Abstract
White adipose tissue (WAT) is becoming widely used in regenerative medicine/cell therapy applications, and its physiological and pathological importance is increasingly appreciated. WAT is a complex organ composed of differentiated adipocytes, stromal mesenchymal progenitors known as adipose stromal cells (ASC), as well as endothelial vascular cells and infiltrating leukocytes. Two-dimensional (2D) culture that has been typically used for studying adipose cells does not adequately recapitulate WAT complexity. Improved methods for reconstruction of functional WAT ex vivo are instrumental for understanding of physiological interactions between the composing cell populations. Here, we used a three-dimensional (3D) levitation tissue culture system based on magnetic nanoparticle assembly to model WAT development and growth in organoids termed adipospheres. We show that 3T3-L1 preadipocytes remain viable in spheroids for a long period of time, while in 2D culture, they lose adherence and die after reaching confluence. Upon adipogenesis induction in 3T3-L1 adipospheres, cells efficiently formed large lipid droplets typical of white adipocytes in vivo, while only smaller lipid droplet formation is achievable in 2D. Adiposphere-based coculture of 3T3-L1 preadipocytes with murine endothelial bEND.3 cells led to a vascular-like network assembly concomitantly with lipogenesis in perivascular cells. Adipocyte-depleted stromal vascular fraction (SVF) of mouse WAT cultured in 3D underwent assembly into organoids with vascular-like structures containing luminal endothelial and perivascular stromal cell layers. Adipospheres made from primary WAT cells displayed robust proliferation and complex hierarchical organization reflected by a matricellular gradient incorporating ASC, endothelial cells, and leukocytes, while ASC quickly outgrew other cell types in adherent culture. Upon adipogenesis induction, adipospheres derived from the SVF displayed more efficient lipid droplet accumulation than 2D cultures. This indicates that 3D intercellular signaling better recapitulates WAT organogenesis. Combined, our studies show that adipospheres are appropriate for WAT modeling ex vivo and provide a new platform for functional screens to identify molecules bioactive toward individual adipose cell populations. This 3D methodology could be adopted for WAT transplantation applications and aid approaches to WAT-based cell therapy.
Introduction
Recovery from various pathological conditions involves tissue remodeling and repair. These processes are integral for healing either after tissue reconstruction surgeries or post-tissue damage often caused by disease. Success of tissue repair relies on stem cells and partially differentiated progenitor cells present in the grafted tissue and/or recruited from endogenous organs.1 Bone marrow is a bona fide source of progenitor cells activated in response to trauma.2 However, because the quantity and ability of the bone marrow progenitors to respond to mobilization stimuli appears to decline with age, the contribution of cells recruited for injury repair is likely to progressively decrease in parallel. Instead, some extramedullary organs, such as white adipose tissue (WAT), have been shown to ectopically accumulate endothelial and hematopoietic progenitors.3 On the other hand, the importance of stromal mesenchymal progenitors, commonly referred to as mesenchymal stromal cells (MSC), in tissue repair has been increasingly appreciated.4,5 MSC had been originally characterized in the bone marrow as fibroblast colony-forming units. MSC are not only capable of differentiating into adipocytes, osteoblasts, and chondrocytes, which has resulted in the term mesenchymal stem cells,6,7 but also support vascularization as trophic pericytic cells and suppress the immune response.8 These combined features have made bone marrow MSC as a cell type of choice for numerous clinical trials that are currently in progress. In the meanwhile, organs such as WAT have been shown as a considerably more abundant reservoir of mesenchymal progenitors.9 This has led to an explosion of interest in the potential of WAT in regenerative medicine and cell therapy applications.10 The potential of using ex vivo engineered WAT for angiogenic tissue grafting has become an emerging concept.11,12
WAT develops throughout the mammalian body in areas of loose connective tissue, such as subcutaneous layers between muscle and dermis. In addition, visceral WAT depots also form around the gut, heart, kidneys, and other internal organs.13 The main cellular components of WAT are adipocytes, the large cells accumulating triglycerides in lipid droplets.9 The remaining cells composing the stromal vascular fraction (SVF) include perivascular adipose stromal cells (ASC) serving as adipocyte progenitors, as well as vascular endothelial cells and infiltrating leukocytes.14,15 We and others have shown that ASC display multipotency and proliferation capacity comparable to those of bone marrow MSC while also serving as pericytes.16–19 ASC promote endothelial proliferation and blood vessel formation at least in part via trophic effects of secreted growth factors, while also displaying marked anti-inflammatory properties.8 These features of WAT have made grafts of adipose tissue fragments or cells (lipotransfer) a promising approach to cosmetic and functional tissue repair.20 In parallel, approaches to de novo tissue engineering based on ASC have been developed.21
With subcutaneous WAT being readily harvestable, hundreds of regenerative therapy clinical trials are underway.22 Obesity is a result of WAT hypertrophy and hyperplasia, with the latter relying on the expansion of ASC and preadipocytes.9 The increased accessibility of progenitor cells has made WAT from obese individuals particularly attractive as a graft source. On the other hand, the emerging association between WAT expansion in obesity and various diseases has alerted for caution.8 One of the key obesity complications is the metabolic syndrome, a medical condition that is a risk and comorbidity factor for insulin resistance, diabetes, dyslipidemia, and cardiovascular disease.23 Recently, obesity has surfaced as the condition associated with risk and progression of a number of cancers.24,25 This has been attributed to the endocrine function of WAT that secrets numerous growth and inflammatory factors and hormones collectively termed adipokines.26 Recent studies have shown that WAT-derived cells can be mobilized27,28 and recruited as a component of tumor microenvironment,29,30 suggesting adipokine activity at the cancer site. Several leukocyte populations, including macrophages and lymphocytes, progressively concentrate in WAT as obesity progresses,31 and their contribution to the complex secretome of WAT may be key in the obesity–cancer association. The migratory capacity and plasticity of monocytes raise the possibility that they may also traffic from WAT to tumors and contribute to cancer in patients. The apparent crosstalk between WAT and cancer confirmed in a number of recent studies and tumorigenesis associated with lipotransfer for cosmetic tissue reconstruction32 sends a clear message that WAT needs to be better investigated for its potential pathological functions.33
The prospective beneficial properties and pathogenesis associated with WAT calls for streamlining efforts in systematic analysis of this tissue, which could be most efficient in ex vivo screening formats. However, a challenge in adipose tissue biology is the lack of adequate culture systems simulating WAT physiology. While adherent cell cultures are widely used to study the process of adipogenesis (differentiation of ASC into adipocytes), two-dimensional (2D) cultures do not recapitulate the native tissue complexity. Indeed, when the SVF cells are plated in conventional culture conditions, endothelial and hematopoietic cells are lost upon cell passaging, while the mesenchymal compartment takes over.15,17,19 In addition, the morphology, immunophenotype, and gene expression profile of cells instantly changes upon plastic attachment of primary cells.8 This has made it difficult to assess the roles of individual WAT cell populations and to identify functional molecules and markers of WAT cells that could be used for therapeutic targeting.
