Summary
The evolutionarily conserved transcriptional cofactor Jab1 plays critical roles in cell differentiation, proliferation, and apoptosis by modulating the activity of diverse factors and regulating the output of various signaling pathways. Although Jab1 can interact with the bone morphogenetic protein (BMP) downstream effector Smad5 to repress BMP signaling in vitro, the role of Jab1 in BMP-mediated skeletogenesis in vivo is still poorly understood. As a key regulator of skeletogenesis, BMP signaling regulates the critical Ihh-Pthrp feedback loop to promote chondrocyte hypertrophy. In this study, we utilized the loxP/Cre system to delineate the specific role of Jab1 in cartilage formation. Strikingly, Jab1 chondrocyte-specific knockout Jab1flox/flox; Col2a1-Cre (cKO) mutants exhibited neonatal lethal chondrodysplasia with severe dwarfism. In the mutant embryos, all the skeletal elements developed via endochondral ossification were extremely small with severely disorganized chondrocyte columns. Jab1 cKO chondrocytes exhibited increased apoptosis, G2 phase cell cycle arrest, and increased expression of hypertrophic chondrocyte markers Col10a1 and Runx2. Jab1 can also inhibit the transcriptional activity of Runx2, a key regulator of chondrocyte hypertrophy. Notably, our study reveals that Jab1 is likely a novel inhibitor of BMP signaling in chondrocytes in vivo. In Jab1 cKO chondrocytes, there was heightened expression of BMP signaling components including Gdf10/Bmp3b and of BMP targets during chondrocyte hypertrophy such as Ihh. Furthermore, Jab1 cKO chondrocytes exhibited an enhanced response to exogenous BMP treatment. Together, our study demonstrates that Jab1 represses chondrocyte hypertrophy in vivo, likely in part by downregulating BMP signaling and Runx2 activity.
Key words: Jab1/Csn5, Chondrodysplasia, BMP, Runx2, COP9 signalosome
Introduction
During development, endochondral ossification forms most of the skeleton, including vertebrae, ribs and long bones. It involves an initial cartilage anlagen within which chondrocytes undergo an elaborate and well-controlled differentiation process (Kronenberg, 2003). The mesenchymal precursors first condense and commit to chondrocyte lineage under master regulator Sox9, followed by the robust secretion of cartilage-specific extracellular matrix including type II collagen and rapid proliferation to form orderly columns. Chondrocytes then stop dividing, exit the cell cycle, and start prehypertrophy with the expression of type X collagen under key regulators Runx2, Runx3 and Indian Hedgehog (Ihh). Subsequently, chondrocytes undergo overt hypertrophy and endochondral ossification occurs when hypertrophic chondrocytes within the cartilaginous model begin to undergo apoptosis in the center of the cartilage. Osteoblasts are recruited here from adjacent perichondrium and secrete bone matrix, forming a primary ossification center (Kronenberg, 2003). Ihh is a master regulator of skeletal development by coordinating chondrocyte proliferation, chondrocyte differentiation, and osteoblast differentiation (Kronenberg, 2003). During endochondral bone development, Ihh is synthesized by prehypertrophic chondrocytes and early hypertrophic chondrocytes. Ihh binds to its receptor Patched-1 (Ptc-1), which triggers a cascade that leads to the activation of target genes including Pthrp under a mechanism still poorly understood. Pthrp acts on its receptor to keep the chondrocyte proliferating and inhibits the production of Ihh. This Ihh/Pthrp negative-feedback loop controls the length of proliferating columns during cartilage growth (Kronenberg, 2003).
The intracellular factor Jab1 (c-Jun activation domain-binding protein-1) interacts with numerous proteins to regulate diverse signaling pathways and cell differentiation processes (Shackleford and Claret, 2010). Jab1 is also the fifth subunit of the evolutionarily conserved proteolysis regulator COP9 signalosome (Csn5) complex (Bech-Otschir et al., 2002; Olma et al., 2009). CSN is similar, both in size and composition, to the lid of the 19S proteasome regulatory particle (Wei and Deng, 2003). CSN has deneddylase activity to regulate the stability of complex Skip1–Cullin–F-box proteins (SCF), a major group of cullin ring E3 ubiquitin ligase (CRL). SCF core component cullin is covalently modified with the ubiquitin-like protein NEDD8. Repeated cycles of NEDD8 conjugation (neddylation) and NEDD8 removal (deneddylation) maintain SCF E3 ubiquitin ligase activity (Wei et al., 2008). By regulating SCF activity, CSN can regulate the stability and activity of numerous cell-cycle proteins such as p27, p53 and transcriptional regulators including IκB-α (Wei et al., 2008; Chamovitz, 2009). Within the whole COP9 signalosome, Jab1/Csn5 is the only subunit possessing a catalytic metal binding metalloprotease motif that is required for removing NEDD8 from the cullin subunit of SCF (Shackleford and Claret, 2010). Thus, Jab1 is required for COP9 signalosome activity.
Jab1 is involved in signaling transduction, cell cycle control, apoptosis, DNA repair, and a vast array of developmental processes by regulating the stability and activity of various transcription factors (Wei et al., 2008; Chamovitz, 2009; Kato and Yoneda-Kato, 2009; Shackleford and Claret, 2010). Furthermore, Jab1 overexpression has been implicated in the initiation and progression of various cancers, suggesting an oncogenic role (Shackleford and Claret, 2010). The constitutive deletion of Jab1 in mice results in early embryonic lethality by E8.5 with impaired proliferation and accelerated apoptosis (Tomoda et al., 2004; Tian et al., 2010), whereas cell type-specific deletions of Jab1 in T-cell, B-cell, or myeloid cells all lead to severe postnatal cell differentiation defects and increased apoptosis (Panattoni et al., 2008; Deng et al., 2011; Sitte et al., 2012). Thus, Jab1 plays essential roles both in general embryogenesis and in the differentiation of specific organs and tissues. However, the specific function of Jab1 in skeletogenesis in vivo was completely unknown prior to this study.
Bone morphogenetic protein (BMP) signaling coordinates all steps of skeletal growth and differentiation during endochondral ossification (Chen et al., 2004; Yoon and Lyons, 2004; Wan and Cao, 2005). BMPs are members of the TGF-β superfamily that activate heterodimeric receptors with serine/threonine kinase activity (Chen et al., 2004). The canonical BMP signaling intracellular effectors Smad1, 5, and 8 are phosphorylated upon various BMP ligands binding to the BMP receptor complex, then dimerize with the coactivator Smad4, translocate to the nucleus, and modulate the expression of target genes (Ross and Hill, 2008). BMP signaling is exquisitely regulated at the levels of ligands, receptors, antagonists, Smads, and a wide variety of Smad-interacting proteins. This produces a large diversity in transcriptional outputs to exert the precise cell context-dependent control during development (Wharton and Derynck, 2009). Mutations in BMP signaling components, including type I BMP receptors BMPR1B and ACVR1, antagonist NOGGIN, and ligands GDF3 and GDF6, lead to skeletal developmental defects such as brachydactyly, fibrodysplasia ossificans progressiva (FOP), chondrodysplasia, and oculo-skeletal anomalies (Asai-Coakwell et al., 2009; Serra and Chang, 2003; Shore et al., 2006; Ye et al., 2010). Mouse genetic studies reveal that various BMP signaling components regulate the critical Ihh-Pthrp feedback loop to promote chondrocyte proliferation, survival, and hypertrophy (Retting et al., 2009). Interestingly, within cartilage, the expression of some key BMP signaling components is at higher levels in prehypertrophic and hypertrophic chondrocytes than in proliferating chondrocytes (Shu et al., 2011). BMP signaling directly increases the expression of Ihh by prehypertrophic chondrocytes to increase chondrocyte proliferation and to promote chondrocyte hypertrophy (Minina et al., 2002; Retting et al., 2009). Furthermore, the combined loss of Smad1, 5 and 8 specifically in chondrocytes results in severe chondrodysplasia with reduced Col10a1 expression, a phenotype very similar to chondrocyte-specific BMP receptors null mice (Retting et al., 2009). Thus, the effect of BMP signaling in embryonic cartilage formation largely depends on Smad 1/5/8. Further analysis reveals that Smad1 and Smad5 are positive and redundant regulators of chondrocyte differentiation, whereas Smad8 is mostly dispensable for cartilage formation (Retting et al., 2009).
The effect of BMP signaling during skeletogenesis ultimately impinges on a complex transcriptional network in which the transcription factors Sox9 and Runx2 play essential roles (Kronenberg, 2003). Runt domain transcription factor Runx2 regulates all the major genes expressed by osteoblasts in tissue culture (Ducy et al., 1997). Runx2 null mice display a complete lack of osteoblast differentiation (Komori et al., 1997; Otto et al., 1997). Moreover, mutations in RUNX2 result in cleidocranial dysplasia (CCD), a dominantly inherited skeletal dysplasia with generalized bone defects (Lee et al., 1997; Mundlos et al., 1997; Zhou et al., 1999). Besides its essential role in osteoblast differentiation, Runx2 is also important for chondrocyte maturation. Continuous expression of Runx2 in mouse proliferating chondrocytes accelerated the hypertrophy (Takeda et al., 2001; Ueta et al., 2001). Conversely, chondrocyte maturation was delayed in some skeletal elements in Runx2−/− mice (Inada et al., 1999; Kim et al., 1999). Runx2 directly binds to the conserved cis-elements in Col10a1 and Ihh promoters, and contributes to their activity in prehypertrophic and hypertrophic chondrocytes (Yoshida et al., 2004; Zheng et al., 2003). Moreover, Runx2 and related Runx3 play an essential and redundant role during chondrocyte hypertrophy. Runx2−/−; Runx3−/− mice show the complete absence of chondrocyte hypertrophy with reduced chondrocyte proliferation and reduced cell size in the embryonic limbs (Yoshida et al., 2004).
