Abstract
Presenting characteristics of bacterial corneal ulcers may suggest particular causative organisms, helping to guide treatment decisions before cultures become available. In this study, we analyze the association between presentation demographic and clinical characteristics, using data collected as part of a randomized, controlled clinical trial. Data for this study were collected as part of the Steroids for Corneal Ulcers Trial, a randomized, placebo-controlled, double-masked trial. All patients had a culture-proven bacterial corneal ulcer. Patient history, clinical examination, and photography were performed in a standardized fashion at enrollment. Analysis of variance or Fisher’s exact test was used to compare characteristics by organism. Univariate logistic regression was used to analyze predictors of the most common organisms. Five hundred patients were enrolled in the trial, of whom 488 were included in this analysis. The most common organism was Streptococcus pneumoniae (N = 248, 51 %) followed by Pseudomonas aeruginosa (N = 110, 23 %). Compared to other organisms, P. aeruginosa was significantly associated with a larger baseline infiltrate/scar size [odds ratio (OR) 1.6, 95 % confidence interval (CI) 1.4–1.8] and deeper infiltrate (OR 2.4, 95 % CI 1.5–3.8). S. pneumoniae was significantly associated with a smaller baseline infiltrate/scar size (OR 0.8, 95 % CI 0.7–0.9) and dacryocystitis (OR 7.3, 95 % CI 4.1–13.3). Nocardia spp. were significantly associated with longer duration of symptoms prior to presentation (OR 1.4, 95 % CI 1.2–1.6), more shallow infiltrate (OR 0.3, 95 % CI 0.2–0.5), and better baseline visual acuity (OR 0.4, 95 % CI 0.2–0.65). Staphylococcus spp. were less likely to be central in location (OR 0.16, 95 % CI 0.08–0.3). Baseline characteristics of bacterial ulcers may suggest the likely etiology and guide early management.
Keywords: Bacteria, Keratitis, Pseudomonas, Risk factors
Introduction
Bacterial keratitis is a potentially blinding infection of the cornea that disproportionately affects the developing world [1]. Many of the same pathogens are found across different regions, although their frequency can vary widely [2–6]. Corneal ulcers due to different bacterial organisms may be associated with variable risk factors, as well as specific demographic and clinical characteristics at presentation. Because microbiological confirmation of diagnosis can take several days and may delay onset of proper antimicrobial treatment, presentation characteristics may be useful in guiding initial management decisions and prognosis. Any relationship between risk factors and specific organisms may also suggest prevention measures. In this study, we evaluated the association between the bacterial organism isolated on culture and various demographic and clinical risk factors, collected prospectively as part of a clinical trial.
Methods
Trial methods
The Steroids for Corneal Ulcers Trial (SCUT, NEI U10-EY015114) is a National Eye Institute supported prospective, randomized, controlled, double-masked clinical trial comparing clinical outcomes in patients with a culture-positive bacterial corneal ulcer receiving topical corticosteroids or placebo as adjunctive therapy. Patients were enrolled at the Aravind Eye Care System (Madurai, Tirunelveli, and Coimbatore, India), the F.I. Proctor Foundation at the University of California, San Francisco, CA, USA, and the Dartmouth–Hitchcock Medical Center, NH, USA. Institutional review board approval was granted by the University of California, San Francisco, Dartmouth Medical School, and the Aravind Eye Care System. This study conformed to the tenets of the Declaration of Helsinki, and informed consent was obtained from all subjects.
Eligible patients had a culture-proven bacterial ulcer and had been on moxifloxacin for at least 48 h. Exclusion criteria included: a history of penetrating keratoplasty in the affected eye, impending perforation, no light perception in the affected eye, vision worse than 6/60 in their fellow eye, bilateral ulcers, age less than 16 years, evidence of fungus or acanthamoeba by culture or smear, or evidence of herpetic keratitis by history or exam. Full inclusion and exclusion criteria have been previously described [7].
Data for this study were collected prospectively as part of the clinical trial, using standardized trial forms. At enrollment, best spectacle-corrected visual acuity (BSCVA) was assessed by masked refractionists on ETDRS charts. Infiltrate/scar size, epithelial defect size, infiltrate depth, and hypopyon were measured by slit lamp examination. Ulcer location was determined by photography. Standardized patient history and demographic information was obtained at the baseline visit.
