidly migrated toward a micropipette loaded with the two chemoattractants (Fig. 7B, top panels). As the cells approached the micropipette, Latrunculin-A was added (Fig. 7B, bottom panels). The formerly polarized cells rounded up and formed stable PHCrac–GFP crescents toward the gradient source. The immobilized unpolarized cells, however, formed random PHCrac–GFP crescents irrespective of the gradient source (Fig. 7B, bottom right). We have never obtained a stable PHCrac–GFP response of an immobilized cell when put in a FA gradient.
To test if this phenomenon was a general property of unpolarized cells, we examined PHCrac–GFP crescent formation in response to cAMP in cells starved for only 4 hours. The 4-hour-starved cells contained a mixed population of unpolarized cells and semi-polarized cells (Fig. 7C, top panel). The unpolarized cells still clearly were undergoing macropinocytosis, while the semi-polarized cells were not. All of the cells at this point were responsive to uniform stimulus (supplementary material Fig. S3) and could chemotax (supplementary material Movie 3) however, the unpolarized cells were not capable of forming a stable PHCrac–GFP crescent toward the cAMP source. Conversely, the semi-polarized cells formed a stable PHCrac–GFP crescent toward the cAMP source (Fig. 7C, bottom panel; supplementary material Movie 4). We also found that the unpolarized cells were resistant to Latrunculin-A treatment at the concentration used to elicit a response in polarized cells and required a 2-fold increase in Latrunculin-A. When the semi-polarized cells were immobilized and eliciting a crescent PHCrac–GFP response, the unpolarized cells were still capable of migrating slightly towards the micropipette at the lower concentrations, and did so with a biased random walk (supplementary material Movie 5). Vegetative cells grown on bacterial lawns used for FA chemotaxis also displayed this resistance to Latrunculin-A and required higher concentrations to be immobilized. The results here suggest that it is an inherent property of unpolarized cells that prevents cells from forming a stable response, and is not a fundamental difference between the cAMP and folic acid signal transduction pathways.
Discussion
The spatial redistribution of proteins is crucial for motility during random pseudopod formation,, as a cell develops a leading edge and rear during directed migration, and also for the establishment of cellular polarity during cytokinesis. What we demonstrate in this paper, and what can be gleaned from other studies examining the dynamic localization of proteins, is that the presence of a signaling or cytoskeletal molecule on the PM in any one of these morphological conditions would also indicate the localization of that same protein in the other states (Funamoto et al., 2002; Iijima and Devreotes, 2002; Janetopoulos and Firtel, 2008; Kortholt and van Haastert, 2008; Rericha and Parent, 2008; Swaney et al., 2010). Thus, Ras activity, as assayed at the leading edge of a migrating cell, would be predicted to be at the poles of dividing cells or on the advancing edges of pseudopods in a randomly migrating cell (Sasaki et al., 2007). Moreover, one can determine with confidence whether a protein is a leading edge or rear protein by stimulating an unpolarized cell with FA and observing the time course of the protein's redistribution to and from the PM (see Fig. 8). We are now isolating membrane fractions with and without chemoattractant stimulation to look at the spatial changes in the proteome (data not shown). The isolation of membrane-associated proteins in response to a uniform stimulus may be a powerful method for isolating leading edge and rear proteins of polarized cells. It will be interesting to see how well this pattern holds up for other dynamic signaling and cytoskeletal molecules and how applicable it will be overall for other eukaryotic systems.
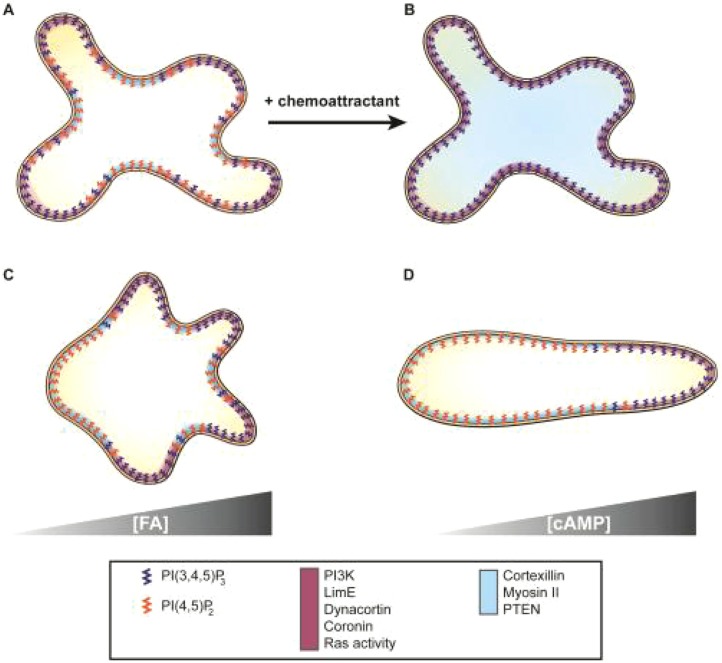
Fig. 8.
Schematic diagram of signaling events. (A) A randomly moving vegetative cell. PtdIns(3,4,5)P3 [PI(3,4,5)P3], PtdIns(4,5)P2 [PI(4,5)P2] and signaling proteins are color coded. Pseudopods are enriched with PtdIns(3,4,5)P3 (dark blue), PI3K, LimE, dynacortin, coronin and Ras activity (maroon signaling and cytoskeletal components). PtdIns(4,5)P2 (orange), cortexillin-1, myosin II and PTEN are localized (light blue) to the membrane areas between the pseudopods. (B) Upon uniform chemoattractant stimulation, PtdIns(4,5)P2 levels decrease and cortexillin-1, myosin II and PTEN delocalize from the membrane and are enriched in the cytoplasm (light blue). In contrast, the other components localize uniformly across the membrane periphery as PtdIns(3,4,5)P3 levels increase. (C) A vegetative cell migrating in a FA gradient. Pseudopods are biased towards the FA source and enriched with PtdIns(3,4,5)P3 and maroon signaling and cytoskeletal components. More PtdIns(4,5)P2, PTEN, cortexillin-1, myosin II and PTEN were found at the plasma membrane away from the FA source. (D) Similar localization was previously observed in polarized cells exposed to a cAMP gradient. PtdIns(3,4,5)P3 localization is based on PHCrac–GFP, while PtdIns(4,5)P2 distribution is based on the localization of PTEN–GFP.