Notably, Jab1 can directly interact with BMP downstream effector Smad5 in immunoprecipitation, and overexpression of Jab1 resulted in an attenuation of BMP-dependent transcriptional responses in chondrocyte culture, suggesting that Jab1 might act as an inhibitor of BMP signaling (Haag and Aigner, 2006). Jab1 can also bind Runx3 and induce Runx3 degradation (Kim et al., 2009). However, almost all of these studies so far have been performed in vitro or in cell culture. Therefore, the physiological relevance of Jab1-BMP signaling interaction and the potential effect of Jab1 on Runx2 expression and activity during cartilage formation were unknown. The conditional deletion of Jab1 in chondrocytes and the analysis of its effect on BMP signaling and Runx2 during chondrocyte differentiation is the most straightforward approach to determine the role of Jab1 during cartilage formation. Hence, in this study, we ablated Jab1 specifically in chondrocytes in mice using the loxP/Cre system and unveiled the critical role of Jab1 in cartilage formation in vivo and the inhibitory effect of Jab1 on Runx2 and BMP signaling, the two key regulators of chondrocyte hypertrophy.
Results
Lethal chondrodysplasia of Jab1flox/flox; Col2a1-Cre mutant mice
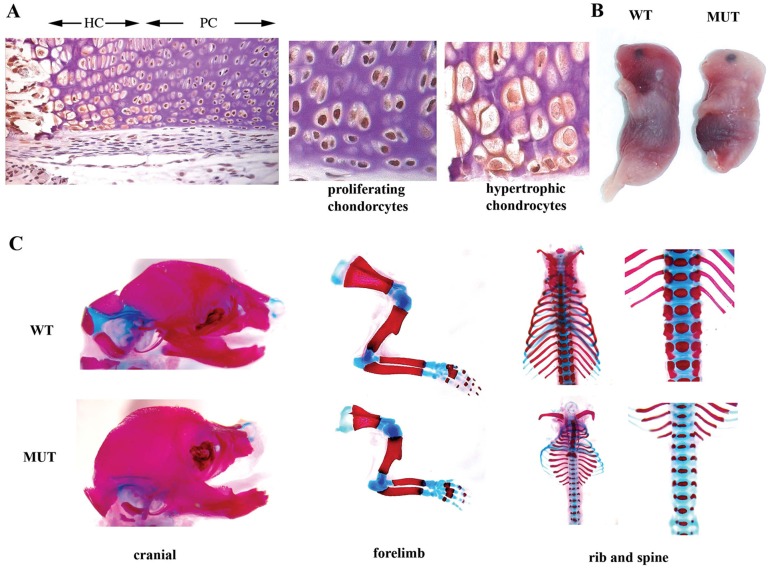
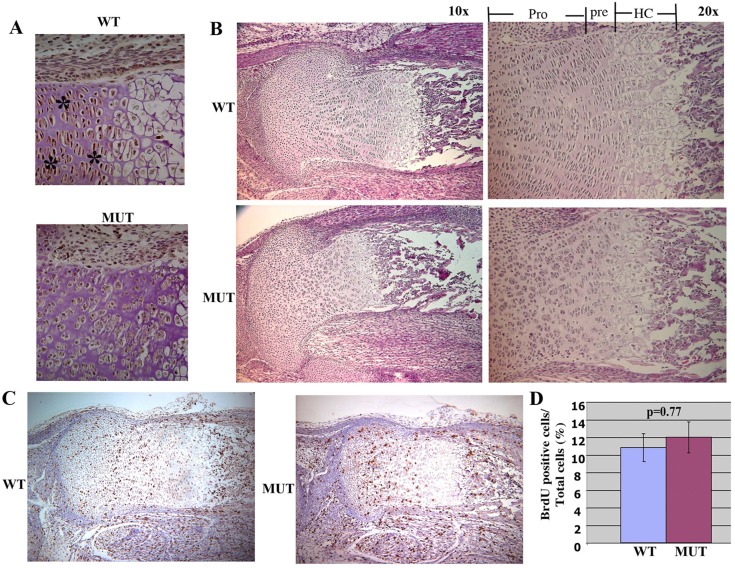
Jab1 is expressed broadly during mouse embryogenesis (Bounpheng et al., 2000; Carrabino et al., 2004). To study Jab1 protein expression in cartilage, we performed immunohistochemistry on E18.5 mouse embryonic sections with an anti-Jab1 antibody. The results show that Jab1 is widely expressed in musculoskeletal tissues, including in chondrocytes (Fig. 1A). Interestingly, Jab1 expression in hypertrophic chondrocytes appeared to be much lower than in proliferating chondrocytes (Fig. 1A). A previous report also described a much weaker JAB1 expression in the hypertrophic zone of human fetal cartilage (Haag and Aigner, 2006). Thus, the distinct Jab1 expression pattern in cartilage suggests that Jab1 might play a role in regulating chondrocyte hypertrophy progression. To circumvent the early embryonic lethality in constitutive Jab1-deficient mice (Tomoda et al., 2004; Tian et al., 2010), we crossed Jab1flox/flox mice (Panattoni et al., 2008) with Col2a1-Cre transgenic mice to delete Jab1 specifically in chondrocytes. The Col2a1-Cre line drives Cre recombinase expression specifically at a high level to differentiating chondrocytes (Ovchinnikov et al., 2000) and has been applied successfully to study the function of various transcription factors in endochondral ossification, including Smad1/5/8 (Retting et al., 2009). While Jab1flox/+; Col2a1-Cre mice appeared grossly normal and were fertile, all Jab1flox/flox; Col2a1-Cre conditionally knockout (cKO) mutant mice died at birth from respiratory distress likely due to the restricted rib cages (Fig. 1B,C). The cKO mutant embryos displayed a very severe and generalized chondrodysplasia at E18.5 (Fig. 1B,C). The mutants featured round heads, short snouts, short trunks, and short limbs, as well as prominent abdomens and protruding tongues (Fig. 1B). Skeletal preparation of E18.5 mutant embryos by alcian blue and alizarin red staining showed that all the skeletal elements that developed via endochondral ossification, such as ribs, limbs, vertebrae, and some craniofacial elements, were much shorter and thinner than the controls (Fig. 1C). The immunostaining confirmed the deletion of Jab1 protein in Jab1 cKO cartilage (Fig. 2A). The histological analysis showed that Jab1flox/flox; Col2a1-Cre mutants had much smaller cartilage elements compared with wild type littermates at E18.5 (Fig. 2B). While wild-type controls had orderly columnar structures of proliferating, prehypertrophic, and hypertrophic chondrocytes, the cKO mutants had severely disorganized chondrocytes (Fig. 2B). Thus, Jab1 is essential for proper endochondral ossification in vivo.
Fig. 1.
Chondrocyte-specific deletion of Jab1 in mice leads to severe and generalized lethal chondrodysplasia. (A) Immunostaining reveals strong Jab1 expression in murine E18.5 femur. PC, proliferating chondrocytes; HC, hypertrophic chondrocytes. (B) Whole-mount Jab1flox/flox; Col2a1-Cre mutant embryos at E18.5. (C) Skeletal preparations with alcian blue staining of cartilage and alizarin red staining of bone show severe and generalized chondrodysplasia in E18.5 Jab1flox/flox; Col2a1-Cre mutants. MUT, Jab1 cKO mutants; WT, wild-type littermates.
Fig. 2.
Severely disorganized chondrocyte columns in Jab1flox/flox; Col2a1-Cre mutants. (A) Immunostaining shows strong Jab1 expression in E18.5 wild-type chondrocytes (indicated by asterisks), but not in Jab1flox/flox; Col2a1-Cre mutants (40×). (B) Hematoxylin and eosin staining of proximal tibia of E18.5 Jab1flox/flox; Col2a1-Cre (cKO) mutants. (C) BrdU labeling of the proximal tibia of E16.5 Jab1flox/flox; Col2a1-Cre mutants (10×). (D) Quantification of BrdU incorporation of E16.5 proximal tibia, showing normal chondrocyte proliferation at E16.5. n = 4 individual mice per group. MUT, Jab1 cKO mutants. WT, wild-type littermates. Pro, proliferating chondrocytes; Pre, prehypertrophic chondrocytes; HC, hypertrophic chondrocytes.
