Abstract
Rationale: Lower socioeconomic status (SES) confers a heightened risk of common cardiovascular and pulmonary diseases and increased mortality. The association of SES with outcomes in patients with pulmonary arterial hypertension (PAH) is less clear.
Objectives: To determine the association between SES and outcomes in patients with PAH.
Methods: We performed a prospective cohort study at a national referral center for patients with PAH in China. Two hundred sixty-two consecutive incident patients aged 18 to 65 years with a diagnosis of idiopathic PAH were recruited between January 2007 and June 2011 and followed up until November 2011. The primary endpoint was all-cause mortality. An SES score for each patient was derived from their educational level, annual household income, occupation, and medical reimbursement rate.
Measurements and Main Results: Patients with a lower SES had higher unadjusted mortality rates, with 3-year survival estimates of 50.1, 70.8, and 86.0% in increasing tertiles of SES (P for trend < 0.001). After adjustment for clinical features, hemodynamics, and type of PAH treatment, the hazard ratios for death were 2.98 (95% confidence interval, 1.51–5.89) in the lowest tertile of SES and 1.80 (95% confidence interval, 0.89–3.63) in the middle tertile of SES compared with the upper tertile (P for trend = 0.006).
Conclusions: A lower SES is strongly associated with a higher risk of death in idiopathic PAH. This association was independent of clinical characteristics, hemodynamics, and treatment. Addressing the health disparities associated with a lower SES may improve the outcomes of patients with PAH.
Keywords: pulmonary arterial hypertension, socioeconomic status, survival
At a Glance Commentary
Scientific Knowledge on the Subject
Lower socioeconomic status (SES) confers a heightened risk of common cardiovascular and pulmonary diseases and increased mortality. The association of SES with outcomes in patients with pulmonary arterial hypertension (PAH) is less clear.
What This Study Adds to the Field
A lower SES was strongly associated with a higher risk of death in idiopathic PAH. This association was independent of clinical characteristics, hemodynamics, and treatment. Addressing the health disparities associated with a lower SES may improve the outcomes of patients with PAH.
Pulmonary arterial hypertension (PAH) is a disease of the small muscular pulmonary arteries characterized by a progressive rise in pulmonary artery pressure and pulmonary vascular resistance (PVR), ultimately resulting in right heart failure and death (1–3). Treatment for PAH includes anticoagulants, diuretics, prostanoids, phosphodiesterase 5 inhibitors, and endothelin receptor antagonists (ERAs) (3–5). Although survival may have improved, patients with PAH still have a greatly increased risk of death, even with approved treatments.
Socioeconomic status (SES) is defined by education, income, occupation, and social status and refers to an individual’s social standing relative to other members of a society (6). A low SES is associated with increased risks of cardiovascular and pulmonary disease and mortality (7–11). However, the association of SES with outcomes in PAH has not been well studied.
Socioeconomic-related inequalities in health and healthcare have increased over recent decades in China (12–14). Approximately 75 to 80% of individuals with low annual household incomes do not carry medical insurance (15), potentially greatly increasing the economic burden of PAH on patients. Wilkens and colleagues reported that each patient with PAH in Germany spends €47,400 per year on average, primarily due to drug costs (16, 17). In the United States, the economic burden of privately ensured patients with PAH is substantial (18). In addition, low educational achievement and occupational exposures are interrelated with economic indicators and may have a synergistic effect on health (19). Therefore, a lower SES could negatively impact outcomes in patients with PAH.
Idiopathic pulmonary arterial hypertension (IPAH) is one of the most common forms of PAH (20, 21). In this study, we explored the associations between SES and clinical worsening and death in IPAH. We hypothesized that a lower SES would be associated with an increased risk of clinical worsening and death.
Methods
Study Design
We performed a prospective cohort study of 268 consecutive patients with IPAH aged 18 to 65 years who had their initial evaluation at the Department of Cardio-Pulmonary Circulation, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, China between January 2007 and June 2011. IPAH was defined using accepted diagnostic criteria (2, 3, 22) based on clinical assessment, right heart catheterization (RHC), echocardiography, spiral-computed tomography of the chest, pulmonary angiography, a ventilation/perfusion lung scan, and complete lung function testing. We excluded heritable PAH and PAH due to connective tissue disease, portal hypertension, or HIV infection, and other forms of pulmonary hypertension, such as pulmonary hypertension due to congenital heart disease, left heart disease, pulmonary disease, sleep-associated disorders, and chronic thromboembolic disease. Six patients had missing data for SES, which left 262 patients with IPAH in the study sample.
The study was approved by local ethics review committees. Written informed consent was obtained from all patients.
Outcomes
The primary outcome was all-cause mortality. Clinical worsening was a secondary endpoint, including death, lung or heart-lung transplantation, hospitalization, or initiation of new active therapy because of worsening PAH. We followed patients until November 1, 2011. Patients lost to follow-up were censored as alive on the last day of contact.
Socioeconomic Measures
Data were collected from patients for the highest educational level achieved, occupation, annual household income, and level of medical reimbursement via a questionnaire at the time of the initial evaluation (6, 23). The highest educational level was divided into three groups: less than high school (low; score = 1), high school graduate or equivalent (medium; score = 2), and college graduate or above (high; score = 3). Occupational levels included manual workers, farmers, or unemployed individuals (low; score = 1); businessmen or clerks (medium; score = 2); and professionals, managers, or government employees (high; score = 3). Annual household income was bracketed into five categories: low (RMB [Ren Min Bi, the official currency of China] < 10,000 [≈$1,500, based on a 2011 exchange rate of 6.469 RMB = $1)]; score = 1), medium-low (RMB, 10,000–30,000 [$1,500–$4,600]; score = 2), medium (RMB, 30,000–50,000 [$4,600–$7,700]; score = 3), medium-high (RMB, 50,000–100,000 [$7,700–$15,500]; score = 4), and high (RMB ≥ 100,000 [≥$15,500]; score = 5). Finally, medical reimbursement was divided into four levels: without reimbursement (score = 1), reimbursement rate less than 30% (low; score = 2), reimbursement rate 30 to 80% (medium; score = 3), and reimbursement rate greater than 80% (high; score = 4).