Microbiological methods
After the slit lamp examination at presentation, corneal scrapings were performed. Two scrapings were smeared for Gram stain and KOH wet mount. Three scrapings were inoculated onto sheep’s blood agar, chocolate agar, and potato dextrose agar or Sabouraud’s agar. The criterion for a positive bacterial culture was growth of the organism on one solid medium at the site of inoculation. For Staphylococcus epidermis and diphtheroids, cultures were considered positive only if moderate growth was seen on at least two solid media or on one solid medium plus a Gram-stained corneal smear [8]. All patients were checked for fungal elements on smear and culture, and any evidence of fungal infection resulted in exclusion. Quality control was performed according to the National Committee for Clinical Laboratory Standards (NCCLS) performance standards, recommendations, guidelines, and reports [NCCLS M100-S10 (M2)] [9].
Statistical methods
Isolates with a mixed infection (≥2 distinct organisms isolated) were excluded from the analysis. Infiltrate/ scar size and epithelial defect size were analyzed as the geometric mean of the longest dimension of the ulcer and the longest perpendicular. Visual acuity in logMAR was used for all statistical analyses. Analysis of variance (ANOVA) for continuous variables or Fisher’s exact test for categorical variables was used to compare characteristics across groups of organism. Univariate logistic regression was performed to analyze the association between baseline demographic and clinical characteristics and the most commonly isolated organisms. A Bonferroni correction was performed for all analyses to adjust for multiple comparisons. For the ANOVAs and Fisher’s exact tests, P ≤ 0.0045 (11 independent comparisons) was set to be the significance level. For the logistic regressions, P ≤ 0.001 (55 independent comparisons) was set to be the significance level. All analyses were conducted in Stata version 10.0 (StataCorp, College Station, TX, USA).
Results
Five hundred patients were enrolled in the trial, all of whom had complete baseline and microbiological data, including a positive bacterial culture. Of these, 6 (1 %) patients were excluded from the analysis due to mixed infection and 6 (1 %) were excluded because the isolate was not an identifiable organism, leaving 488 patients available for this analysis. Of these, 474 (97 %) were enrolled in India. The median age of patients enrolled in this trial was 53 years (interquartile range 40–61), and 225 were female (46 %). Slightly less than half of the patients were agricultural workers (N = 211, 43 %, Table 1).
Table 1.
Presentation clinical and demographic characteristics
| Organism | N | Proportion of cases from India, N (%) | Infiltrate sizea, median (IQR)b | Acuity (BSCVA)c, median (IQR) | >33 % infiltrate depthd | Tear duct obstruction, N (%) | Central location, N (%) | Duration of symptomse, median (IQR) | Agef, median (IQR) | Female gender, N (%) | Hypopyon, N (%) | Agricultural work, N (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacillus spp. | 1 | 1 (100 %) | 2.75 | 0.54 | 1 (100 %) | 0 | 1 (100 %) | 6 | 40 | 0 | 0 | 0 |
