Abstract
Background. Because many human papillomavirus (HPV) infections are transient, rates of transmission may be miscalculated if the interval between testing spans several months. We examined rates of concordance and transmission in heterosexual couples over short intervals.
Methods. Twenty-five adult couples were enrolled and sampled for HPV DNA from the genitals, hand, and mouth 5 times over a 6-week period, including 24 hours after sexual intercourse and after 48 hours of abstinence. Concordance and transmission patterns were described.
Results. Concordance between the couple's genital sites ranged from 64% to 95% for at least 1 HPV type. The highest rates of concordance were observed 24 hours after sexual intercourse. A similar peak in concordance was not seen between genital and nongenital anatomic sites. Transmission rates for female genital to male genital ranged from 26.8 to 187.5 per 100 person-months and for male genital to female genital from 14.5 to 100 per 100 person-months.
Conclusions. High rates of concordance shortly after intercourse suggest that some DNA detections in the genital area are contaminants from a partner and not established HPV infections. Female-to-male transmission appeared more common than male-to-female transmission.
Keywords: heterosexual transmission, HPV
Human papillomavirus (HPV), the cause of cervical and other anogenital cancers, is a well-known sexually transmissible infection. High prevalence in both men and women suggests that HPV is easily transmitted. In the United States and many developed countries, young women have the highest prevalence rates, with up to 25% having cervical HPV DNA detected. Natural history studies show that young women have numerous incident infections, further suggesting easy transmission. Most of these infections spontaneously clear over time. Interestingly, prevalence rates rapidly decrease after age 25 and plateau around 35 years of age [1]. This decrease is thought to occur through development of a cell-mediated immune response resulting in rapid clearance if a woman is reexposed [2–4]. Prevalence patterns in men are different than in women. Prevalence rates of HPV DNA detection in male genitals are around 50%, with prevalence rates appearing to remain steady through all age groups [5]. Differences between prevalence rates in men and women may be due to several reasons including that men rapidly clear HPV infections through innate immune mechanisms without developing a cell-mediated immune response that could offer protection during future exposures [6, 7]. These observations strongly suggest that transmission patterns will differ by gender.
When interpreting transmission patterns, epidemiology studies primarily depend on detection of DNA. However, DNA detection does not necessarily imply “infection.” Rather, it may reflect contamination from the partner after shedding squamous epithelial cells infected with HPV. The HPV may never gain access to the basal epithelial cell layer, which is considered a requirement for establishing infection [7].
Few studies have examined HPV transmission in longitudinal cohorts [8, 9]. Interpretation of results is difficult because transmission patterns may differ between monogamous partners vs concurrent or serial partners. Intervals between visits also influence the calculable rate of transmission, for the virus can come and go numerous times if the interval is long. In this study, we specifically examined the rate of HPV concordance and transmission in monogamous heterosexual couples over a short observational period with short interval testing.
METHODS
Women participating in an ongoing HPV study who had an incident HPV infection were eligible for this study. The parent study was initiated in 1990 and again in 2000 and recruited women aged 13–21 years of age; details have been published elsewhere [10, 11]. Women were seen every 4 months for HPV DNA testing and cervical cytology.
Recruitment design for this study has been published in detail [12]. Women were eligible if they had an incident HPV infection (ie, a new HPV type not detected at the previous visit), were 18 years or older, and had a partner willing to participate. Incident infection criteria were used to increase the chance of enrolling couples in whom transmission of HPV had recently occurred. Additional criteria included report of a single heterosexual relationship of at least 3 months’ duration, normal cytology at the most recent visit, and no current history of genital warts. Eligibility for men included reported monogamy with the study female partner for at least 3 months, age 18 years or older, no genital warts, and no current use of medication in the genital area. Men and women were consented separately according to the Institutional Review Boards (IRBs) of the University of California, San Francisco and San Francisco State University. Twenty-five couples were enrolled.
A baseline study visit (V1) was scheduled within 8 weeks of the parent study visit, which defined the incident HPV detection. Partners were seen on the same day for HPV sampling. In separate rooms, each partner completed a self-administered questionnaire on sexual habits. Female samples for HPV DNA were obtained from the intra-anal canal, by placing a swab 2 cm into the canal, and vulva and vagina using separate polyester swabs for each site. Cervical samples were obtained using normal saline wash [12]. Male samples were obtained from the glans (including corona sulcus), shaft, inner foreskin if applicable, scrotum, and perianal area using separate exfoliation paper followed by wetted polyester swabs for each site. The perianal area included the anal opening and 2-cm radius of surrounding skin. In men and women, a sample was obtained from the palmar surface of the dominant hand, using exfoliation and wetted polyester swab, and from the buccal mucosa and tongue using a cytobrush. Male perianal samples were obtained because a small, unpublished pilot study suggested male nonparticipation if intra-anal samples were requested. Because women provided intra-anal samples during the parent study, this continued during the transmission study. All samples were immediately placed into separate vials of sample transport media (Qiagen Inc, Valencia, CA).