The SES score assigned to each patient was the sum of education, annual household income, occupation, and medical reimbursement scores, as previously described (24–26). For ease of presentation and interpretation, we analyzed the study sample after stratifying by approximate tertiles of the score distribution (Figure E1 in the online supplement): less than or equal to 5 (n = 73), 6 to 7 (n = 90), and greater than or equal to 8 (n = 99).
Clinical Characteristics
Demographic and anthropometric data, place of residence, World Health Organization functional class (WHO FC), comorbid conditions, and other variables were collected at baseline. Transthoracic echocardiography was performed in all patients. The 6-minute walk distance test was performed according to the recommendations of the American Thoracic Society (27).
Hemodynamic evaluation by RHC was performed at baseline as previously described (28, 29). The mean right atrial pressure, mean pulmonary arterial pressure, pulmonary capillary wedge pressure, and cardiac output (calculated by thermodilution) were measured. The cardiac index was calculated as cardiac output in liters per minute divided by body surface area in square meters. An acute pulmonary vasodilator challenge was performed during RHC as previously described (29, 30), and a positive vasodilator response was defined as greater than or equal to 10 mm Hg decrease in mean pulmonary arterial pressure to less than 40 mm Hg with a preserved cardiac index (2).
Statistical Analyses
Demographic features and baseline clinical characteristics were reported as means, medians, or proportions, as appropriate. Differences across SES tertiles were assessed using one-way analysis of variance or a Kruskal-Wallis test for continuous and logistic regression analysis for categorical variables. Survival rate was assessed by the Kaplan-Meier method. Cox proportional regression analysis was used to estimate the hazard ratios and the 95% confidence intervals for the association between SES and outcomes. We forced age and sex into the models. Other variables believed to have clinical importance and those with P less than 0.20 in the univariable analysis were considered as confounders. We retained confounding variables that changed the coefficient of SES by 15% or more in the final model. Adjusted survival curves were generated from the Cox models using the corrected group prognosis method (31). P values less than 0.05 were considered significant. The main analysis was performed using SPSS (Statistic Package for Social Science, Chicago, IL) version 14, and SAS (Statistical Analysis System, SAS Institute, Cary, NC) Version 8.2.
Results
The mean age of the patients was 36.1 (12.0) years (median, 33 yr; interquartile range, 27–45 yr); 71.0% were women, and 56.1% came from the southern part of China. Almost one-half (47.3%) of the patients reported less than a high school education, and 56.5% reported an annual household income less than RMB 30,000 (≈$4,600). Most patients (88.9%) had a less than 30% medical reimbursement rate, and 71.0% were unemployed, a farmer, or a laborer.
Age, sex, body mass index, and place of residence were similar across the tertiles of SES (Table 1). Tobacco and alcohol use were uncommon and not associated with SES. Coronary artery disease, diabetes, hypertension, hypercholesterolemia, and renal insufficiency were also rare. There were no statistically significant differences in the 6-minute walk distance (P = 0.29) and WHO FC across (P = 0.18) tertiles. The median time from symptoms to diagnosis was approximately 2 years in all categories (P = 0.47). Hemodynamics were similar across the groups; however, patients with a higher SES were more likely to have acute vasoreactivity (P = 0.03).
TABLE 1.
BASELINE CHARACTERISTICS ACROSS THE SOCIOECONOMIC STATUS TERTILES
| Socioeconomic Status Tertiles |
||||
| Characteristic | Low (n = 73) | Middle (n = 90) | High (n = 99) | P Value* |
| Age, mean (± SD), yr | 35 (11) | 36 (13) | 37 (12) | 0.65 |
| Female, n (%) | 51 (69.9) | 67 (74.4) | 68 (68.7) | 0.81 |
| BMI, mean (± SD), kg/m2 | 21.7 (2.8) | 21.9 (3.1) | 22.4 (3.7) | 0.35 |
| Place of residence, n (%) | 0.19 | |||
| North China | 38 (52.1) | 36 (40.0) | 41 (41.4) | |
| South China | 35 (47.9) | 54 (60.0) | 58 (58.6) | |
| Tobacco use, n (%) | 6 (8.2) | 6 (6.7) | 7 (7.1) | 0.79 |
| Alcohol use, n (%) | 2 (2.7) | 1 (1.1) | 4 (4.0) | 0.54 |
| Comorbid conditions, n (%) | ||||
| Coronary artery disease | 0 | 0 | 2 (2.0) | 1.00 |
| Diabetes | 1 (1.4) | 1 (1.1) | 1 (1.0) | 0.83 |