When cells were stimulated with a uniform concentration of FA, Ras and PI3K were activated, the PM levels of PtdIns(3,4,5)P3 increased, and the cytoskeletal proteins LimE, dynacortin and coronin were all recruited to the inner leaflet of the PM. Conversely, PTEN, myosin II, and cortexillin-1 dissociate from the PM in response to a uniform stimulus of FA (Fig. 1). The same responses were found in the pseudopods of cells migrating in a FA gradient (Fig. 2). The localization and translocation of the proteins listed above in unpolarized cells are similar to the published results for developed cells in response to cAMP (Funamoto et al., 2002; Iijima and Devreotes, 2002; Janetopoulos and Firtel, 2008; Kortholt and van Haastert, 2008; Rericha and Parent, 2008; Swaney et al., 2010). Our results demonstrate that the major signal transduction proteins, lipids, and cytoskeletal elements function similarly in both types of directed migration.
In previous studies, PTEN, myosin II and cortexillin I were shown to act as mechanosensors that control cell shape during cytokinesis (Pramanik et al., 2009; Ren et al., 2009). We show, for the first time, that cortexillin-1 also responds dynamically to a uniform chemoattractant stimulus. The system appears to require cortexillin I to stably anchor the actin filaments so that the myosin motor can generate tension during cytokinesis (Ren et al., 2009). The same mechanism may operate at the rear of cell during migration. Moreover, PTEN and cortexillin I have PtdIns(4,5)P2-binding domains, which suggest that these proteins are posterior and furrow residing proteins (Iijima et al., 2004; Stock et al., 1999). Conversely, dynacortin becomes highly enriched in cortical protrusions and at leading edge of polarized cells and likely contributes to actin assembly (Girard et al., 2004; Kabacoff et al., 2007; Robinson et al., 2002).
Given the similarity of FA and cAMP-mediated chemotaxis, we analyzed several suspected chemotaxis mutants that have been characterized mainly in response to cAMP. The protein Ras G regulates PI3K and thus PtdIns(3,4,5)P3 synthesis, which recruits and activates PKBA. Ras C regulates the TORC2 pathway, which activates PBKR1 and PKBA to a lesser extent (Kamimura et al., 2008). These activated protein kinases phosphorylate a number of actin regulatory proteins such as Talin B, PI5K, Ras GefS and RhoGap. It has been shown that that Ras C and G are required for cAMP-stimulated development and chemotaxis. It is not surprising that cells lacking Ras G or both Ras C and G had poor cAMP responses, given that there was virtually no upregulation of the cAMP receptor in these cells (Bolourani et al., 2006). Interestingly, while the RBDRaf–GFP construct is supposed to be recruited to activated Ras (Kortholt et al., 2011), it has not been previously used as a biosensor in the Ras mutant backgrounds of D. discoideum. We observed the localization of PHCrac–GFP, PI3K–GFP and RBDRaf–GFP during random motility in vegetative WT, Ras C null, Ras G null, and Ras C/G null cells. In Ras G and Ras C/G null mutants RBDRaf, PI3K and PHCrac membrane translocations were reduced compared to WT and the Ras C null mutant. This suggests that Ras G predominantely regulates PI3K activity, as has been shown for cAMP-mediated responses. Given that there is still some low level of PI3K activity in our assays, there may be other Ras proteins, such as Rap1, or Ras D or Ras B, that regulate PI3K and PtdIns(3,4,5)P3 levels. Ras D and Ras B have been shown to have increased expression levels in Ras G nulls, and Ras D has also been shown to go up in CG nulls (personal communication, Huaqing Cai, Devreotes lab, Department of Cell Biology, Johns Hopkins University School of Medicine). However, these redundant and possibly compensatory Ras proteins do not apparently bind RBDRaf–GFP. In contrast, our PKB substrate phosphorylation data showed Ras G contributed little to overall substrate phosphorylation. Ras C and Ras C/G null mutants showed a decreased ability to phosphorylate numerous PKB substrates in response to FA. These data suggests that Ras C is important for PKB activity and that Ras G is important for PtdIns(3,4,5)P3 production in response to FA. Since Ras C predominantly activates PKBR1, these results are consistent to those during cAMP signaling which demonstrated that PKBR1 is the major PKB isoform in cAMP signaling regulating PKB substrate phosphorylation (Kamimura et al., 2008). We also tested the ability of Ras mutants to elicit chemotaxis in a FA gradient. Our results demonstrate that there were no statistical differences between the Ras mutants and WT in migration speed, chemotactic index and persistence even though Ras and PI3K activity were clearly inhibited. Thus, Ras C and G are dispensable for FA chemotaxis. This is in stark contrast to the previously published FA and cAMP chemotaxis data of Ras C, G and CG nulls (Bolourani et al., 2006, Kortholt et al., 2011; Bolourani et al., 2010). In the previous reports, the Ras mutants had a severe FA and cAMP chemotaxis defect and cAMP developmental defect. However, in our experiments, we found no difference between the Ras C and G mutants and wild-type chemotaxis toward FA or cAMP. However, we did observe cytokinesis and developmental defects in the Ras CG nulls, which are consistent with the published data. One possible reason for the differences we see in the FA chemotaxis is that we obtain highly chemotactic cells when they are grown in the presence of Klebsiella aerogenes, whereas the previous reports had grown cells axenically in HL-5 medium prior to testing (Bolourani et al., 2010). We do find that Ras CG nulls require ‘coaxing’ to polarize properly, but they are clearly able to chemotax to cAMP. We do not find this surprising given that PKBA/PKBR1 double nulls also chemotax to both FA and cAMP. Future studies should be directed towards determining if Ras CG nulls have difficulty migrating in shallow gradients of cAMP, as has been reported for mutants lacking PI3 kinase activity (Takeda et al., 2007; van Haastert et al., 2007). However, we caution that these studies may be difficult to interpret since the cells do not polarize properly.