| Corynebacterium spp. | 5 | 4 (80 %) | 3.29 (3.12, 3.38) | 0.42 (0.28, 1.7) | 3 (60 %) | 0 | 3 (60 %) | 4 (4, 7) | 53 (35, 56) | 3 (60 %) | 1 (20 %) | 1 (20 %) |
| Mycobacteria spp. | 1 | 1 (100 %) | 5.91 | 1.7 | 1 (100 %) | 0 | 1 (100 %) | 60 | 45 | 1 (100 %) | 1 (100 %) | 0 |
| Nocardia spp. | 55 | 55 (100 %) | 2.65 (1.91, 4.08) | 0.34 (0.1, 1.2) | 16 (29 %) | 3 (5 %) | 42 (76 %) | 10 (4, 15) | 48 (40, 60) | 25 (45 %) | 14 (25 %) | 26 (47 %) |
| Staphylococcus aureus | 13 | 11 (85 %) | 1.65 (1.2, 2.49) | 0.40 (0.26, 0.84) | 5 (38 %) | 0 | 8 (62 %) | 4 (3, 15) | 42 (27, 57) | 5 (38 %) | 4 (31 %) | 4 (31 %) |
| Coagulase-negative Staphylococcus | 22 | 22 (100 %) | 2.25 (1.25, 3.78) | 0.59 (0.34, 1.1) | 11 (50 %) | 3 (14 %) | 12 (55 %) | 8.5 (3, 12) | 45 (40, 60) | 11 (50 %) | 6 (27 %) | 5 (23 %) |
| S. pneumoniae | 248 | 248 (100 %) | 2.55 (1.90, 3.66) | 0.86 (0.42, 1.7) | 139 (56 %) | 81 (32 %) | 226 (91 %) | 4 (3, 7) | 56.5 (48, 65) | 128 (52 %) | 135 (54 %) | 135 (54 %) |
| Streptococcus, viridans group | 11 | 9 (82 %) | 2.50 (1.50, 6.26) | 1.2 (0.56, 1.7) | 6 (55 %) | 3 (27 %) | 9 (82 %) | 10 (4, 21) | 59 (46, 66) | 4 (36 %) | 6 (54 %) | 5 (46 %) |
| Enterobacter spp. | 2 | 2 (100 %) | 1.74 (1.70, 1.79) | 0.47 (0.1, 0.84) | 1 (50 %) | 0 | 2 (100 %) | 2 (1, 3) | 27.5 (22, 33) | 0 | 0 | 0 |
| Klebsiella spp. | 3 | 3 (100 %) | 2.10 (1.41, 2.12) | 0.62 (0.62, 0.66) | 1 (33 %) | 2 (66 %) | 3 (100 %) | 4 (3, 7) | 60 (60, 65) | 0 | 3 (100 %) | 1 (33 %) |
| Moraxella spp. | 14 | 10 (71 %) | 2.05 (1.50, 3.74) | 0.73 (0.6, 0.9) | 6 (43 %) | 0 | 11 (79 %) | 5.5 (3, 7) | 45 (24, 55) | 7 (50 %) | 7 (50 %) | 1 (7 %) |
| Pseudomonas spp. (non-aeruginosa) | 3 | 3 (100 %) | 4.68 (1.50, 6.12) | 1.7 (0.52, 1.7) | 1 (33 %) | 0 | 3 (100 %) | 4 (4, 7) | 63 (60, 75) | 0 | 1 (33 %) | 0 |
| P. aeruginosa | 110 | 105 (95 %) | 3.80 (2.50, 5.60) | 1.26 (0.46, 1.7) | 78 (71 %) | 4 (4 %) | 101 (92 %) | 3.5 (2, 6) | 43 (30, 54) | 41 (37 %) | 57 (52 %) | 33 (30 %) |
| Pg | 0.01 | <0.001 | 0.0006 | <0.001 | <0.001 | <0.001 | 0.0001 | <0.001 | 0.16 | <0.001 | 0.0002 | |
| Total | 488 | 474 (97 %) | 2.74 (1.96, 4.09) | 0.84 (0.36, 1.7) | 269 (55 %) | 96 (20 %) | 422 (87 %) | 4 (3, 7) | 53 (40, 61) | 225 (46 %) | 235 (48 %) | 211 (43 %) |
Geometric mean of the longest dimension and the longest perpendicular to that dimension, in mm
Interquartile range
BSCVA, in logMAR
Measured as: 0–33 % depth; >33 % depth
In days
In years
ANOVA for continuous variables, Fisher’s exact test for categorical variables.
Bold indicates comparisons that were significant after Bonferroni correction
The most commonly isolated organisms included Streptococcus pneumoniae (N = 248, 51 %), Pseudomonas aeruginosa (N = 110, 23 %), and Nocardia spp. (N = 55, 11 %, Fig. 1). Organisms were different by country (P = 0.01), but did not reach statistical significance with correction for multiple comparisons. Eight patients (2 %) were contact lens wearers, seven of whom had a P. aeruginosa ulcer (88 %, P = 0.01). Of 488 patients, 235 had a hypopyon at presentation (48 %).
Fig. 1.
