Abstract
Endothelial progenitor cell (EPC)-capturing techniques have led to revolutionary strategies that can improve the performance of cardiovascular implant devices and engineered tissues by enhancing re-endothelialization and angiogenesis. However, these strategies are limited by controversies regarding the phenotypic identities of EPCs as well as their inability to target and prevent the other afflictions associated with current therapies, namely, thrombosis and neointimal hyperplasia. Therefore, the goal of this study was to study the efficacy of a bioinspired multifunctional nanomatrix in recruiting and promoting the differentiation of EPCs toward an endothelial lineage. The bioinspired nanomatrix combines multiple components, including self-assembled peptide amphiphiles (PAs) that include cell adhesive ligands, nitric oxide (NO)-producing donors, and enzyme-mediated degradable sequences to achieve an endothelium-mimicking character. In this study, human peripheral blood mononuclear cells (PBMNCs) were isolated and cultured on the bioinspired multifunctional nanomatrix. Initial cell adhesion, lectin staining, acetylated low-density lipoprotein uptake, and expression of endothelial markers, including CD31, CD34, von Willebrand Factor, and VEGFR2, were analyzed. The results from this study indicate that the NO releasing bioinspired multifunctional nanomatrix promotes initial adhesion of EPCs when compared to control surfaces. The expression of endothelial markers is also increased on the bioinspired multifunctional nanomatrix, suggesting that it directs the differentiation of EPCs toward an endothelial phenotype. The bioinspired nanomatrix therefore provides a novel biomaterial-based platform for capturing as well as directing EPC behavior. Therefore, this study has the potential to positively impact the patency of cardiovascular devices such as stents and vascular grafts as well as enhanced angiogenesis for ischemic or engineered tissues.
Introduction
Endothelial progenitor cells (EPCs) are immature bone marrow-derived cells that circulate in the blood and possess the ability to proliferate, migrate, and differentiate into endothelial cells, but have not yet acquired the characteristics of mature endothelial cells. Currently, VEGFR2/CD133 or CD34/VEGFR2 double positive cells are commonly considered to be EPCs.1–5 EPCs have been found to be clinically significant in cardiovascular pathologies. They participate in endothelial homeostasis and promote formation of new blood vessels.6,7 They are mobilized in cases of tissue ischemia and home to sites of nascent neovascularization.4 Increased cardiovascular risk factors and development of atherosclerosis have been correlated with reduced EPC circulation.8–10 Significantly, coronary and arterial pathologies show improvement when treated with EPC-based therapies.11–14 Since their discovery,5 increasing attempts have been made to devise therapeutic strategies utilizing these cells to promote vasculogenesis and re-endothelialization in ischemic tissues and injured blood vessels, respectively.
The potential of EPCs gives them an especially great significance in cardiovascular implants such as stents, vascular grafts, and heart valves. The pathophysiology of adverse events in cardiovascular implant therapies consists of interactions between the cellular components of the blood vessels, blood, and the components of cardiovascular implants. Given the sheer number of these procedures performed every year, there remains a need to further improve the clinical performance of these devices. It is widely accepted that the re-establishment of a healthy endothelium is the ideal approach to achieve this aim.15 To this effect, several EPC capture strategies have been explored and they can be broadly divided into four categories: antibodies,16–18 peptides,19–21 selectins,2–24 and magnetic molecules.25 These techniques, especially the use of anti-CD34 antibodies on metallic stents, have shown great promise.26
Therefore, despite providing evidence for feasibility of EPC capture techniques, these strategies suffer from several limitations and unanswered questions.27–29 The safety of these devices has been questioned, as it was estimated that 99.6% of the cells attracted to anti-CD34 antibodies were not EPCs.30 Recent studies show that certain populations CD34+ cells can also differentiate into a smooth muscle cell phenotype, which can increase neointimal hyperplasia that is undesirable for stent patency.31,32 This is consistent with studies that show unselected bone marrow cells can differentiate into smooth muscle cells33 and lead to increased calcification.34 A similar increase in neointimal proliferation has also been found with anti-CD34 antibody-coated vascular grafts.35 Therefore, whether this method of EPC capture provides improvement over currently available technology has been questioned. The cause of these limitations can be tracked down to the controversy regarding the nature and the effectiveness of EPCs. EPCs are currently defined by the presence of a group of markers, including CD34, CD133, VE-cadherin, CD31, VEGFR2, and c-Kit.1,3,5,10,36,37 VEGFR2/CD133 or CD34/VEGFR2 double-positive cells are commonly considered to be EPCs.1,2,4,5 This lack of clear definition hinders the development of clear and effective strategies to capture EPCs and points toward the need for multifunctional strategies. It also prevents the accurate interpretation of the results from different studies utilizing different strategies.
Nevertheless, the need for re-establishment of endothelium in cardiovascular implant therapies such as stenting warrants further research on effective EPC-capturing strategies. The complexity of events that lead to adverse events such as restenosis and thrombosis, however, necessitate the successful incorporation of the EPC-capturing technique into an overall multifunctional strategy that simultaneously prevents all the limitations faced by conventional cardiovascular implants, including inflammation, restenosis, and thrombosis. Mimicking the native endothelium, which consists of a layer of endothelial cells lying on a nanofibrillar extracellular matrix, presents such a strategy that has great potential in increasing the clinical patency of stents. The endothelium also releases soluble factors to regulate vascular tone, particularly nitric oxide (NO), which is critical for maintaining vascular cell homeostasis.38 The design and development of biomaterials that mimic the native endothelium can therefore lead to improved EPC-capturing techniques, which in turn can result in improved clinical outcomes.
Therefore, the goal of this study is to study the effect of a bioinspired multifunctional nanomatrix that mimics the native endothelium on the cellular behavior of EPCs. It is hypothesized that the NO-releasing bioinspired multifunctional nanomatrix promotes the adhesion and the differentiation of EPCs toward an endothelial phenotype. The bioinspired nanomatrix consists of self-assembled peptide amphiphiles (PAs). PAs are an emerging class of versatile peptide-based biomaterials that comprise hydrophilic peptide chains covalently linked to a hydrophobic alkyl groups. The amphiphilicity of PAs drives their self-assembly in aqueous media without the use of organic solvents and therefore extends their applicability to a variety of implant devices. The peptide sequence allows the incorporation of cellular cues and therefore allows the design of multifunctional PAs that elicit specific responses from specific cell types. In this study, the peptide chain consists of a matrix metalloproteinase-2 (MMP-2) degradable sequence (Gly-Thr-Ala-Gly-Leu-Ile-Gly-Glu; GTAGLIGQ) linked to either an endothelial cell adhesive ligand (Tyr-Ile-Gly-Ser-Arg; YIGSR) or an NO-producing donor polylysine sequence (KKKKK). The MMP-2 degradable sequence allows degradation of the bioinspired nanomatrix by the cells to promote their migration.39 YIGSR is derived from laminin, which is a major component of the endothelial basement membrane. Previous studies have shown that incorporation of YIGSR promotes the adhesion and spreading of endothelial cells.40–45 NO is a critical cardiovascular regulator and is implicated in the mobilization, recruitment, and differentiation of EPCs.46,47 In native tissues, NO release from the endothelium via endothelial nitric oxide synthase (eNOS) stimulation has been shown to be the fundamental step in mobilization of EPCs into circulation.48 However, the understanding of physiological effect of NO on EPCs remains incomplete. In addition to a biomimetic multifunctional approach, an added significance of this study is the attempt to elucidate the potential of NO from donors in promoting recruitment and differentiation of EPCs, of which there are no conclusive studies.