Increased apoptosis and altered cell cycle progression in Jab1flox/flox; Col2a1-Cre mutants
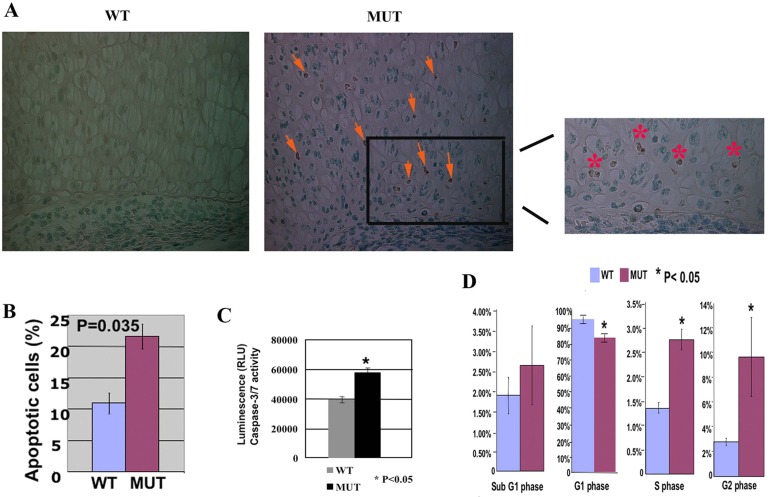
Cell proliferation analysis via BrdU labeling revealed no significant proliferation differences at E18.5 between Jab1 cKO mutants and controls (Fig. 2C,D). This is consistent with the notion that Jab1 is not essential for overall proliferation in differentiating cells (Panattoni et al., 2008). However, TUNEL labeling experiments showed more positive staining of apoptotic cells in Jab1 cKO mutant limbs than in control littermates at E18.5 (Fig. 3A). Annexin V-based apoptosis flow cytometry analysis confirmed a one-fold increase in the apoptosis rate in primary rib chondrocytes of Jab1 cKO mutants compared with control littermates at E18.5 (Fig. 3B). Moreover, Jab1 cKO chondrocytes had higher activity of caspase-3/7, a major facilitator of apoptosis (Fig. 3C). Jab1 can modulate specific steps of cell cycle progression in T cell differentiation (Panattoni et al., 2008). Our flow cytometry cell cycle analysis of primary chondrocytes showed that chondrocytes of Jab1 cKO mutants also accumulated at the G2 phase at higher rates than in the controls (Fig. 3D).
Fig. 3.
Increased apoptosis and abnormal cell cycle progression in Jab1flox/flox; Col2a1-Cre mutants. (A) TUNEL staining of apoptosis in E18.5 distal femur (40×). Significant apoptosis was detected in the mutant, whereas there was no detectable TUNEL staining in the wild-type control. Arrows point to the positive staining of apoptosis in proliferating chondrocytes. The right panel shows the enlarged image of the boxed area in the middle panel, with asterisks indicating the positive staining of apoptosis in the mutant. (B) Quantification of annexin V stained cells showed increased apoptosis in Jab1 cKO primary rib chondrocytes. (C) Increased caspase-3/7 activity in Jab1 cKO primary chondrocytes. E18.5 primary chondrocytes were treated with etoposide for 24 hours to induce apoptosis and then subjected to Caspase-Clo3/7 assay for quantification of caspase-3/7 activity as apoptosis index. (D) Quantification of flow cytometry indicates altered cell cycle progression with significant G2 phase arrest in Jab1 cKO chondrocytes. MUT, Jab1 cKO mutants. WT, wild-type littermates. n = 3 for each group.
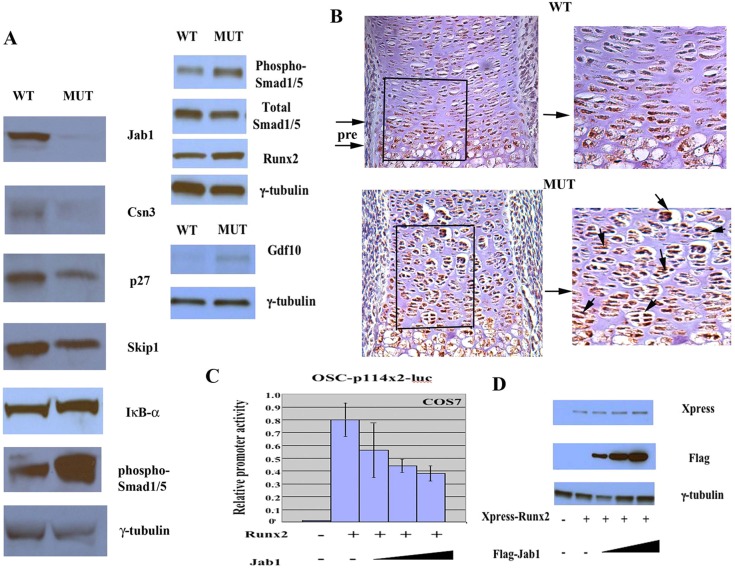
Next, we performed the western blot analysis of extracted proteins from primary chondrocytes to determine the effect of loss of Jab1 in chondrocytes on the protein stability of various putative Jab1 targets (Fig. 4A) (Shackleford and Claret, 2010). Interestingly, the expression of IkB-α, a major downstream target of Jab1 in T cell differentiation (Panattoni et al., 2008), was not significantly changed in the mutants. Thus, Jab1 is likely to have distinct downstream targets in different cell types during development. The expression of Csn3, another key component of COP9 signalosome, appeared to be downregulated, suggesting that Jab1 is essential for the integrity of COP9 holocomplex. The expressions of p27 and Skip1 were both decreased in Jab1 cKO chondrocytes. p27 is a key cell cyclin kinase inhibitor (Shackleford and Claret, 2010). Skip1, a S-phase kinase-associated protein, is an adaptor between cullin ring E3 ubiquitin ligases and COP9 signalosome and affects cell survival and cell cycle (Shackleford and Claret, 2010). The decreased expression of p27 and Skip1 correlated with altered cell cycle progression and increased apoptosis. Importantly, western blot analysis revealed increased phospho-Smad1/5 expression, but no drastic change in total Smad1/5 expression, in E18.5 Jab1 cKO mutant chondrocytes (Fig. 4A). This suggests that Jab1 might negatively regulate Smad1/5-mediated canonical BMP signaling during chondrocyte differentiation.
Fig. 4.
Jab1 is a negative regulator of chondrocyte hypertrophy. (A) Western blot analysis of Jab1flox/flox; Col2a1-Cre primary rib chondrocytes. (B) Immunohistochemical staining of Runx2 in distal tibia of E16.5 Jab1flox/flox; Col2a1-Cre mutants. Pre, prehypertrophic zone. The right panels are the enlarged images of the boxed areas in the left panels. There was strong ectopic Runx2 expression (brown staining indicated by arrows) in the mutant. MUT, Jab1flox/flox; Col2a1-Cre mutants; WT, wild-type littermates. (C) Jab1 represses Runx2 transcriptional activity in vitro. COS7 cells were transfected with the Runx2-responsive osteocalcin reporter OSC-p114x2-luc and expression plasmids for Runx2 (0.25 µg) and Jab1 in increasing amounts (0.25 µg, 0.5 µg and 1.0 µg). (D) Jab1 does not affect Runx2 stability in transient transfection. COS7 cells were co-transfected with 0.25 µg Xpress tagged-Runx2 expression plasmid and increasing amounts of Flag-tagged Jab1 expression plasmid (0.25 µg, 0.5 µg and 1.0 µg). Whole cell extract was collected 24 hours later and subjected to western blot analysis with indicated antibodies.
Increased chondrocyte hypertrophy in Jab1flox/flox; Col2a1-Cre mutant mice
Strikingly, at E16.5, the distinct and tightly packed layers of prehypertrophic chondrocytes in wild-type mice were already missing in Jab1 cKO mutants. Instead, there were disorganized and abnormally large clusters of chondrocytes in Jab1 cKO mutants (Fig. 4B). Furthermore, some of the abnormally large clusters of chondrocytes adjacent to hypertrophic chondrocytes expressed Runx2, a positive regulator of chondrocyte hypertrophy (Fig. 4B). Western blot analysis also revealed slightly increased Runx2 expression in Jab1 cKO chondrocytes (Fig. 4A). To test whether Jab1 has any direct effect on Runx2 transcriptional activity, Jab1 and Runx2 expression plasmids were co-transfected with an OSC-114x2-luc reporter into COS7 cells that do not express any Runx proteins (Ducy et al., 1999). OSC-114x2-luc contains two tandem repeats of a 114-bp osteocalcin enhancer fragment fused to the luciferase reporter gene (Zhou et al., 2006). The activity of this osteocalcin enhancer fragment is partly dependent on the presence of one Runx2-binding OSE2 element (Ducy and Karsenty, 1995; Zhou et al., 2006). While Runx2 alone activated the reporter significantly, the co-transfection of Jab1 decreased its activity by almost 50% (Fig. 4C). Therefore, Jab1 could be a negative regulator of Runx2 activity. However, increased Jab1 expression did not affect Runx2 protein stability in transient transfection (Fig. 4D). Therefore, the effect of Jab1 on Runx2 is likely to be context-dependent and remains to be further investigated. Interestingly, a recent report showed that Jab1 can also repress the transcriptional activity of highly related Runx3 in cell culture (Kim et al., 2009).