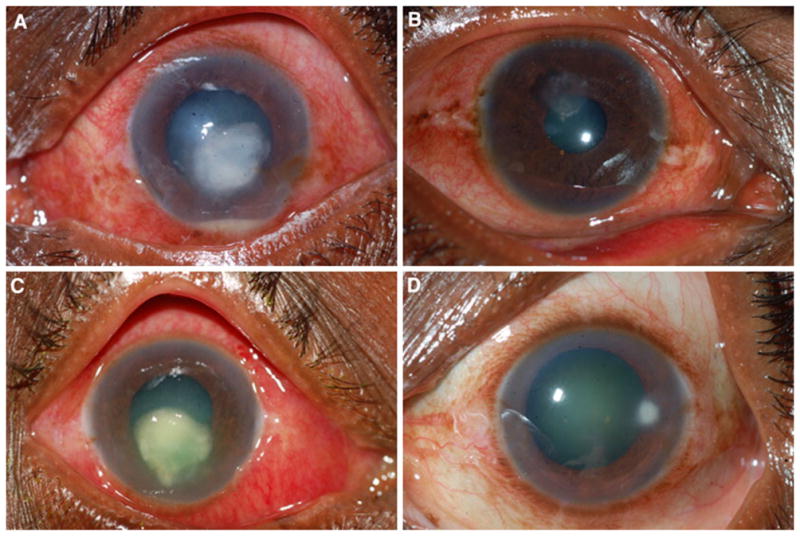
a S. pneumoniae corneal ulcer with a hypopyon; b Nocardia spp. ulcer; c severe P. aeruginosa ulcer; d peripheral Staphylococcus spp. ulcer
Characteristics that were significantly different across categories of organism are presented in Table 1, and included infiltrate size at baseline (P<0.001), acuity at baseline (P = 0.0006), infiltrate depth at baseline (P = 0.0002), dacryocystitis (P<0.001), central location (P<0.001), durations of symptoms (P = 0.0001), age (P<0.001), hypopyon (P<0.001), and employment as an agricultural worker (P = 0.0002). There were no significant differences in gender (P = 0.16), ocular surface disease (P = 0.53), or injuries due to vegetative matter (P = 0.26), wood (P = 0.30), or metal (P = 0.09) among ulcers caused by different bacteria.
Pseudomonas aeruginosa was significantly associated with a larger baseline infiltrate (OR 1.6, 95 % CI 1.4–1.8) and deeper infiltrate (OR 2.4, 95 % CI 1.5–3.8) compared to non-P. aeruginosa ulcers (Table 2). S. pneumoniae was significantly associated with a smaller baseline infiltrate/scar size (OR 0.8, 95 % CI 0.7–0.9), shorter duration of symptoms prior to presentation (OR 0.65, 95 % CI 0.5–0.8), older age (OR 1.5, 95 % CI 1.3–1.7), dacryocystitis (OR 7.3, 95 % CI 4.1–13.1), and agricultural work (OR 2.7, 95 % CI 1.8–3.9). Nocardia spp. was significantly associated with longer duration of symptoms prior to presentation (OR 1.4, 95 % CI 1.2–1.6), more shallow infiltrate (OR 0.3, 95 % CI 0.2–0.5), and better baseline visual acuity (OR 0.4, 95 % CI 0.2–0.65). Staphylococcus spp. ulcers were more likely to be peripheral in location (OR 0.16, 95 % CI 0.08–0.3).
Table 2.
Univariate logistic regression for baseline characteristics predicting organism
| Characteristic | S. pneumoniae, OR (95 % CI) | P. aeruginosa, OR (95 % CI) | Nocardia spp., OR (95 % CI) | Staphylococcus spp., OR (95 % CI) | Moraxella spp., OR (95 % CI) |
|---|---|---|---|---|---|
| P value | P value | P value | P value | P value | |
| Infiltrate size | 0.78 (0.70–0.87) | 1.58 (1.39–1.80) | 0.98 (0.83–1.16) | 0.70 (0.53–0.91) | 0.80 (0.54–1.15) |
| P < 0.001 | P < 0.001 | P = 0.81 | P = 0.009 | P = 0.22 | |
| Dacryocystitis | 7.28 (4.05–13.08) | 0.12 (0.042–0.33) | 0.21 (0.064–0.69) | 0.36 (0.11–1.21) | N/a |
| P < 0.001 | P < 0.001 | P = 0.009 | P = 0.099 | ||
| Duration of symptoms prior to presentation (by week) | 0.65 (0.51–0.82) | 0.59 (0.41–0.87) | 1.38 (1.18–1.62) | 1.22 (1.05–1.41) | 0.83 (0.43–1.60) |
| P < 0.001 | P = 0.007 | P < 0.001 | P = 0.009 | P = 0.57 | |
| Age (by decade) | 1.48 (1.30–1.68) | 0.69 (0.59–0.79) | 0.90 (0.75–1.07) | 0.83 (0.66–1.03) | 0.67 (0.47–0.95) |
| P < 0.001 | P < 0.001 | P = 0.24 | P = 0.095 | P = 0.025 | |
| Depth (0: 0–33 %; 1: > 33–100 %) | 1.09 (0.76–1.56) | 2.39 (1.51–3.77) | 0.29 (0.16–0.54) | 0.67 (0.33–1.33) | 0.60 (0.20–1.76) |
| P = 0.63 | P < 0.001 | P < 0.001 | P = 0.25 | P = 0.36 | |
| Location (0 = peripheral; 1 = non-peripheral) | 1.09 (1.38–4.16) | 1.92 (0.92–4.03) | 0.48 (0.24–0.97) | 0.16 (0.08–0.34) | 0.54 (0.15–2.01) |
| P = 0.002 | P = 0.083 | P = 0.04 | P < 0.001 | P = 0.36 | |
| Baseline visual acuity | 1.26 (0.95–1.67) | 1.66 (1.18–2.33) | 0.39 (0.24–0.65) | 0.45 0.25–0.81 |
0.70 (0.30–1.65) |
| P = 0.10 | P = 0.003 | P < 0.001 | P = 0.008 | P = 0.41 | |
| Female gender | 1.57 (1.10–2.26) | 0.63 (0.41–0.97) | 0.97 (0.55–1.71) | 0.98 0.49–1.96 |
1.17 (0.41–3.40) |
| P = 0.012 | P = 0.036 | P = 0.92 | P = 0.96 | P = 0.77 | |
| Contact lens wearer | N/a | 25.6 (3.12–210.61) | N/a | N/a | 5.13 (0.59–44.79) |