Here, to establish a tissue culture model simulating the complex intercellular interactions of WAT components, we took advantage of a recently reported three-dimensional (3D) cell culturing system based on magnetic levitation.34 By facilitating aggregation of different populations of adipose cells using a magnetic nanoparticle assembly and designed magnetic fields, we achieved consistent generation of organoids termed adipospheres that display key properties of endogenous WAT. We demonstrate that this 3D culturing system allows the retention of multicellular WAT complexity and partial vascularization concomitant with efficient cell proliferation and differentiation of preadipocytes.
Materials and Methods
Cell culture
3T3-L1 preadipocyte cells and bEND.3 endothelial cells were purchased from the American Type Tissue Collection. To obtain green fluorescent protein (GFP)-labeled bEND.3, cells were transduced with lentivirus harboring GFP generated using the Lenti-X HTX system,19 according to the manufacturer's protocol (Clontech). Cells were 2D cultured in the Dulbecco's modified Eagle medium (DMEM) containing 10% (v/v) fetal bovine serum (FBS), 100 U/mL of penicillin, and 100 mg/mL of streptomycin to obtain ∼80% confluency. For coculture, counted cells were mixed at the ratio of 95:5 (3T3-L1:bEND.3-GFP) before levitation.
Preparation of mouse primary SVF
The SVF from mixed subcutaneous and intraperitoneal WAT was isolated by enzymatic digestion as described.19 Nucleated cells were counted and plated on uncoated culture plate overnight in DMEM/10% FBS (v/v) to obtain ∼80% cell confluency for levitation culture setup or to 100% confluency for control 2D cultures.
Magnetic cell levitation
Three-dimensional levitation cell cultures were based on previously established methodology34 and were set up using the 6- or 24-well Bio-Assembler™ kit (Nano3D Biosciences™, Inc.) consisting of nanoshuttle (NS) solution and a 6-well or 24-well plate magnetic drive. The NS is a nanoparticle assembly of iron oxide (Fe2O3) and gold (Au) nanoparticles cross-linked with poly-l-lysine to promote cellular uptake. The 6- or 24-well plates used for 3D culture were flat bottom ultralow attachment plates (Costar®, 3471 or 3473, respectively). As shown in Figure 1A, indicated numbers of cultured or primary cells were mixed with 8 μL of NS per cm2 plate area (or ∼10,000 cells per μL of NS) and placed in a standard CO2 cell culture incubator (37°C, 5% CO2 [v/v] in air) for 12 h in standard adherent culture conditions. Cells were then trypsinized to detach adherent cells from plate and to obtain single-cell suspension. After trypsin inactivation with serum, cells were counted, centrifuged, and seeded into a multiwell ultralow-attachment plate. The medium volume in each well was 1.0 mL and 0.3 mL for the 6-well and 24-well plate, respectively. A magnetic drive was immediately placed above the culture to magnetically levitate the cells and guide them to aggregate within hours of levitation. The cells stay levitated just below the meniscus at the center of the well, where they self-assemble into spheroids. Levitated spheroids were incubated in a CO2 incubator until analysis at indicated time points. Cultures can be easily visualized while levitating with standard inverted microscopes by just positioning the culture plates on the microscope stand without removing the magnet drive and by allowing light to be transmitted through the magnet opening (magnet drives are made of ring magnets to allow light transmission). Furthermore, because 3D cultures are magnetized, the same magnet drives that are used to enable magnetic levitation can be used to facilitate medium exchange throughout the 3D cell culturing process. This is accomplished by removing the magnet drive from the top and placing it at the bottom of the 6- or 24-well tissue culture plate. After medium exchange, magnet drive can be placed back on the top of the culture to resume levitation.
FIG. 1.