| Hypertension | 6 (8.2) | 8 (8.9) | 12 (12.1) | 0.38 |
| Hypercholesterolemia | 0 | 0 | 3 (3.0) | 0.99 |
| Renal insufficiency | 0 | 0 | 2 (2.0) | 1.00 |
| Obesity (BMI > 30 kg/m2) | 0 | 0 | 4 (4.0) | 0.99 |
| Chronic lung disease | 1 (1.4) | 4 (4.4) | 2 (2.0) | 0.88 |
| 6MWD, mean (± SD), m | 353 (99) | 363 (106) | 378 (104) | 0.29 |
| WHO FC, n (%) | 0.18 | |||
| Class I and II | 20 (27.4) | 31 (34.4) | 37 (37.4) | |
| Class III and IV | 53 (72.6) | 59 (65.6) | 62 (62.6) | |
| Time from onset of symptoms to diagnosis, median (IQR), mo | 25 (8–43) | 24 (12–61) | 24 (8–48) | 0.47 |
| BMPR2 mutation, n (%) | 15 (20.5) | 20 (22.2) | 14 (14.1) | 0.25 |
| Pericardial effusion, n (%) | 23 (31.5) | 13 (14.4) | 20 (20.2) | 0.11 |
| Hemodynamic parameters, mean (± SD) | ||||
| mRAP, mm Hg | 9 (6) | 7 (6) | 8 (6) | 0.29 |
| mPAP, mm Hg | 65 (13) | 63 (15) | 63 (16) | 0.56 |
| PVR, Wood Units | 18 (8) | 17 (8) | 16 (7) | 0.37 |
| Cardiac index, L/min/m2 | 2.3 (0.8) | 2.3 (0.8) | 2.4 (0.9) | 0.56 |
| Acute vasoreactivity, n (%) | 0 | 3 (3.3) | 7 (7.1) | 0.03 |
| Targeted therapy, n (%) | ||||
| PDE5 inhibitors | 47 (64.4) | 56 (62.2) | 66 (66.7) | 0.72 |
| Sildenafil | 34 (46.6) | 41 (45.6) | 51 (51.5) | 0.49 |
| Vardenafil | 13 (17.8) | 15 (16.7) | 15 (15.2) | 0.64 |
| ERA (bosentan) | 8 (11.0) | 17 (18.9) | 27 (27.3) | 0.009 |
| Prostanoids | 7 (9.6) | 12 (13.3) | 17 (17.2) | 0.90 |
| Iloprost | 4 (5.5) | 6 (6.7) | 7 (7.1) | 0.68 |
| Beraprost | 8 (11.0) | 5 (5.6) | 7 (7.1) | 0.39 |
| Combination therapy | 11 (15.1) | 21 (23.3) | 27 (27.3) | 0.06 |
| No targeted therapy, n (%) | 9 (12.3) | 13 (14.4) | 9 (9.1) | 0.47 |
| Clinical trial, n (%) | 26 (35.6) | 25 (27.8) | 32 (32.3) | 0.71 |
| No. of visits, median (IQR) | 2 (1–3) | 1 (1–2) | 1 (1–2) | 0.22 |
| Readmission patients (n, %) | 41(56.2) | 42 (46.7) | 47 (47.5) | 0.29 |
| Reevaluated with RHC | 20 (48.8) | 23 (54.8) | 31 (66.0) | 0.10 |
| Reevaluated with DE | 38 (92.7) | 39 (92.9) | 43 (91.5) | 0.83 |
| Education, n (%) | <0.001 | |||
| Low | 68 (93.2) | 47 (52.2) | 9 (9.1) | |
| Medium | 5 (6.8) | 39 (43.3) | 33 (33.3) | |
| High | 0 | 4 (4.4) | 57 (57.6) | |
| Annual household income, n (%) | <0.001 | |||
| Low | 32 (43.8) | 4 (4.4) | 2 (2.0) | |
| Medium-low | 41 (56.2) | 57 (63.3) | 12 (12.1) | |
| Medium | 0 | 25 (27.8) | 49 (49.5) | |
| Medium-high | 0 | 4 (4.4) | 27 (27.3) | |
| High | 0 | 0 | 9 (9.1) | |
| Medical reimbursement insurance, n (%) | <0.001 | |||
| Without | 66 (90.4) | 59 (65.6) | 42 (42.4) | |
| Low | 7 (9.6) | 22 (24.4) | 37 (37.4) | |
| Medium | 0 | 9 (10.0) | 15 (15.2) | |
| High | 0 | 0 | 5 (5.1) | |
| Occupation, n (%) | <0.001 | |||
| Low | 71 (97.3) | 78 (86.7) | 37 (37.4) | |
| Medium | 2 (2.7) | 12 (13.3) | 58 (58.6) | |
| Higher | 0 | 0 | 4 (4.0) | |
Definition of abbreviations: 6MWD = six-minute walk test distance; BMI = body mass index; BMPR2 = bone morphogenetic protein type II receptor gene; DE = Doppler echocardiography; ERA = endothelin receptor antagonist; IQR = interquartile range; mRAP = mean right atrial pressure; mPAP = mean pulmonary arterial pressure; PDE5 = phosphodiesterase type 5; PVR = pulmonary vascular resistance; RHC = right heart catheterization; WHO FC = World Health Organization functional classification.
P values represent the results of one-way analysis of variance or Kruskal-Wallis test for continuous variables and logistic regression analysis for categorical variables.
Approximately 90% of the patients in each tertile of SES received targeted therapy for PAH after the baseline evaluation. Most patients in each group received a phosphodiesterase 5 inhibitor, but those with a higher SES were more likely to receive bosentan than those with a lower SES (P = 0.009). A combination of the three types of therapy was somewhat more commonly used in the higher SES groups (P = 0.06). Approximately 30% of the study sample was enrolled in a clinical trial of PAH therapy, which did not differ between the SES tertiles. There were no significant differences in the number of visits and assessments at our center over the period of the study.
There were 517.8 person-years of follow-up time, and the median follow-up duration was 21 months (interquartile range, 10–35 mo). Six patients (2.3%) were lost to follow-up: three were low SES patients, two were middle SES patients, and one was from the highest SES group. Sixty-four patients died during follow-up; 62 of these (96.9%) were cardiopulmonary deaths. One low-SES patient and one high-SES patient died due to other causes.