At each visit, couples were counseled regarding condom use per IRB guidelines; HPV sampling and questionnaire data were collected as described for V1. After V1, couples were scheduled for visit 2 (V2) and asked to have vaginal intercourse 24 hours before V2. After V2, couples were asked to abstain from all sexual interaction, specifically sexual intercourse, and return for a third visit (V3) 48 hours after V2. After V3, couples returned 2 weeks (V4) and 6 weeks (V5) after V2. Couples were asked to engage in their normal sexual behaviors following V3. HPV detection used the Roche Linear Array HPV Genome Typing Test (Roche Molecular Systems, Pleasanton, CA), which tests for 37 different HPV types [12].
ANALYSIS
Genital category for women included results from the vulvar, vaginal, and cervical samples and for men included scrotal, shaft, coronal, and foreskin samples; the anogenital category included genital and perianal/intra-anal sample results. Type-specific concordance between partners’ same and different anatomic sites was determined at each visit. Positive concordance occurred when at least one HPV type was found in common at the sites examined. Concordance was determined by gender because males and females had different rates of type-specific infections. Concordance for all HPV types detected was also calculated. Negative concordance occurred when each partner's sites were negative for all HPV types.
A couple was considered at risk for transmission if, during a single visit, a partner (the positive partner) had one or more HPV types not detected in the other partner (the negative, at-risk partner). Transmission was defined to occur when one or more HPV types detected in the positive partner was detected at the next visit in the previously negative, at-risk partner. Transmission rates are expressed as the number of transmission events per 100 person-months, with the rate denominator equaling the total number of months the study couples were exposed to HPV between visits. Rates were calculated for male-to-female and female-to-male transmission between each subsequent visit (eg, V1 to V2, V2 to V3) for all anatomic sites except oral because few oral infections were detected. In addition, overall anogenital transmission rates were calculated with the rate numerator being accumulative anogenital to anogenital transmission events from V1 through all subsequent visits.
RESULTS
Each of the 25 couples enrolled completed all study visits. All couples reported vaginal intercourse within 24 hours of V2 and reported abstinence from vaginal intercourse between V2 and V3. Twenty-three couples were monogamous for the duration of the study. One male partner reported nonmonogamous heterosexual sex before V4, and one female partner from a different couple reported nonmonogamous heterosexual sex before V5. Demographic and behavioral characteristics at baseline of the 25 couples have been described elsewhere [12]. Mean age of the men was 25.5 years (SD, 6.1) and of the women was 22·6 years (SD, 3.4). The median number of months of reported monogamy for men was 12 (interquartile range [IQR], 8.7–36) and for women was 16.5 (IQR, 7.2–36). The median number of months in which couples reported they were in a sexual relationship with their partner was 26 (IQR, 8.5–42) for men and 32 for women (IQR, 9–49). Men reported a median of 12 (IQR, 5–20) lifetime sexual partners, and women reported a median of 7 (IQR, 4.5–13).