The setup and test of magnetic levitation system for adipocyte culture. (A) Subsequent steps of the levitation 3D cell culture setup where the steps are as follows: cells and magnetic particles are placed into a culture plate well; a magnet driver is placed above this well; cells levitate to the meniscus; and finally, cells aggregate/self-assemble into a spheroid (see the Materials and Methods section for details). (B) Representative wells with spheroids of 3T3-L1 preadipocytes initiated by seeding 2.4×105 cells and cultured in a basal (left) or adipogenesis induction (right) medium for 14 days. (C) Spheroids of 3T3-L1 preadipocytes display buoyancy (right tube, solid arrow) in phosphate-buffered saline 14 days postinduction of adipogenesis due to lipid accumulation. The tube on the left shows a control spheroid cultured without induction at the bottom of the tube (no buoyancy, hollow arrow). (D) Phase-contrast micrographs of 3T3-L1 spheroids initiated by seeding 2.4×105 cells after 8 days of levitation in either a basal (left) or adipogenesis induction medium (right) with peripheral adipocytes containing large lipid droplets indicated (arrows). (E) Paraffin sections of spheroids in (D) subjected to immunofluorescence with perilipin antibodies (red), indicating lipid droplet maturation in adipocytes composing the spheroid upon culture in the adipogenesis induction medium. (F) Frozen sections of spheroids in (D) stained with Oil Red O (red arrows), indicating lipid accumulation in adipocytes differentiating in spheroid upon culture in an adipogenesis induction medium. Scale bar: 100 μm. 3D, three-dimensional. Color images available online at www.liebertpub.com/tec
Adipogenesis induction
All 2D or 3D cultures levitated for 1 day were induced for adipogenenic differentiation with a conventional medium19 consisting of 0.5 mM isobutylmethyxanthine, 1 μM dexamethasone, 0.2 mM indomethacin, and 1.7 μM insulin (all from Sigma-Aldrich) in DMEM/10% FBS (v/v) for 72 h. After this, the induction medium was replaced with DMEM/10% FBS containing 1.7 μM insulin, which was subsequently replaced every 2 days until analysis at the indicated time points.
Cell analysis by immunofluorescence
Cell cultures were fixed in 4% paraformaldehyde for 30 min, washed with phosphate-buffered saline (PBS), and then processed for whole-mount immunofluorescence analysis or embedded for frozen or paraffin sectioning. For whole-mount immunostaining, cultures were permeabilized with 0.3% Triton-X in PBS for 20 min. Whole-mount samples were magnetically held at the bottom of the slide by placing a magnet (magnet drive from Bio-Assember) under the microwell carrying the sample. For section analysis, citrate buffer-based antigen retrieval (Thermo Scientific) was performed before washing with 0.3% Triton-X/PBS. After blocking in a serum-free Sea block buffer (Thermo Scientific) for 30 min, samples were exposed to primary antibodies (12–16 h at 4°C), and secondary antibodies (2 h at room temperature) in PBS/0.01% Triton-X. The following primary antibodies were used: 1:100 rabbit anti-perillipin (Cell Signaling), 1:200 goat anti-GFP (GenTex), 1:100 rabbit anti-CD31 and 1:50 goat anti-CD31 (Santa Cruz Biotechnology), 1:200 goat anti-decorin (R&D Systems), 1:100 rabbit anti-Ki67 (Neomarkers), 1:50 rabbit anti-platelet-derived growth factor receptor-β (PDGFRβ; Epitomics), and 1:100 rat anti-CD45 (eBioscience). Secondary immunoglobulin G used was as follows: donkey Alexa 488-conjugated (Invitrogen) and Cy3-conjugated (Jackson Laboratories). Nuclei were stained with Hoechst 33258 or TOPRO3 (Invitrogen). Fluorescence images were acquired with Olympus IX70 and Magnafire software (Olympus). Confocal images were acquired with TCS SP5 and LAS AF software (Leica).
Oil Red O staining on spheroids
Frozen sections of spheroids were fixed in 10% formalin for 30 min. Sections were rinsed with water and washed once with 60% isopropanol for 30 s, and then covered with filtered Oil Red O solution (three parts of 0.3% Oil Red O [Sigma]/isopropanol stock solution with two parts water) for 10 min. Sections were rinsed with water and mounted, and bright-field images were acquired.
Results
Magnetic levitation and differentiation of 3T3-L1 cells in spheroids
To test whether magnetic nanoparticles and levitation can be used for assembly of 3D adipose tissue cultures, we first took advantage of the mouse 3T3-L1 cell line19 conventionally used to study adipogenesis. We used polylysine-based magnetic nanoparticle assembly to start 3D cultures from 60,000, 120,000, and 240,000 cells based on the technique illustrated in Figure 1A. Spheroids successfully formed in every case (Fig. 1B) and remained stable in culture in the presence of magnetic field for the period of up to 45 days, after which they were analyzed. As reported previously, 3T3-L1 cells detached and died upon reaching confluence unless adipogenesis was induced. In contrast, as revealed by analysis of spheroid sections, 3D-cultured 3T3-L1 cells remained viable at the periphery, although cell death was observed in the center of the sphere when more than 2.5×105 cells were aggregated and cultured extensively after onset or levitation (data not shown). This observation suggests that intercellular communication between cells grown in spheroids may differ from that achievable in conventional adherent culture setting.
To determine whether magnetic levitation-based cultures allow cells to undergo lipogenesis, we induced adipose differentiation in 3T3-L1 spheroids. After 14 days of differentiation, spheroids maintained in an adipogenesis induction medium were buoyant in PBS, indicating lipid accumulation (Fig. 1C). Spheroids in the induction medium were noticeably larger after 14 days after adipogenesis initiation, with peripheral adipocytes visible by phase-contrast micrography (Fig. 1D). We performed immunofluorescence analysis on paraffin sections of spheroids, which detected robust expression of perilipin-1, a marker of mature lipid droplets, in an adipogenesis induction medium (Fig. 1E). Oil Red-O staining of adiposphere frozen-sections confirmed lipid accumulation upon adipogenesis induction, but not in undifferentiated spheroids (Fig. 1F).