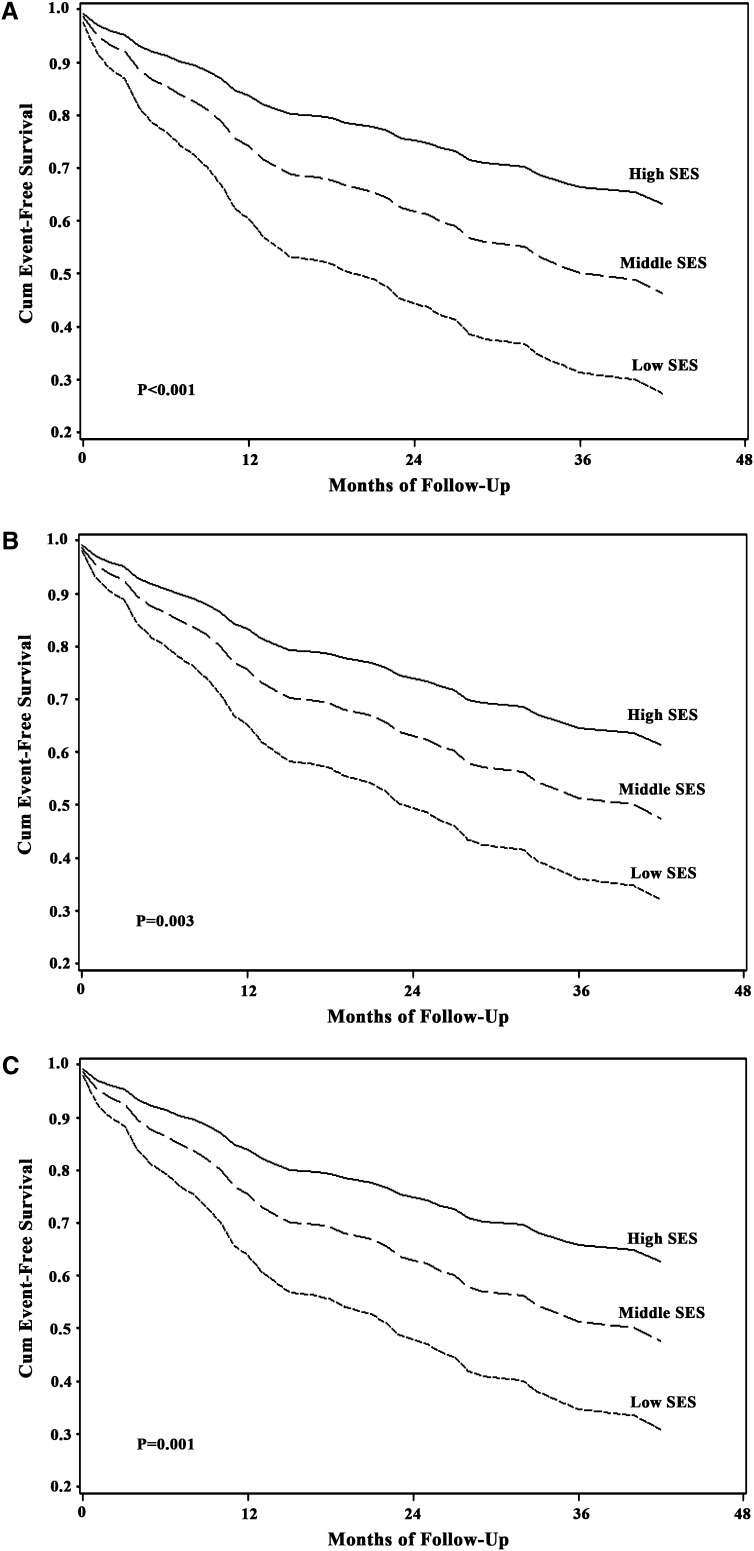
The 1-year overall survival was 84.8%, and the 3-year survival was 71.1%. Patients with a lower SES had higher unadjusted mortality rates, with 3-year survival estimates of 50.1, 70.8, and 86.0% in increasing tertiles of SES (P for trend < 0.001). The SES level was significantly associated with the risk of death (Table 2, Figure 1). A lower SES was associated with a higher all-cause mortality rate after adjustment for age and sex (P < 0.001) (Table 2, Figure 1A). This association persisted after the addition of WHO FC and PVR (P = 0.008) (Table 2, Figure 1B). Further adjustment for the use of targeted medical treatments (or their combination) did not affect the results (P = 0.006) (Table 2, Figure 1C). In all models, patients in the lowest tertile of SES had almost triple the risk of death of those in the highest tertile. Additional adjustment for the presence of a pericardial effusion, acute vasoreactivity, and other covariates did not change these results.
TABLE 2.
HAZARD RATIOS OF ALL-CAUSE MORTALITY ASSOCIATED WITH SOCIOECONOMIC STATUS TERTILES
| Socioeconomic Status Tertiles |
||||
| Adjustment | Low (n = 73) | Middle (n = 90) | High (n = 99) | P for Trend |
| Deaths (n, %) | 30 (41.4) | 21 (23.3) | 13 (13.1) | |
| Model 1* | 3.65 (1.88–7.02) | 1.88 (0.94–3.76) | 1.0 | <0.001 |
| Model 2† | 2.87 (1.47–5.62) | 1.89 (0.94–3.80) | 1.0 | 0.008 |
| Model 3‡ | 2.98 (1.51–5.89) | 1.79 (0.89–3.63) | 1.0 | 0.006 |
Data are presented as hazard ratio (95% confidence interval) unless otherwise stated.
Model 1: adjusted for age and sex.
Model 2: Model 1 plus adjustment for World Health Organization functional classification and pulmonary vascular resistance.
Model 3: Model 2 plus adjustment for phosphodiesterase type 5 inhibitors, endothelin receptor antagonists, and prostanoids.
Figure 1.
Multivariable adjusted survival curves for death stratified by socioeconomic status (SES) tertiles. (A) Adjusted for age and sex. (B) Adjusted for age, sex, World Health Organization functional classification, and pulmonary vascular resistance. (C) Adjusted for age, sex, World Health Organization functional classification, pulmonary vascular resistance, and use of phosphodiesterase type 5 inhibitors, endothelin receptor antagonists, and prostanoids. Cum = cumulative.