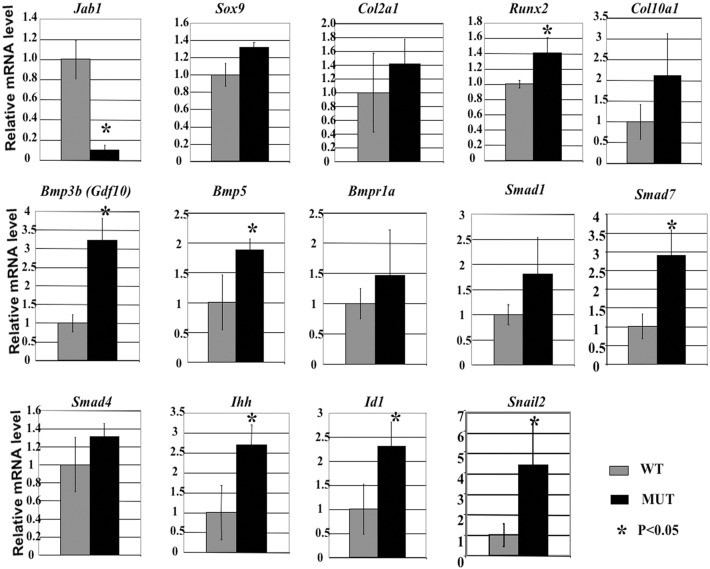
To investigate the consequence of the loss of Jab1 in differentiating chondrocytes at the transcriptional level, we performed real-time RT-PCR analysis in E18.5 Jab1 cKO and wild-type littermate chondrocytes, using an Osteogenesis PCR Array. PCR Array is a technology combining the profiling capability of microarray with real-time PCR performance. Primary rib chondrocytes from E18.5 Jab1 cKO mutants and wild-type littermates were collected and subjected to the analysis. Our results showed that while proliferating chondrocyte markers Col2a1 and Sox9 expressions were not significantly changed, chondrocyte hypertrophy-associated genes such as Col10a1, Runx2 were among the most severely dysregulated genes (Fig. 5). The expression of Runx2 was upregulated by 40% in mutant chondrocytes. Col10a1 is the most-specific hypertrophic chondrocyte marker (Zheng et al., 2003) and its expression was upregulated by one fold (Fig. 5). The changes in these genes suggested increased chondrocyte hypertrophy in Jab1 cKO mutants.
Fig. 5.
Increased BMP signaling and altered chondrocyte differentiation in Jab1flox/flox; Col2a1-Cre mutant chondrocytes. Real-time qualitative RT-PCR assay for BMP signaling components and chondrocyte differentiation markers in primary chondrocytes from E18.5 Jab1flox/flox; Col2a1-Cre mutants. The data represent the mean ± s.d. from 3–5 mice per genotype. MUT, Jab1flox/flox; Col2a1-Cre mutants; WT, wild-type littermates. The gene expression changes in some cases were not statistically significant with large SD, likely due to the heterogeneity of each mouse embryo sample and also the limited numbers of Jab1 cKO mutant samples analyzed.
Upregulated expression of BMP signaling components in Jab flox/flox; Col2a1-Cre mutant chondrocytes
Our PCR array analysis also revealed that the expression of some BMP signaling components was upregulated in Jab1 cKO chondrocytes, including Bmp3b (also named Gdf10), Bmp5, Bmpr1a, Smad1, Smad4 and Smad7 (Fig. 5). Western blot analysis also showed increased Gdf10 protein expression in Jab1 cKO chondrocytes (Fig. 4A). To investigate the effect of Jab1 on BMP downstream targets in endochondral ossification, we performed real-time quantitative RT-PCR to study the changes in direct BMP target genes Ihh, Id1 and Snail 2 during chondrocyte hypertrophy. Indeed, the expressions of all three were significantly upregulated in the mutants (Fig. 5). Thus, Jab1 deficiency likely leads to dysregulated BMP signaling and accelerated hypertrophy in chondrocytes. This indicates that Jab1 might repress BMP signaling during chondrocyte differentiation. Interestingly, when wild-type chondrocytes were treated with BMP7, they exhibited similar cellular phenotypes as those of Jab1 cKO cells, namely significantly increased apoptosis and G2 arrest of the cell cycle (supplementary material Figs S1, S3). Furthermore, cell cycle analysis by flow cytometry revealed significant differences, in the G1, S and G2 phases of the cell cycle, between BMP7-treated chondrocytes versus small molecule BMP inhibitor LDN-193189-treated wild-type primary chondrocytes (supplementary material Fig. S2). Thus, BMP signaling has to be tightly regulated during chondrocyte differentiation to ensure proper cell cycle progression and survival.
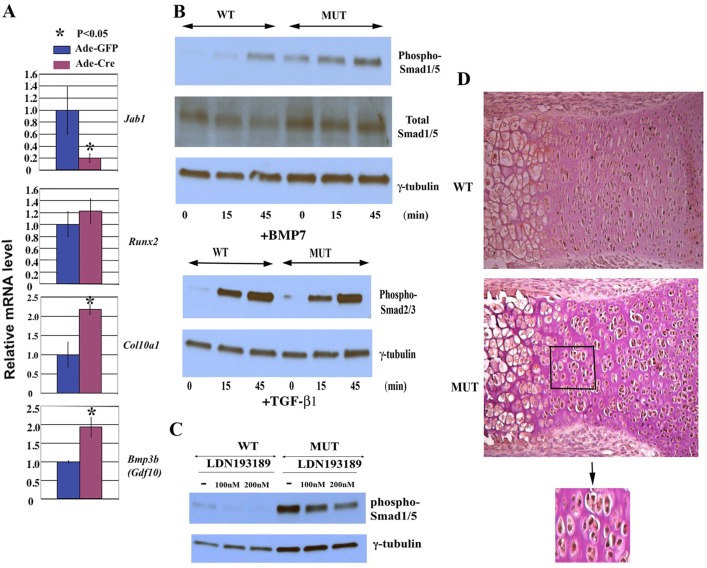
To determine whether the increase in chondrocyte hypertrophic markers and BMP signaling components is due to the cell autonomous effect of Jab1 inactivation in chondrocytes, we isolated primary rib chondrocytes from Jab1flox/flox mice. The cells were infected with adenovirus expressing Cre recombinase (Ade-Cre) or a control virus expressing GFP (Ade-GFP). Real-time RT-PCR analysis revealed a significant increase in Col10a1 and Bmp3b/Gdf10 expression in cells infected with Ade-Cre (Fig. 6A). Thus the inhibitory effect of Jab1 on chondrocyte hypertrophy and BMP signaling is likely to be cell autonomous.
Fig. 6.
Upregulated BMP signaling in Jab1-deficient chondrocytes. (A) Real-time RT-PCR analysis showed increased Runx2, Col10a1 and Bmp3b/Gdf10 expression in Jab1flox/flox primary rib chondrocytes infected with Ade-Cre in comparison with those infected with Ade-GFP. (B) Increased response to BMP signaling, but not TGF-β signaling, in Jab1flox/flox; Col2a1-Cre mutants. Primary chondrocytes were serum-starved for 12 hours before BMP7 stimulation (100 ng/ml) (top panel) or TGF-β1 (2 ng/ml) (lower panel) for the indicated times, and then subjected to immunoblotting as indicated. (C) The small molecule BMP inhibitor LDN-193189 can partially block the increased BMP signaling in Jab1flox/flox; Col2a1-Cre mutants in a dose-dependent manner. Primary chondrocytes were cultured in DMEM medium containing 10% FBS and indicated amounts of LDN-193189 for 24 hours before being subjected to immunoblotting. (D) Increased immunostaining of phospho-Smad1/5 in E17.5 proximal tibia of the Jab1flox/flox; Col2a1-Cre mutant (20×). Bottom panel, enlarged image of the boxed area in the middle panel. MUT, Jab1flox/flox; Col2a1-Cre mutants; WT, wild-type littermates.
Enhanced BMP signaling response in Jab1 flox/flox; Col2a1-Cre mutant chondrocytes
Upon binding of BMP ligands, BMP receptors phosphorylate Smad1/5 to initiate downstream events. Notably, western blot analysis of extracted proteins from primary chondrocytes revealed increased phospho-Smad1/5 expression in E18.5 Jab1 cKO mutants (Fig. 4A). To further analyze the BMP signaling response in cKO mutant chondrocytes, cultured primary chondrocytes were serum-starved for 12 hours, then stimulated by recombinant BMP-7 for 15 and 45 minutes, respectively. The cell extracts were then subjected to immunoblotting with an anti-phospho (activated)-Smad1/5 antibody. Indeed, Jab1 cKO mutant chondrocytes had upregulated expression of phospho-Smad1/5 compared with wild-type controls, both before and after exogenous BMP7 treatment (Fig. 6B, top panel). This suggests that Jab1flox/flox; Col2a1-Cre mutant chondrocytes have an increased canonical BMP signaling response.
Jab1 was previously reported to interact with coactivator Smad4 and inhibitory Smad7, two key modulators of TGF-β signaling (Wan et al., 2002; Kim et al., 2004). To determine the status of the response of closely related TGF-β signaling in Jab1 cKO mutants, cultured primary chondrocytes were serum-starved for 12 hours, treated with TGF-β1 for 15 and 45 minutes, respectively, and then were subjected to immunoblotting with an antibody against phospho (activated)-Smad2/3 antibody, the key downstream effector of TGF-β signaling (Ross and Hill, 2008). In contrast to the altered phospho-Smad1/5 expression, there was no drastic phospho-Smad2/3 expression change in the Jab1 cKO mutants versus the controls (Fig. 6B, lower panel). This indicates that Jab1 cKO mutant chondrocytes have no gross change in TGF-β signaling response in culture.