CONCORDANCE AT VISITS
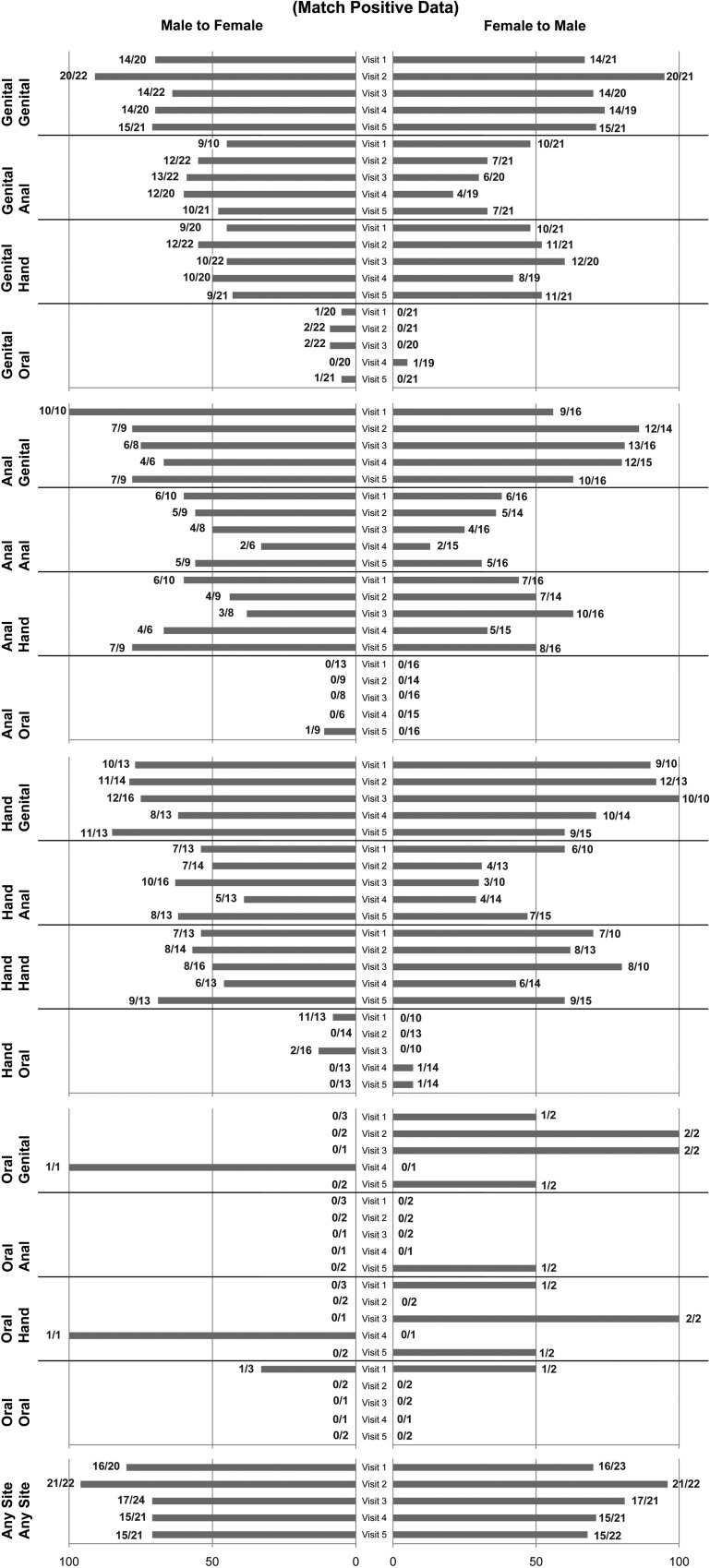
Comparing female-to-male concordance, 102 of 125 visits were positive for HPV in female genital samples. Of 102 samples, 77 visits showed that the male partner shared at least one type. Of 23 visits with HPV-negative samples, 12 visits showed that the male partner also had HPV-negative genital samples. Comparing male-to-female concordance, 105 of 125 visits were positive for HPV in male genital samples. Of 105 samples, 77 visits showed that the female partner shared at least one type. Of 20 visits with HPV-negative samples, 12 visits showed that the female partner also had HPV-negative genital samples. The percentage of couples with positive and negative concordance between anatomic sites at each visit is depicted in Figure 1. The highest rate of positive concordance (95%) between female and male genital samples occurred at V2 (24 hours after intercourse) compared with the preceding and subsequent visits, which exhibited 67%–74% positive concordance.
Figure 1.
Positive and negative concordance of human papillomavirus (HPV) between partners at each visit by anatomic site. Because males and female partners differed in the HPV detected at each visit, concordance is shown by gender. Bars to the right represent percent concordance when comparing male samples to that of the female; numbers at the end represent the number of females with HPV (denominator) and the number of males having at least one HPV type shared with the female (numerator). Bars to the left represent percent concordance when comparing female samples to that of the male; numbers at the end of the bar represent the number of males with HPV (denominator) and the number of females having at least one HPV type shared with the male (numerator). Negative concordance reflects percent concordance that both couples were HPV negative from the specific anatomic site. Anal sample for women refers to the intra-anal and for men refers to the perianal samples.
Rates of detecting the woman's genital HPV type in the partner's hand ranged from 48% to 60% at any one visit and from 21% to 48% in the partner's perianal area. Detection of the woman's genital HPV type was less common in the partner's oral cavity, ranging from 0% to 5%. A similar peak of genital–genital concordance at V2 was not observed for the partner's nongenital sites. Only 4–6 women had no genital HPV detected at one of the visits; among these women, 40%–75% had a male partner with negative concordance.
