Abstract
Bitterness and irritation elicited by pharmaceutically active molecules remain problematic for pediatric medications, fortified foods and dietary supplements. Few effective methods exist for reducing these unpalatable sensations, negatively impacting medication compliance and intake of beneficial phytonutrients. A physicochemical approach to masking these sensations may be the most successful approach for generalizability to a wide range of structurally and functionally unique compounds. Here, solutions of the non-steroidal anti- inflammatory drug, ibuprofen, were prepared in milk products with varying fat content. Our hypothesis, based on other reports of similar phenomena, was that increasing the fat content would cause ibuprofen to selectively partition into the fat phase, thereby reducing interaction with sensory receptors and decreasing adversive sensations. Quantification of the aqueous concentration of ibuprofen was performed using an isocratic HPLC method coupled with an external standard curve. Sensory testing showed a modest but significant decrease (~20%) in irritation ratings between the skim milk (0% fat) and the half-and-half (11% fat) samples, indicating that increased fat may contribute to a reduced sensory response. Bitterness was not reduced, remaining constant over all fat levels. The HPLC results indicate a constant amount of ibuprofen remained in the aqueous phase regardless of fat level, so a simple partitioning hypothesis cannot explain the reduced irritancy ratings. Association of ionized ibuprofen with continuous phase solutes such as unabsorbed protein should be explored in future work.
Introduction
The acceptability and palatability of oral pediatric medications is an ongoing problem for parents and medical professionals. Rejection of unpalatable medications compromises compliance with medical regimes and can lead to harm of the child [1]. Unfortunately, many biologically or pharmaceutically active compounds taste bitter and/or irritate the mouth or throat, making these issues important for adults, either directly or indirectly. Similar issues also confront food manufacturers who wish to fortify foods with bioactive ingredients. It is widely accepted in the chemosensory literature that suppression of bitter tastes can occur through one of three mechanisms. Central cognitive suppression relies on the actual perception of an opponent taste quality for the suppression to occur. The best illustration of this effect was shown in a series of experiments by Lawless [2]. In one, sucrose sweetness was inhibited by the bitterness of phenylthiocarbamide (PTC) for tasters of PTC, but not for non-tasters. In a separate split-tongue experiment, the bitterness of quinine on one side of the tongue was decreased by 20% when sucrose was flowed over the other side. Together these suggest a central mechanism for suppression that is not explained by chemical interactions at the receptor, as the sucrose and quinine were physically separated in the second experiment.
Alternatively, peripheral suppression, which implies modification of binding at the receptor either through altering the shape of the receptor (as has been shown for sodium, lithium, and zinc ions [3, 4]) or direct antagonism of the receptor (e.g. [5]). Note that these are both independent of a perceived taste quality, like ‘saltiness.’ The specificity of direct receptor antagonism is simultaneously desirable and problematic. This approach may have limited practical utility because many bitter compounds activate more than one receptor, so even if you were to successfully antagonize a single bitter receptor, other receptors may provide functional recovery, eliciting an undesirable sensory response [6]. Interestingly, neither central nor peripheral suppression have been wholly effective for all bitter molecules and even a mixture of approaches may fall short at providing real applicability to the food and pharmaceutical industries, as shown by Keast and colleagues [7]. Thus, it is not surprising that the current methods used in current pediatric formulations (addition of sweeteners such as sucrose, glycerin, sugar alcohols, or high intensity sweeteners) have been relatively unsuccessful in increasing the palatability of liquid pharmaceuticals.
As an alternative, using what we know about the physical characteristics of the target molecule (i.e. polarity), we may be able to either provide a physical barrier between the agonist and receptor or manipulate its ability to access the receptor by providing an environment that is more “attractive” than the aqueous salivary layer surrounding the receptor. This last method makes intuitive sense to food scientists, as similar techniques are applied to control flavor release and moisture migration in foods. We believe this technique may also have the most utility for the reduction of bitter and irritating sensations across a number of structurally and functionally diverse pharmaceutical agents.