To determine the underlying mechanism of enhanced BMP signaling in Jab1 cKO chondrocytes, we treated primary chondrocytes with the small molecule inhibitor LDN-193189. As a selective inhibitor of BMP type I receptor kinases, LDN-193189 can potently inhibit BMP signaling response such as phospho-Smad1/5 expression, but not TGF-β signaling response in cell culture (Yu et al., 2008). Moreover, LDN-193189 can reduce heterotopic ossification in a mouse model of fibrodysplasia ossificans progressiva (FOP) (Yu et al., 2008). Indeed, LDN-193189 can partially reverse the increased phospho-Smad1/5 expression in Jab1flox/flox; Col2a1-Cre mutant chondrocytes in a dose-dependent manner (Fig. 6C). In light of the enhanced expression of BMP signaling components including Gdf10/Bmp3b, Bmp5 and Bmpr1a (Fig. 5), the upregulated BMP signaling in Jab1 cKO mice is likely to be in part due to increased BMP ligands and/or enhanced BMP receptor activity. Furthermore, LDN-193189 reduced the caspase-3/7 activity in primary Jab1flox/flox chondrocytes infected with adenovirus expressing Cre recombinase (Ade-Cre), but not in Jab1flox/flox chondrocytes infected with a control virus expressing GFP (Ade-GFP) (supplementary material Fig. S3). Thus, increased apoptosis in Jab1-deficient chondrocytes might be due in part to upregulated BMP signaling activity.
Consistent with the above ex vivo culture experiments, immunostaining of phospho-Smad1/5 showed that phospho-Smad1/5 protein expression was upregulated in the long bones of Jab1 cKO embryos at E17.5 compared with wild-type littermates (Fig. 6D). In the wild-type cartilage, phospho-Smad1/5 expression was mainly restricted to proliferating and prehypertrophic chondrocytes as previously reported (Retting et al., 2009). In Jab1 cKO mutants, phospho-Smad1/5 expression appeared to be more intense, especially in the clusters of chondrocytes of abnormally large size adjacent to the hypertrophic zone (Fig. 6D).
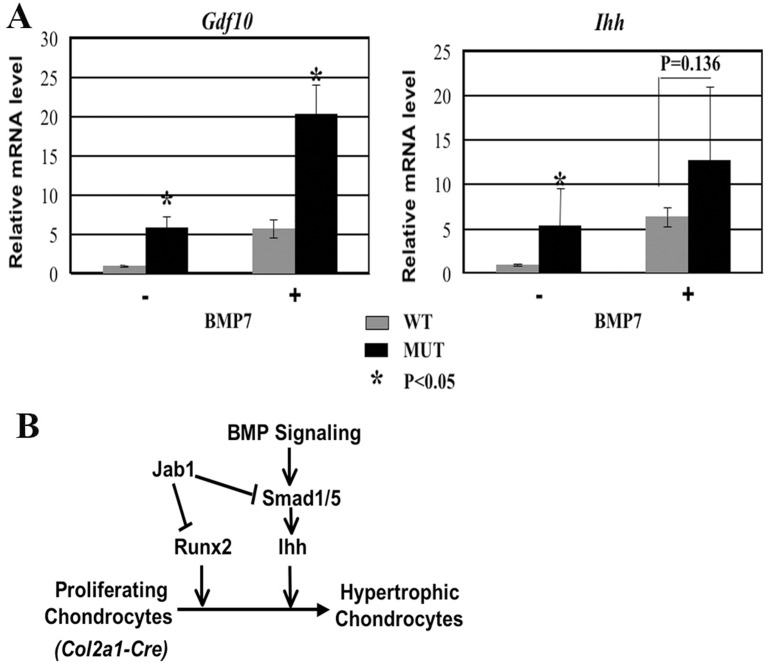
Lastly, we performed real-time RT-PCR analysis to determine whether Jab1 cKO chondrocytes have a more robust response to BMP ligand stimulation. Indeed, both before and after BMP7 stimulation, cultured Jab1 cKO mutant primary chondrocytes had increased expression of BMP signaling component Gdf10 and increased expression of the marker of prehypertrophic chondrocytes, Ihh (Fig. 7A). In light of the established role of canonical BMP signaling and Runx2 in promoting chondrocyte hypertrophy in part by upregulating Ihh expression, our studies collectively suggest that Jab1 negatively regulates chondrocyte hypertrophy in part by specifically inhibiting canonical BMP signaling and possibly downregulating Runx2 activity (Fig. 7B).
Fig. 7.
Upregulated BMP signaling response in Jab1flox/flox; Col2a1-Cre mutant chondrocytes. (A) Increased BMP signaling response in Jab1 cKO chondrocytes. E18.5 primary chondrocytes were cultured for 2 days, serum-starved overnight, and then treated with BMP7 (100 ng/ml) for 7 hours before being subjected to real-time RT-PCR analysis. n = 3 per group. (B) Proposed model for the integration of Jab1, Runx2 and BMP signaling during chondrocyte differentiation.
Discussion
Our study is the first one to demonstrate the essential and novel roles of Jab1 in chondrocyte differentiation, especially its effect on BMP signaling and Runx2 activity in cartilage formation (Fig. 7B). We report here that the chondrocyte-specific deletion of Jab1 in mice led to neonatal lethal chondrodysplasia with severe dwarfism and disorganized chondrocyte columns (Figs 1, 2). Canonical BMP signaling, through the phosphorylation of its effectors Smad1/5 proteins and the subsequent activation of downstream targets, regulates the critical Ihh-Pthrp feedback loop to promote chondrocyte proliferation, survival, and hypertrophy (Retting et al., 2009; Song et al., 2009). A very recent mouse genetic study revealed that Smad6, an inhibitory Smad of BMP signaling, represses chondrocyte hypertrophy (Estrada et al., 2011), reconfirming that BMP signaling has to be finely tuned and tightly controlled during development to ensure proper cartilage formation and growth. Our study is the first one to investigate the specific effect of Jab1 on the canonical BMP signaling in vivo. Our results demonstrate that Jab1 is likely to be a novel inhibitor of canonical BMP signaling in chondrocytes based on the following observations: (1) Gene expression profiles reveal heightened expression of BMP ligands (Gdf10, BMP5) and BMP targets and chondrocyte differentiation markers (Ihh, Snail2) in Jab1 cKO chondrocytes; (2) The expression of BMP downstream effector phospho- (activated form) Smad1/5 is upregulated in Jab1 cKO cartilage; and (3) Jab1 cKO primary chondrocytes exhibit an enhanced response to exogenous BMP treatment. Jab1 was previously reported to directly bind to Smad5 to negatively regulate BMP signaling in human chondrocyte culture, although the mechanism was not entirely clear (Haag and Aigner, 2006). Conversely, our results consistently showed that BMP signaling was upregulated in Jab1 cKO cartilage.
The mechanism of Jab1-mediated BMP signaling inhibition during chondrocyte differentiation is likely to be complex and multi-layered. The upregulated BMP signaling may be in part due to the increased expression of BMP ligands and/or enhanced BMP receptor activity since a small molecule BMP inhibitor LDN-193189 can partially reversed enhanced phospho-Smad1/5 expression (Fig. 6C). Jab1 can modulate TGF-β/BMP signaling by direct interaction with various Smads. Jab1 binds Smad4 and induces its ubiquitylation for degradation. Ectopic expression of Jab1 decreased endogenous Smad4 steady levels and inhibited TGF-β-induced gene transcription (Wan et al., 2002). On the other hand, Jab1 can associate constitutively with inhibitory Smad7 (Kim et al., 2004). Overexpression of Jab1 promotes Smad7 degradation and enhances TGF-β-mediated transcriptional activity. These conflicting results are likely due to the different cells used in the studies and may very likely also reflect the complex role of Jab1 in TGF-β/BMP signaling. Thus, it will be important to study the interaction between various Smads and Jab1 in diverse cell type differentiation.
Transcription factor Runx2 is essential for chondrocyte maturation (Schroeder et al., 2005). In our study, Jab1 deficiency in differentiating chondrocytes resulted in increased Runx2 expression at both the mRNA and the protein levels (Fig. 4A, Fig. 5), indicating that Jab1 might be a negative regulator of Runx2 during chondrocyte differentiation. Furthermore, Jab1 can repress Runx2 transcriptional activity (Fig. 4C). This suggests that accelerated chondrocyte hypertrophy in Jab1 cKO mutants might in part be Runx2-mediated, and it will be critical to further elucidate the interaction between Jab1 and Runx2 during chondrocyte differentiation.
Jab1 cKO mutant chondrocytes exhibit accelerated apoptosis and G2 cell cycle arrest (Fig. 3). These defects might cause, at least in part, the shorter and smaller cartilage in Jab1 cKO mutants (Fig. 2A). Jab1-containing CSN complex affects the DNA-damage checkpoint control pathway at multiple points (Wei et al., 2008; Shackleford and Claret, 2010). Interestingly, when Jab1 was conditionally knocked out in T cells in mice, the Jab-null T cells also displayed reduced survival and delayed cell cycle progression at G2-M stage (Panattoni et al., 2008). The G2 arrest in Jab1 cKO cells might be associated with a DNA damage-repair defect since Jab1 is reported to interact with the key DNA repair Rad9-Rad1-Hus1 (9-1-1) complex and other DNA repair machinery (Huang et al., 2007). Furthermore, the loss of Jab1 in mouse embryonic cells and osteosarcoma cells is associated with an upregulated p53-dependent DNA double-break repair capability in those cells (Tian et al., 2010). Thus, it will be interesting to investigate the status of the DNA repair apparatus in Jab1 cKO mutant chondrocytes in the future.