| P = 0.003 | P = 0.14 | ||||
| Presence of hypopyon | 1.65 (1.16–2.36) | 1.21 (0.79–1.85) | 0.33 (0.17–0.62) | 0.41 0.19–0.86 |
1.08 (0.37–3.12) |
| P = 0.006 | P = 0.38 | P = 0.001 | P = 0.02 | P = 0.89 | |
| Agricultural work | 2.67 (1.84–3.88) | 0.48 (0.31–0.76) | 1.20 (0.69–2.11) | 0.43 (0.20–0.94) | 0.097 (0.013–0.75) |
| P < 0.001 | P = 0.002 | P = 0.52 | P = 0.034 | P = 0.03 |
Bold cells indicate comparisons that were significant after Bonferroni correction
Discussion
Presentation clinical signs and symptoms may help distinguish between causative organisms in bacterial keratitis. Because it generally takes several days for microbiological diagnosis, clinical signs which are predictive of the causative organism would be useful to ensure that proper treatment is started as soon as possible. Previously, it has been shown that there may be a predictive utility of clinical signs and symptoms in determining the etiologic agent in bacterial conjunctivitis [10]. In the current study, we found that presentation characteristics, including risk factors and markers of severity, were significantly different between organisms causing the ulcer.
Pseudomonas aeruginosa caused the most severe ulcers on presentation. These ulcers were deeper and had significantly larger infiltrate sizes than non-pseudomonas ulcers. Median visual acuity in these ulcers was approximately 4 lines worse relative to the entire cohort, which was statistically significant across groups of organisms. In this study, the duration of symptoms prior to presentation for P. aeruginosa ulcers was shorter than the overall study population, which may have been due to patients recognizing the need for care more urgently due to the aggressive nature of the pathogen and the resultant large degree of vision loss. These findings are consistent with the notoriety of P. aeruginosa as a particularly virulent and rapidly-progressing pathogen [11].
Patients with ulcers caused by Nocardia spp. had a significantly delayed presentation compared to the entire study population. In these patients, the median baseline visual acuity was nearly 5 lines better than the overall population. Nocardia keratitis has been characterized as having a slow course [12], which may explain the extended duration of symptoms and better relative visual acuity at presentation. Nocardia spp. are relatively rare isolates from keratitis, especially outside of India [12]. In this study, it was the third most commonly isolated organism; however, there were no Nocardia spp. isolated in the United States. Previous reports have shown that patients with Nocardia keratitis who present within 15 days of symptom development have the highest rate of recovery [13]. In our study, a quarter of patients presented after 15 days of symptom duration. It is possible these patients could have benefitted from earlier presentation, indicating that despite slow progression, treatment should be started promptly. Nocardia keratitis has been reported to have similar presenting characteristics to fungal keratitis, which can confuse the initial diagnosis prior to microbiological confirmation of the etiologic organism [12, 13]. In this study, no clinical signs that are typically characteristic of fungal keratitis were reported for Nocardia keratitis (such as ring infiltrate, feathery borders, endothelial plaque, etc.). Nocardia keratitis was associated with a superficial infiltrate, as has been reported in the literature [13]. Nocardia keratitis has been associated with corneal trauma in previous series, although we were unable to confirm this here [12, 13].