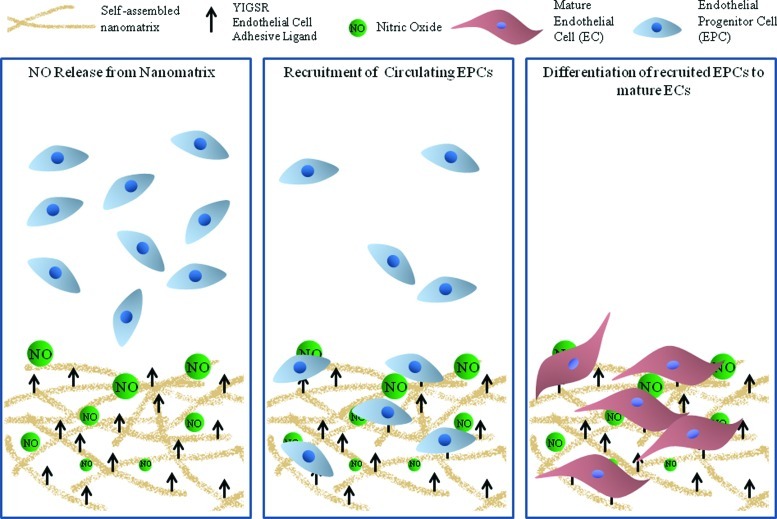
In summary, despite the promise of recently developed strategies to overcome the challenges of current cardiovascular therapies, there remain many emerging concerns and limited success. It has to be recognized that capturing EPCs is only the initial aspect of EPC-based therapeutic techniques, and is therefore critical to develop a multifunctional strategy. The presence of endothelial cell adhesive ligands, NO-producing donors, and enzyme-mediated degradable sequence endow this nanomatrix with a multifunctional, endothelium-mimicking character as shown in Figure 1. This nanomatrix is a novel biomaterial-based approach to capturing EPCs that provides a unique alternative to current techniques. The significance of this study lies in the design of a strategy to not just capture the EPCs, but to direct their endothelial differentiation, and therefore allowing their integration into the endothelium. The outcome of this study will also provide insight into the effect of NO on EPCs. The multifunctional biomaterial based approach to capturing EPCs will allow the utilization of the potential of EPCs toward vascularization of engineered tissues and ischemic tissues. Introduction of EPCs has shown increased capillary density and improved left ventricular ejection fraction in myocardial infarction models,49–51 and increased clinical performance in hind limb ischemia models,11,12,52 cerebral ischemia model,53 as well as in diabetic neuropathy.54 However, retention of cells remains a critical issue, and therefore a bioinspired nanomatrix can greatly improve the patency of these therapies. This study can therefore greatly impact the field of cardiovascular therapies by leading to improved clinical performance.
FIG. 1.
The bioinspired multifunctional nanomatrix is composed of a YIGSR cell adhesive ligand, nitric oxide (NO) producing donors and enzyme-mediated degradable sites. The presence of the Tyr-Ile-Gly-Ser-Arg (YIGSR) ligand and NO release recruit endothelial progenitor cell (EPCs) and promote their differentiation toward an endothelial phenotype. Color images available online at www.liebertpub.com/tec
Materials and Methods
Preparation of a bioinspired multifunctional nanomatrix
The bioinspired nanomatrix was prepared as previously described.55,56 Briefly, the two PAs, PA-YIGSR (CH3-(CH2)14-CONH-GTAGLIGQ-YIGSR) and PA-KKKKK (CH3-(CH2)14-CONH-KKKKK), were synthesized using Fmoc chemistry. The peptide chains were first synthesized on an Aapptech Apex 396 peptide synthesizer (Aapptech, KY). They were then alkylated with palmitic acid at the N-terminals using a mixture of o-benzotriazole-N,N,N,N-tetramethyluroniumhexafluorophosphate (HBTU), diisopropylethylamine (DiEA), and dimethylformamide (DMF). Cleavage was performed with a cleavage cocktail comprising trifluoroacetic acid, deionized water, triisopropylsilane, and anisole. The PAs were then precipitated and lyophilized, and the mass was confirmed via matrix-assisted laser desorption/ionization–time of flight.
PA-YIGSR contained an MMP-2 degradable sequence (GTAGLIGQ) linked to an endothelial cell adhesive ligand (YIGSR). PA-KKKKK contained the MMP-2 degradable sequence linked to a polylysine NO-producing donor (KKKKK). The PAs were dissolved in water at a concentration of 1 wt% and a pH of 7. The two PAs were mixed in a 9:1 ratio to form PA-YK.55,56 About 250 μL of 0.1 wt% PA-YK was added to each well in a 24-well plate and incubated at room temperature for 24 h. The increasing concentration of PA-YK with evaporation of water causes self-assembly of PA-YK into uniform nanofibers with diameters ranging from 8 to 10 nm. PA-YK was then reacted with pure NO to form PA-YK-NO. PA-YK-NO was then allowed to self-assemble by water evaporation to form nanomatrix coatings similar to PA-YK. In all subsequent experiments, PA-YK served as a control surface.
Isolation of human endothelial progenitor cells
Human endothelial progenitor cells (EPCs) were obtained from peripheral blood of healthy, consenting donors as previously described.11,12 The protocols used in this study have been approved by the Institutional Review Board (IRB). Human peripheral blood mononuclear cells (PBMNCs) were isolated using Histopaque-1077 (Sigma). Fifteen microliters of 2-fold magnetic-activated cell sorting (MACS) buffer diluted donor blood was added to 15 mL of Histopaque-1077 in a centrifuge tube, and centrifuged at 400 g for 30 min at room temperature. The supernatant was then discarded, and the opaque interface containing human PBMNCs was transferred to a new tube and suspended in MACS buffer. After mixing by gentle pipetting with a Pasteur pipette, the mixture was centrifuged again. After centrifugation, the supernatant was discarded, and the cell pellet was resuspended in media. Isolated human PBMNCs were suspended in endothelial growth medium-2 Bulletkit, containing endothelial basal media, 5% fetal bovine serum, human epidermal growth factor, fibroblast growth factor-B, vascular endothelial growth factor (VEGF), insulin-like growth factor, and ascorbic acid. Cells were seeded at a density of 80,000 cells per cm2 on PA-coated wells (24 well) and incubated at 37°C and 5% carbon dioxide.
Evaluation of initial PBMNC adhesion
Human PBMNCs were seeded at 80,000 cells/cm2 on PA-YK-NO and PA-YK. Tissue culture polystyrene (TCP) was used as a control surface. To evaluate initial adhesion at 24 h, the cells were washed three times with phosphate buffered saline and then stained by 4′,6-diamidino-2-phenylindole (DAPI) (Molecular Probes) and imaged by fluorescent microscopy. The number of cells/cm2 were evaluated by using the NIS elements imaging software.