In summary, our study provides novel insights into the critical regulation of skeletogenesis by Jab1 during mouse development. BMP signaling is important for all aspects of skeletal formation and regeneration, including fracture healing and articular cartilage repair. BMP2 and BMP7 have been used for bone regeneration in over one million patients worldwide for long-bone non-unions and acute fractures, and for improving the success of spinal fusions. Our study here establishes that Jab1 is an essential factor for proper endochondral ossification in vivo, likely in part by inhibiting canonical BMP signaling (Fig. 7B). It will be important to further understand the underlying mechanism of Jab1 regulation of BMP signaling during skeletogenesis, which may lead to the development of novel therapies to treat fractures, osteoarthritis, and skeletal disorders associated with abnormalities in BMP signaling. Additionally, Jab1flox/flox; Col2a1-Cre mutant mice can serve as an excellent model to study the pathogenesis of human lethal chondrodysplasias. With the rapid advances in genomics tools, further study of the Jab1flox/flox; Col2a1-Cre mutant skeletal phenotype can facilitate the diagnosis and treatment of lethal chondrodysplasias that might be associated with dysregulated JAB1 expression or activity.
Materials and Methods
Mouse breeding and genotyping
All mice were maintained and housed at the Case Western Reserve University animal facility under standard conditions. All animal protocols have been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Case Western Reserve University. The Jab1 conditional allele Jab1flox/flox mice (Panattoni et al., 2008) were crossed with Col2a1-Cre transgenic mice (Ovchinnikov et al., 2000) to generate Jab1flox/flox; Col2a1-Cre conditional knockout (cKO) mutants. All mice were maintained on C57/BL6J background. All controls were Cre negative wild-type littermates of Jab1 cKO mutants. Mouse genotyping was performed by PCR using GoTaq Flexi DNA polymerase (Promega, Madison, WI, USA) with the following primer pairs: for floxed Jab1 allele, forward primer 5′-GGTCAGAAAGCTAGGCCTAAGAAGG-3′, reverse primer 5′-GGCATGCATCACCATTTTCAGTAG-3′ (wild-type allele, 350 bp; floxed Jab1 allele, 450 bp); for Col2a1-Cre transgene, forward primer 5′-TGCAACGAGTGATGAGGTTCG-3′, reverse primer 5′-CATGTTTAGCTGGCCCAAATGT-3′.
Embryo processing, BrdU labeling and immunohistochemistry
The noon of the plugging day was counted as E 0.5 for the embryo's age. Embryos were collected at indicated times. Mouse skeletal preparation was made with alcian blue staining of cartilage and alizarin red staining of bone as described (Zhou et al., 2006). The tissue sections were stained with hematoxylin and eosin for morphological analyses. For immunohistochemistry, the sections were first heated in a steamer for 10 minutes in 1×Antigen Unmasking Solution (Vector Laboratories, Burlingame, CA, USA). The endogenous peroxidase activity was quenched with 3% H2O2 for 10 minutes. After PBS rinsing, the sections were incubated in normal serum blocker buffer, which contained 3% normal serum, 0.1% BSA, and 0.1% Triton X-100 in PBS, for 20 minutes. The sections were then incubated in specific primary antibodies overnight at 4°C, followed by incubation with biotinylated secondary antibody for 1 hour at room temperature. Sections were subsequently treated with avidin-biotin-peroxidase complex (ABC) reagent for 1 hour at room temperature, followed by incubation with DAB peroxidase substrate solution (Vector Laboratories). Afterwards, sections were counterstained with hematoxylin QS nuclear counterstaining solution (Vector Laboratories), and covered with VectaMount Permanent Mounting Medium (Vector Laboratories). For bromodeoxyuridine (BrdU) labeling, the mice pregnant with E16.5 embryos were intraperitoneally injected with BrdU labeling reagent (Invitrogen, Carlsbad, CA, USA). 4 hours after injection, embryos were harvested, fixed in 10% formalin overnight, sectioned, and analyzed for BrdU incorporation with a BrdU staining kit (Invitrogen). All images were captured with a digital camera (DM500, Leica Microsystems, Buffalo Grove, IL, USA) using Leica Application Suite 1.3 software, under Leica DM6000B and DM IRB microscopes.
Apoptosis analysis by annexin V staining and TUNEL staining
Annexin V staining was performed using a Vybrant Apoptosis Assay Kit (#V13241, Invitrogen) according to the manufacturer's instruction. Cultured mouse rib chondrocytes were harvested, washed in cold PBS, and stained using Alexa Fluor 488-Annexin V and propidium iodide (PI) to identify apoptotic cells per the manufacturer's instruction. The stained cells were analyzed by flow cytometry, and apoptotic rate was determined by scoring apoptotic (Annexin V-FITC+, PI−) cells.
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining was performed with ApopTag Plus Peroxidase In Situ Apoptosis Detection Kit (Chemicon, Billerica, MA, USA) according to the manufacturer's instruction. The sections were pretreated in freshly diluted proteinase K (20 µg/ml) for 15 minutes at room temperature. Endogenous peroxidase was quenched in 3% hydrogen peroxide for 5 minutes at room temperature. The sections were then incubated with the TUNEL reaction mixture in a humidified chamber at 37°C for 1 hour. Anti-digoxigenin conjugate was applied on the sections, followed by incubation in a humidified chamber for 30 minutes at room temperature. Peroxidase substrate was then applied on the sections to develop signal. Afterwards the slides were counterstained with methyl green and mounted with VectaMount Permanent Mounting Medium (Vector Laboratories).
Caspase-Glo3/7 assay and MTT assay
Primary chondrocytes were seeded in a 24-well plate at a density of 25,000 per well. The next day, cells were treated with 100 ng/ml BMP7 or 200 nM BMP inhibitor LDN-193189 for 24 hours when indicated. Subsequently, 500 µM etoposide (Sigma, St Louis, MO) were added to induce apoptosis. Twenty-four hours later, apoptosis was measured with a luminescent Caspase-Glo3/7 assay according to the manufacturer's instructions (Promega, Madison, WI).
For the MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) assay to evaluate cell viability and proliferation, primary chondrocytes were seeded in a 24-well plate at a density of 25,000 per well. The next day, cells were treated with BMP7 or LDN-193189 when indicated. After another 48-hour culture, the MTT labeling reagent (#M6494, Invitrogen, CA) was added, and the spectrophotometric absorbance at 570 nm was measured with a microplate reader.
Transfection assay and cell cycle analysis with propidium iodide (PI)
Transient transfection assays in COS7 cells with Jab1 and Runx2 expression plasmids and OSC-p114x2-luc reporter plasmids were performed as described (Zhou et al., 1999). For cell cycle analysis, cultured primary rib chondrocytes from Jab1 cKO mutants and control littermates were washed in ice-cold PBS, fixed in 90% ethanol at −20°C overnight, resuspended in 20 µg/ml of RNase and incubated at 37°C for 30 minutes. Cells were then chilled on ice for 10 minutes, followed by incubation in 50 µg/ml of PI solution in PBS at 4°C for 60 minutes. Each sample was then passed through a cell strainer tube, and analyzed by FACS Calibur flow cytometer (BD Biosciences, San Jose, CA, USA) at Case Flow Cytometry Core Facility.
Real-time quantitative RT-PCR
Mouse primary rib chondrocytes were collected as described (Lefebvre et al., 1994) and cultured in DMEM medium containing 10% FBS, 2 mM L-glutamine, and 1% penicillin/streptomycin before RNA extraction. For adenovirus infection, primary rib chondrocytes of postnatal day 3 Jab1flox/flox mice were seeded in 12-well plates at a density of 50,000 cells/cm2 and infected with adenovirus expressing Cre (Ade-Cre) or green fluorescent protein (Ade-GFP) (Gene Transfer Vector Core, University of Iowa) at a multiplicity of infection of 500. RNA was extracted 5 days later for real-time RT-PCR analysis.
For real-time quantitative RT-PCR analysis, total RNA was extracted using TRIzol Reagent (Invitrogen) and PureLink RNA Mini Kit (Invitrogen) per manufacturer's instruction. RNA was reverse transcribed to first-strand cDNA using an iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA, USA). Real-time PCR was performed with the Applied Biosystems (Carlsbad, CA, USA) 7500 real-time PCR detection system, using an Osteogenesis PCR Array (#PAMM-026, SABiosciences, Valencia, CA, USA). For the genes that were not included in the array, Gdf10, Id1, Ihh, Jab1, Smad7, and Snail2, the Power SYBR Green PCR Master Mix (Applied Biosystems) was used for real-time RT-PCR as described (Liang et al., 2012) with gene-specific primers listed in supplementary material Table S1. Levels of gene expression were determined with the comparative cycle threshold (ΔΔCt) method. The mRNA level of each gene was normalized to the level of Gapdh mRNA, and the relative fold change of gene expression in Jab1 cKO mutant chondrocytes versus control littermate chondrocytes was presented with control values arbitrarily designated as 1. Each reaction was performed in triplicate and repeated on at least three independent samples per genotype to ensure the reproducibility of the data.