Since we established that the Ras C and G proteins function similarly in both polarized and unpolarized cells, we were interested in testing mutants lacking phospholipase C (PLC). PLC nulls should have aberrant phosphoinositide levels, which might affect polarity and also cause developmental problems. Previously, it was reported that PLC null cells showed very weak changes in PM PtdIns(3,4,5)P3 levels upon cAMP stimulation (Kortholt et al., 2007). These observations concluded that, with levels of PtdIns(4,5)P2 not changing much in the absence of PLC, PTEN would remain associated with the PM and bias the PtdIns(4,5)P2/PtdIns(3,4,5)P3 balance towards even more PtdIns(4,5)P2. This would dampen any effect of PI3K activity. Furthermore, in cells overexpressing PLC, PTEN was found mostly in the cytoplasm, because the overall abundance of PM PtdIns(4,5)P2 was lowered. When uniformly stimulated with FA, we observed a very weak dissociation of PTEN–GFP from the PM in PLC null cells, consistent with previously published results of cells stimulated with cAMP (Kortholt et al., 2007). On the other hand, we found a strong dissociation of PTEN–GFP from the PM in PLC null cells stimulated with cAMP. This was not significantly different from PTEN–GFP in PTEN null cells. These data suggest that PLC does affect the localization of PTEN by shifting the PM balance towards more PtdIns(4,5)P2 and this can be seen in PLC null cells stimulated with the weak chemoattractant FA. However, starved PLC null cells that are stimulated with cAMP can still lower PtdIns(4,5)P2 via other pathways, such as PI3K. It is important to note that highly polarized cells, whether they be wild-type or PLC nulls, will localize PTEN to the very rear of the cell and do not show much of a redistribution of PTEN to the cytosol when stimulated with cAMP. We found that PLC null cells polarize earlier than wild-type cells, and this may have contributed to the conflicting report in the earlier study. We did find that overexpression of PLC did apparently lower PM PtdIns(4,5)P2 levels and redistributed the PTEN into the cytosol, as previously reported (Kortholt et al., 2007). As is often typical of a negative regulator, overexpression of the protein has a stronger phenotype than deleting it.
Finally, we examined the morphology and behavior of unpolarized and polarized cells during gradient sensing and gradient switching. Most polarized cells make big U-turns when the chemotactic source is moved to the rears of the cells. In contrast, vegetative cells extend multiple pseudopods that tend to be oriented towards the initial FA source. When the gradient is switched, unpolarized cells stop immediately and extend pseudopods towards the new direction of the gradient. This result demonstrates that the apparent biased random walk of unpolarized cells is more efficient at rapid changing of direction than fully polarized cells. This may be a useful characteristic so that feeding D. discoideum can rapidly reorient to the correct direction of food sources. Unpolarized cells can also generate phagosomes along the entire periphery of the cell.
We next tested the ability of unpolarized cells treated with Latrunculin-A to form a stable PHCrac–GFP crescent toward a FA source. When cytoskeletal actin organization was disrupted by Latrunculin-A, PtdIns(3,4,5)P3 oscillations were observed all over the PM, regardless of the direction of the gradient. However, in polarized cells, the PtdIns(3,4,5)P3 crescent was stably localized toward the high side of the cAMP gradient (Fig. 7B). We also tested the ability of 4-hour-starved cells to form a stable PHCrac–GFP crescent toward a cAMP gradient (Fig. 7C; supplementary material Movie 4). Highly unpolarized cells, whether responding to FA or cAMP, are unable to make stabilized PtdIns(3,4,5)P3 responses. We speculate that these underlying ‘random’ crescents in vegetative and in 4-hour-starved unpolarized cells are generated by an oscillatory mechanism that is independent of the heterotrimeric G proteins and can only be biased to some extent by the chemotactic signal transduction system. It has been demonstrated that the enzymes regulating phosphoinositide levels are regulated quite normally during random motility and during cytokinesis in cells lacking heterotrimeric function (,Sasaki et al., 2007). Polarized cells appear to be able to suppress these basal oscillations and focus the PtdIns(3,4,5)P3 synthesis in the direction of the gradient and distribute PTEN at the rear of the cell.
Latrunculin-A-treated unpolarized cells in general may struggle to be able to make a stable response, because the internal motility oscillator is always in the background. We speculate that unpolarized cells are highly sensitive to stochastic signaling fluctuations within the cell – this is why their PM is so active, and may also explain why they can move and change directions quickly when the gradient is switched. The stochastic ‘excitation’ that produces these oscillations may be biased by the FA gradient so that the random pseudopods occur more often in the correct direction. However, it is also possible that the pseudopods are reinforced or stabilized in the correct direction and that FA-mediated signaling is not capable, by itself, of producing a pseudopod (Insall and Andrew, 2007). Instead, FA-mediated signaling can only modulate an existing pseudopod initiation process. We suggest that cells responding to FA are still performing spatial sensing and integrate the signal across the entire periphery of the cell. We speculate that this increases the probability of random pseudopods on the high side of the gradient. This phenomenon is not limited to FA gradient sensing. Unpolarized cells that have been starved for a few hours can chemotax to cAMP, but are also unable to give a stable crescent response in a cAMP gradient when treated with Latrunculin-A. Cell polarity likely requires feedback mechanisms that are reinforced after upregulation of chemosensory components, the addition of a chemoattractant, and the silencing of this stochastic signaling. This same inhibitory mechanism is likely at work as cells become quiescent at the onset of mitotic metaphase just prior to cytokinesis, but without an external cue, the cells round up and await mitosis. We were unable to find a signaling mutant other than cells lacking the heterotrimeric G proteins that could not migrate to FA gradients. These findings suggest that cells that divide and undergo random motility will also likely be capable of chemotaxis. Therefore, screens using conditional mutants may be necessary to find other core regulatory elements involved in chemotaxis.
Materials and Methods
Materials
Folic acid (FA) was obtained from Fischer scientific, Latrunculin-A from Molecular Probes, cAMP and LY294002 from Sigma-Aldrich, and anti-phospho-PKB substrate antibody from Cell Signaling Technology. Ras C, Ras G and Ras C/G null cells were described previously (Bolourani et al., 2006). Cell lines were acquired from the Gerald Weeks labs twice, Dicty base once, and once from the Van Haastert lab with the same results. The Ras C/G double null mutant cells need to be pulsed with 60 nM cAMP or they will not develop properly. In experiments described here, PTEN–GFP was expressed in the PLC null cell lines previously described (Kortholt et al., 2007). PTEN–GFP was also expressed in the original PLC nulls (not shown) with similar results (Drayer et al., 1994). Plasmids have all been described previously: PHCrac-GFP (Parent et al., 1998), PI3K2-GFP, PTEN-GFP, coronin-GFP, GFP-myosin II (Janetopoulos et al., 2005), RBDRaf-GFP (Kortholt et al., 2011), LimE-RFP (Clarke et al., 2006) and GFP-dynacortin and GFP-cortexillin-1 (Girard et al., 2004). All of the fusion proteins are full length and GFP or RFP fusions do not appear to affect the target protein function as determined in the cited references.
Cell culture
Cells were grown in axenic or non-axenic conditions as required for assay. For axenic culture, cells were grown in HL5 medium (ForMedium) at 22°C. For non-axenic culture, cells were grown with Klebsiella aerogenes (KA) on an SM agar plate overnight at 22°C. Transformants were maintained in 40 µg/ml G418 (RPI) or 50 µg/ml hygromycin (Sigma-Aldrich), or both as required.