To confirm the endothelial nature of the cells recruited by PA-YK-NO, they were subjected to lectin staining and ac-LDL uptake as previously performed.12 Human PBMNCs were seeded on PA-YK-NO at 80,000 cells/cm2. PA-YK and TCP were used as controls. At 7 and 14 days, the cells were incubated in media containing 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine labeled acetylated low-density lipoprotein (DiI-Ac-LDL) (Biomedical Technologies) for 2 h at 37°C. Then, the cells were fixed with 4% paraformaldehyde. Then, the cells were incubated with 1:200 Fluorescein Isothiocyanate (FITC)-conjugated Ulex Europaeus Agglutinin-1 Lectin (Sigma) and 1:40,000 DAPI. The cells were then be imaged and analyzed by NIS elements software. Cells triple-positive for Ac-LDL uptake, Lectin staining, and DAPI were imaged as EPCs.
Evaluation of EPC differentiation
Effect of NO released from PA-YK-NO on cell differentiation was studied by analyzing the change in cell surface markers, including CD45, CD34, CD31, VEGFR2, and von Willebrand Factor (vWF), using flow cytometry as previously done.11,12 Human PBMNCs were seeded on PA-YK and PA-YK-NO. TCP was used as a control surface. After 7 and 14 days in culture, cells were detached using Accutase and incubated for 30 min at 4°C with primary antibodies (FITC-anti-CD45, Phycoerythrin (PE)-anti-CD34, PE-anti-CD31, PE-anti-VEGFR2, and PE-anti-vWF). Isotype identical antibodies served as controls. The cells were fixed in 4% paraformaldehyde and subjected to quantitative flow cytometry.
Statistical analysis
Each study was performed independently at least three times. Statistical significance was obtained by using one-way analysis of variance (ANOVA) (SPSS) to compare the data. Within ANOVA, Tukey multiple comparisons test was performed to detect significant differences between pairs. A value of p<0.05 was considered to be statistically significant.
Results and Discussion
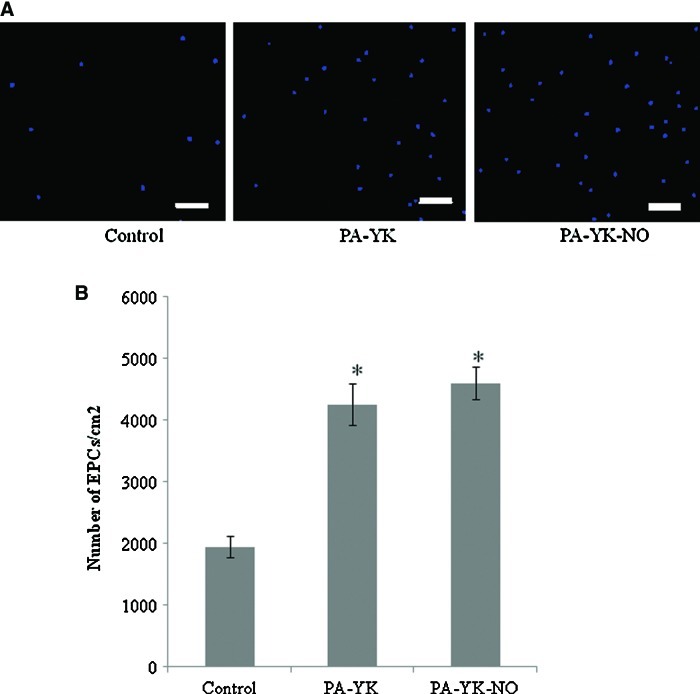
The bioinspired multifunctional nanomatrix was prepared as previously described.55,56 As previously performed,55 the ratio of PA-YIGSR to PA-KKKKK was optimized based on endothelial cell adhesion. Briefly, endothelial cells were cultured on increasing ratios of PA-YIGSR and PA-KKKKK and cell number was evaluated at 4 h. The greatest adhesion was found on 9:1 mixture of PA-YIGSR and PA-KKKKK. This was called PA-YK and was reacted with pure NO to obtain PA-YK-NO. PA-YK-NO is the NO-releasing endothelium-mimicking nanomatrix. Human EPCs were successfully isolated from the blood of consenting volunteers as described previously.11,12 The EPCs isolated in this study may be considered “early” EPCs.57 While “late” EPCs exhibit greater proliferative capabilities, “early” EPCs show limited proliferation, but greater secretion of pro-angiogenic factors. Initial cell adhesion was evaluated by staining with DAPI and the results are shown in Figure 2A. From Figure 2A, it is evident that there are a greater number of cells with PA-YK-NO and PA-YK when compared to the uncoated TCP control surface. After 24 h, cell number was analyzed using NIS Elements software and these results are shown in Figure 2B. The number of PBMNCs was significantly higher on PA-YK-NO and PA-YK when compared to TCP. There was no difference between PA-YK-NO and PA-YK. This shows that the bioinspired nanomatrix has the ability to promote the adhesion of EPCs. It can also be inferred that the presence of YIGSR cell adhesive ligand is promoting the adhesion and retention of these cells.
FIG. 2.
Initial adhesion of EPCs. (A) 4′,6-diamidino-2-phenylindole staining on Tissue culture plate (TCP), PA-YK, and PA-YK-NO. Scale bar=50 μm. (B) Quantitative analysis of initial adhesion on TCP, PA-YK and PA-YK-NO. The number of EPCs was significantly higher on PA-YK-NO and PA-YK when compared to TCP (*p<0.05). Color images available online at www.liebertpub.com/tec
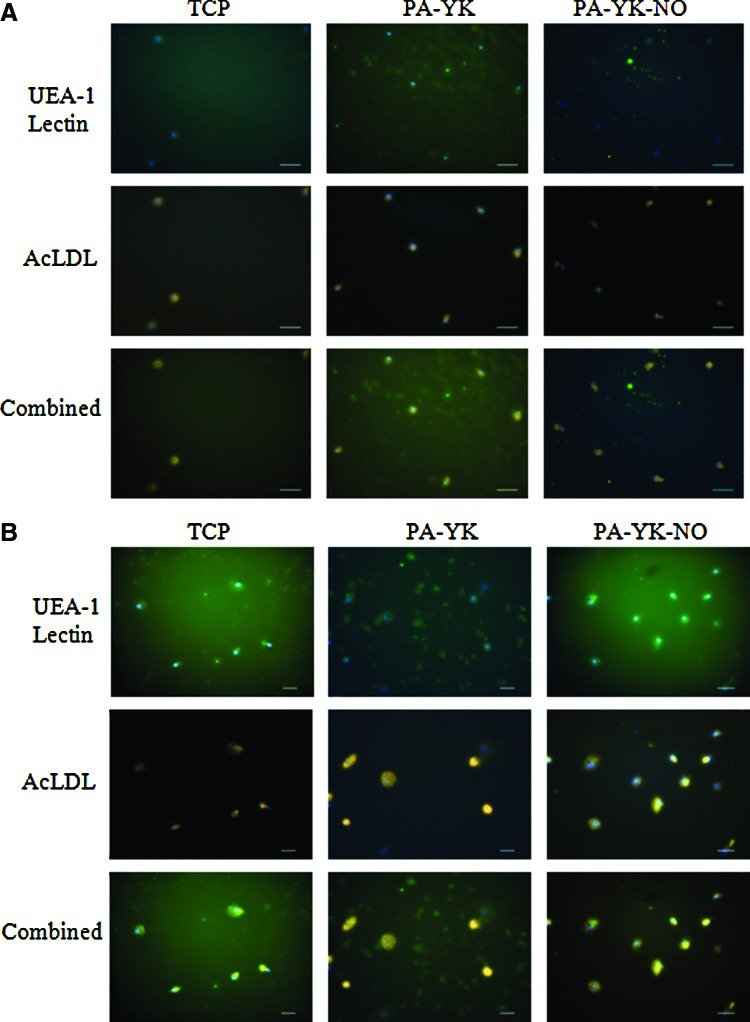
To evaluate whether these circulating cells recruited by the bioinspired nanomatrix possessed endothelial character, they were assayed for uptake of 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine labeled acetylated low-density lipoprotein (Dil-ac-LDL) and staining by FITC-conjugated Ulex Europaeus Agglutinin-1 Lectin. Uptake of ac-LDL and Ulex lectin staining is characteristic of endothelial cells. As shown in Figure 3, the cells recruited by the nanomatrices showed ac-LDL uptake as well as lectin staining at day 7 and day 14, thereby showing that they possessed endothelial characteristics. Since these cells were originally PBMNCs that were recruited, it can be inferred that they were indeed EPCs. This is a significant result as the population of true EPCs in peripheral blood is very small. However, in cases of acute vascular trauma, a 50-fold increase in EPC population has been reported2 and the recruitment of these cells is critical for patency of cardiovascular devices.