When examining concordance between the female anal site and the male partner, a similar pattern was observed. The rate of positive concordance with the partner's genitals ranged from 56% to 86%; hand, from 33% to 63%; perianal area, from 13% to 38%; and oral cavity, 0%.
Of the women with HPV detected in the hand, the rate of positive concordance with their partner's genitals ranged from 60% to 100%; hand, from 36% to 80%; perianal area, from 29% to 60%; and oral cavity, from 0% to 7%. Oral HPV in women at any visit was rare. Last, at any site examined, at least one HPV found in the women was found in 68% to 91% of partners, with a peak at V2 of 95%.
Figure 1 demonstrates concordance rates by visit for male HPV status by site of infection. Among the men with HPV detected in their genital area, 91% of their partners had positive concordance at V2 compared with the preceding and succeeding visits, when the positive concordance rate ranged from 64% to 71%.
Rates of detecting male-genital HPV in the partner's hand ranged from 45% to 64% and in the partner's anal area from 50% to 64%. As mentioned, oral HPV in women was rare. Of the 3–5 men who were HPV negative in the genital area, 90%–100% had a female partner who was also negative in the genital area.
Among the men with perianal HPV detected at one of the visits, the rate of positive concordance with their partner's genitals ranged from 67% to 100%; hand, from 38% to 78%; anal site, from 33% to 60%; and oral cavity, 0%.
Among the men who had HPV detected in the hand, the rate of positive concordance with their partner's genitals ranged from 62% to 85%; hand, from 46% to 69%; anal area, from 50% to 63%; and oral cavity, from 0% to 13%. Oral detection in men was rare during all visits. Last, at any site examined, at least one HPV found in the men was also found in 71%–95% of female partners.
We examined exact match rates for all HPV types detected in one person. Of 116 visits in which HPV was detected at any site in one partner, only 17 (15%) visits detected identical HPV types somewhere in the other partner. Of 113 visits in which HPV was detected in one partner's genital area, 19 (17%) visits had an identical match for all HPV types in the other partner's genital area. Other areas of HPV detection in one partner that produced identical matches for all HPV types in the other partner in the same anatomic area are as follows: anal/perianal, 7 of 93 (8%) visits; hand, 11 of 89 (12%) visits; and oral cavity, 1 of 17 (6%) visits.
TRANSMISSION RATES
Table 1 shows transmission events from partners’ genital sites between visits. Transmission rates from female genitals to male genitals ranged from 26.9 to 187.5 per 100 person-months, with the lowest transmission rate observed between V4 and V5 (comprising the longest interval between visits). The highest transmission rates occurred between V1 and V2 as well as V2 and V3, which had the shortest intervals. A similar pattern emerged for male-to-female genital transmission, with the highest transmission rate observed (100 per 100 person-months) between V2 and V3 and the lowest between V4 and V5 (14.5 per 100 person-months). At each visit, the transmission rate from female to male was higher than from male to female.
Table 1.
HPV Transmission Rates per 100 Person-Months From Male to Female and Female to Male
| Visit 1 to 2 |
Visit 2 to 3 |
Visit 3 to 4 |
Visit 4 to 5 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male Site | Female Site | n/X | Rate | 95% CI | n/X | Rate | 95% CI | n/X | Rate | 95% CI | n/X | Rate | 95% CI |
| Transmission Rate From Male to Female | |||||||||||||
| Genital | Genital | 2/12 | 87.0 | (10.5–314.1) | 1/15 | 100.0 | (2.5–557.2) | 2/15 | 32.8 | (4.0–118.4) | 2/15 | 14.5 | (1.8–52.4) |