Random motility assay
Vegetative cells grown with Klebsiella aerogenes (KA) bacteria overnight were harvested from the SM plate and washed three times with development buffer (DB) (5 mM Na2HPO4 5 mM KH2PO4, 1 mM CaCl2, 2 mM MgCl2, pH to 6.5) by centrifugation at 1000 rpm for 5 minutes to isolate D. discoideum in the absence of bacteria. Washed cells were seeded onto a chambered microscope slide in 2 ml of DB and allowed to adhere for 15 minutes. Images were captured on a Zeiss-Axiovert 200 M microscope using SlideBook5 software. FITC and Narrow Band Cy3 Cubes for 3I Marianas workstation were used for GFP and RFP fluorescence.
Uniform folic acid stimulation
Cells were prepared using the same protocol as described for the random motility assay. A micropipette containing 100 µM FA was used to stimulate cells. The micropipette was lowered after the fifth frame during a 30-frame time-lapse acquisition. Fluorescent frames were acquired every 1.5 seconds and imaged using a 40× PlanNeofluar 1.3 NA oil objective.
Analysis of biosensor translocation to the plasma membrane
Mean fluorescent intensity of the cytosol and membrane before and during translocation was measured using Slidebook5 software. The membrane to cytosol ratio before and during translocation was calculated using the following formula (membrane-background/cytosol-background). The percentage change of intensity during translocation was calculated by the following formula: (membrane to cytosol ratio after translocation − membrane to cytosol ratio before translocation)/(membrane to cytosol ratio before translocation)×100. Membrane and cytosol regions were manually traced prior to and after FA addition.
FA and cAMP chemotaxis assays
D. discoideum cells (5×105) were centrifuged and pelleted at 1500 rpm for ∼2 minutes in a 15 ml conical tube. Cells were then resuspended in 500 µl HL5 (no antibiotics). One inoculation loop full of KA bacteria grown freshly the night before was added to the conical tube. The D. discoideum and KA bacteria were resuspended by vortexing and subsequently spread on fresh SM plate. When we analyzed the Ras mutants, we mixed WT Ax2 cells expressing PHCrac–GFP and mutant cells on the same SM bacterial plate. Our control cell line expressing PHCrac–GFP were carefully examined and were expressing the GFP fusion protein near 100%. Data was not included if both cells failed to chemotaxis. As Ras mutants were created in the JH10 background, we also raced AX2 PHCrac–GFP cells against non-fluorescent JH10s and found no difference in chemotaxis. FA needs to be prepared properly for the chemotaxis assays to work well. One must add just enough NaOH to dissolve FA. We added 5.5 mg FA to 12.5 ml dH2O, and then 13.5 µl 2 N NaOH to make a 1.25 mM stock FA solution. FA was centrifuged at high speed in a microcentrifuge to eliminate any undissolved FA or large particulates that could clog the micropipette. A micropipette containing 100 µM folic was placed in the same focal plane as the cells and phase contrast and fluorescent frames were acquired with an interval of 15 seconds for 30 minutes using a 40× 1.3 NA oil objective. In the case of the mixed chemotaxis assay, polarized cells were mixed with vegetative cells in a chambered microscope slide. Polarized cells were prepared as mentioned previously (Kamimura et al., 2008). For polarity reversals and U-turn experiments, a micropipette containing 6 µl of both 10 µM cAMP and 100 µM FA was placed at one position in the field of view until the cells move toward the source. The micropipette was then shifted to a distant position in the same field of view and polarity reversals and U-turns of cells were monitored. During image acquisition, 60 frames were captured with an interval of 15 seconds at position 1 and then 60 more frames were collected at micropipette position 2 for each individual movie.
Average speed, chemotactic index and persistence calculation
Average speed was calculated as the total distance traveled by the cell divided by time using Slidebook5 software. To calculate chemotactic index, the cosine of the angle between the direction of movement and the direction of chemoattractant gradient was determined. The X, Y coordinates of micropipette, start and end positions of the cell were calculated by Slidebook5 software. From these values, the length of each side of the triangle was determined. The cosine of the angle was calculated by plugging in the length of each face in the standard law of cosine formula. Persistence was calculated as the shortest linear distance between the start point and end point of the migration path divided by the total distance traveled by the cell. Linear distance was derived from XY coordinates of start and end point of cell and total distance from XY coordinates every position from start to end (Cai et al., 2010).
Detection of PKB substrate phosphorylation by western blotting
Vegetative Cells grown on KA plates were prepared as described above. The cells were stimulated with 50 µM folic acid for 15 seconds then lysed by heating at 95°C for 5 minutes in 1× SDS sample buffer. Unstimulated control cells were aliquoted before stimulation and treated as above. For stimulating polarized cells, 1 µM cAMP was used. Cell lysate equivalent to 4×104 cells were loaded into SDS-PAGE and transferred using the Invitrogen transblot apparatus. The blot was probed using rabbit anti phospho-PKB substrate antibody (Cell Signaling Technology) (Kamimura et al., 2008) and goat anti-rabbit secondary IR680 antibody (Li-Cor). The blot was visualized using Odyssey (Li-Cor) IR detecting instrument.
Analysis of phospho-PKB substrates using ImageJ
The intensity of all bands in each lane of the blot (Fig. 4C) was measured using ImageJ. The intensity for WT was normalized to 100. The response of Ras and α4 mutants was calculated as 100Y/X, where X is the intensity for WT and Y the intensity for Ras mutants.
Directional sensing of PH–GFP in Latrunculin-A-treated vegetative and polarized cells exposed to a gradient containing a mixture of cAMP and folic acid
Vegetative cells were prepared as described above. Polarized cells were prepared as described above with the only difference being starved cells were pulsed for 5 hours with cAMP. For the chemotaxis assay, the cAMP and FA competent cells were mixed in a 1∶1 ratio and added to a one-well chamber for observation under the microscope. 6 µl of final 10 µM cAMP and 100 µM folic acid mixture was added to a glass micropipette. The cells were observed under a 40× oil 1.3 NA immersion objective using a Zeiss Axiovert 200 M fluorescence microscope. Cells were captured migrating toward the mixed chemoattractant pipette using a Cool Snap CCD camera and Slidebook software. Once both cell types were close to the micropipette (∼4 minutes from when the pipette was lowered), 10 µM Latrunculin-A was added to the cells. The cells became round within 2 minutes of Latrunculin-A treatment.