FIG. 3.
UEA-1 Lectin staining and acetylated low-density lipoprotein (ac-LDL) uptake on TCP, PA-YK, and PA-YK-NO on day 7 (A) and day 14 (B). Lectin staining and LDL uptake show that the cells have an endothelial character. Scale bar=50 μm. Color images available online at www.liebertpub.com/tec
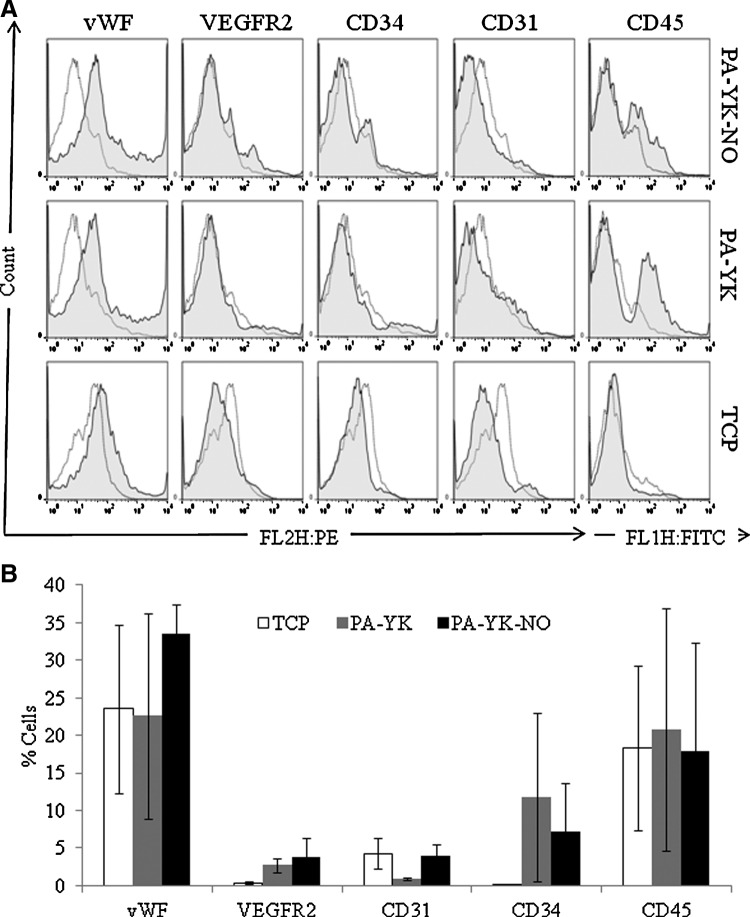
To further analyze EPC differentiation, the cells were treated with fluorochrome tagged antibodies for CD31, vWF, CD34, VEGFR2 and CD45 at days 7 and 14 and analyzed by flow cytometry. Figure 4 displays the expression of endothelial markers at the 7 day time point while expression of the same markers at day 14 is shown in Figure 5. CD31, CD34, VEGFR2, and vWF are endothelial markers. It is evident that there is an increased expression of these endothelial markers on PA-YK-NO when compared to controls at day 7 as well as day 14. When quantified, the percentage of cells expressing CD34, VEGFR2, and vWF was significantly higher on PA-YK when compared to TCP. However, the expression of these markers was significantly increased on PA-YK-NO, the NO releasing bioinspired nanomatrix when compared to the control substrates, as shown in Figure 5B (day 14). There was no statistical difference on day 7 (Fig. 4B). This shows that over the 2 weeks, the cells that have been recruited are directed toward endothelial phenotype. The expression of VEGFR2 is especially important, as recent studies show elevated levels of VEGF in cases of acute vascular trauma.2 This is also consistent with the role of VEGF as a promoter of angiogenesis and vasculogenesis via interactions with VEGFR2. VEGF is also thought to regulate the mobilization of EPCs as evidenced by the correlation of elevated blood EPC levels with serum VEGF levels in animal models and clinical pilot trials.2,58,59 Interestingly, the expression of CD45 was lower on PA-YK-NO when compared to control surfaces at day 14. At day 7, there was no difference in the expression of CD45 on PA-YK-NO when compared to the controls. CD45 is a cell surface marker found commonly on circulating blood cells and is indicative of an inflammatory phenotype. From these results, it can be inferred that PA-YK-NO is limiting the differentiation of the recruited cells into an inflammatory phenotype. This is consistent with previous studies have shown that NO may have an anti-inflammatory effect60,61 and therefore contributes to the patency of PA-YK-NO coated devices.
FIG. 4.
(A) Flow cytometric analysis of endothelial markers on PA-YK-NO, PA-YK, and TCP on day 7. (B) Quantitative analysis of expression of endothelial markers on day 7.
FIG. 5.
(A) Flow cytometric analysis of endothelial markers on PA-YK-NO, PA-YK, and TCP on day 14. (B) Quantitative analysis of expression of endothelial markers at 14 days. Expression of von Willebrand Factor (vWF), VEGFR2, and CD34 was significantly higher on PA-YK-NO when compared to control samples (*p<0.05). Expression of vWF, VEGFR2, and CD34 was significantly higher on PA-YK when compared to TCP (#p<0.05). Expression of CD45 was significantly reduced on PA-YK-NO (**p<0.05).