Vasculature simulation in magnetic levitation culture
Next, we tested whether magnetic levitation-based 3D conditions enable coculture of distinct cell types. Interaction of adipose stromal and endothelial cells is integral for the formation of the basement membrane and mature vasculature.35 We therefore investigated the assembly of 3T3-L1 preadipocytes cocultured with murine endothelial bEND.3 cells that have been previously shown to form vascular networks when cocultured with ASC in 2D conditions.36 To enable identification of endothelial structures among stromal cells, we stably transduced bEND.3 cells with lentivirus constitutively expressing GFP. Mixed 3T3-L1 and bEND.3-GFP cells readily formed spheroids (Fig. 2A, left); an identical cell admixture plated at confluency in 2D formed a layer also displaying cell heterogeneity (Fig. 2A, right). Fluorescent microscopy on cultured cells revealed formation of circular endothelial structures in spheroids (Fig. 2B, left), while in adherent culture, only clusters of aggregated bEND.3-GFP cells within the 3T3-L1 cell mass were observed (Fig. 2B, right). As 3T3-L1 adipospheres, upon adipogenesis induction, spheroids formed by 3T3-L1 and bEND.3-GFP cells accumulated lipids microscopically visible in differentiating adipocytes (Fig. 2C). Whole-mount immunofluorescence analysis of adipospheres revealed large perilipin-positive lipid droplets clustering around GFP-positive networks formed by bEND.3 cells (Fig. 2D). These data indicate that magnetic levitation makes it possible to simultaneously simulate vascularization and lipogenesis in cultured tissue composed of endothelial cells and mesenchymal progenitors.
FIG. 2.

Preadipocytes and endothelial cells cooperate in 3D coculture. 3T3-L1 preadipocytes (2.25×105 cells) and GFP-expressing mouse bEND.3 endothelial cells (2.5×104 cells) were used to coseed levitation (3D) or adherent (2D) cultures. (A) Phase-contrast micrographs after 8 days of 3D (left) or 2D (right) culture in an adipogenesis induction medium. (B) GFP fluorescence (green) of vessel-like structures (indicated) composed by bEND.3 cells in 3D culture contrasted with nonuniform clustering of minimally organized bEND.3 cells in 2D culture. (C) Phase-contrast micrographs of 3D cells after 14 days of culture. Uninduced spheroids (left) and adipogenesis-induced spheroids (right) with large adipocytes indicated. (D) Whole mounts of spheroids formed by 3T3-L1 preadipocytes (2.25×105 cells) and GFP-expressing mouse bEND.3 endothelial cells (2.5×104 cells) 14 days postadipogenesis induction were subjected to immunofluorescence with perilipin antibodies (red arrows) and GFP antibodies (green arrows), indicating lipid droplet maturation in adipocytes clustering around bEND.3 vessel-like structures (arrows). DNA stained blue. Scale bar: 50 μm. GFP, green fluorescent protein; 2D, two-dimensional. Color images available online at www.liebertpub.com/tec
Having confirmed the functionality of the magnetic levitation in application to cell lines, we have proceeded to test it for simulation of WAT organogenesis from primary cells. Cells derived from mouse WAT by enzymatic digestion were used to isolate the SVF containing ASC, endothelium, and infiltrating leukocytes as described previously.19,30 Spheroids readily formed upon levitation of disperse SVF preincubated with NS and have been maintained for as long as 21 days without signs of decomposition. Cells isolated from the spheroids after 21 days displayed viability and phenotype diversity representative of the original SVF (data not shown). Paraffin sections of spheroids revealed striking hierarchical organization with distinct capsule and internal large vessel-like structures (Fig. 3). Immunofluorescence analysis with anti-CD31 antibodies confirmed that the lumens of these vascular cavities in spheroids were lined by endothelial cells (Fig. 3A). Moreover, immunofluorescence with antibodies against decorin, a protein abundantly secreted by stromal cells in WAT,19 demonstrated perivascular localization of ASC (Fig. 3B). Secreted decorin deposited within the extracellular matrix (ECM) was also abundant in other spheroid compartments. Our data show that magnetic levitation allows endothelial cells and mesenchymal cells of WAT to assemble into structures recapitulating in vivo interactions between these distinct cell types.
FIG. 3.
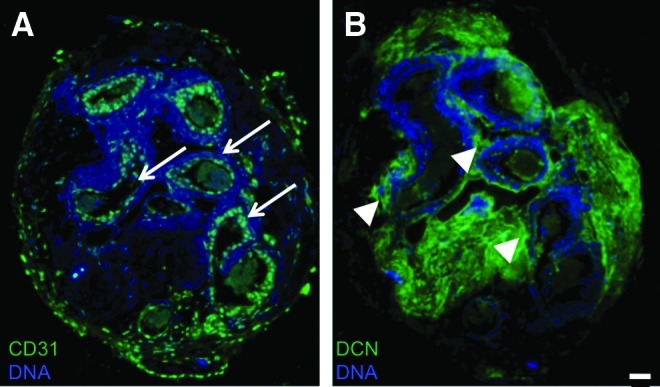
Vascularization in adipospheres formed by primary WAT cells. The SVF of mouse visceral WAT (6×104 cells) was used to start 3D levitation cultures. Paraffin sections of spheroids harvested 8 days after adipogenesis induction were subjected to immunofluorescence with antibodies against CD31 (A) or decorin (DCN) (B) secreted by stromal cells. Secondary antibody signal is in green. Note lumen formation by endothelial (CD31+) cells (arrows) surrounded by DCN+ stroma (arrowheads). DNA stained blue. Scale bar: 50 μm. WAT, white adipose tissue; SVF, stromal vascular fraction. Color images available online at www.liebertpub.com/tec
Simulation of WAT cellular hierarchy in magnetic levitation culture
To identify the potential advantages offered by the magnetic levitation method, as opposed to conventional adherent culture, 3D and confluent 2D cultures formed by identical numbers of SVF cells from the same WAT preparation were systematically compared. Cells were cultured in the same medium for the same period of time and fixed using the same protocol for 2D and 3D cultures to allow for adequate marker expression comparison. Whole-mount spheroid and adherent cultures were subjected to confocal immunofluorescence. Vascular network formation analyzed with two distinct CD31 antibodies was markedly more robust in spheroids compared to 2D cultures (Fig. 4A–C). Our quantification data show that cultured WAT SVF display significantly more CD31+ network branch points in spheroids compared to 2D cultured cells (Supplementary Fig. S1A; Supplementary Data are available online at www.liebertpub.com/tec).