Directional sensing of PH–GFP in Latrunculin-A-treated 4-hour-starved cells exposed to cAMP gradient
WT cells expressing PH–GFP were starved for 1 hour and subsequently pulsed with 60 nM cAMP for 3 hours. Chemotaxis assay was performed as described above. 1 µM Latrunculin-A was added initially and then ramped up to 10 µM during the course of the 30-minute movie until all cells were round.
Supplementary Material
Acknowledgments
We thank the people referenced to in the Materials and Methods section for providing us plasmids. We thank Dictybase and the Van Haastert lab for providing the PLC mutants, and Dictybase and the Weeks lab and Arjan Kortholt for providing various Ras mutants. We also thank Rachel C. Wright for helping produce the model figure, Dawit Johwar for assistance in editing movies, and Ryan Khodadadi for help in editing the manuscript.
Footnotes
Funding
This work was supported by the National Institutes of Health [grant number RO1GM080370 to K.S., C.J. and G.W.]. K.J.A. was supported by Faculty Development Leave from Texas A&M University. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.113415/-/DC1
References
- Abe F., Maeda Y. (1994). Precise expression of the cAMP receptor gene, CAR1, during transition from growth to differentiation in Dictyostelium discoideum. FEBS Lett. 342, 239–241 10.1016/0014-5793(94)80509-1 [DOI] [PubMed] [Google Scholar]
- Andrew N., Insall R. H. (2007). Chemotaxis in shallow gradients is mediated independently of PtdIns 3-kinase by biased choices between random protrusions. Nat. Cell Biol. 9, 193–200 10.1038/ncb1536 [DOI] [PubMed] [Google Scholar]
- Bernstein R., van Driel R., Rossier C. (1981). Regulatory interactions of cyclic AMP and folic acid during differentiation of Dictyostelium discoideum. Adv. Cycl. Nucl. Res. 14, 705 [Google Scholar]
- Bolourani P., Spiegelman G. B., Weeks G. (2006). Delineation of the roles played by RasG and RasC in cAMP-dependent signal transduction during the early development of Dictyostelium discoideum. Mol. Biol. Cell 17, 4543–4550 10.1091/mbc.E05-11-1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolourani P., Spiegelman G., Weeks G. (2010). Ras proteins have multiple functions in vegetative cells of Dictyostelium. Eukaryot. Cell 9, 1728–1733 10.1128/EC.00141-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner J. T. (1971). Aggregation and differentiation in the cellular slime molds. Annu. Rev. Microbiol. 25, 75–92 10.1146/annurev.mi.25.100171.000451 [DOI] [PubMed] [Google Scholar]
- Bonner J. (1978). The life cycle of the cellular slime molds. Nat. Hist. 87, 70–79 [Google Scholar]
- Bozulic L., Hemmings B. A.(2009). PIKKing on PKB: regulation of PKB activity by phosphorylation. Curr. Opin. Cell Biol. 21, 256–261 10.1016/j.ceb.2009.02.002 [DOI] [PubMed] [Google Scholar]
- Cai H., Das S., Kamimura Y., Long Y., Parent C. A., Devreotes P. N. (2010). Ras-mediated activation of the TORC2-PKB pathway is critical for chemotaxis. J. Cell Biol. 190, 233–245 10.1083/jcb.201001129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charest P. G., Shen Z., Lakoduk A., Sasaki A. T., Briggs S. P., Firtel R. A. (2010). A Ras signaling complex controls the RasC-TORC2 pathway and directed cell migration. Dev. Cell 18, 737–749 10.1016/j.devcel.2010.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. Y., Insall R. H., Devreotes P. N. (1996). Signaling through chemoattractant receptors in Dictyostelium. Trends Genet. 12, 52–57 10.1016/0168-9525(96)81400-4 [DOI] [PubMed] [Google Scholar]
- Chubb J. R., Wilkins A., Wessels D. J., Soll D. R., Insall R. H. (2002). Pseudopodium dynamics and rapid cell movement in Dictyostelium Ras pathway mutants. Cell Motil. Cytoskeleton 53, 150–162 10.1002/cm.10064 [DOI] [PubMed] [Google Scholar]
- Chung C. Y., Potikyan G., Firtel R. A. (2001). Control of cell polarity and chemotaxis by Akt/PKB and PI3 kinase through the regulation of PAKa. Mol. Cell 7, 937–947 10.1016/S1097-2765(01)00247-7 [DOI] [PubMed] [Google Scholar]
- Clarke M., Müller–Taubenberger A., Anderson K. I., Engel U., Gerisch G. (2006). Mechanically induced actin-mediated rocketing of phagosomes. Mol. Biol. Cell 17, 4866–4875 10.1091/mbc.E06-04-0365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hostos E. L., Bradtke B., Lottspeich F., Guggenheim R., Gerisch G. (1991). Coronin, an actin binding protein of Dictyostelium discoideum localized to cell surface projections, has sequence similarities to G protein beta subunits. EMBO J. 10, 4097–4104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit R., Rinke de Wit T. (1986). Developmental regulation of the folic acid chemosensory system in Dictyostelium discoideum. Dev. Biol. 118, 385–391 10.1016/0012-1606(86)90008-4 [DOI] [Google Scholar]
- Devreotes P. N. (1983). The effect of folic acid on cAMP-elicited cAMP production in Dictyostelium discoideum. Dev. Biol. 95, 154–162 10.1016/0012-1606(83)90014-3 [DOI] [PubMed] [Google Scholar]
- Devreotes P., Janetopoulos C. (2003). Eukaryotic chemotaxis: distinctions between directional sensing and polarization. J. Biol. Chem. 278, 20445–20448 10.1074/jbc.R300010200 [DOI] [PubMed] [Google Scholar]
- Devreotes P. N., Zigmond S. H. (1988). Chemotaxis in eukaryotic cells: a focus on leukocytes and Dictyostelium. Annu. Rev. Cell Biol. 4, 649–686 10.1146/annurev.cb.04.110188.003245 [DOI] [PubMed] [Google Scholar]
- DiNitto J. P., Lambright D. G. (2006). Membrane and juxtamembrane targeting by PH and PTB domains. Biochim. Biophys. Acta 1761, 850–867 10.1016/j.bbalip.2006.04.008 [DOI] [PubMed] [Google Scholar]
- Dormann D., Kim J. Y., Devreotes P. N., Weijer C. J. (2001). cAMP receptor affinity controls wave dynamics, geometry and morphogenesis in Dictyostelium. J. Cell Sci. 114, 2513–2523 [DOI] [PubMed] [Google Scholar]