From these experiments, it is evident that the PA-YK-NO nanomatrix is promoting the initial adhesion of EPCs and it is directing the differentiation of these EPCs into an endothelial phenotype. Further, it is clear that the presence of the YIGSR cell adhesive ligand is promoting the initial cell adhesion of these cells. Previous studies have demonstrated that laminin derived YIGSR promotes the adhesion and spreading of endothelial cells.40 However, NO released by PA-YK-NO is promoting the differentiation of these cells into an endothelial phenotype. NO is a critical cardiovascular regulator and it has been shown to positively influence the recruitment and differentiation of EPCs. More importantly, the release of NO may promote the mobilization of EPCs from the bone marrow. While several factors such as G-CSF, GM-CSF, and VEGF initiate the mobilization of EPCs,7,62,63 NO released by the endothelium via endothelial eNOS has been shown to be a critical regulator in all their mechanisms of action.47 Increased NO bioavailability leads to cleavage of intercellular adhesions between stem cells and stromal cells of bone marrow by proteinases, causing the mobilization of EPCs into circulation.48 eNOS transcription enhancer treated bone marrow cells exhibited improved migration and neovascularization in a hind-limb ischemia model, while the use of an eNOS inhibitor prevented such function.64 This is also consistent with cases of impaired eNOS function, as seen in diabetes mellitus where reduced EPC mobilization has been observed.65 Thus, mimicking the native endothelium by releasing NO not only recruits and differentiates EPCs; it further increases EPC availability for accelerating the process of endothelialization.
EPC capture strategies have gained attention recently due to the potential of EPCs to increase clinical patency of cardiovascular devices. Immobilization of antibodies for known EPC markers has been the most attractive strategy so far.18,26 In animal studies, the EPC-capturing stents demonstrated endothelialization within 24–48 h after implantation, and this allowed earlier discontinuation of anti-platelet therapy. This technology has been further studied in clinical studies, and has proven to be feasible.16,66,67 In one clinical study, it was found that thrombosis was minimal at 30 days and 6 months while neointimal hyperplasia was seen at rates similar to BMS.16 However, the actual clinical effectiveness was questioned when a case of restenosis and late stent thrombosis was reported in an EPC capture stent roughly 5 months after implantation.68 Recent reports also suggest that while the technology is feasible, it was lacking in efficacy as it could not prevent neointimal hyperplasia and restenosis.27–29 These strategies are clearly limited by the controversy regarding the nature of EPCs and this underlines the need for further research into characterization of EPCs. These strategies also focus on endothelialization and fail to consider the other limitations of current devices. This highlights the need for a multifunctional strategy. Recent studies have attempted a combination of EPC capture techniques.24,69 In one study, EPC-capturing anti-CD34 antibody was used on a sirolimus eluting stent.70 While the results were promising, the effectiveness of the strategy remains in doubt.71,72 A bioinspired endothelium mimicking approach may be able to prevent all the limitations of these devices by providing the right microenvironment for the adhesion of specific cell types and by regulating cellular behavior.
In summary, a self-assembled bioinspired multifunctional nanomatrix has been developed that contains endothelial cell adhesive ligands, NO producing donors and enzyme-mediated degradable sites. Previous studies have shown that this bioinspired nanomatrix releases NO in a desirable biphasic manner,55,56 and previous studies have shown that the amount of NO released is comparable to cumulative NO released by endothelial cells.55,73 It promotes the adhesion and proliferation of endothelial cells, but limits smooth muscle cell proliferation and platelet adhesion.55,56 This bioinspired nanomatrix promotes the adhesion of EPCs and it has been shown to promote endothelial differentiation of EPCs. In vivo this NO release may therefore promote the recruitment and differentiation of EPCs, while YIGSR is expected to improve retention of cells under shear stress. The novelty of this approach lies in its difference from all other EPC-capturing strategies as it uses an endothelium mimicking biomaterial to capture EPCs. Further research on EPC biology is warranted for effective utilization of their potential and will lead to better and improved EPC-capturing techniques. However, taking into perspective the current ambiguity and controversy regarding the phenotypic identity of EPCs and their potential to differentiate into other cell types, merely capturing EPCs does not represent the true endpoint of an EPC based therapy. It is also critical to regulate and direct the endothelial differentiation of EPCs and promote their integration into the endothelium. The ability of this nanomatrix to recruit EPCs and promote their endothelial differentiation by mimicking the native endothelium is therefore the true significance of this study. In addition, this bioinspired nanomatrix allows the successful incorporation of an EPC based therapy into a multifunctional strategy that targets all the limitations of current devices including restenosis, thrombosis, and neointimal hyperplasia. The simple water based self-assembly of the nanomatrix without the use of organic solvents also allows the extension of EPC based techniques to the newly developing field of bioabsorbable stents.74–77 The biomaterial based approach to EPC therapy also allows the extension of this technology to improve vascularization of engineered tissues and ischemic tissues. Angiogenesis remains a critical requirement in cases of ischemia, pathologies such as diabetic neuropathy, and tissue engineered constructs. This nanomatrix therefore has great potential as a novel biomaterial for a broad range of cardiovascular applications and it can positively impact the performance of devices such as stents, vascular grafts, and heart valves as well as ischemic and engineered tissues through enhanced angiogenesis.
Acknowledgments
The authors express their gratitude to Joan Harshberger (RN) and Enid Keyser for the use of Flow cytometry facilities. This study was supported by the Wallace H Coulter Foundation (HWJ), Caroline P Ireland Research Scholarship and the American Heart Association Predoctoral Fellowship (10PRE3500024) (AA), UAB PREP scholarship and the NIH (GM086256) (CPA).
Disclosure Statement
No competing financial interests exist.