One hundred five patients had clinical worsening events during follow-up, including 40 hospitalizations, 26 changes of therapy (10 were low-SES patients, 10 were middle-SES patients, and 6 were from the highest SES group), and 39 deaths. No patient received a lung or heart-lung transplant. For patients with more than one event, only the first event was used in the analysis. The level of SES was significantly associated with the risk of clinical worsening (Table 3, Figure 2). A lower SES was associated with a higher risk of clinical worsening after adjustment for age and sex (P < 0.001) (Table 3, Figure 2A). This association was somewhat weaker but persisted after adjustment for WHO FC and PVR (P = 0.003) (Table 3, Figure 2B). If anything, further adjustment for the use of targeted medical treatments (or their combination) strengthened the association (P = 0.001) (Table 3, Figure 2C). In all models, patients in the lowest tertile of SES had more than double the risk of clinical worsening of those in the highest tertile. Additional adjustment for the presence of a pericardial effusion, acute vasoreactivity, and other covariates did not change these results.
TABLE 3.
HAZARD RATIOS OF CLINICAL WORSENING EVENTS ASSOCIATED WITH SOCIOECONOMIC STATUS TERTILES
| Socioeconomic Status Tertiles |
||||
| Adjustment | Low (n = 73) | Middle (n = 90) | High (n = 99) | P for Trend |
| Events (n, %) | 47 (64.4) | 35 (38.9) | 27 (27.3) | |
| Model 1* | 2.85 (1.75–4.65) | 1.68 (1.00–2.80) | 1.0 | <0.001 |
| Model 2† | 2.37 (1.44–3.91) | 1.68 (1.01–2.81) | 1.0 | 0.003 |
| Model 3‡ | 2.60 (1.56–4.35) | 1.76 (1.04–2.96) | 1.0 | 0.001 |
Data are presented as hazard ratio (95% confidence interval) unless otherwise stated. Clinical worsening was defined by the combined endpoint of death, lung transplantation, hospitalization, or the addition of or switch to other active therapies because of worsening pulmonary arterial hypertension or right heart failure.
Model 1: adjusted for age and sex.
Model 2: Model 1 plus adjustment for World Health Organization functional classification and pulmonary vascular resistance.
Model 3: Model 2 plus adjustment for phosphodiesterase type 5 inhibitors, endothelin receptor antagonists and prostanoids.
Figure 2.
Multivariable adjusted survival curves for clinical worsening stratified by socioeconomic status (SES) tertiles. (A) Adjusted for age and sex. (B) Adjusted for age, sex, World Health Organization functional classification, and pulmonary vascular resistance. (C) Adjusted for age, sex, World Health Organization functional classification, pulmonary vascular resistance, and use of phosphodiesterase type 5 inhibitors, endothelin receptor antagonists, and prostanoids. Cum = cumulative.
Discussion
This prospective cohort study has shown that a lower SES is associated with higher mortality and increased risk of clinical worsening in patients with IPAH. These associations were independent of clinical features and hemodynamics at baseline. Although patients with IPAH with a lower SES may have used different initial medical treatment regimens, the findings persisted despite adjustment for the therapies used and other clinical variables, showing that they did not account for the findings. The highest SES group had more vasoreactivity than the other groups. However, the differences in outcome persisted after adjustment for acute vasoreactivity, suggesting that vasoreactivity did not explain the health disparities related to SES in patients with PAH. Adjustment for WHO FC and PVR attenuated the mortality and clinical worsening risks somewhat, indicating that differences in the severity of IPAH may explain some (but not all) of the increased risk associated with a lower SES.
To our knowledge, no published prospective studies have evaluated the impact of SES on PAH. Our findings document the serious implications for patients with PAH with a low SES. Components of SES that could lead to disparities in health include the social environment, psychology, behavior, and physical environment (32). PAH is a rare disease, which is best diagnosed and treated in certain tertiary care centers in China, nearly all of which are located in urban settings. Community hospitals commonly lack the expertise and technology to care for these patients. The ability and timeliness of accessing specialty care could lead to patients in rural lower SES settings visiting their PAH physician less often and later in the course of their illness than urban residents with a higher SES (33, 34). The increased risk associated with the lowest SES was somewhat explained by factors such as WHO FC and PVR, suggesting that delay in evaluation was a possible cause. However, we found that the time from symptom onset to initial evaluation and the number of visits and assessments were similar at all levels of SES, and the differences in outcome persisted despite adjustment for baseline hemodynamics, suggesting that there are other factors that accounted for the increased risk in the lower SES tertile.
Effective therapies for patients with PAH are not universally available in China (35–38). Most hospitals do not routinely stock PAH-targeted medicines, such as bosentan, iloprost, and vardenafil. Although there are some patient assistance programs (39), which may reduce costs, these are only available at a few centers in large cities such as Shanghai, Peking, and Guangzhou. Patients may have to travel long distances to reach these hospitals to obtain refills of their medications. This inequitable distribution of public services (33) could lead to an increasing risk of death and clinical worsening for lower-SES patients.
In 2009, the National Bureau of Statistics of China estimated that the mean annual household income was only RMB 5,153(≈$800) per person in rural areas and RMB 17,175 (≈$2,700) per person in urban areas (40). The out-of-pocket cost for an average hospital admission is almost equivalent to China’s per capita annual income (41). In 2003, 14.9% of urban and 21.5% of rural residents had financial difficulties in accessing healthcare, and 69% of urban and 93% of rural residents were discharged early from the hospital (42). PAH-targeted therapies impose a tremendous economic burden on Chinese patients as they are not covered by insurance. The annual costs for a single patient are RMB 21,400 to 35,640 (≈$3,300–$5,500) for sildenafil, RMB 237,600 (≈$36,700) for bosentan, and RMB 600,000 (≈$ 92,700) for iloprost. Even in the charitable drug programs, patients must pay nearly RMB 52,100 (≈$8,100) for bosentan and RMB 60,200 (≈$9,300) for iloprost per year. These financial barriers may limit patients’ access to health services and appropriate treatment, imposing a disproportionate burden on those with a lower SES (14, 41). There was a significant difference in ERA use among tertiles of SES, which might be partly responsible for the disparities. However, ERAs have not shown a significant impact on survival, and the lowest SES group still had three times the risk of death of the highest SES group after adjustment for ERA use. This suggests that it is unlikely that this dramatic difference in outcome would only be attributable to ERAs. Differential adherence to prescribed PAH treatment could account for our results; however, adherence is notoriously difficult to measure and was not assessed in this study.