- Dormann D., Weijer G., Dowler S., Weijer C. J. (2004). In vivo analysis of 3-phosphoinositide dynamics during Dictyostelium phagocytosis and chemotaxis. J. Cell Sci. 117, 6497–6509 10.1242/jcs.01579 [DOI] [PubMed] [Google Scholar]
- Drayer A. L., Van der Kaay J., Mayr G. W., Van Haastert P. J. (1994). Role of phospholipase C in Dictyostelium: formation of inositol 1,4,5-trisphosphate and normal development in cells lacking phospholipase C activity. EMBO J. 13, 1601–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J., Park J., Cron P., Hess D., Hemmings B. A. (2004). Identification of a PKB/Akt hydrophobic motif Ser-473 kinase as DNA-dependent protein kinase. J. Biol. Chem. 279, 41189–41196 10.1074/jbc.M406731200 [DOI] [PubMed] [Google Scholar]
- Funamoto S., Meili R., Lee S., Parry L., Firtel R. A. (2002). Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. Cell 109, 611–623 10.1016/S0092-8674(02)00755-9 [DOI] [PubMed] [Google Scholar]
- Girard K. D., Chaney C., Delannoy M., Kuo S. C., Robinson D. N. (2004). Dynacortin contributes to cortical viscoelasticity and helps define the shape changes of cytokinesis. EMBO J. 23, 1536–1546 10.1038/sj.emboj.7600167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertin D. A., Stevens D. M., Thoreen C. C., Burds A. A., Kalaany N. Y., Moffat J., Brown M., Fitzgerald K. J., Sabatini D. M.(2006). Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev. Cell 11, 859–871 10.1016/j.devcel.2006.10.007 [DOI] [PubMed] [Google Scholar]
- Hadwiger J. A. (2007). Developmental morphology and chemotactic responses are dependent on G alpha subunit specificity in Dictyostelium. Dev. Biol. 312, 1–12 10.1016/j.ydbio.2007.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadwiger J. A., Srinivasan J. (1999). Folic acid stimulation of the Galpha4 G protein-mediated signal transduction pathway inhibits anterior prestalk cell development in Dictyostelium. Differentiation 64, 195–204 10.1046/j.1432-0436.1999.6440195.x [DOI] [PubMed] [Google Scholar]
- Huang Y. E., Iijima M., Parent C. A., Funamoto S., Firtel R. A., Devreotes P. (2003). Receptor-mediated regulation of PI3Ks confines PI(3,4,5)P3 to the leading edge of chemotaxing cells. Mol. Biol. Cell 14, 1913–1922 10.1091/mbc.E02-10-0703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima M., Devreotes P. (2002). Tumor suppressor PTEN mediates sensing of chemoattractant gradients. Cell 109, 599–610 10.1016/S0092-8674(02)00745-6 [DOI] [PubMed] [Google Scholar]
- Iijima M., Huang Y. E., Luo H. R., Vazquez F., Devreotes P. N. (2004). Novel mechanism of PTEN regulation by its phosphatidylinositol 4,5-bisphosphate binding motif is critical for chemotaxis. J. Biol. Chem. 279, 16606–16613 10.1074/jbc.M312098200 [DOI] [PubMed] [Google Scholar]
- Insall R., Andrew N. (2007). Chemotaxis in Dictyostelium: how to walk straight using parallel pathways. Curr. Opin. Microbiol. 10, 578–581 10.1016/j.mib.2007.10.004 [DOI] [PubMed] [Google Scholar]
- Janetopoulos C., Devreotes P. (2006). Phosphoinositide signaling plays a key role in cytokinesis. J. Cell Biol. 174, 485–490 10.1083/jcb.200603156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janetopoulos C., Firtel R. A. (2008). Directional sensing during chemotaxis. FEBS Lett. 582, 2075–2085 10.1016/j.febslet.2008.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janetopoulos C., Jin T., Devreotes P. (2001). Receptor-mediated activation of heterotrimeric G-proteins in living cells. Science 291, 2408–2411 10.1126/science.1055835 [DOI] [PubMed] [Google Scholar]
- Janetopoulos C., Ma L., Devreotes P. N., Iglesias P. A. (2004). Chemoattractant-induced phosphatidylinositol 3,4,5-trisphosphate accumulation is spatially amplified and adapts, independent of the actin cytoskeleton. Proc. Natl. Acad. Sci. USA 101, 8951–8956 10.1073/pnas.0402152101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janetopoulos C., Borleis J., Vazquez F., Iijima M., Devreotes P. (2005). Temporal and spatial regulation of phosphoinositide signaling mediates cytokinesis. Dev. Cell 8, 467–477 10.1016/j.devcel.2005.02.010 [DOI] [PubMed] [Google Scholar]
- Jowhar D., Wright G., Samson P. C., Wikswo J. P., Janetopoulos C. (2010). Open access microfluidic device for the study of cell migration during chemotaxis. Integr. Biol. (Camb) 2, 648–658 10.1039/c0ib00110d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabacoff C., Xiong Y., Musib R., Reichl E. M., Kim J., Iglesias P. A., Robinson D. N. (2007). Dynacortin facilitates polarization of chemotaxing cells. BMC Biol. 5, 53 10.1186/1741-7007-5-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kae H., Lim C. J., Spiegelman G. B., Weeks G. (2004). Chemoattractant-induced Ras activation during Dictyostelium aggregation. EMBO Rep. 5, 602–606 10.1038/sj.embor.7400151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimura Y., Devreotes P. N. (2010). Phosphoinositide-dependent protein kinase (PDK) activity regulates phosphatidylinositol 3,4,5-trisphosphate-dependent and -independent protein kinase B activation and chemotaxis. J. Biol. Chem. 285, 7938–7946 10.1074/jbc.M109.089235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimura Y., Xiong Y., Iglesias P. A., Hoeller O., Bolourani P., Devreotes P. N. (2008). PIP3-independent activation of TorC2 and PKB at the cell’s leading edge mediates chemotaxis. Curr. Biol. 18, 1034–1043 10.1016/j.cub.2008.06.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh M., Chen G., Roberge E., Shaulsky G., Kuspa A. (2007). Developmental commitment in Dictyostelium discoideum. Eukaryotic Cell 6, 2038–2045 10.1128/EC.00223-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesbeke F., van Haastert P., de Wit R., Snaar–Jagalska B. (1990). Chemotaxis to cyclic AMP and folic acid is mediated by different G-proteins in Dictyostelium discoideum. J. Cell Sci. 96, 668–673 content/96/4/668.short [Google Scholar]