References
- 1.Shi Q. Rafii S. Wu M.H. Wijelath E.S. Yu C. Ishida A., et al. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92:362. [PubMed] [Google Scholar]
- 2.Gill M. Dias S. Hattori K. Rivera M.L. Hicklin D. Witte L., et al. Vascular trauma induces rapid but transient mobilization of VEGFR2(+)AC133(+) endothelial precursor cells. Circ Res. 2001;88:167. doi: 10.1161/01.res.88.2.167. [DOI] [PubMed] [Google Scholar]
- 3.Peichev M. Naiyer A.J. Pereira D. Zhu Z. Lane W.J. Williams M., et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952. [PubMed] [Google Scholar]
- 4.Asahara T. Masuda H. Takahashi T. Kalka C. Pastore C. Silver M., et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 5.Asahara T. Murohara T. Sullivan A. Silver M. van der Zee R. Li T., et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 6.Werner N. Priller J. Laufs U. Endres M. Bohm M. Dirnagl U., et al. Bone marrow-derived progenitor cells modulate vascular reendothelialization and neointimal formation: effect of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition. Arterioscler Thromb Vasc Biol. 2002;22:1567. doi: 10.1161/01.atv.0000036417.43987.d8. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi T. Kalka C. Masuda H. Chen D. Silver M. Kearney M., et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 8.Fadini G.P. Miorin M. Facco M. Bonamico S. Baesso I. Grego F., et al. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol. 2005;45:1449. doi: 10.1016/j.jacc.2004.11.067. [DOI] [PubMed] [Google Scholar]
- 9.Vasa M. Fichtlscherer S. Aicher A. Adler K. Urbich C. Martin H., et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 10.Hill J.M. Zalos G. Halcox J.P. Schenke W.H. Waclawiw M.A. Quyyumi A.A., et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 11.Kim H. Cho H.J. Kim S.W. Liu B. Choi Y.J. Lee J., et al. CD31+ cells represent highly angiogenic and vasculogenic cells in bone marrow: novel role of nonendothelial CD31+ cells in neovascularization and their therapeutic effects on ischemic vascular disease. Circ Res. 2010;107:602. doi: 10.1161/CIRCRESAHA.110.218396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim S.W. Kim H. Cho H.J. Lee J.U. Levit R. Yoon Y.S. Human peripheral blood-derived CD31+ cells have robust angiogenic and vasculogenic properties and are effective for treating ischemic vascular disease. J Am Coll Cardiol. 2010;56:593. doi: 10.1016/j.jacc.2010.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tateishi-Yuyama E. Matsubara H. Murohara T. Ikeda U. Shintani S. Masaki H., et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360:427. doi: 10.1016/S0140-6736(02)09670-8. [DOI] [PubMed] [Google Scholar]
- 14.Assmus B. Schachinger V. Teupe C. Britten M. Lehmann R. Dobert N., et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI) Circulation. 2002;106:3009. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 15.Kipshidze N. Dangas G. Tsapenko M. Moses J. Leon M.B. Kutryk M., et al. Role of the endothelium in modulating neointimal formation: vasculoprotective approaches to attenuate restenosis after percutaneous coronary interventions. J Am Coll Cardiol. 2004;44:733. doi: 10.1016/j.jacc.2004.04.048. [DOI] [PubMed] [Google Scholar]
- 16.Aoki J. Serruys P.W. van Beusekom H. Ong A.T. McFadden E.P. Sianos G., et al. Endothelial progenitor cell capture by stents coated with antibody against CD34: the HEALING-FIM (Healthy Endothelial Accelerated Lining Inhibits Neointimal Growth-First In Man) Registry. J Am Coll Cardiol. 2005;45:1574. doi: 10.1016/j.jacc.2005.01.048. [DOI] [PubMed] [Google Scholar]
- 17.Garg S. Duckers H.J. Serruys P.W. Endothelial progenitor cell capture stents: will this technology find its niche in contemporary practice? Eur Heart J. 2010;31:1032. doi: 10.1093/eurheartj/ehp591. [DOI] [PubMed] [Google Scholar]
- 18.Vaughn M.W. Kuo L. Liao J.C. Effective diffusion distance of nitric oxide in the microcirculation. Am J Physiol. 1998;274:H1705. doi: 10.1152/ajpheart.1998.274.5.H1705. [DOI] [PubMed] [Google Scholar]
- 19.Veleva A.N. Heath D.E. Cooper S.L. Patterson C. Selective endothelial cell attachment to peptide-modified terpolymers. Biomaterials. 2008;29:3656. doi: 10.1016/j.biomaterials.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 20.Blindt R. Vogt F. Astafieva I. Fach C. Hristov M. Krott N., et al. A novel drug-eluting stent coated with an integrin-binding cyclic Arg-Gly-Asp peptide inhibits neointimal hyperplasia by recruiting endothelial progenitor cells. J Am Coll Cardiol. 2006;47:1786. doi: 10.1016/j.jacc.2005.11.081. [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann J. Paul A. Harwardt M. Groll J. Reeswinkel T. Klee D., et al. Immobilized DNA aptamers used as potent attractors for porcine endothelial precursor cells. J Biomed Mater Res A. 2008;84:614. doi: 10.1002/jbm.a.31309. [DOI] [PubMed] [Google Scholar]
- 22.Suuronen E.J. Zhang P. Kuraitis D. Cao X. Melhuish A. McKee D., et al. An acellular matrix-bound ligand enhances the mobilization, recruitment and therapeutic effects of circulating progenitor cells in a hindlimb ischemia model. Faseb J. 2009;23:1447. doi: 10.1096/fj.08-111054. [DOI] [PubMed] [Google Scholar]
- 23.Narasipura S.D. Wojciechowski J.C. Charles N. Liesveld J.L. King M.R. P-Selectin coated microtube for enrichment of CD34+ hematopoietic stem and progenitor cells from human bone marrow. Clin Chem. 2008;54:77. doi: 10.1373/clinchem.2007.089896. [DOI] [PubMed] [Google Scholar]
- 24.Behjati M. Kazemi M. Hashemi M. Zarkesh-Esfahanai S.H. Bahrami E. Hashemi-Beni B., et al. Double-chimera proteins to enhance recruitment of endothelial cells, their progenitor cells. Int J Cardiol. 2012 doi: 10.1016/j.ijcard.2012.04.094. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Pislaru S.V. Harbuzariu A. Agarwal G. Witt T. Gulati R. Sandhu N.P., et al. Magnetic forces enable rapid endothelialization of synthetic vascular grafts. Circulation. 2006;114:I314. doi: 10.1161/CIRCULATIONAHA.105.001446. [DOI] [PubMed] [Google Scholar]
- 26.Kutryk M. Kuliszewski M. In vivo endothelial progenitor cell seeding for the accelerated endothelialization of endovascular devices. Am J Cardiol. 2003;92:941. [Google Scholar]
- 27.van Beusekom H.M. Ertas G. Sorop O. Serruys P.W. van der Giessen W.J. The Genous endothelial progenitor cell capture stent accelerates stent re-endothelialization but does not affect intimal hyperplasia in porcine coronary arteries. Catheter Cardiovasc Interv. 2012;79:231. doi: 10.1002/ccd.22928. [DOI] [PubMed] [Google Scholar]