SES also has behavioral components, potentially affecting the likelihood of enacting health-related activities (43) such as scheduled medication-taking and exercise. Behaviors rely on knowledge and access to information, such as a person’s perceptions of risk and severity, self-efficacy beliefs, and outcome expectations. Patients with a lower SES may lack education and health literacy and therefore may have behavior-related risk factors for worse outcomes (44). It may more difficult for lowest-SES patients to comply with treatment or to remain active, leading to deconditioning.
In addition, a life-threatening disease and expensive treatment costs may result in anxiety and depression, which could especially affect the low SES group (45, 46). These mental illnesses increase as the severity of PAH progresses (47) and may contribute to the treatment outcome (48–50). Finally, environmental exposures (which are occupation related) and diet (such as sodium consumption) may differ among individuals at different SES levels.
Our findings suggest the need for reducing social gradients and improving healthcare services for patients with PAH, especially those with a disadvantaged SES. Increased affordability of therapies via increased access to medical insurance, reduced costs of medical care, or supplemented income among the low SES population might lessen the disparities shown (24). Fortunately, Chinese authorities have launched major reforms of the healthcare system to address these problems (42).
Different countries have different national conditions, whereas socioeconomic differences in health tend to occur in all countries (51). Although this study is from a developing country, the association between lower SES and poorer health outcomes in patients with chronic disease is well documented in developed countries (7–9). Furthermore, economic burden (18), inequitable distribution of public services (34), adherence to treatment, and other factors may also exist in developed countries. The magnitude of the differentials in health and mortality can vary across countries with distinct economic, political, and social policies (51). Further studies need to be performed in Western countries, and we hope our study will serve as the nidus for future research to better define the mechanisms explaining the risks associated with a low SES. Further research of the social and environmental risk factors that may account for these differences (which likely exist in other countries) could result in interventions to lessen the disparities in outcome for patients with a lower SES. Physicians should appreciate the potential risks associated with a low SES and possibly target these individuals to reduce their risks via effective and equitable secondary prevention.
This study has several limitations. First, the cohort was recruited from a single, tertiary-care center for pulmonary vascular disease. Many patients with PAH, especially those in rural areas, only have access to community hospitals or small clinics, which may have caused selection bias in our study sample. However, as a national and specialized referral center, we have close ties with community hospitals and draw many patients with suspected PAH from these hospitals, lessening the potential bias. Second, personal SES information was self-reported at baseline and could change over time. This could result in misclassification but would likely bias to the null. In this case, the associations between SES and outcome may be even stronger than we have shown. Third, we defined hospitalization or initiation of new target drugs as clinical worsening, which may depend on the SES. Patients with lower SES may not be hospitalized and receive additional therapy as frequently as patients with higher SES due to financial disadvantages, which may also bias the results toward the null. In this case, the associations between SES and clinical worsening may be even stronger than shown. Finally, we did not collect data on neighborhood SES, which could have important modifying effects on the link between individual SES and outcomes. Despite this, it is likely that individual SES indicators should serve, at least partly, as a proxy for unmeasured characteristics of the environment.
In conclusion, we found that patients with PAH with a lower SES have an increased risk for mortality and clinical worsening independently of clinical characteristics and other risk factors. We believe that policy leaders need to be aware of this difference and urgently develop policies to reduce the burden on patients with PAH, and physicians should consider the potential impact in lower-SES patients.
Supplementary Material
Acknowledgments
The authors thank all investigators who participated in this study. They also thank the patients who participated in the study.
Footnotes
Funded by the Program for Shanghai Outstanding Academic Leader, the “Dawn” Program of Shanghai Education Committee (DPSEC-10SG25), the Program of New Century Excellent Talents in University (NCET-10-0630), Shen Kang Major Joint Research Project on Emerging Technology in Municipal Hospital (shdc-12010102), the Innovation Program of Shanghai Municipal Education Commission (09ZZ35), and National Institutes of Health grant K24 HL103844.
The funding sources provided financial support for the study but played no role in the study design or conduct, data collection and analysis, or the decision to submit the manuscript for publication.