Streptococcus pneumoniae was the most commonly isolated organism in this study, present in approximately half of the corneal ulcers. Dacryocystitis had a 7.3-fold increased odds of being associated with a S. pneumoniae corneal ulcer. Chronic dacryocystitis has been well documented to be associated with pneumococcal keratitis [14, 15]. Presence of hypopyon has also been reported as a clinical sign of pneumococcal keratitis [15], and was present in over half of the pneumococcal ulcers in our study, but this association was not significant after correction for multiple comparisons. The infiltrate size at baseline was smaller in pneumococcal ulcers in this study compared to non-pneumococcal ulcers. However, the ulcers were more likely to be central in location, and the median visual acuity was poor at presentation. Pneumococcal ulcers appeared to be less severe than P. aeruginosa ulcers at presentation; median baseline visual acuity in P. aeruginosa ulcers was nearly 4 lines worse, and the infiltrate size was larger.
Risk factors for developing an ulcer varied significantly between different subtypes of bacterial ulcers. Agricultural work was significantly associated with pneumococcal ulcers, and P. aeruginosa ulcers appeared to be less common in agricultural workers, although this was not significant after multiple comparisons correction. Previously, agricultural work has been shown to be associated with Nocardia keratitis, but we did not replicate that finding in this study [12]. While very few contact lens wearers were enrolled, the vast majority of those enrolled had a P. aeruginosa ulcer. Previous reports have shown that contact lens use is associated with P. aeruginosa keratitis [16]. In our study, contact lens use was an approximately 20-fold risk factor for pseudomonas, but this association did not reach statistical significance after multiple comparisons correction.
This study presents a large series of prospectively collected corneal ulcers with standardized characteristics recorded for each patient. All study assessments were conducted by certified personnel according to a specific protocol. The large size of this series allows us to make comparisons between the major groups of organisms enrolled. However, due to the nature of the inclusion and exclusion criteria as part of the clinical trial, some biases may be introduced into this analysis. Impending perforation, patients with a history of penetrating keratoplasty in the affected eye, patients with a history of a corneal scar in the affected eye, and patients less than 16 years of age at enrollment were all excluded from this trial. We do not have information on these ulcers, and therefore cannot make comparisons. We therefore cannot assess whether there are differences in etiology in perforated or nearly perforated ulcers, or if certain organisms are more likely to cause keratitis in patients younger than 16 years of age. In addition, the standardized forms on which data was collected for the trial were designed to collect information such as the infiltrate/scar size and depth of the ulcer for the main analyses of the trial. Finally, the same clinician did not evaluate each patient in this trial. Therefore, it is possible that some intra-observer variability was introduced to our analyses. To minimize this bias, all clinicians, refractionists, and microbiologists who collected data during the trial were specifically certified for the trial, and regular site visits were made to re-certify all investigators and ensure that trial protocols were being followed. In addition, every tenth patient received repeat BSCVA measurements and clinical examinations from different refractionists and examiners. These repeated measurements showed high inter-rater reliability (κ = 0.99).
Organism-specific characteristics, such as wreath-like infiltrate for Nocardia spp., stromal lysis and round-shaped infiltrate in P. aeruginosa, or an active spreading edge in S. pneumoniae, may be useful in distinguishing between different etiologic organisms in bacterial keratitis. However, since this study was not planned prospectively, data regarding these characteristics were not collected in this trial, and therefore we could not analyze them. Previous studies have suggested that such signs can distinguish between organisms in fungal keratitis [17]. Further work should be done assessing the role of these specific signs in distinguishing between causative organisms in bacterial keratitis in a standardized, masked fashion.
The vast majority of patients enrolled in this trial were contact lens non-wearers in India. There were differences across groups of organisms isolated in each country. There were no S. pneumoniae isolates from the United States. P. aeruginosa was the most commonly isolated organism in the United States, and was the second most commonly isolated organism overall in the study. A small number of ulcers from the United States were enrolled in this trial, making comparisons of ulcers between the United States and India difficult. Previous reports of etiologic agents of bacterial keratitis in the United States and globally vary widely [6, 18–20]. This study enrolled a large number of ulcers that were diverse in severity and etiology. While numbers were too small to make comparisons with all organisms, we found that there exist significant differences in baseline characteristics among the top five isolates.