| Genital | Hand | 1/17 | 33.3 | (0.8–185.7) | 0/18 | 0.0 | (0, 0) | 1/21 | 11.7 | (0.3–65) | 1/17 | 6.4 | (0.2–35.5) |
| Genital | Perianal | 0/17 | 0.0 | (0, 0) | 0/19 | 0.0 | (0, 0) | 1/18 | 13.7 | (0.3–76.3) | 1/16 | 6.8 | (0.2–37.8) |
| Hand | Genital | 1/5 | 93.8 | (2.4–522.3) | 0/5 | 0.0 | (0, 0) | 0/8 | 0.0 | (0, 0) | 1/6 | 17.5 | (0.4–97.7) |
| Hand | Hand | 0/10 | 0.0 | (0–0) | 0/8 | 0.0 | (0, 0) | 0/14 | 0.0 | (0, 0) | 1/9 | 11.8 | (0.3–65.5) |
| Hand | Perianal | 0/10 | 0.0 | (0, 0) | 0/11 | 0.0 | (0, 0) | 0/12 | 0.0 | (0, 0) | 0/11 | 0.0 | (0, 0) |
| Peri-anal | Genital | 0/2 | 0.0 | (0, 0) | 0/2 | 0.0 | (0, 0) | 0/2 | 0.0 | (0, 0) | 0/2 | 0.0 | (0, 0) |
| Peri-anal | Hand | 0/6 | 0.0 | (0, 0) | 0/5 | 0.0 | (0, 0) | 0/5 | 0.0 | (0, 0) | 0/3 | 0.0 | (0, 0) |
| Peri-anal | Perianal | 0/6 | 0.0 | (0, 0) | 0/5 | 0.0 | (0, 0) | 0/4 | 0.0 | (0, 0) | 0/5 | 0.0 | (0, 0) |
| Transmission Rate From Female to Male | |||||||||||||
| Genital | Genital | 5/15 | 187.5 | (60.9–437.6) | 1/8 | 187.5 | (4.7–1044.7) | 2/12 | 41.7 | (5.0–150.5) | 3/12 | 26.9 | (5.5–78.5) |
| Genital | Hand | 2/16 | 70.6 | (8.5–255) | 2/17 | 171.4 | (20.8–619.3) | 3/16 | 45.7 | (9.4–133.5) | 3/17 | 18.9 | (3.9–55.4) |
| Genital | Perianal | 2/19 | 56.1 | (6.8–202.6) | 1/19 | 76.9 | (1.9–428.6) | 1/20 | 12.2 | (0.3–68.2) | 2/18 | 11.9 | (1.4–43.1) |
| Hand | Genital | 0/4 | 0.0 | (0, 0) | 0/5 | 0.0 | (0, 0) | 0/5 | 0.0 | (0, 0) | 1/6 | 18.0 | (0.5–100.1) |
| Hand | Hand | 0/5 | 0.0 | (0, 0) | 1/8 | 176.5 | (4.5–983.2) | 0/5 | 0.0 | (0, 0) | 0/10 | 0.0 | (0, 0) |
| Hand | Perianal | 0/5 | 0.0 | (0, 0) | 1/10 | 142.9 | (3.6–795.9) | 0/9 | 0.0 | (0, 0) | 0/12 | 0.0 | (0, 0) |
| Perianal | Genital | 2/8 | 122.4 | (14.8–442.3) | 1/5 | 272.7 | (6.9–1519.5) | 0/7 | 0.0 | (0, 0) | 0/7 | 0.0 | (0, 0) |
| Perianal | Hand | 1/10 | 50.8 | (1.3–283.3) | 1/9 | 157.9 | (4.0–879.7) | 0/10 | 0.0 | (0, 0) | 2/12 | 18.0 | (2.2–64.9) |
| Perianal | Perianal | 1/10 | 57.7 | (1.5–321.4) | 1/12 | 120.0 | (3.0–668.6) | 0/16 | 0.0 | (0, 0) | 0/14 | 0.0 | (0, 0) |
Abbreviations: n, no. of previously negative, at-risk partners with discordant HPV type detected at the later visit; X, no. of negative, at-risk partners at the earlier visit; 95% CI, 95% confidence interval. Rate is calculated per 100 person-months. 95% CI was calculated using Poisson distribution.
Male-to-female transmission rates between other anatomic sites were uncommon (Table 1). In part, few females were at risk because the male was either HPV negative at the hand or perianal site or the female had already shared all HPV types. In contrast, female-to-male transmission was higher between all anatomic sites, particularly between V1 and V2 and V2 and V3. Transmissions from female genitals to the partner's hand and perianal areas were almost as high as transmissions from female-to-male genitals. Transmissions from female anal to male genital and from female hand to male perianal areas were similar to transmissions from female-to male genitals. As in men, transmission from female hand to other male anatomic sites was less common, mostly because female hand samples were often negative and therefore did not place men at risk.
The overall transmission rate for female anogenital (genital and anal sites combined) to male anogenital areas between V1 and the other visits was 21.35 per 100 person-months, and the overall transmission rate for male-to-female transmission was 9.23 per 100 person-months (Table 2). Of the 2 at-risk women who had an anogenital transmission event at V2, one had the transmitted type (HPV53) persist to V5. Of the 8 at-risk men who had an anogenital transmission event between V1 and V2, 2 had the transmitted type (HPV53 and HPV89, respectively) persist to V5. Only one couple remained HPV negative throughout all visits.