FIG. 4.

Cell composition, organization, and proliferation in primary adipospheres. The SVF of mouse visceral WAT (3.5×105 cells) was used to start levitation (3D) or adherent (2D) cultures. At day 4 postadipogenesis induction, 3D whole mounts and 2D cultures were fixed in 4% paraformaldehyde and processed for immunostaining with antibodies against indicated markers and the corresponding secondary antibodies (red/green). Note increased frequency of proliferating Ki67+ cells (arrows) (A) and hematopoietic (CD45+) cells (B) in spheroid culture compared to 2D. In (B), arrows indicate leukocytes (green) in magnified insets; arrowheads indicate areas of dead cells trapping antibodies. The gradient of CD140b+ stromal cells (C) enriched in outside layers (*) and robust vascular network (CD31+) organization (A–C) are evident in 3D. DNA stained blue. Spheroid edge is on the left. Scale bar: 100 μm. Color images available online at www.liebertpub.com/tec
Consistent with the data from sections (Fig. 3), the phenotypes of vascular-like structures were different depending on their location within the spheroid (Fig. 4). Analysis of Ki-67 expression revealed cell proliferation in both 3D and 2D cultures, with areas of more intensive cell division observed in peripheral areas of spheroids (Fig. 4A). In general, Ki-67 positive cells were more concentrated in areas of CD31-positive cell networks. Tissue analysis with antibodies against a pan-leukocyte marker CD45 revealed an abundance of hematopoietic cells in spheroids, while in adherent cultures, they were predominantly lost (Fig. 4B). Comparatively, higher CD31 expression by endothelium than by leukocytes allowed us to distinguish the two cell types in spheroids. Simulating their in vivo localization, leukocytes were localized in association with CD31-positive endothelium in the spheroids. To identify ASC, we used antibodies against CD140b, also known as PDGFR-β, a marker of mesenchymal cells. As expected, CD140b and CD31 signals were mutually exclusive (Supplementary Fig. S1B). Formation of stromal/vascular network was observed in spheroids, in particular in subperipheral layers (Fig. 4C).
Finally, we investigated whether levitated spheroids can serve to model WAT development. Identical numbers of SVF cells were used to establish spheroid cultures and confluent 2D cultures, after which adipogenesis was induced. Robust lipid droplet formation was detected by perilipin immunofluorescence in spheroid whole-mounts 4 days postinduction, while only traces of lipid accumulation were observed in adherent culture at this point (Fig. 5A). The obvious difference in lipid droplet size remained at 8 days postinduction (Fig. 5A). Importantly, in adipospheres, adipocytes formed continuous tissue mass, while only islets of differentiating adipocytes were detectable in adherent 2D culture. Both 3D and 2D cultures were compatible with retention of endothelial cells and their assembly into networks during differentiation; however, endothelial aggregates detected with CD31 antibody were better organized in 3D. At day 14 postinduction, lipid droplets in adipocytes observed in adiposphere culture were comparable to those in WAT derived from adult WAT of a C57BL/6 mouse (Fig. 5B). Combined, our data show that organoids formed via magnetic levitation of cells recapitulate key properties of endogenous WAT.
FIG. 5.

Adipogenesis in adipospheres made from primary WAT cells. The SVF of mouse visceral WAT (3.5×105 cells) was used to start levitation (3D) or adherent (2D) cultures. After 4 and 8 days (A) or 14 days (B) of adipogenesis induction, whole mounts were subjected to confocal immunofluorescence with perilipin antibodies (red arrows) and CD31 antibodies (green arrows). Larger and more numerous perilipin+ lipid droplets are observed in 3D (A). Comparison of differentiated adipospheres to endogenous mouse WAT whole mount (B) identifies similar sizes of adipocytes and comparable appearance of CD31+ vessels. DNA stained blue. Spheroid edge is on the left. Scale bar: 100 μm. Color images available online at www.liebertpub.com/tec
Discussion
Although 2D cell culture has enabled the progress in understanding of the mechanisms driving adipogenesis, lipogenesis, and lipolysis in adipocyte progenitors, other cellular components of WAT have remained relatively understudied. An obstacle in basic research and drug discovery programs focusing on WAT has been the lack of physiologically relevant ex vivo tissue culture platforms. Here, to simulate the complex 3D architecture of WAT, we aggregated different adipose cell populations in levitation culture based on magnetic field. By using 3T3-L1 preadipocytes as a model, we show that while in 2D these cells do not survive confluency unless adipogenesis is induced, they remain viable indefinitely upon integration within the spheroid 3D structure. Both 3T3-L1- and primary WAT-derived cells grown in adipospheres displayed more robust lipid droplet formation and maturation upon adipogenesis induction than cells in adherent monolayer. According to our comprehensive analysis, the levitated organoids maintain multicellular complexity of endogenous WAT, unlike 2D cultures that are prone to loss of leukocytes. Importantly, adipocyte differentiation is compatible with proliferation and hierarchical organization of cells within adipospheres. Vessel-like structures observed in SVF adipospheres were often larger than venules and arterioles of WAT, and no clear lumens were observed. Nevertheless, our data indicating that adipospheres to an extent simulate vascularization take place in endogenous WAT represent a significant advance for the field of tissue engineering. Combined, our data indicate that magnetically levitated cultures recapitulate key components of WAT organogenesis.