Author Contributions: W.-H.W., L.Y., and F.-H.P. contributed to the study design and performance, data acquisition, analysis and interpretation, and preparation of the manuscript. J.Y. was the research nurse in this study; she and L.G. contributed to study performance and data acquisition. L.-L.Z. and D.L. contributed to statistical analysis of the data. X.J. contributed to data acquisition and manuscript preparation; J.L. and J.-M.Q. contributed to manuscript editing and revision. S.M.K. contributed to data analysis and interpretation, manuscript editing, and revision. Z.-C.J. contributed to the study conception and design, revising the article critically for important intellectual content, and final approval of the version to be published. All authors had full access to all study data and had final responsibility for the decision to submit for publication. All have reviewed the manuscript and approved the final version for submission.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201207-1290OC on December 6, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Rubin LJ. Primary pulmonary hypertension. N Engl J Med 1997;336:111–117 [DOI] [PubMed] [Google Scholar]
- 2.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc., and the Pulmonary Hypertension Association. J Am Coll Cardiol 2009;53:1573–1619 [DOI] [PubMed] [Google Scholar]
- 3.Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009;30:2493–2537 [DOI] [PubMed] [Google Scholar]
- 4.O’Callaghan DS, Savale L, Montani D, Jais X, Sitbon O, Simonneau G, Humbert M. Treatment of pulmonary arterial hypertension with targeted therapies. Nat Rev Cardiol 2011;8:526–538 [DOI] [PubMed] [Google Scholar]
- 5.Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med 2004;351:1425–1436 [DOI] [PubMed] [Google Scholar]
- 6.Clark AM, DesMeules M, Luo W, Duncan AS, Wielgosz A. Socioeconomic status and cardiovascular disease: risks and implications for care. Nat Rev Cardiol 2009;6:712–722 [DOI] [PubMed] [Google Scholar]
- 7.Avendano M, Kunst AE, Huisman M, Lenthe FV, Bopp M, Regidor E, Glickman M, Costa G, Spadea T, Deboosere P, et al. Socioeconomic status and ischaemic heart disease mortality in 10 Western European populations during the 1990s. Heart 2006;92:461–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shishehbor MH, Litaker D, Pothier CE, Lauer MS. Association of socioeconomic status with functional capacity, heart rate recovery, and all-cause mortality. JAMA 2006;295:784–792 [DOI] [PubMed] [Google Scholar]
- 9.Salomaa V, Niemela M, Miettinen H, Ketonen M, Immonen-Raiha P, Koskinen S, Mahonen M, Lehto S, Vuorenmaa T, Palomaki P, et al. Relationship of socioeconomic status to the incidence and prehospital, 28-day, and 1-year mortality rates of acute coronary events in the FINMONICA myocardial infarction register study. Circulation 2000;101:1913–1918 [DOI] [PubMed] [Google Scholar]
- 10.Prescott E, Vestbo J. Socioeconomic status and chronic obstructive pulmonary disease. Thorax 1999;54:737–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Najera-Ortiz JC, Sanchez-Perez HJ, Ochoa-Diaz H, Arana-Cedeno M, Lezama MS, Mateo MM. Demographic, health services and socio-economic factors associated with pulmonary tuberculosis mortality in Los Altos region of Chiapas, Mexico. Int J Epidemiol 2008;37:786–795 [DOI] [PubMed] [Google Scholar]
- 12.Wu J, Liu Y, Rao K, Sun Q, Qian J, Li Z. Education-related gender differences in health in rural china. Am J Public Health 2004;94:1713–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanibuchi T, Nakaya T, Murata C. Socio-economic status and self-rated health in East Asia: a comparison of China, Japan, South Korea and Taiwan. Eur J Public Health 2012;22:47–52 [DOI] [PubMed] [Google Scholar]
- 14.Tang S, Meng Q, Chen L, Bekedam H, Evans T, Whitehead M. Tackling the challenges to health equity in China. Lancet 2008;372:1493–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang LD, Ma XW. Centre for Health Statistics and Information, Ministry of Health. An analysis report of national health services survey in 2003. Beijing: Peking Union Medical University Press; 2004: p. 86.
- 16.Wilkens H, Grimminger F, Hoeper M, Stahler G, Ehlken B, Plesnila-Frank C, Berger K, Resch A, Ghofrani A. Burden of pulmonary arterial hypertension in Germany. Respir Med 2010;104:902–910 [DOI] [PubMed] [Google Scholar]
- 17.Hoeper MM, Faulenbach C, Golpon H, Winkler J, Welte T, Niedermeyer J. Combination therapy with bosentan and sildenafil in idiopathic pulmonary arterial hypertension. Eur Respir J 2004;24:1007–1010 [DOI] [PubMed] [Google Scholar]
- 18.Kirson NY, Birnbaum HG, Ivanova JI, Waldman T, Joish V, Williamson T. Excess costs associated with patients with pulmonary arterial hypertension in a us privately insured population. Appl Health Econ Health Policy 2011;9:293–303 [DOI] [PubMed] [Google Scholar]
- 19.Winkleby MA, Jatulis DE, Frank E, Fortmann SP. Socioeconomic status and health: how education, income, and occupation contribute to risk factors for cardiovascular disease. Am J Public Health 1992;82:816–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med 2006;173:1023–1030 [DOI] [PubMed] [Google Scholar]
- 21.Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, Frost A, Barst RJ, Badesch DB, Elliott CG, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 2010;122:164–172 [DOI] [PubMed] [Google Scholar]
- 22.Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, Elliott CG, Gaine SP, Gladwin MT, Jing ZC, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2009;54:S43–S54 [DOI] [PubMed] [Google Scholar]
- 23.Kaplan GA, Keil JE. Socioeconomic factors and cardiovascular disease: a review of the literature. Circulation 1993;88:1973–1998 [DOI] [PubMed] [Google Scholar]
- 24.Niu S, Zhao D, Zhu J, Liu J, Liu Q, Liu J, Wang W, Smith SJ. The association between socioeconomic status of high-risk patients with coronary heart disease and the treatment rates of evidence-based medicine for coronary heart disease secondary prevention in China: results from the Bridging the Gap on CHD Secondary Prevention in China (BRIG) project. Am Heart J 2009;157:709–715 [DOI] [PubMed] [Google Scholar]