Table 2.
Rate of Overall Anogenital Type-Specific Human Papillomavirus (HPV) Transmission per 100 Person-Months Detected Between Visit 1 and All Subsequent Visits
| Transmitter | Receiver | No. of Transmission Events | Days of Observation | Rate per 100 person-months | 95% CI |
|---|---|---|---|---|---|
| Male | Female | 2 | 650 | 9.23 | 1.12–33.34 |
| Female | Male | 6 | 843 | 21.35 | 7.8–46.48 |
Abbreviation: 95% CI, 95% confidence interval.
For the 2 couples in whom one partner reported nonmonogamous sex, we initially looked for new types introduced to the couple after the nonmonogamous sex occurred and then looked for transmission of the new type to the other partner. In the couple in whom the man reported nonmonogamous vaginal intercourse and receiving oral sex before V4, the male partner acquired HPV52 in the genital area at V4, which persisted to V5. His partner did not acquire HPV52 at V4 or V5. In the couple in whom the woman reported nonmonogamous vaginal intercourse and giving/receiving oral sex before V5, the woman acquired HPV62 in her genital area and HPV54 in her anal area. The male partner did not acquire either of these HPV types at V5.
DISCUSSION
Sharing of HPV was extremely high among a population of couples in whom the women had a recent incident HPV infection. All couples reported monogamy during the initial weeks of the study, and all but 2 reported monogamy throughout the observation period. The highest rates of concordance in the genital areas of the couples in whom at least one HPV type was shared occurred at V2 shortly after the couples had sexual intercourse. When the couples returned 48 hours later without having had sexual intercourse, the concordance rate dropped to the previously observed rate, suggesting that some DNA detections in the genital area are contaminants from the partner (ie, partner cells infected with HPV DNA). Most notably, the concordance rate—whether matched from the male's or female's HPV status—was relatively similar over the 6 weeks, ranging from 70% to 86% at any one visit. Perfect concordance was low, with <20% showing perfect concordance for all HPV types detected at any site.
Our observed transmission rates were extremely high—up to 300-fold higher than other published studies. Burchell et al [8] recently followed 179 couples prospectively and found a male-to-female transmission rate of 3.5 per 100 person-months and a female-to-male rate of 4.0 per 100 person-months. One of the main differences of their study design was inclusion of only one follow-up period approximately 5 months after the first visit. Second, our study chose women with known incident infection; hence, recent transmission of HPV had already occurred. Hernandez et al [13] reported a similar low male-to-female genital transmission rate (penis/scrotum to cervix) of 4.5 per 100 person-months. In this study, couples were followed at 2-month intervals over an average of 7.5 months. The higher transmission rates reported in our study likely occurred because the intervals between visits were quite short, ranging from 48 hours to 2 weeks. Decline in the rate of transmission paralleled increases in the time interval, with a peak rate of 100–428 per 100 person-months for the 48-hour interval to the lowest rate of 14–25 per 100 person-months for the 2-week interval. Interestingly, the highest rate of transmission was observed during the shortest interval when couples were asked to abstain from sexual intercourse. The presence of any transmission event during this abstinent period underscores the complexity of understanding transmission dynamics. Events may have stemmed from sexual acts other than intercourse, such as hand to genital or auto-inoculation from other infected sites, or detection may be dependent on viral dynamics (ie, time from exposure to established infection). Based on animal studies and studies in women who were recently sexually active [14, 15], time between exposure and established, replicative infection likely takes weeks to months. Detection of HPV DNA that is not an established infection may lead to overestimations of HPV transience and lead to higher transmission rate estimations when measured over short intervals. The rapid appearance of HPV accentuates the complexity of determining the transmission rates of virus that result in infection vs transmission rates that result in transient detection. Hernandez et al [9] reported several instances in which transmission appeared to occur as a form of self-inoculation (ie, hand to cervix, hand to penis, anus to genital). The high rates of transmission observed in our study support the model developed by Bogaards et al, who estimated a per-partnership transmission probability of close to 100% [16].