- 28.Belardi J.A. Albertal M. Genous endothelial progenitor cell capturing stent: thrombus-resistant but vulnerable to restenosis. Catheter Cardiovasc Interv. 2011;78:196. doi: 10.1002/ccd.23284. [DOI] [PubMed] [Google Scholar]
- 29.den Dekker W.K. Houtgraaf J.H. Onuma Y. Benit E. de Winter R.J. Wijns W., et al. Final results of the HEALING IIB trial to evaluate a bio-engineered CD34 antibody coated stent (GenousStent) designed to promote vascular healing by capture of circulating endothelial progenitor cells in CAD patients. Atherosclerosis. 2011;219:245. doi: 10.1016/j.atherosclerosis.2011.06.032. [DOI] [PubMed] [Google Scholar]
- 30.Avci-Adali M. Perle N. Ziemer G. Wendel H.P. Current concepts and new developments for autologous in vivo endothelialisation of biomaterials for intravascular applications. Eur Cell Mater. 2011;21:157. doi: 10.22203/ecm.v021a13. [DOI] [PubMed] [Google Scholar]
- 31.Shimizu K. Sugiyama S. Aikawa M. Fukumoto Y. Rabkin E. Libby P., et al. Host bone-marrow cells are a source of donor intimal smooth- muscle-like cells in murine aortic transplant arteriopathy. Nat Med. 2001;7:738. doi: 10.1038/89121. [DOI] [PubMed] [Google Scholar]
- 32.Simper D. Stalboerger P.G. Panetta C.J. Wang S. Caplice N.M. Smooth muscle progenitor cells in human blood. Circulation. 2002;106:1199. doi: 10.1161/01.cir.0000031525.61826.a8. [DOI] [PubMed] [Google Scholar]
- 33.Caplice N.M. Bunch T.J. Stalboerger P.G. Wang S. Simper D. Miller D.V., et al. Smooth muscle cells in human coronary atherosclerosis can originate from cells administered at marrow transplantation. Proc Natl Acad Sci U S A. 2003;100:4754. doi: 10.1073/pnas.0730743100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoon Y.S. Park J.S. Tkebuchava T. Luedeman C. Losordo D.W. Unexpected severe calcification after transplantation of bone marrow cells in acute myocardial infarction. Circulation. 2004;109:3154. doi: 10.1161/01.CIR.0000134696.08436.65. [DOI] [PubMed] [Google Scholar]
- 35.Rotmans J.I. Heyligers J.M. Verhagen H. Velema E. Nagtegaal M. Kleijn D.D, et al. In vivo cell seeding with anti-CD34 antibodies successfully accelerates endothelialization but stimulates intimal hyperplasia in porcine arteriovenous expanded polytetrafluoroethylene grafts. Circulation. 2005;112:12. doi: 10.1161/CIRCULATIONAHA.104.504407. [DOI] [PubMed] [Google Scholar]
- 36.George J. Shmilovich H. Deutsch V. Miller H. Keren G. Roth A. Comparative analysis of methods for assessment of circulating endothelial progenitor cells. Tissue Eng. 2006;12:331. doi: 10.1089/ten.2006.12.331. [DOI] [PubMed] [Google Scholar]
- 37.Hristov M. Erl W. Weber P.C. Endothelial progenitor cells: mobilization, differentiation, and homing. Arterioscler Thromb Vasc Biol. 2003;23:1185. doi: 10.1161/01.ATV.0000073832.49290.B5. [DOI] [PubMed] [Google Scholar]
- 38.Saavedra J. Fitzhugh A. Keefer L. Chapter 24: Diazeniumdiolates (Formerly NONOates) in Cardiovscular Research and Potential Clinical Applications. In: Loscalzo J., editor; Vita J.A., editor. Nitric Oxide and the Cardiovascular System. Totowa, NJ: Humana Press; 2000. [Google Scholar]
- 39.Jun H.W. Yuwono V. Paramonov S.E. Hartgerink J.D. Enzyme-mediated degradation of peptide-amphiphile nanofiber networks. Adv Mater. 2005;17:2612. [Google Scholar]
- 40.Andukuri A. Minor W.P. Kushwaha M. Anderson J.M. Jun H.W. Effect of endothelium mimicking self-assembled nanomatrices on cell adhesion and spreading of human endothelial cells and smooth muscle cells. Nanomedicine. 2010;6:289. doi: 10.1016/j.nano.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vaughn M.W. Huang K.T. Kuo L. Liao J.C. Erythrocyte consumption of nitric oxide: competition experiment and model analysis. Nitric Oxide. 2001;5:18. doi: 10.1006/niox.2000.0328. [DOI] [PubMed] [Google Scholar]
- 42.Huang K.T. Han T.H. Hyduke D.R. Vaughn M.W. Van Herle H. Hein T.W., et al. Modulation of nitric oxide bioavailability by erythrocytes. Proc Natl Acad Sci U S A. 2001;98:11771. doi: 10.1073/pnas.201276698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song J. Kim H. Rhee M. Chae I. Sohn D. Oh B., et al. Effect of hypercholesterolemia on the sequential changes of apoptosis and proliferation after balloon injury to rabbit iliac artery. Atherosclerosis. 2000;150:309. doi: 10.1016/s0021-9150(99)00384-6. [DOI] [PubMed] [Google Scholar]
- 44.Liao J.C. Hein T.W. Vaughn M.W. Huang K.T. Kuo L. Intravascular flow decreases erythrocyte consumption of nitric oxide. Proc Natl Acad Sci U S A. 1999;96:8757. doi: 10.1073/pnas.96.15.8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu X. Samouilov A. Lancaster J.R., Jr. Zweier J.L. Nitric oxide uptake by erythrocytes is primarily limited by extracellular diffusion not membrane resistance. J Biol Chem. 2002;277:26194. doi: 10.1074/jbc.M201939200. [DOI] [PubMed] [Google Scholar]
- 46.Chu L. Jiang Y. Hao H. Xia Y. Xu J. Liu Z., et al. Nitric oxide enhances Oct-4 expression in bone marrow stem cells and promotes endothelial differentiation. Eur J Pharmacol. 2008;591:59. doi: 10.1016/j.ejphar.2008.06.066. [DOI] [PubMed] [Google Scholar]
- 47.Aicher A. Heeschen C. Mildner-Rihm C. Urbich C. Ihling C. Technau-Ihling K., et al. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- 48.Heissig B. Hattori K. Dias S. Friedrich M. Ferris B. Hackett N.R., et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kamihata H. Matsubara H. Nishiue T. Fujiyama S. Amano K. Iba O., et al. Improvement of collateral perfusion and regional function by implantation of peripheral blood mononuclear cells into ischemic hibernating myocardium. Arterioscler Thromb Vasc Biol. 2002;22:1804. doi: 10.1161/01.atv.0000039168.95670.b9. [DOI] [PubMed] [Google Scholar]
- 50.Kawamoto A. Gwon H.C. Iwaguro H. Yamaguchi J.I. Uchida S. Masuda H., et al. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001;103:634. doi: 10.1161/01.cir.103.5.634. [DOI] [PubMed] [Google Scholar]
- 51.Kawamoto A. Tkebuchava T. Yamaguchi J. Nishimura H. Yoon Y.S. Milliken C., et al. Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation. 2003;107:461. doi: 10.1161/01.cir.0000046450.89986.50. [DOI] [PubMed] [Google Scholar]
- 52.Kalka C. Masuda H. Takahashi T. Kalka-Moll W.M. Silver M. Kearney M., et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci U S A. 2000;97:3422. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Z.G. Zhang L. Jiang Q. Chopp M. Bone marrow-derived endothelial progenitor cells participate in cerebral neovascularization after focal cerebral ischemia in the adult mouse. Circ Res. 2002;90:284. doi: 10.1161/hh0302.104460. [DOI] [PubMed] [Google Scholar]