While the advantages of levitated adipospheres over adherent monolayers are apparent, more studies will be necessary to determine the difference of this technique from alternative approaches to 3D culture. In recent years, a number of 3D cell culture techniques have been developed to simulate complex tissue organization.37 While some tissues are capable of forming organoids spontaneously,38 others (including WAT) require scaffolds for the cells to integrate in an organotypic manner. Most efforts have focused on biopolymer scaffolds with Matrigel or other ECM-based platforms being commonly used for tissue microenvironment simulation.39 More rigid, sponge-like scaffolds made from materials such as hydroxyapatite, or synthetic organic polymers casted using foaming agents, have also been introduced.40,41 While artificial matrices are often used in tissue modeling, the relevance of tissue composition achieved with their help to in vivo settings has remained questionable. Recently, matrix scaffolds based on decellularized native tissues have gained popularity.42 To generate substantial tissue amounts, agitation-based bioreactors have been implemented.43 Such setups allow for expansion of certain cell types in the context of perfusion simulating vasculature. However, they are cumbersome and have other shortcomings, such as inability to retain key cell types composing the endogenous organ and difficulty of processing for subsequent in vivo application.
The initial attempts to simulate WAT organogenesis have been based on the ceiling culture method.44 Notable progress in 3D tissue engineering has been recently achieved in mimicking the in vivo WAT microenvironment.20,45–48 However, so far reported studies have been based on scaffolds and custom-made bioreactors that do not allow for easy translation.12,49,50 Three-dimensional aqueous-derived silk scaffolds have been previously used to coculture endothelial cells and human ASC that could be induced to undergo adipogenesis.51 It has been shown that tissues engineered ex vivo based on scaffolds can become vascularized upon implantation into a host.40 It was also reported that grafting of human ASC grown in spheroids is similarly followed by in vivo angiogenesis.52 Interestingly, a recent study demonstrated that scaffold-free spheroids preserve multilineage potential of MSC.53 While these studies represent important advances, the capacity to recapitulate and integrate the multiplicity of cell types composing WAT in vivo has not been demonstrated to date, thus making our combined results an important step forward.
The approach to 3D culture by magnetic levitation has been previously established based on magnetized phage hydrogels seeded with tumor and neural stem cells.34 Here, we tested the application of the magnetic levitation setup that does not depend on the phage hydrogel. In our phage-free method, NS cellular uptake is necessary for cells to be levitated and guided together to self-assemble into 3D spheroids. However, as cells proliferate in the primer, most of their progeny in the resulting spheroid end up nanoparticle-free, and the NS often localize in the ECM within the 3D structure. Therefore, because the NS stays within the levitated spheroid, cultures do not have to be replenished with NS to continue the levitation process as the culture assembles and cells proliferate. As a part of this work, we compared cell proliferation, differentiation, morphology, and lipid distribution in 2D cultures with and without the presence of NS. Besides the initially observed dark coloration of cells due to NS uptake, no changes in cell proliferation or differentiation were detected in 2D cultures upon NS exposure (Supplementary Fig. S2). While the NS are biocompatible and in this study no adverse effects were detected for cells treated with NS either in 2D or 3D, possible nanoparticle effects in downstream applications are yet to be ruled out.
A unique feature of the magnetic levitation approach is the expedited timeline of 3D spheroid formation driven by magnetic field without the loss of cell populations upon passive cell propagation in conditions favoring spheroid formation. While simpler approaches to spheroid culture in an agitated medium and/or low-adherence have been reported for many cell types, including ASC,52,54,55 the rationale behind using magnetic levitation for WAT-derived cells was to enforce retention of cell types other than ASC composing the organ, which tend to be dominated by ASC and progressively lost in conventional culture settings. Furthermore, the magnetic levitation method assures the cell–cell interaction between different cell types at the onset of levitation by magnetically guiding cells together, in contrast to relying on random cell interactions when using an agitated medium and/or low-adherence methods.
This study showed that levitated cell spheroids are feasible for long-term multicellular studies and recapitulate relative cell positioning more closely than 2D culture. An important advantage of this system is the dependence of cell adherence on autocrine ECM molecules rather than on artificial substrates serving as a foundation of other 3D culture designs. We have not ruled out that some of the polylysine used for assembly might remain external to cells and contributes to cell interaction. However, compared to many other systems, such a minimal tissue composition manipulation gives primary tissue cells an opportunity to better re-establish their endogenous organ microenvironment. While normal WAT does appear largely homogeneous on the millimeter scale, like any other tissue, it faces interactions with other tissues. This is relevant in both normal conditions and pathology: for example, tumors tend to be surrounded by WAT, which may be key in cancer progression. While investigating that this is beyond the scope of this initial report, in future studies, magnetic guidance will indeed likely to be useful for aggregating tissue components with distinct cell contents in a desired orientation to model intercellular interactions.
While this study focused on adipose tissue culture, our results have important implications for research pursued toward engineering of other tissues. They prove the principle that forced aggregation of cells secreting an endogenous matrix in 3D can be used as an approach to re-establish native tissue architecture. It remains to be determined whether NS-based levitation can be applied to other organs composed of different types of cells requiring different medium compositions for maintenance. In the future, careful comparison of magnetic levitation with other 3D cell culture platforms will help to define the nuances of each approach and identify appropriate technologies for specific applications.