Notably, the transmission rate in our study from females to males was higher than from males to females at every visit. Hernandez et al [9] also observed higher female-to-male transmission rates of 17.4 per 100 person-months—similar to our transmission rate if the rate was calculated over the entire 6-week period. The lack of detecting differences by gender in the study by Burchell et al [8] may be due to the long interval between visits and the single follow-up visit. Some evidence suggests that men acquire more transient infections than women [17] that do not result in development of a memory immune response, thereby placing men at higher risk for reinfection. Supporting this position are observations that HPV DNA detection is more common in men than in women at almost any age, whereas antibodies are much lower in men at any age [5, 17–20]. The observed higher transmission rate from females to males is different than that observed for other viral sexually transmitted infections (STIs) [21–23]. However, comparisons are difficult because HPV is primarily a mucosal infection, whereas other viral STIs result in systemic infections.
The low concordance rate between male perianal samples and female intra-anal samples is worth noting. Lower concordance rates might be explained by differences in sampling. However, the genital–perianal and genital–intra-anal concordance rates between couples were very similar. Similar concordance was also found between the man's hand and his partner's intra-anal sample and genital and intra-anal samples. Concordance was slightly lower for the woman's hand and the man's perianal HPV types, which may not be surprising because finger–anal sex or penile–anal sex would introduce those perianal HPV types into the anal canal. However, because intra-anal swabs are passed through the perianal area, contamination from the perianal area is also possible. Consistent with several studies, HPV was commonly detected in hands of both men and women in our study [9, 12, 24]. Hand-to-hand or hand-to-other site transmission was lower than genital-to-genital transmission, underscoring the important role of sexual intercourse in transmitting HPV. However, the high rates of concordance support the contention that the hand may provide a means for HPV to move between partners during sexual activity.
This study is unique in that we selected women based on an incident HPV infection in hopes of examining the initial transmission dynamics for new infections. However, given the extremely high transmission rates observed, it is likely that these HPV types were introduced into the relationship at some other point. Although the short observation intervals in this study allowed us to closely examine these high rates of HPV DNA transmission, they did not allow us to examine whether any infections remained established. Clearly, further studies are needed to continue our understanding of HPV transmission. High rates of HPV transmission exist among heterosexual couples, with a predominance of female-to-male transmission. HPV DNA transmission can be detected even during periods of abstinence from sexual intercourse, thus highlighting the complexity of HPV transmission dynamics.
Notes
Acknowledgments. The authors would like to thank Anthony Kung for manuscript preparation.
Financial support. This work was supported by the National Cancer Institute (grant R37 CA51323); Maternal Child Health branch (grant T71MC00003); American Cancer Society (grant 92-026-12); and by a research grant from the Investigator-Initiated Studies Program of Merck & Co, Inc. The opinions expressed in this article are those of the authors and do not necessarily represent those of Merck & Co., Inc. These studies were carried out in part in the Pediatric Clinical Research Center, Moffitt Hospital, University of California San Francisco with funds provided by the National Center for Research Resources, US Public Health Service (grant 5 M01 RR-01271).
Potential conflicts of interest. The authors do not have a commercial or other association that might pose a conflict of interest (eg, pharmaceutical stock ownership, consultancy, advisory board membership, relevant patents, or research funding). All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Burchell AN, Winer RL, de Sanjose S, Franco EL. Chapter 6: Epidemiology and transmission dynamics of genital HPV infection. Vaccine. 2006;24(Suppl 3):S3/52–61. doi: 10.1016/j.vaccine.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 2.Wentzensen N, Rodriguez AC, Viscidi R, et al. A competitive serological assay shows naturally acquired immunity to human papillomavirus infections in the Guanacaste Natural History Study. J Infect Dis. 2011;204:94–102. doi: 10.1093/infdis/jir209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trottier H, Ferreira S, Thomann P, et al. Human papillomavirus infection and reinfection in adult women: the role of sexual activity and natural immunity. Cancer Res. 2010;70:8569–77. doi: 10.1158/0008-5472.CAN-10-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez AC, Schiffman M, Herrero R, et al. Low risk of type-specific carcinogenic HPV re-appearance with subsequent cervical intraepithelial neoplasia grade 2/3. Int J Cancer. 