- 25.Ezeamama AE, Viali S, Tuitele J, McGarvey ST. The influence of socioeconomic factors on cardiovascular disease risk factors in the context of economic development in the Samoan archipelago. Soc Sci Med 2006;63:2533–2545 [DOI] [PubMed] [Google Scholar]
- 26.Singh RB, Beegom R, Mehta AS, Niaz MA, De AK, Mitra RK, Haque M, Verma SP, Dube GK, Siddiqui HM, et al. Social class, coronary risk factors and undernutrition, a double burden of diseases, in women during transition, in five Indian cities. Int J Cardiol 1999;69:139–147 [DOI] [PubMed] [Google Scholar]
- 27.ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111–117 [DOI] [PubMed] [Google Scholar]
- 28.Sitbon O, Humbert M, Jais X, Ioos V, Hamid AM, Provencher S, Garcia G, Parent F, Herve P, Simonneau G. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation 2005;111:3105–3111 [DOI] [PubMed] [Google Scholar]
- 29.Jing ZC, Jiang X, Han ZY, Xu XQ, Wang Y, Wu Y, Lv H, Ma CR, Yang YJ, Pu JL. Iloprost for pulmonary vasodilator testing in idiopathic pulmonary arterial hypertension. Eur Respir J 2009;33:1354–1360 [DOI] [PubMed] [Google Scholar]
- 30.Schrader BJ, Inbar S, Kaufmann L, Vestal RE, Rich S. Comparison of the effects of adenosine and nifedipine in pulmonary hypertension. J Am Coll Cardiol 1992;19:1060–1064 [DOI] [PubMed] [Google Scholar]
- 31.Ghali WA, Quan H, Brant R, van Melle G, Norris CM, Faris PD, Galbraith PD, Knudtson ML. Comparison of 2 methods for calculating adjusted survival curves from proportional hazards models. JAMA 2001;286:1494–1497 [DOI] [PubMed] [Google Scholar]
- 32.Adler NE, Boyce T, Chesney MA, Cohen S, Folkman S, Kahn RL, Syme SL. Socioeconomic status and health. The challenge of the gradient. Am Psychol 1994;49:15–24 [DOI] [PubMed] [Google Scholar]
- 33.Anand S, Fan VY, Zhang J, Zhang L, Ke Y, Dong Z, Chen LC. China’s human resources for health: quantity, quality, and distribution. Lancet 2008;372:1774–1781 [DOI] [PubMed] [Google Scholar]
- 34.van Dis J. MSJAMA. Where we live: health care in rural vs urban America. JAMA 2002;287:108. [PubMed] [Google Scholar]
- 35.Zhang R, Dai LZ, Xie WP, Yu ZX, Wu BX, Pan L, Yuan P, Jiang X, He J, Humbert M, et al. Survival of Chinese patients with pulmonary arterial hypertension in the modern treatment era. Chest 2011;140:301–309 [DOI] [PubMed] [Google Scholar]
- 36.Jing ZC, Yu ZX, Shen JY, Wu BX, Xu KF, Zhu XY, Pan L, Zhang ZL, Liu XQ, Zhang YS, et al. Vardenafil in pulmonary arterial hypertension: a randomized, double-blind, placebo-controlled study. Am J Respir Crit Care Med 2011;183:1723–1729 [DOI] [PubMed] [Google Scholar]
- 37.Jing ZC, Strange G, Zhu XY, Zhou DX, Shen JY, Gu H, Yang ZK, Pan X, Xiang MX, Yao H, et al. Efficacy, safety and tolerability of bosentan in Chinese patients with pulmonary arterial hypertension. J Heart Lung Transplant 2010;29:150–156 [DOI] [PubMed] [Google Scholar]
- 38.Xu XQ, Jing ZC, Zhang JH, Wu Y, Wang Y, Jiang X, Wang ZX, Sun YG, Pu JL, Yang YJ. The efficacy and safety of sildenafil in Chinese patients with pulmonary arterial hypertension. Hypertens Res 2009;32:911–915 [DOI] [PubMed] [Google Scholar]
- 39.China Charity Federation: the Tracleer project [accessed 2012 May 1]. Available from: http://www.tpapchina.org/
- 40.National Bureau of Statistics of China. 2009 [accessed 2012 May 1]. Available from: http://www.stats.gov.cn/english/statisticaldata/yearlydata/
- 41.Hu S, Tang S, Liu Y, Zhao Y, Escobar ML, de Ferranti D. Reform of how health care is paid for in China: challenges and opportunities. Lancet 2008;372:1846–1853 [DOI] [PubMed] [Google Scholar]
- 42.Liu Y, Rao K, Wu J, Gakidou E. China’s health system performance. Lancet 2008;372:1914–1923 [DOI] [PubMed] [Google Scholar]
- 43.Adler NE, Ostrove JM. Socioeconomic status and health: what we know and what we don’t. Ann N Y Acad Sci 1999;896:3–15 [DOI] [PubMed] [Google Scholar]
- 44.Lantz PM, House JS, Lepkowski JM, Williams DR, Mero RP, Chen J. Socioeconomic factors, health behaviors, and mortality: results from a nationally representative prospective study of US adults. JAMA 1998;279:1703–1708 [DOI] [PubMed] [Google Scholar]
- 45.Ansseau M, Fischler B, Dierick M, Albert A, Leyman S, Mignon A. Socioeconomic correlates of generalized anxiety disorder and major depression in primary care: the GADIS II study (Generalized Anxiety and Depression Impact Survey II). Depress Anxiety 2008;25:506–513 [DOI] [PubMed] [Google Scholar]
- 46.Lorant V, Deliege D, Eaton W, Robert A, Philippot P, Ansseau M. Socioeconomic inequalities in depression: a meta-analysis. Am J Epidemiol 2003;157:98–112 [DOI] [PubMed] [Google Scholar]
- 47.Lowe B, Grafe K, Ufer C, Kroenke K, Grunig E, Herzog W, Borst MM. Anxiety and depression in patients with pulmonary hypertension. Psychosom Med 2004;66:831–836 [DOI] [PubMed] [Google Scholar]
- 48.Herrmann C, Brand-Driehorst S, Buss U, Ruger U. Effects of anxiety and depression on 5-year mortality in 5,057 patients referred for exercise testing. J Psychosom Res 2000;48:455–462 [DOI] [PubMed] [Google Scholar]
- 49.Barefoot JC, Helms MJ, Mark DB, Blumenthal JA, Califf RM, Haney TL, O’Connor CM, Siegler IC, Williams RB. Depression and long-term mortality risk in patients with coronary artery disease. Am J Cardiol 1996;78:613–617 [DOI] [PubMed] [Google Scholar]
- 50.Frasure-Smith N, Lesperance F, Habra M, Talajic M, Khairy P, Dorian P, Roy D. Elevated depression symptoms predict long-term cardiovascular mortality in patients with atrial fibrillation and heart failure. Circulation 2009;120:134–140 [DOI] [PubMed] [Google Scholar]
- 51.National Research Council (US) Panel on Understanding Divergent Trends in Longevity in High-Income Countries; Crimmins EM, Preston SH, Cohen B, editors. Explaining divergent levels of longevity in high-income countries. Washington DC: National Academies Press (US); 2011. 9, The role of inequality [accessed 2012 Oct 1]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK62362/ [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.