Supplementary Material
Acknowledgments
Glauco R. Souza received support from the National Science Foundation (NSF) SBIR award and the State of Texas Emerging Technology Fund (ETF). Research in the Kolonin laboratory is supported by the grants R01DK088131 and 1R21DK090752 from the NIH. Research in the Souza laboratory is supported by Phase I (0945954) and Phase II (1127551) SBIR grant awards from NSF IIP Division of Industrial Innovation and Partnerships and the State of Texas Emerging Technology Fund (ETF).
Disclosure Statement
Glauco R. Souza is employed by Nano3D Biosciences™, Inc.
References
- 1.Caplan A.I. Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9:11. doi: 10.1016/j.stem.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papayannopoulou T. Scadden D.T. Stem-cell ecology and stem cells in motion. Blood. 2008;111:3923. doi: 10.1182/blood-2007-08-078147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han J., et al. Adipose tissue is an extramedullary reservoir for functional hematopoietic stem and progenitor cells. Blood. 2010;115:957. doi: 10.1182/blood-2009-05-219923. [DOI] [PubMed] [Google Scholar]
- 4.Bianco P. Robey P.G. Simmons P.J. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolonin M.G. Simmons P.J. Combinatorial stem cell mobilization. Nat Biotechnol. 2009;27:252. doi: 10.1038/nbt0309-252. [DOI] [PubMed] [Google Scholar]
- 6.Pittenger M.F., et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 7.Prockop D.J. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 8.Kolonin M.G. Evans K.W. Mani S.A. Gomer R.H. Alternative origins of stroma in normal organs and disease. Stem Cell Res. 2012;8:312. doi: 10.1016/j.scr.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daquinag A.C. Zhang Y. Kolonin M.G. Vascular targeting of adipose tissue as an anti-obesity approach. Trends Pharmacol Sci. 2011;32:300. doi: 10.1016/j.tips.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Choi J.H., et al. Adipose tissue engineering for soft tissue regeneration. Tissue Eng Part B Rev. 2010;16:413. doi: 10.1089/ten.teb.2009.0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scherberich A. Muller A.M. Schafer D.J. Banfi A. Martin I. Adipose tissue-derived progenitors for engineering osteogenic and vasculogenic grafts. J Cell Physiol. 2010;225:348. doi: 10.1002/jcp.22313. [DOI] [PubMed] [Google Scholar]
- 12.Guven S., et al. Engineering of large osteogenic grafts with rapid engraftment capacity using mesenchymal and endothelial progenitors from human adipose tissue. Biomaterials. 2011;32:5801. doi: 10.1016/j.biomaterials.2011.04.064. [DOI] [PubMed] [Google Scholar]
- 13.Ailhaud G. Grimaldi P. Negrel R. Cellular and molecular aspects of adipose tissue development. Annu Rev Nutr. 1992;12:207. doi: 10.1146/annurev.nu.12.070192.001231. [DOI] [PubMed] [Google Scholar]
- 14.Hausman D.B. DiGirolamo M. Bartness T.J. Hausman G.J. Martin R.J. The biology of white adipocyte proliferation. Obes Rev. 2001;2:239. doi: 10.1046/j.1467-789x.2001.00042.x. [DOI] [PubMed] [Google Scholar]
- 15.Zuk P.A., et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 16.Rodeheffer M.S. Birsoy K. Friedman J.M. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135:240. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 17.Traktuev D., et al. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102:77. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- 18.Tang W., et al. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daquinag A.C. Zhang Y. Amaya-Manzanares F. Simmons P.J. Kolonin M.G. An isoform of decorin is a resistin receptor on the surface of adipose progenitor cells. Cell Stem Cell. 2011;9:74. doi: 10.1016/j.stem.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 20.Park H., et al. Three-dimensional hydrogel model using adipose-derived stem cells for vocal fold augmentation. Tissue Eng Part A. 2010;16:535. doi: 10.1089/ten.TEA.2009.0029. [DOI] [PubMed] [Google Scholar]
- 21.Gomillion C.T. Burg K.J. Stem cells and adipose tissue engineering. Biomaterials. 2006;27:6052. doi: 10.1016/j.biomaterials.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 22.Gimble J.M. Bunnell B.A. Casteilla L. Jung J.S. Yoshimura K. Phases I-III clinical trials using adult stem cells. Stem Cells Int. 2011;2010:604. doi: 10.4061/2010/604713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lazar M.A. How obesity causes diabetes: not a tall tale. Science. 2005;307:373. doi: 10.1126/science.1104342. [DOI] [PubMed] [Google Scholar]
- 24.Renehan A.G. Tyson M. Egger M. Heller R.F. Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 25.Flegal K.M. Graubard B.I. Williamson D.F. Gail M.H. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298:2028. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- 26.Park J. Euhus D.M. Scherer P.E. Paracrine and endocrine effects of adipose tissue on cancer development and progression. Endocr Rev. 2011;32:550. doi: 10.1210/er.2010-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bellows C.F. Zhang Y. Simmons P.J. Khalsa A.S. Kolonin M.G. Influence of BMI on level of circulating progenitor cells. Obesity. 2011;19:1722. doi: 10.1038/oby.2010.