- 54.Jeong J.O. Kim M.O. Kim H. Lee M.Y. Kim S.W. Ii M., et al. Dual angiogenic and neurotrophic effects of bone marrow-derived endothelial progenitor cells on diabetic neuropathy. Circulation. 2009;119:699. doi: 10.1161/CIRCULATIONAHA.108.789297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kushwaha M. Anderson J.M. Bosworth C.A. Andukuri A. Minor W.P. Lancaster J.R., Jr., et al. A nitric oxide releasing, self assembled peptide amphiphile matrix that mimics native endothelium for coating implantable cardiovascular devices. Biomaterials. 2010;31:1502. doi: 10.1016/j.biomaterials.2009.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Andukuri A. Kushwaha M. Tambralli A. Anderson J.M. Dean D.R. Berry J.L., et al. A hybrid biomimetic nanomatrix composed of electrospun polycaprolactone and bioactive peptide amphiphiles for cardiovascular implants. Acta Biomater. 2011;7:225. doi: 10.1016/j.actbio.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cho H.J. Kim H.S. Lee M.M. Kim D.H. Yang H.J. Hur J., et al. Mobilized endothelial progenitor cells by granulocyte-macrophage colony-stimulating factor accelerate reendothelialization and reduce vascular inflammation after intravascular radiation. Circulation. 2003;108:2918. doi: 10.1161/01.CIR.0000097001.79750.78. [DOI] [PubMed] [Google Scholar]
- 58.Asahara T. Bauters C. Pastore C. Kearney M. Rossow S. Bunting S., et al. Local delivery of vascular endothelial growth factor accelerates reendothelialization and attenuates intimal hyperplasia in balloon-injured rat carotid artery. Circulation. 1995;91:2793. doi: 10.1161/01.cir.91.11.2793. [DOI] [PubMed] [Google Scholar]
- 59.Kalka C. Tehrani H. Laudenberg B. Vale P.R. Isner J.M. Asahara T., et al. VEGF gene transfer mobilizes endothelial progenitor cells in patients with inoperable coronary disease. Ann Thorac Surg. 2000;70:829. doi: 10.1016/s0003-4975(00)01633-7. [DOI] [PubMed] [Google Scholar]
- 60.Kubes P. Suzuki M. Granger D.N. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991;88:4651. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harrison C.B. Drummond G.R. Sobey C.G. Selemidis S. Evidence that nitric oxide inhibits vascular inflammation and superoxide production via a p47phox-dependent mechanism in mice. Clin Exp Pharmacol Physiol. 2010;37:429. doi: 10.1111/j.1440-1681.2009.05317.x. [DOI] [PubMed] [Google Scholar]
- 62.Aicher A. Zeiher A.M. Dimmeler S. Mobilizing endothelial progenitor cells. Hypertension. 2005;45:321. doi: 10.1161/01.HYP.0000154789.28695.ea. [DOI] [PubMed] [Google Scholar]
- 63.Wojakowski W. Kucia M. Kazmierski M. Ratajczak M.Z. Tendera M. Circulating progenitor cells in stable coronary heart disease and acute coronary syndromes: relevant reparatory mechanism? Heart. 2008;94:27. doi: 10.1136/hrt.2006.103358. [DOI] [PubMed] [Google Scholar]
- 64.Sasaki K. Heeschen C. Aicher A. Ziebart T. Honold J. Urbich C., et al. Ex vivo pretreatment of bone marrow mononuclear cells with endothelial NO synthase enhancer AVE9488 enhances their functional activity for cell therapy. Proc Natl Acad Sci U S A. 2006;103:14537. doi: 10.1073/pnas.0604144103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thum T. Fraccarollo D. Schultheiss M. Froese S. Galuppo P. Widder J.D., et al. Endothelial nitric oxide synthase uncoupling impairs endothelial progenitor cell mobilization and function in diabetes. Diabetes. 2007;56:666. doi: 10.2337/db06-0699. [DOI] [PubMed] [Google Scholar]
- 66.Miglionico M. Patti G. D'Ambrosio A. Di Sciascio G. Percutaneous coronary intervention utilizing a new endothelial progenitor cells antibody-coated stent: a prospective single-center registry in high-risk patients. Catheter Cardiovasc Interv. 2008;71:600. doi: 10.1002/ccd.21437. [DOI] [PubMed] [Google Scholar]
- 67.Co M. Tay E. Lee C.H. Poh K.K. Low A. Lim J., et al. Use of endothelial progenitor cell capture stent (Genous Bio-Engineered R Stent) during primary percutaneous coronary intervention in acute myocardial infarction: intermediate- to long-term clinical follow-up. Am Heart J. 2008;155:128. doi: 10.1016/j.ahj.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 68.Rossi M.L. Zavalloni D. Gasparini G.L. Mango R. Belli G. Presbitero P. The first report of late stent thrombosis leading to acute myocardial infarction in patient receiving the new endothelial progenitor cell capture stent. Int J Cardiol. 2010;141:e20. doi: 10.1016/j.ijcard.2008.11.134. [DOI] [PubMed] [Google Scholar]
- 69.Xu H. Nguyen K.T. Brilakis E.S. Yang J. Fuh E. Banerjee S. Enhanced endothelialization of a new stent polymer through surface enhancement and incorporation of growth factor-delivering microparticles. J Cardiovasc Transl Res. 2012;5:519. doi: 10.1007/s12265-012-9381-8. [DOI] [PubMed] [Google Scholar]
- 70.Nakazawa G. Granada J.F. Alviar C.L. Tellez A. Kaluza G.L. Guilhermier M.Y., et al. Anti-CD34 antibodies immobilized on the surface of sirolimus-eluting stents enhance stent endothelialization. JACC Cardiovasc Interv. 2010;3:68. doi: 10.1016/j.jcin.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 71.Imanishi T. Kobayashi K. Kuki S. Takahashi C. Akasaka T. Sirolimus accelerates senescence of endothelial progenitor cells through telomerase inactivation. Atherosclerosis. 2006;189:288. doi: 10.1016/j.atherosclerosis.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 72.Banerjee S. Xu H. Fuh E. Nguyen K.T. Garcia J.A. Brilakis E.S., et al. Endothelial progenitor cell response to antiproliferative drug exposure. Atherosclerosis. 2012;225:91. doi: 10.1016/j.atherosclerosis.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 73.Vaughn M.W. Kuo L. Liao J.C. Estimation of nitric oxide production and reaction rates in tissue by use of a mathematical model. Am J Physiol. 1998;274:H2163. doi: 10.1152/ajpheart.1998.274.6.H2163. [DOI] [PubMed] [Google Scholar]
- 74.Tamai H. Igaki K. Kyo E. Kosuga K. Kawashima A. Matsui S., et al. Initial and 6-month results of biodegradable poly-l-lactic acid coronary stents in humans. Circulation. 2000;102:399. doi: 10.1161/01.cir.102.4.399. [DOI] [PubMed] [Google Scholar]
- 75.Serruys P.W. Ormiston J.A. Onuma Y. Regar E. Gonzalo N. Garcia-Garcia H.M., et al. A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2-year outcomes and results from multiple imaging methods. Lancet. 2009;373:897. doi: 10.1016/S0140-6736(09)60325-1. [DOI] [PubMed] [Google Scholar]
- 76.Ormiston J.A. Serruys P.W. Bioabsorbable coronary stents. Circ Cardiovasc Interv. 2009;2:255. doi: 10.1161/CIRCINTERVENTIONS.109.859173. [DOI] [PubMed] [Google Scholar]
- 77.Garg S. Serruys P.W. Coronary stents: looking forward. J Am Coll Cardiol. 2010;56:S43. doi: 10.1016/j.jacc.2010.06.008. [DOI] [PubMed] [Google Scholar]