- Kim J. Y., Borleis J. A., Devreotes P. N. (1998). Switching of chemoattractant receptors programs development and morphogenesis in Dictyostelium: receptor subtypes activate common responses at different agonist concentrations. Dev. Biol. 197, 117–128 10.1006/dbio.1998.8882 [DOI] [PubMed] [Google Scholar]
- Kortholt A., van Haastert P. J. (2008). Highlighting the role of Ras and Rap during Dictyostelium chemotaxis. Cell. Signal. 20, 1415–1422 10.1016/j.cellsig.2008.02.006 [DOI] [PubMed] [Google Scholar]
- Kortholt A., King J. S., Keizer–Gunnink I., Harwood A. J., Van Haastert P. J. (2007). Phospholipase C regulation of phosphatidylinositol 3,4,5-trisphosphate-mediated chemotaxis. Mol. Biol. Cell 18, 4772–4779 10.1091/mbc.E07-05-0407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortholt A., Bolourani P., Rehmann H., Keizer–Gunnink I., Weeks G., Wittinghofer A., Van Haastert P. J. (2010). Rap/phosphatidylinositol 3-kinase pathway controls pseudopod formation. Mol Biol Cell. 21, 936–945 Epub 2010 Jan 20.A 10.1091/mbc.E09-03-0177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortholt A., Kataria R., Keizer–Gunnink I., Van Egmond W. N., Khanna A., Van Haastert P. J. (2011). Dictyostelium chemotaxis: essential Ras activation and accessory signalling pathways for amplification. EMBO Rep. 12, 1273–1279 10.1038/embor.2011.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A., Pupillo M., Gundersen R., Miake–Lye R., Devreotes P. N., Firtel R. A. (1989). Regulation and function of G alpha protein subunits in Dictyostelium. Cell 57, 265–275 10.1016/0092-8674(89)90964-1 [DOI] [PubMed] [Google Scholar]
- Kumagai A., Hadwiger J. A., Pupillo M., Firtel R. A. (1991). Molecular genetic analysis of two G alpha protein subunits in Dictyostelium. J. Biol. Chem. 266, 1220–1228 [PubMed] [Google Scholar]
- Lazennec G., Richmond A. (2010). Chemokines and chemokine receptors: new insights into cancer-related inflammation. Trends Mol. Med. 16, 133–144 10.1016/j.molmed.2010.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X-H., Buggey J., Kimmel A. R. (2010). Chemotactic activation of Dictyostelium AGC-family kinases AKT and PKBR1 requires separate but coordinated functions of PDK1 and TORC2. J. Cell Sci. 123, 983–992 10.1242/jcs.064022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P. (2002). Inflammation in atherosclerosis. Nature 420, 868–874 10.1038/nature01323 [DOI] [PubMed] [Google Scholar]
- Loomis W. F. (1979). Biochemistry of Aggregation in Dictyostelium. A review. Dev. Biol. 70, 1–12 10.1016/0012-1606(79)90002-2 [DOI] [PubMed] [Google Scholar]
- Ma L., Janetopoulos C., Yang L., Devreotes P. N., Iglesias P. A. (2004). Two complementary, local excitation, global inhibition mechanisms acting in parallel can explain the chemoattractant-induced regulation of PI(3,4,5)P3 response in dictyostelium cells. Biophys. J. 87, 3764–3774 10.1529/biophysj.104.045484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda M., Firtel R. A. (1997). Activation of the mitogen-activated protein kinase ERK2 by the chemoattractant folic acid in Dictyostelium. J. Biol. Chem. 272, 23690–23695 10.1074/jbc.272.38.23690 [DOI] [PubMed] [Google Scholar]
- Maeda Y., Mayanagi T., Amagai A. (2009). Folic acid is a potent chemoattractant of free-living amoebae in a new and amazing species of protist, Vahlkampfia sp. Zoolog. Sci. 26, 179–186 10.2108/zsj.26.179 [DOI] [PubMed] [Google Scholar]
- Manahan C. L., Iglesias P. A., Long Y., Devreotes P. N. (2004). Chemoattractant signaling in dictyostelium discoideum. Annu. Rev. Cell Dev. Biol. 20, 223–253 10.1146/annurev.cellbio.20.011303.132633 [DOI] [PubMed] [Google Scholar]
- Meili R., Ellsworth C., Lee S., Reddy T. B. K., Ma H., Firtel R. A. (1999). Chemoattractant-mediated transient activation and membrane localization of Akt/PKB is required for efficient chemotaxis to cAMP in Dictyostelium. EMBO J. 18, 2092–2105 10.1093/emboj/18.8.2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne J. L., Caterina M. J., Devreotes P. N. (1997). Random mutagenesis of the cAMP chemoattractant receptor, cAR1, of Dictyostelium. Evidence for multiple states of activation. J. Biol Chem. 272, 2069–2076 10.1074/jbc.272.4.2069 [DOI] [PubMed] [Google Scholar]
- Moore M. A. (2001). The role of chemoattraction in cancer metastases. Bioessays 23, 674–676 10.1002/bies.1095 [DOI] [PubMed] [Google Scholar]
- Niggli V. (2003). Microtubule-disruption-induced and chemotactic-peptide-induced migration of human neutrophils: implications for differential sets of signalling pathways. J. Cell Sci. 116, 813–822 10.1242/jcs.00306 [DOI] [PubMed] [Google Scholar]
- Pan P., Hall E. M., Bonner J. T. (1972). Folic acid as second chemotactic substance in the cellular slime moulds. Nat. New Biol. 237, 181–182 [DOI] [PubMed] [Google Scholar]
- Parent C. A., Devreotes P. N. (1996). Constitutively active adenylyl cyclase mutant requires neither G proteins nor cytosolic regulators. J. Biol. Chem. 271, 18333–18336 10.1074/jbc.271.31.18333 [DOI] [PubMed] [Google Scholar]
- Parent C. A., Devreotes P. N. (1999). A cell’s sense of direction. Science 284, 765–770 10.1126/science.284.5415.765 [DOI] [PubMed] [Google Scholar]
- Parent C. A., Blacklock B. J., Froehlich W. M., Murphy D. B., Devreotes P. N. (1998). G protein signaling events are activated at the leading edge of chemotactic cells. Cell 95, 81–91 10.1016/S0092-8674(00)81784-5 [DOI] [PubMed] [Google Scholar]
- Park H. T., Wu J., Rao Y. (2002). Molecular control of neuronal migration. Bioessays 24, 821–827 10.1002/bies.10141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D. D., Haynes B. F. (2001). Leukocyte homing to synovium. Curr. Dir. Autoimmun.. 3, 133–167 10.1159/000060517 [DOI] [PubMed] [Google Scholar]