Western blotting
For western blotting (immunoblotting), total cellular protein was extracted from mouse primary rib chondrocytes in the lysis buffer containing 50 mM Tris–HCl (pH 8.0), 150 mM NaCl, 1% NP-40, 1 mM DTT, and 1 mM PMSF, supplemented with Halt Protease and Phosphatase Inhibitor Cocktail (Thermo Fisher Scientific Inc., Waltham, MA, USA). 10 µg of proteins were separated by 4–15% Tris–HCl SDS-PAGE, and transferred onto 0.2 µm PVDF nitrocellulose membrane (Bio-Rad). The membrane was then blotted with 5% non-fat dry milk buffered saline (pH 7.6) containing 0.1% Tween-20, followed by incubation in the primary antibodies for 2 hours at room temperature or overnight at 4°C. The membrane was then incubated with horseradish peroxidase-conjugated secondary antibody for 1 hour at room temperature. The signal was detected by ECL western blotting detection reagents (GE Healthcare Life Sciences, Piscataway, NJ, USA). Afterwards, the membrane was stripped of antibodies with Restore Plus Western Blot Stripping Buffer (Thermo Scientific) before being re-blotted with another primary antibody. For the analysis of BMP and TGF-β signaling response, cultured primary chondrocytes were serum-starved for 12 hours, then stimulated with BMP7 (100 ng/ml, Peprotech, Rocky Hill, NJ, USA) and TGF-β1(2 ng/ml, Peprotech) for indicated times respectively, followed by western blot analysis with an anti-phospho-Smad1/5 antibody and an anti-phospho-Smad2/3 antibody respectively. For the effect of BMP inhibitor LDN-193189 (Selleckchem, Houston, TX), primary rib chondrocytes were cultured in DMEM medium containing 10% FBS and indicated amounts of LDN-193189 for 24 hours before western blotting analysis. Primary antibodies for immunohistochemistry and western blotting in this study are listed in supplementary material Table S2.
Statistical analysis
Results were presented as mean ± standard deviations (SD). Statistical analysis was performed by Student's t-test to compare the differences between the Jab1 cKO mutant mice and the wild-type littermates (n = 3–5). P<0.05 is considered to be statistically significant.
Supplementary Material
Acknowledgments
We thank Dr Richard R. Behringer for Col2a1-Cre transgenic mice, Teresa Pizzuto for expert histology work, Dr Shunichi Murakami for critical reading of the manuscript and Valerie Schmedlen for editorial assistance. The Cytometry and Imaging Microscope Core Facility of the Case Comprehensive Cancer Center was supported by grant number P30 CA43703 from the National Institutes of Health.
Footnotes
Funding
This work is supported by the National Institutes of Health (NIH) [grant numbers R03-DE019190 to G.Z., R03-DE019190-1A1S1 to G.Z., T32 AR07505 to L.B.]; a pilot grant from The Cleveland Clinic Musculoskeletal Core Center [NIAMS Core Center grant number P30 AR-050953 to G.Z.]; and Case CCC Institutional Research grant [grant number 119999-IRG-91-022-18-IRG to G.Z.]. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.113795/-/DC1
References
- Asai–Coakwell M., French C. R., Ye M., Garcha K., Bigot K., Perera A. G., Staehling–Hampton K., Mema S. C., Chanda B., Mushegian A.et al. (2009). Incomplete penetrance and phenotypic variability characterize Gdf6-attributable oculo-skeletal phenotypes. Hum. Mol. Genet. 18, 1110–1121 10.1093/hmg/ddp008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bech–Otschir D., Seeger M., Dubiel W. (2002). The COP9 signalosome: at the interface between signal transduction and ubiquitin-dependent proteolysis. J. Cell Sci. 115, 467–473 [DOI] [PubMed] [Google Scholar]
- Bounpheng M. A., Melnikova I. N., Dodds S. G., Chen H., Copeland N. G., Gilbert D. J., Jenkins N. A., Christy B. A. (2000). Characterization of the mouse JAB1 cDNA and protein. Gene 242, 41–50 10.1016/S0378-1119(99)00525-9 [DOI] [PubMed] [Google Scholar]
- Carrabino S., Carminati E., Talarico D., Pardi R., Bianchi E. (2004). Expression pattern of the JAB1/CSN5 gene during murine embryogenesis: colocalization with NEDD8. Gene Expr. Patterns 4, 423–431 10.1016/j.modgep.2004.01.005 [DOI] [PubMed] [Google Scholar]
- Chamovitz D. A. (2009). Revisiting the COP9 signalosome as a transcriptional regulator. EMBO Rep. 10, 352–358 10.1038/embor.2009.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Zhao M., Mundy G. R. (2004). Bone morphogenetic proteins. Growth Factors 22, 233–241 10.1080/08977190412331279890 [DOI] [PubMed] [Google Scholar]
- Deng Z., Pardi R., Cheadle W., Xiang X., Zhang S., Shah S. V., Grizzle W., Miller D., Mountz J., Zhang H. G. (2011). Plant homologue constitutive photomorphogenesis 9 (COP9) signalosome subunit CSN5 regulates innate immune responses in macrophages. Blood 117, 4796–4804 10.1182/blood-2010-10-314526 [DOI] [PubMed] [Google Scholar]
- Ducy P., Karsenty G. (1995). Two distinct osteoblast-specific cis-acting elements control expression of a mouse osteocalcin gene. Mol. Cell. Biol. 15, 1858–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducy P., Zhang R., Geoffroy V., Ridall A. L., Karsenty G. (1997). Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89, 747–754 10.1016/S0092-8674(00)80257-3 [DOI] [PubMed] [Google Scholar]
- Ducy P., Starbuck M., Priemel M., Shen J., Pinero G., Geoffroy V., Amling M., Karsenty G. (1999). A Cbfa1-dependent genetic pathway controls bone formation beyond embryonic development. Genes Dev. 13, 1025–1036 10.1101/gad.13.8.1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada K. D., Retting K. N., Chin A. M., Lyons K. M. (2011). Smad6 is essential to limit BMP signaling during cartilage development. J. Bone Miner. Res. 26, 2498–2510 10.1002/jbmr.443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haag J., Aigner T. (2006). Jun activation domain-binding protein 1 binds Smad5 and inhibits bone morphogenetic protein signaling. Arthritis Rheum. 54, 3878–3884 10.1002/art.22261 [DOI] [PubMed] [Google Scholar]
- Huang J., Yuan H., Lu C., Liu X., Cao X., Wan M. (2007). Jab1 mediates protein degradation of the Rad9-Rad1-Hus1 checkpoint complex. J. Mol. Biol. 371, 514–527 10.1016/j.jmb.2007.05.095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada M., Yasui T., Nomura S., Miyake S., Deguchi K., Himeno M., Sato M., Yamagiwa H., Kimura T., Yasui N.et al. (1999). Maturational disturbance of chondrocytes in Cbfa1-deficient mice. Dev. Dyn. 214, 279–290 [DOI] [PubMed] [Google Scholar]
- Kato J. Y., Yoneda–Kato N. (2009). Mammalian COP9 signalosome. Genes Cells 14, 1209–1225 10.1111/j.1365-2443.2009.01349.x [DOI] [PubMed] [Google Scholar]
- Kim I. S., Otto F., Zabel B., Mundlos S. (1999). Regulation of chondrocyte differentiation by Cbfa1. Mech. Dev. 80, 159–170 10.1016/S0925-4773(98)00210-X [DOI] [PubMed] [Google Scholar]
- Kim B. C., Lee H. J., Park S. H., Lee S. R., Karpova T. S., McNally J. G., Felici A., Lee D. K., Kim S. J. (2004). Jab1/CSN5, a component of the COP9 signalosome, regulates transforming growth factor beta signaling by binding to Smad7 and promoting its degradation. Mol. Cell. Biol. 24, 2251–2262 10.1128/MCB.24.6.2251-2262.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. H., Choi J. K., Cinghu S., Jang J. W., Lee Y. S., Li Y. H., Goh Y. M., Chi X. Z., Lee K. S., Wee H.et al. (2009). Jab1/CSN5 induces the cytoplasmic localization and degradation of RUNX3. J. Cell. Biochem. 107, 557–565 10.1002/jcb.22157 [DOI] [PubMed] [Google Scholar]
- Komori T., Yagi H., Nomura S., Yamaguchi A., Sasaki K., Deguchi K., Shimizu Y., Bronson R. T., Gao Y. H., Inada M.et al. (1997). Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89, 755–764 10.1016/S0092-8674(00)80258-5 [DOI] [PubMed] [Google Scholar]
- Kronenberg H. M. (2003). Developmental regulation of the growth plate. Nature 423, 332–336 10.1038/nature01657 [DOI] [PubMed] [Google Scholar]
- Lee B., Thirunavukkarasu K., Zhou L., Pastore L., Baldini A., Hecht J., Geoffroy V., Ducy P., Karsenty G. (1997). Missense mutations abolishing DNA binding of the osteoblast-specific transcription factor OSF2/CBFA1 in cleidocranial dysplasia. Nat. Genet. 16, 307–310 10.1038/ng0797-307 [DOI] [PubMed] [Google Scholar]
- Lefebvre V., Garofalo S., Zhou G., Metsäranta M., Vuorio E., De Crombrugghe B. (1994). Characterization of primary cultures of chondrocytes from type II collagen/beta-galactosidase transgenic mice. Matrix Biol. 14, 329–335 10.1016/0945-053X(94)90199-6 [DOI] [PubMed] [Google Scholar]