In conclusion, in this study of presentation characteristics of etiologic agents of bacterial keratitis, we found that P. aeruginosa ulcers were the most severe, with the largest and deepest infiltrates, and the worst presentation visual acuity. Contact lens use was associated with P. aeruginosa ulcers. Pneumococcal ulcers were less severe, although still had significant visual loss at presentation. Concomitant dacryocystitis was highly predictive of a pneumococcal ulcer. Nocardia ulcers had the best presenting visual acuity, but also were the most delayed in presentation. This series will help guide initial management decisions based on presenting characteristics, prior to microbiological diagnosis.
Acknowledgments
We would like to thank the patients who enrolled in the SCUT trial and their families, as well as the research staff at each of the trial sites. We are also grateful for the invaluable guidance and advice of the SCUT data and safety monitoring board: Marian Fisher, Ph.D. (chair), Anthony Aldave, M.D., Donald Everett, M.A., Jacqueline Glover, Ph.D., K. Ananda Kannan, M.D., Steven Kymes, Ph.D., G.V.S. Murthy, M.D., and Ivan Schwab, M.D. Funding for the SCUT was from the National Eye Institute, U10 EY015114. Dr. Acharya is supported by a National Eye Institute K23EY017897 grant and a Research to Prevent Blindness Award. The Department of Ophthalmology at UCSF is supported by a core grant from the National Eye Institute, EY02162, an unrestricted grant from Research to Prevent Blindness, New York, NY, and That Man May See, Inc., San Francisco, CA, USA.
Footnotes
Conflicts of interest None of the authors has any conflicts of interest to declare.
Contributor Information
Jeena Mascarenhas, Aravind Eye Care System, Madurai, India.
Muthiah Srinivasan, Aravind Eye Care System, Madurai, India.
Michael Chen, Department of Ophthalmology, University of California San Francisco, San Francisco, CA, USA.
Revathi Rajaraman, Aravind Eye Care System, Coimbatore, India.
Meenakshi Ravindran, Aravind Eye Care System, Tirunelveli, India.
Prajna Lalitha, Aravind Eye Care System, Madurai, India.
Catherine E. Oldenburg, F.I. Proctor Foundation, University of California San Francisco, San Francisco, CA, USA
Kathryn J. Ray, F.I. Proctor Foundation, University of California San Francisco, San Francisco, CA, USA
David V. Glidden, Department of Epidemiology & Biostatistics, University of California San Francisco, San Francisco, CA, USA
Stephanie Costanza, F.I. Proctor Foundation, University of California San Francisco, San Francisco, CA, USA.
Thomas M. Lietman, Department of Ophthalmology, University of California San Francisco, San Francisco, CA, USA. F.I. Proctor Foundation, University of California San Francisco, San Francisco, CA, USA. Department of Epidemiology & Biostatistics, University of California San Francisco, San Francisco, CA, USA
Nisha R. Acharya, Email: nisha.acharya@ucsf.edu, Department of Ophthalmology, University of California San Francisco, San Francisco, CA, USA. F.I. Proctor Foundation, University of California San Francisco, San Francisco, CA, USA. F.I. Proctor Foundation, University of California San Francisco, Room S309 513 Parnassus Avenue, San Francisco, CA 94143-0412, USA.