2012;131:1874–81. doi: 10.1002/ijc.27418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nyitray AG, da Silva RJ, Baggio ML, et al. The prevalence of genital HPV and factors associated with oncogenic HPV among men having sex with men and men having sex with women and men: the HIM study. Sex Transm Dis. 2011;38:932–40. doi: 10.1097/OLQ.0b013e31822154f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu B, Viscidi RP, Wu Y, et al. Prevalent serum antibody is not a marker of immune protection against acquisition of oncogenic HPV16 in men. Cancer Res. 2012;72:676–85. doi: 10.1158/0008-5472.CAN-11-0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stanley MA. Epithelial cell responses to infection with human papillomavirus. Clin Microbiol Rev. 2012;25:215–22. doi: 10.1128/CMR.05028-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burchell AN, Coutlee F, Tellier PP, Hanley J, Franco EL. Genital transmission of human papillomavirus in recently formed heterosexual couples. J Infect Dis. 2011;204:1723–9. doi: 10.1093/infdis/jir644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernandez BY, Wilkens LR, Zhu X, et al. Transmission of human papillomavirus in heterosexual couples. Emerg Infect Dis. 2008;14:888–94. doi: 10.3201/eid1406.070616.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moscicki AB, Shiboski S, Hills NK, et al. Regression of low-grade squamous intra-epithelial lesions in young women. Lancet. 2004;364:1678–83. doi: 10.1016/S0140-6736(04)17354-6. [DOI] [PubMed] [Google Scholar]
- 11.Moscicki AB, Ma Y, Jonte J, et al. The role of sexual behavior and human papillomavirus persistence in predicting repeated infections with new human papillomavirus types. Cancer Epidemiol Biomarkers Prev. 2010;19:2055–65. doi: 10.1158/1055-9965.EPI-10-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Widdice LE, Breland DJ, Jonte J, et al. Human papillomavirus concordance in heterosexual couples. J Adolesc Health. 2010;47:151–9. doi: 10.1016/j.jadohealth.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernandez BY, Barnholtz-Sloan J, German RR, et al. Burden of invasive squamous cell carcinoma of the penis in the United States, 1998–2003. Cancer. 2008;113:2883–91. doi: 10.1002/cncr.23743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnston KB, Monteiro JM, Schultz LD, et al. Protection of beagle dogs from mucosal challenge with canine oral papillomavirus by immunization with recombinant adenoviruses expressing codon-optimized early genes. Virology. 2005;336:208–18. doi: 10.1016/j.virol.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 15.Winer RL, Lee SK, Hughes JP, Adam DE, Kiviat NB, Koutsky LA. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol. 2003;157:218–26. doi: 10.1093/aje/kwf180. [DOI] [PubMed] [Google Scholar]
- 16.Bogaards JA, Coupe VM, Xiridou M, Meijer CJ, Wallinga J, Berkhof J. Long-term impact of human papillomavirus vaccination on infection rates, cervical abnormalities, and cancer incidence. Epidemiology. 2011;22:505–15. doi: 10.1097/EDE.0b013e31821d107b. [DOI] [PubMed] [Google Scholar]
- 17.Giuliano AR, Lee JH, Fulp W, et al. Incidence and clearance of genital human papillomavirus infection in men (HIM): a cohort study. Lancet. 2011;377:932–40. doi: 10.1016/S0140-6736(10)62342-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bauer HM, Ting Y, Greer CE, et al. Genital human papillomavirus infection in female university students as determined by a PCR-based method. JAMA. 1991;265:472–7. [PubMed] [Google Scholar]
- 19.Rumbold AR, Tan SE, Condon JR, et al. Investigating a cluster of vulvar cancer in young women: a cross-sectional study of genital human papillomavirus prevalence. BMC Infect Dis. 2012;12:243. doi: 10.1186/1471-2334-12-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markowitz LE, Sternberg M, Dunne EF, McQuillan G, Unger ER. Seroprevalence of human papillomavirus types 6, 11, 16, and 18 in the United States: National Health and Nutrition Examination Survey 2003–2004. J Infect Dis. 2009;200:1059–67. doi: 10.1086/604729. [DOI] [PubMed] [Google Scholar]
- 21.Mertz GJ, Benedetti J, Ashley R, et al. Risk factors for sexual transmission of genital herpes. Ann Int Med. 1992;116:197–202. doi: 10.7326/0003-4819-116-3-197. [DOI] [PubMed] [Google Scholar]
- 22.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921–9. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 23.Fideli US, Allen SA, Musonda R, et al. Virologic and immunologic determinants of heterosexual transmission of human immunodeficiency virus type 1 in Africa. AIDS Res Hum Retroviruses. 2001;17:901–10. doi: 10.1089/088922201750290023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winer RL, Hughes JP, Feng Q, et al. Detection of genital HPV types in fingertip samples from newly sexually active female university students. Cancer Epidemiol Biomarkers Prev. 2010;19:1682–5. doi: 10.1158/1055-9965.EPI-10-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]