- Prabhu Y., Eichinger L. (2006). The Dictyostelium repertoire of seven transmembrane domain receptors. Eur. J. Cell Biol. 85, 937–946 10.1016/j.ejcb.2006.04.003 [DOI] [PubMed] [Google Scholar]
- Pramanik M. K., Iijima M., Iwadate Y., Yumura S. (2009). PTEN is a mechanosensing signal transducer for myosin II localization in Dictyostelium cells. Genes Cells 14, 821–834 10.1111/j.1365-2443.2009.01312.x [DOI] [PubMed] [Google Scholar]
- Prassler J., Murr A., Stocker S., Faix J., Murphy J., Marriott G. (1998). DdLIM is a cytoskeleton-associated protein involved in the protrusion of lamellipodia in Dictyostelium. Mol. Biol. Cell 9, 545–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pupillo M., Kumagai A., Pitt G. S., Firtel R. A., Devreotes P. N. (1989). Multiple alpha subunits of guanine nucleotide-binding proteins in Dictyostelium. Proc. Natl. Acad. Sci. USA 86, 4892–4896 10.1073/pnas.86.13.4892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y., Effler J. C., Norstrom M., Luo T., Firtel R. A., Iglesias P. A., Rock R. S., Robinson D. N. (2009). Mechanosensing through cooperative interactions between myosin II and the actin crosslinker cortexillin I. Curr. Biol. 19, 1421–1428 10.1016/j.cub.2009.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rericha E. C., Parent C. A. (2008). Steering in quadruplet: the complex signaling pathways directing chemotaxis. Sci. Signal. 1, pe26 10.1126/scisignal.122pe26 [DOI] [PubMed] [Google Scholar]
- Rickert P., Weiner O. D., Wang F., Bourne H. R., Servant G. (2000). Leukocytes navigate by compass: roles of PI3Kgamma and its lipid products. Trends Cell Biol. 10, 466–473 10.1016/S0962-8924(00)01841-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D. N., Ocon S. S., Rock R. S., Spudich J. A. (2002). Dynacortin is a novel actin bundling protein that localizes to dynamic actin structures. J. Biol. Chem. 277, 9088–9095 10.1074/jbc.M112144200 [DOI] [PubMed] [Google Scholar]
- Sarbassov D. D., Guertin D. A., Ali S. M., Sabatini D. M. (2005). Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307, 1098–1101 10.1126/science.1106148 [DOI] [PubMed] [Google Scholar]
- Sasaki A. T., Firtel R. A. (2009). Spatiotemporal regulation of Ras-GTPases during chemotaxis. Methods Mol. Biol. 571, 333–348 10.1007/978-1-60761-198-1_23 [DOI] [PubMed] [Google Scholar]
- Sasaki A. T., Janetopoulos C., Lee S., Charest P. G., Takeda K., Sundheimer L. W., Meili R., Devreotes P. N., Firtel R. A. (2007). G protein-independent Ras/PI3K/F-actin circuit regulates basic cell motility. J. Cell Biol. 178, 185–191 10.1083/jcb.200611138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnemann J., Aichem A., Schlatterer C. (1998). Dissection of the cAMP induced cytosolic calcium response in Dictyostelium discoideum: the role of cAMP receptor subtypes and G protein subunits. FEBS Lett. 436, 271–276 10.1016/S0014-5793(98)01139-9 [DOI] [PubMed] [Google Scholar]
- Stock A., Steinmetz M. O., Janmey P. A., Aebi U., Gerisch G., Kammerer R. A., Weber I., Faix J. (1999). Domain analysis of cortexillin I: actin-bundling, PIP(2)-binding and the rescue of cytokinesis. EMBO J. 18, 5274–5284 10.1093/emboj/18.19.5274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaney K. F., Huang C-H., Devreotes P. N. (2010). Eukaryotic chemotaxis: a network of signaling pathways controls motility, directional sensing, and polarity. Annu. Rev. Biophys. 39, 265–289 10.1146/annurev.biophys.093008.131228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K., Sasaki A. T., Ha H., Seung H. A., Firtel R. A. (2007). Role of phosphatidylinositol 3-kinases in chemotaxis in Dictyostelium. J. Biol. Chem. 282, 11874–11884 10.1074/jbc.M610984200 [DOI] [PubMed] [Google Scholar]
- van Haastert P. J., Bosgraaf L. (2009). The local cell curvature guides pseudopodia towards chemoattractants. Hfsp J. 3, 282–286 10.2976/1.3185725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Haastert P. J., Postma M. (2007). Biased random walk by stochastic fluctuations of chemoattractant-receptor interactions at the lower limit of detection. Biophys. J. 93, 1787–1796 10.1529/biophysj.107.104356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Haastert P. J., De Wit R. J., Konijn T. M. (1982). Antagonists of chemoattractants reveal separate receptors for cAMP, folic acid and pterin in Dictyostelium. Exp. Cell Res. 140, 453–456 10.1016/0014-4827(82)90139-2 [DOI] [PubMed] [Google Scholar]
- van Haastert P. J., Keizer–Gunnink I., Kortholt A. (2007). Essential role of PI3-kinase and phospholipase A2 in Dictyostelium discoideum chemotaxis. J. Cell Biol. 177, 809–816 10.1083/jcb.200701134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkerke–Van Wijk I., Kim J. Y., Brandt R., Devreotes P. N., Schaap P. (1998). Functional promiscuity of gene regulation by serpentine receptors in Dictyostelium discoideum. Mol. Cell. Biol. 18, 5744–5749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams H. P., Harwood A. J. (2003). Cell polarity and Dictyostelium development. Curr. Opin. Microbiol. 6, 621–627 10.1016/j.mib.2003.10.008 [DOI] [PubMed] [Google Scholar]
- Wu L. J., Valkema R., Van Haastert P. J. M., Devreotes P. N. (1995). The G protein beta subunit is essential for multiple responses to chemoattractants in Dictyostelium. J. Cell Biol. 129, 1667–1675 10.1083/jcb.129.6.1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Goswami M., Sawai S., Cox E. C., Hereld D. (2007). Regulation of G protein-coupled cAMP receptor activation by a hydrophobic residue in transmembrane helix 3. Mol. Microbiol. 65, 508–520 10.1111/j.1365-2958.2007.05803.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.