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bellows C.F. Zhang Y. Chen J. Frazier M.L. Kolonin M.G. Circulation of progenitor cells in obese and lean colorectal cancer patients. Cancer Epidemiol Biomarkers Prev. 2011;20:2461. doi: 10.1158/1055-9965.EPI-11-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klopp A.H., et al. Omental adipose tissue-derived stromal cells promote vascularization and growth of endometrial tumors. Clin Cancer Res. 2012;18:771. doi: 10.1158/1078-0432.CCR-11-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y., et al. White adipose tissue cells are recruited by experimental tumors and promote cancer progression in mouse models. Cancer Res. 2009;69:5259. doi: 10.1158/0008-5472.CAN-08-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weisberg S.P., et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petit J.Y., et al. Locoregional recurrence risk after lipofilling in breast cancer patients. Ann Oncol. 2012;23:582. doi: 10.1093/annonc/mdr158. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y. Bellows C.F. Kolonin M.G. Adipose tissue-derived progenitor cells and cancer. World J Stem Cells. 2010;2:103. doi: 10.4252/wjsc.v2.i5.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Souza G.R., et al. Three-dimensional tissue culture based on magnetic cell levitation. Nat Nanotechnol. 2010;5:291. doi: 10.1038/nnano.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kolonin M.G. Tissue-specific targeting based on markers expressed outside endothelial cells. Adv Genet. 2009;67:61. doi: 10.1016/S0065-2660(09)67003-6. [DOI] [PubMed] [Google Scholar]
- 36.Thangarajah H., et al. IFATS collection: adipose stromal cells adopt a proangiogenic phenotype under the influence of hypoxia. Stem Cells. 2009;27:266. doi: 10.1634/stemcells.2008-0276. [DOI] [PubMed] [Google Scholar]
- 37.Haycock J.W. 3D cell culture: a review of current approaches and techniques. Methods Mol Biol. 2011;695:1. doi: 10.1007/978-1-60761-984-0_1. [DOI] [PubMed] [Google Scholar]
- 38.Suga H., et al. Self-formation of functional adenohypophysis in three-dimensional culture. Nature. 2011;480:57. doi: 10.1038/nature10637. [DOI] [PubMed] [Google Scholar]
- 39.Dutta R.C. Dutta A.K. Cell-interactive 3D-scaffold; advances and applications. Biotechnol Adv. 2009;27:334. doi: 10.1016/j.biotechadv.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 40.Wiggenhauser P.S., et al. Engineering of vascularized adipose constructs. Cell Tissue Res. 2012;327:747. doi: 10.1007/s00441-011-1226-2. [DOI] [PubMed] [Google Scholar]
- 41.Carletti E. Motta A. Migliaresi C. Scaffolds for tissue engineering and 3D cell culture. Methods Mol Biol. 2011;695:17. doi: 10.1007/978-1-60761-984-0_2. [DOI] [PubMed] [Google Scholar]
- 42.Song J.J. Ott H.C. Organ engineering based on decellularized matrix scaffolds. Trends Mol Med. 2011;17:424. doi: 10.1016/j.molmed.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 43.Timmins N.E., et al. Three-dimensional cell culture and tissue engineering in a T-CUP (tissue culture under perfusion) Tissue Eng. 2007;13:2021. doi: 10.1089/ten.2006.0158. [DOI] [PubMed] [Google Scholar]
- 44.Toda S., et al. Adipose tissue-organotypic culture system as a promising model for studying adipose tissue biology and regeneration. Organogenesis. 2009;5:50. doi: 10.4161/org.5.2.8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hong L. Peptan I.A. Colpan A. Daw J.L. Adipose tissue engineering by human adipose-derived stromal cells. Cells Tissues Organs. 2006;183:133. doi: 10.1159/000095987. [DOI] [PubMed] [Google Scholar]
- 46.Flynn L. Woodhouse K.A. Adipose tissue engineering with cells in engineered matrices. Organogenesis. 2008;4:228. doi: 10.4161/org.4.4.7082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hemmrich K., et al. Autologous in vivo adipose tissue engineering in hyaluronan-based gels—a pilot study. J Surg Res. 2008;144:82. doi: 10.1016/j.jss.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 48.Shanti R.M., et al. In vitro adipose tissue engineering using an electrospun nanofibrous scaffold. Ann Plast Surg. 2008;61:566. doi: 10.1097/SAP.0b013e31816d9579. [DOI] [PubMed] [Google Scholar]
- 49.Scherberich A. Galli R. Jaquiery C. Farhadi J. Martin I. Three-dimensional perfusion culture of human adipose tissue-derived endothelial and osteoblastic progenitors generates osteogenic constructs with intrinsic vascularization capacity. Stem Cells. 2007;25:1823. doi: 10.1634/stemcells.2007-0124. [DOI] [PubMed] [Google Scholar]
- 50.Gerlach J.C., et al. Adipogenesis of human adipose-derived stem cells within three-dimensional hollow fiber-based bioreactors. Tissue Eng Part C Methods. 2012;18:54. doi: 10.1089/ten.tec.2011.0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kang J.H. Gimble J.M. Kaplan D.L. In vitro 3D model for human vascularized adipose tissue. Tissue Eng Part A. 2009;15:2227. doi: 10.1089/ten.tea.2008.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bhang S.H., et al. Angiogenesis in ischemic tissue produced by spheroid grafting of human adipose-derived stromal cells. Biomaterials. 2011;32:2734. doi: 10.1016/j.biomaterials.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 53.Baraniak P.R. McDevitt T.C. Scaffold-free culture of mesenchymal stem cell spheroids in suspension preserves multilineage potential. Cell Tissue Res. 2012;347:701. doi: 10.1007/s00441-011-1215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sanchez-Bustamante C.D. Frey U. Kelm J.M. Hierlemann A. Fussenegger M. Modulation of cardiomyocyte electrical properties using regulated bone morphogenetic protein-2 expression. Tissue Eng Part A. 2008;14:1969. doi: 10.1089/ten.tea.2007.0302. [DOI] [PubMed] [Google Scholar]
- 55.Verseijden F., et al. Adult human bone marrow- and adipose tissue-derived stromal cells support the formation of prevascular-like structures from endothelial cells in vitro. Tissue Eng Part A. 2010;16:101. doi: 10.1089/ten.TEA.2009.0106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