- Liang B., Cotter M. M., Chen D., Hernandez C. J., Zhou G. (2012). Ectopic expression of SOX9 in osteoblasts alters bone mechanical properties. Calcif. Tissue Int. 90, 76–89 10.1007/s00223-011-9550-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minina E., Kreschel C., Naski M. C., Ornitz D. M., Vortkamp A. (2002). Interaction of FGF, Ihh/Pthlh, and BMP signaling integrates chondrocyte proliferation and hypertrophic differentiation. Dev. Cell 3, 439–449 10.1016/S1534-5807(02)00261-7 [DOI] [PubMed] [Google Scholar]
- Mundlos S., Otto F., Mundlos C., Mulliken J. B., Aylsworth A. S., Albright S., Lindhout D., Cole W. G., Henn W., Knoll J. H.et al. (1997). Mutations involving the transcription factor CBFA1 cause cleidocranial dysplasia. Cell 89, 773–779 10.1016/S0092-8674(00)80260-3 [DOI] [PubMed] [Google Scholar]
- Olma M. H., Roy M., Le Bihan T., Sumara I., Maerki S., Larsen B., Quadroni M., Peter M., Tyers M., Pintard L. (2009). An interaction network of the mammalian COP9 signalosome identifies Dda1 as a core subunit of multiple Cul4-based E3 ligases. J. Cell Sci. 122, 1035–1044 10.1242/jcs.043539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto F., Thornell A. P., Crompton T., Denzel A., Gilmour K. C., Rosewell I. R., Stamp G. W., Beddington R. S., Mundlos S., Olsen B. R.et al. (1997). Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 89, 765–771 10.1016/S0092-8674(00)80259-7 [DOI] [PubMed] [Google Scholar]
- Ovchinnikov D. A., Deng J. M., Ogunrinu G., Behringer R. R. (2000). Col2a1-directed expression of Cre recombinase in differentiating chondrocytes in transgenic mice. Genesis 26, 145–146 [DOI] [PubMed] [Google Scholar]
- Panattoni M., Sanvito F., Basso V., Doglioni C., Casorati G., Montini E., Bender J. R., Mondino A., Pardi R. (2008). Targeted inactivation of the COP9 signalosome impairs multiple stages of T cell development. J. Exp. Med. 205, 465–477 10.1084/jem.20070725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retting K. N., Song B., Yoon B. S., Lyons K. M. (2009). BMP canonical Smad signaling through Smad1 and Smad5 is required for endochondral bone formation. Development 136, 1093–1104 10.1242/dev.029926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S., Hill C. S. (2008). How the Smads regulate transcription. Int. J. Biochem. Cell Biol. 40, 383–408 10.1016/j.biocel.2007.09.006 [DOI] [PubMed] [Google Scholar]
- Schroeder T. M., Jensen E. D., Westendorf J. J. (2005). Runx2: a master organizer of gene transcription in developing and maturing osteoblasts. Birth Defects Res. C Embryo Today 75, 213–225 10.1002/bdrc.20043 [DOI] [PubMed] [Google Scholar]
- Serra R., Chang C. (2003). TGF-beta signaling in human skeletal and patterning disorders. Birth Defects Res. C Embryo Today 69, 333–351 10.1002/bdrc.10023 [DOI] [PubMed] [Google Scholar]
- Shackleford T. J., Claret F. X. (2010). JAB1/CSN5: a new player in cell cycle control and cancer. Cell Div. 5, 26 10.1186/1747-1028-5-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore E. M., Xu M., Feldman G. J., Fenstermacher D. A., Cho T. J., Choi I. H., Connor J. M., Delai P., Glaser D. L., LeMerrer M.et al. (2006). A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat. Genet. 38, 525–527 10.1038/ng1783 [DOI] [PubMed] [Google Scholar]
- Shu B., Zhang M., Xie R., Wang M., Jin H., Hou W., Tang D., Harris S. E., Mishina Y., O'Keefe R. J.et al. (2011). BMP2, but not BMP4, is crucial for chondrocyte proliferation and maturation during endochondral bone development. J. Cell Sci. 124, 3428–3440 10.1242/jcs.083659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitte S., Gläsner J., Jellusova J., Weisel F., Panattoni M., Pardi R., Gessner A. (2012). JAB1 is essential for B cell development and germinal center formation and inversely regulates Fas ligand and Bcl6 expression. J. Immunol. 188, 2677–2686 10.4049/jimmunol.1101455 [DOI] [PubMed] [Google Scholar]
- Song B., Estrada K. D., Lyons K. M. (2009). Smad signaling in skeletal development and regeneration. Cytokine Growth Factor Rev. 20, 379–388 10.1016/j.cytogfr.2009.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S., Bonnamy J. P., Owen M. J., Ducy P., Karsenty G. (2001). Continuous expression of Cbfa1 in nonhypertrophic chondrocytes uncovers its ability to induce hypertrophic chondrocyte differentiation and partially rescues Cbfa1-deficient mice. Genes Dev. 15, 467–481 10.1101/gad.845101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L., Peng G., Parant J. M., Leventaki V., Drakos E., Zhang Q., Parker–Thornburg J., Shackleford T. J., Dai H., Lin S. Y.et al. (2010). Essential roles of Jab1 in cell survival, spontaneous DNA damage and DNA repair. Oncogene 29, 6125–6137 10.1038/onc.2010.345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoda K., Yoneda–Kato N., Fukumoto A., Yamanaka S., Kato J. Y. (2004). Multiple functions of Jab1 are required for early embryonic development and growth potential in mice. J. Biol. Chem. 279, 43013–43018 10.1074/jbc.M406559200 [DOI] [PubMed] [Google Scholar]
- Ueta C., Iwamoto M., Kanatani N., Yoshida C., Liu Y., Enomoto–Iwamoto M., Ohmori T., Enomoto H., Nakata K., Takada K.et al. (2001). Skeletal malformations caused by overexpression of Cbfa1 or its dominant negative form in chondrocytes. J. Cell Biol. 153, 87–100 10.1083/jcb.153.1.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan M., Cao X. (2005). BMP signaling in skeletal development. Biochem. Biophys. Res. Commun. 328, 651–657 10.1016/j.bbrc.2004.11.067 [DOI] [PubMed] [Google Scholar]
- Wan M., Cao X., Wu Y., Bai S., Wu L., Shi X., Wang N., Cao X. (2002). Jab1 antagonizes TGF-beta signaling by inducing Smad4 degradation. EMBO Rep. 3, 171–176 10.1093/embo-reports/kvf024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei N., Deng X. W. (2003). The COP9 signalosome. Annu. Rev. Cell Dev. Biol. 19, 261–286 10.1146/annurev.cellbio.19.111301.112449 [DOI] [PubMed] [Google Scholar]
- Wei N., Serino G., Deng X. W. (2008). The COP9 signalosome: more than a protease. Trends Biochem. Sci. 33, 592–600 10.1016/j.tibs.2008.09.004 [DOI] [PubMed] [Google Scholar]
- Wharton K., Derynck R. (2009). TGFbeta family signaling: novel insights in development and disease. Development 136, 3691–3697 10.1242/dev.040584 [DOI] [PubMed] [Google Scholar]
- Ye M., Berry–Wynne K. M., Asai–Coakwell M., Sundaresan P., Footz T., French C. R., Abitbol M., Fleisch V. C., Corbett N., Allison W. T.et al. (2010). Mutation of the bone morphogenetic protein GDF3 causes ocular and skeletal anomalies. Hum. Mol. Genet. 19, 287–298 10.1093/hmg/ddp496 [DOI] [PubMed] [Google Scholar]
- Yoon B. S., Lyons K. M. (2004). Multiple functions of BMPs in chondrogenesis. J. Cell. Biochem. 93, 93–103 10.1002/jcb.20211 [DOI] [PubMed] [Google Scholar]
- Yoshida C. A., Yamamoto H., Fujita T., Furuichi T., Ito K., Inoue K., Yamana K., Zanma A., Takada K., Ito Y.et al. (2004). Runx2 and Runx3 are essential for chondrocyte maturation, and Runx2 regulates limb growth through induction of Indian hedgehog. Genes Dev. 18, 952–963 10.1101/gad.1174704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P. B., Deng D. Y., Lai C. S., Hong C. C., Cuny G. D., Bouxsein M. L., Hong D. W., McManus P. M., Katagiri T., Sachidanandan C.et al. (2008). BMP type I receptor inhibition reduces heterotopic [corrected] ossification. Nat. Med. 14, 1363–1369 10.1038/nm.1888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q., Zhou G., Morello R., Chen Y., Garcia–Rojas X., Lee B. (2003). Type X collagen gene regulation by Runx2 contributes directly to its hypertrophic chondrocyte-specific expression in vivo. J. Cell Biol. 162, 833–842 10.1083/jcb.200211089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G., Chen Y., Zhou L., Thirunavukkarasu K., Hecht J., Chitayat D., Gelb B. D., Pirinen S., Berry S. A., Greenberg C. R.et al. (1999). CBFA1 mutation analysis and functional correlation with phenotypic variability in cleidocranial dysplasia. Hum. Mol. Genet. 8, 2311–2316 10.1093/hmg/8.12.2311 [DOI] [PubMed] [Google Scholar]
- Zhou G., Zheng Q., Engin F., Munivez E., Chen Y., Sebald E., Krakow D., Lee B. (2006). Dominance of SOX9 function over RUNX2 during skeletogenesis. Proc. Natl. Acad. Sci. USA 103, 19004–19009 10.1073/pnas.0605170103 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.