References
- 1.Whitcher J, Srinivasan M, Upadhyay M. Corneal blindness: a global perspective. Bull World Health Organ. 2001;79:214–221. [PMC free article] [PubMed] [Google Scholar]
- 2.Bharathi MJ, Ramakrishnan R, Meenakshi R, Padmavathy S, Shivakumar C, Srinivasan M. Microbial keratitis in South India: influence of risk factors, climate, and geographical variation. Ophthalmic Epidemiol. 2007;14(2):61–69. doi: 10.1080/09286580601001347. [DOI] [PubMed] [Google Scholar]
- 3.Dunlop A, Wright E, Howlader S, Nazrul I, Husain R, McClellan K, et al. Suppurative corneal ulceration in Bangladesh. Aust NZ J Ophthalmol. 1994;22(2):105–110. doi: 10.1111/j.1442-9071.1994.tb00775.x. [DOI] [PubMed] [Google Scholar]
- 4.Keay L, Edwards K, Naduvilath T, Taylor H, Snibson G, Forde K, et al. Microbial keratitis: predisposing factors and morbidity. Ophthalmology. 2006;113:109–116. doi: 10.1016/j.ophtha.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 5.Leck A, Thomas P, Hagan M, Kaliamurthy J, Ackuaku E, John M, et al. Aetiology of suppurative corneal ulcers in Ghana and South India, and epidemiology of fungal keratitis. Br J Ophthalmol. 2002;86:1211–1215. doi: 10.1136/bjo.86.11.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varaprasathan G, Miller K, Lietman T, Whitcher J, Cevallos V, Okumoto M, et al. Trends in the etiology of infectious corneal ulcers at the F.I. Proctor Foundation. Cornea. 2004;23:360–364. doi: 10.1097/00003226-200405000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Srinivasan M, Mascarenhas J, Rajaraman R, Ravindran M, Lalitha P, Glidden DV, Ray KJ, Hong KC, Oldenburg CE, Lee SM, Zegans ME, McLeod SD, Lietman TM, Acharya NR the Steroids for Corneal Ulcers Trial Group . The Steroids for Corneal Ulcers Trial (SCUT): study design and baseline characteristics. Arch Ophthalmol. 2012;130(2):151–157. doi: 10.1001/archophthalmol.2011.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilhelmus K, Liesegang T, Osato M, Jone D. Laboratory diagnosis of ocular infections. American Society for Microbiology Press; Washington: 1994. [Google Scholar]
- 9.National Committee for Clinical Laboratory Standards (NCCLS) NCCLS document M100-S10. 10. NCCLS; Wayne, PA: 2000. Performance standards for antimicrobial susceptibility testing. [Google Scholar]
- 10.Rietveld P, ter Riet G, Bindels P, Sloos J, van Weert H. Predicting bacterial cause in infectious conjunctivitis: a cohort study on informativeness of combinations of signs and symptoms. BMJ. 2004;329:206–210. doi: 10.1136/bmj.38128.631319.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krachmer J, Mannis M, Holland E, editors. Cornea: cornea and external disease: clinical diagnosis and management. Mosby; New York: 1997. [Google Scholar]
- 12.Sridhar M, Sharma S, Reddy M, Mruthyunjay P, Rao G. Clinicomicrobiological review of Nocardia keratitis. Cornea. 1998;17(1):17–22. doi: 10.1097/00003226-199801000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Lalitha P, Tiwari M, Prajna N, Gilpin C, Prakash K, Srinivasan M. Nocardia keratitis: species, drug sensitivities, and clinical correlation. Cornea. 2007;26(3):255–259. doi: 10.1097/ICO.0b013e318033d853. [DOI] [PubMed] [Google Scholar]
- 14.Aasuri M, Reddy M, Sharma S, Rao G. Co-occurrence of pneumococcal keratitis and dacryocystitis. Cornea. 1999;18(3):273–276. doi: 10.1097/00003226-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Parmar P, Salman A, Kalavathy M, Jesudasan C, Thomas P. Pneumococcal keratitis: a clinical profile. Clin Exp Ophthalmol. 2003;31:44–47. doi: 10.1046/j.1442-9071.2003.00598.x. [DOI] [PubMed] [Google Scholar]
- 16.Green M, Apel A, Stapleton F. Risk factors and causative organisms in microbial keratitis. Cornea. 2008;27(1):22–27. doi: 10.1097/ICO.0b013e318156caf2. [DOI] [PubMed] [Google Scholar]
- 17.Oldenburg C, Prajna N, Lalitha P, Krishnan T, Mascarenhas J, Vaitilingam C, et al. Clinical signs in dematiaceous and hyaline fungal keratitis. Br J Ophthalmol. 2011;95(5):750–751. doi: 10.1136/bjo.2010.198648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bourcier T, Thomas F, Borderie V, Chaumeil C, Laroche L. Bacterial keratitis: predisposing factors, clinical and microbiological review of 300 cases. Br J Ophthalmol. 2003;87:834–838. doi: 10.1136/bjo.87.7.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green M, Apel A, Stapleton F. A longitudinal study of trends in keratitis in Australia. Cornea. 2008;27(1):33–39. doi: 10.1097/ICO.0b013e318156cb1f. [DOI] [PubMed] [Google Scholar]
- 20.Srinivasan M, Gonzales C, George C, Cevallos V, Mascarenhas J, Asokan B, et al. Epidemiology and aetiological diagnosis of corneal ulceration in Madurai, South India. Br J Ophthalmol. 1997;81:965–971. doi: 10.1136/bjo.81.11.965. [DOI] [PMC free article] [PubMed] [Google Scholar]


