Abstract
Mutations in the lamin A/C (LMNA) gene cause significant disruption to skeletal and myocardial muscle, as well as nervous tissue. We describe a case illustrating varied manifestations of a LMNA mutation and the implications for diagnosis and management. We turn to several family studies that describe considerable phenotypic variation arising from LMNA mutations. The discussion focuses on educating the reader in recognition of potential presentations of LMNA mutations.
Background
Up to 50% of idiopathic dilated cardiomyopathies (IDC) are thought to have a familial aetiology, and mutations in the LMNA gene are responsible for 10% of familial cases.1 The LMNA gene codes for lamin proteins A and C which are involved in nuclear architecture and transcription.1 As well as dilated cardiomyopathy (DCM), mutations in the LMNA gene are responsible for a range of conditions including Emery-Dreifuss muscular dystrophy (EDMD), limb girdle muscular dystrophy (LGMD), partial lipodystrophy and cardiac conduction defects.1 Current evidence suggests considerable overlap between these conditions.1 We outline the case of a man with a novel LMNA mutation (435delG), to aid recognition and management of these patients.
Case presentation
A 62-year-old male farmer was admitted with worsening breathlessness. The patient was known to have familial dilated cardiomyopathy due to a novel LMNA mutation (435delG) and had been admitted to hospital on numerous occasions with similar symptoms. He was taking maximal medical therapy for heart failure in addition to three antiarrhythmic agents, due to previous episodes of ventricular tachycardia (figure 1). He had an implantable cardioverter-defibrillator (ICD) and biventricular pacemaker. His identical twin brother had the same LMNA mutation and had recently died of cardiac failure while awaiting a transplant. The twins’ father had died of congestive cardiac failure aged 63, and their grandfather died at age 61 of an embolic stroke with known cardiac hypertrophy and failure.
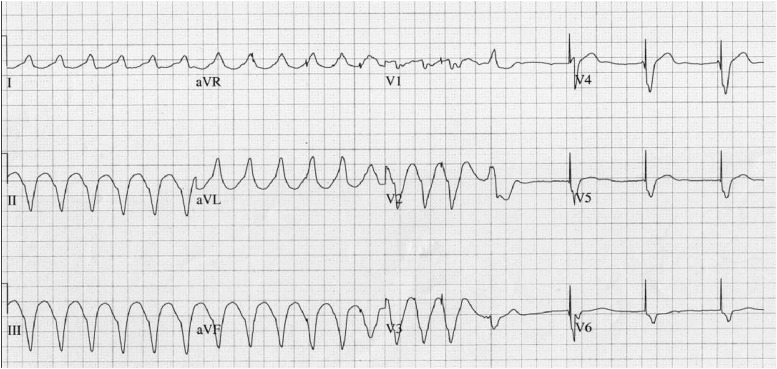
Figure 1.
Patient's ECG showing ventricular tachyarrythmia with self-termination and subsequent pacemaker activity.
Investigations
On examination he was thin, with skin discolouration secondary to long term amiodarone therapy. He was in congestive cardiac failure. Echocardiography revealed a severely dilated and impaired left ventricle, with an ejection fraction of 18%. The thickness of the posterior left ventricular wall was at the upper limit of normal (11 mm). There was reduced right ventricular function, severe bi-atrial dilatation and pulmonary hypertension with an estimated pulmonary artery pressure of 36 mm Hg plus right atrial pressure.
Outcome and follow-up
In spite of adequate diuresis, he remained persistently breathless and exhausted. He had been given a continuous positive airway pressure (CPAP) machine, 2 years back for obstructive sleep apnoea, though no documentation relating to this could be obtained. Overnight oximetry at this stage revealed up to 80 desaturations/h (SaO2 as low as 54%), with mean duration of 15 s.
Two weeks into admission he developed type two respiratory failure secondary to pneumonia, requiring a period of ventilation on the intensive care unit. Right-sided pneumonia raised suspicion of aspiration, and indeed the patient admitted to difficulty in swallowing, which had been progressing over a period of 2 years. Video fluoroscopy findings were in keeping with neuromuscular weakness.
It emerged that he had also been experiencing increased difficulty in walking, and neurological examination revealed quadriceps wasting, fasciculation and markedly reduced lower limb power. Reflexes were absent and plantars downgoing. Nerve conduction studies were normal. A neurology opinion concluded that the weakness of respiratory, bulbar and limb muscles, was a consequence of previously unrecognised skeletal muscle disease. The patient declined a muscle biopsy and died 2 weeks later.
Discussion
Studies have identified 200 lamin A/C (LMNA) mutations resulting in dilated cardiomyopathy (DCM).1 Mutations may be spontaneous, or inherited in an autosomal dominant fashion. Penetrance and phenotypic expression are variable.1
The traditional perception that different LMNA mutations cause separate diseases is being replaced by an appreciation of considerable overlap between conditions. In this case study there was a delay in recognising skeletal myopathy in a patient with familial DCM.
In a 35 member family affected by DCM, limb girdle muscular dystrophy (LGMD) and sudden cardiac death, muscle weakness was not a presenting symptom, despite being present in 27% of patients at the time of diagnosis.2 In another study involving a 72 member family with DCM, cardiac disease was found to precede skeletal muscle weakness. The mean age for cardiac involvement was 37 years (±7.9) but for neurological involvement was 44 years (±0.8).3
The apparent delay between cardiac and skeletal muscle involvement may reflect difficulty in detecting early signs of skeletal muscle weakness, particularly where there is a low index of suspicion on the part of the clinician.
Learning points.
Evaluation of a patient with unexplained heart failure should include assessment for conduction disease, skeletal myopathy and an appropriate detailed family history.
Muscle weakness may develop simultaneously with cardiac disease but is recognised later. Clinicians should be aware of the overlap between these conditions to avoid a delay in diagnosis, or misdiagnosis.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Malhotra R, Mason PK. Lamin A/C deficiency as a cause of familial dilated cardiomyopathy. Current Opin Cardiol 2009;24:203–8 [DOI] [PubMed] [Google Scholar]
- 2.Antoniades L, Eftychiou C, Kyriakides T, et al. Malignant mutation in the lamin A/C gene causing progressive conduction system disease and early sudden death in a family with mild form of limb-girdle muscular dystrophy. J Intervent Card Electrophysiol 2007;19:1–7 [DOI] [PubMed] [Google Scholar]
- 3.Charniot JC, Desnos M, Zerhouni K, et al. Severe dilated cardiomyopathy and quadriceps myopathy due to lamin A/C gene mutation: a phenotypic study. Eur J Heart Failure 2006;3:249–56 [DOI] [PubMed] [Google Scholar]