There are a variety of ways to physicochemical encapsulate or block a bitterant or irritant from interacting with a receptor. Cyclodextrins have been used to form complexes that allow hydrophobic molecules to enter a protective pocket on the inside of the cyclodextrin, while sugar molecules on the outside make the overall complex water-soluble [8]. More simply yet is an interesting phenomenon where the introduction of fat into the system may increase or decrease the amount of perceived bitterness. For the bitter compound caffeine, adding fat has been shown to intensify the bitter taste [9]; while for quinine, increasing the fat content of the sample causes a reduction in bitterness [10]. This may be expected due to the relative hydrophobicity of the two compounds. Caffeine is predominantly water-soluble (LogP =−0.07 [11]) and as such, may selectively partition into the aqueous phase of the sample, creating a local concentration of caffeine in the aqueous salivary environment that is much higher than the ‘true’ molarity of the solution. Alternatively, we would expect quinine, which is predominantly hydrophobic (LogPs of 2.82 and 3.52 [12]), to partition selectively into the fat phase thereby reducing access to the aqueous boundary layer adjacent to the receptor. Additionally, increasing the fat content in an emulsion system was also shown to significantly increase the detection threshold of quinine, regardless of the type of fat used [13]. This thinking is not new, as Lawless and students suggested a similar explanation for why capsaicin thresholds were significantly higher when presented in soybean oil, and suprathreshold ratings of irritation were significantly lower in oil [14]. While theoretically appealing, these reports have postulated these mechanistic explanations without empirically quantifying the concentration of the target compound in each phase. Here, we provide HPLC data to quantitate the amount of ibuprofen that remains in the aqueous phase of the ibuprofen-dairy solutions after a 24-hour equilibration period, to gain additional insight into the relationship between fat concentration and bitterness and chemesthetic intensity. Our motivation for using dairy products was two fold. First, commercially available milk is a stable emulsion, precluding the need to formulate a model system. Second, milk is readily available in different fat levels, meaning that a successful result would provide caregivers an immediately deployable means to improve oral medication palatability.
In a pilot study where untrained participants (n=28) were asked to make single time point ratings of ‘overall irritation’ and ‘bitterness’ from ibuprofen in milk products with increasing fat content, no significant effect of fat was seen for either attribute, though a trend for lower irritation with increasing fat content was visible. Upon further inspection of the data, there was evidence of a clear first-position bias [15]. That is, regardless of the nature of the sample, the first sample presented to the participant was rated as the most intense of the series. Seventy percent of participants gave the first sample their highest rating on a generalized Labeled Magnitude Scale (gLMS), where only 1/3rd would be expected by chance. We concluded that the novelty of ibuprofen/dairy samples had to be overcome before panelists were capable of making unbiased judgments in a rating task. We attribute this response to the incongruity associated with these compounds being presented in milk, which is under normal circumstances considered a bland or even refreshing beverage. In their textbook, Lawless and Heymann [15] suggest a possible remedy for first position effects may be to present a ‘dummy’ sample first to absorb the psychological effects before proceeding with the test stimuli of interest. We decided to use this approach in a follow-up experiment where the first sample presented to each participant was quinine in whole milk. Quinine was chosen as the dummy stimulus because it is bitter and unpleasant, but not irritating. Our intention was to reduce fatigue by presenting a non-chemesthetic compound.
Materials and Methods
Subjects
Reportedly healthy, non-smoking adults (n=50; 13 men; aged 18–45 years) were recruited from the Penn State community. Procedures were approved by the local Institutional Review Board, written informed consent was obtained, and participants were paid for their time. All sensory data were collected one-on-one by the lead author at the Sensory Evaluation Center at Penn State.
Stimuli
All samples were prepared in commercially available skim milk, whole milk, and half-and half purchased from Penn State’s Berkey Creamery. The stimuli were 10 mL samples of 2.50% (w/v) (121.2 mM) USP grade ibuprofen sodium (Fluka, CAS# 31121-93-4) and 0.41 mM kosher quinine hydrochloride (SAFC, CAS# 6119-47-4) in milk. We considered including a water only control to rule out effects of dairy proteins, but decided against it, both because the sample would be visually distinct from the other 4 samples, and because the absence of lactose and dairy volatiles in the water only condition would fundamentally alter perception of the sample [16]. Commercial half-and-half does contain a small amount of disodium phosphate as an emulsifier. How this addition may affect the partitioning behavior of ibuprofen was not determined in this study
The total solids and fat content of the samples were determined using CEM SMART System5 Moisture/Solids Analyzer and CEM Trac Fat Analysis System (Mathews, NC) following manufacturers instructions, and are provided in Table 1. The ibuprofen concentration used was chosen based on work in our laboratory, which indicated this concentration would give irritation ratings between ‘moderate’ and ‘strong’ on a generalized Labeled Magnitude Scale (gLMS) [17]. All samples were held in 30 mL plastic medicine cups, at 4 C until presented to the participant. All samples were presented in randomized order and labeled with random 3-digit blinding codes. A dummy ‘warm-up’ sample containing quinine, and three test samples (total n=4) were presented per session. Replicates were not obtained due to concerns regarding maximal daily dosing.
Table 1.
Physical composition of milk products. Top shows milk products presented to panelists 1–34, where bottom was presented to panelists 35–50. Two batches were required due to the length of the experiment and shelf life of the dairy products.
| Product | Total Solids (%) | Fat (%) |
|---|---|---|
| Skim milk | 9.06 | 0.12 |
| Whole milk | 12.01 | 3.40 |
| Half-n- half | 18.70 | 10.58 |
| Product | Total Solids (%) | Fat (%) |
|---|---|---|
| Skim milk | 9.04 | 0.36 |
| Whole milk | 11.98 | 3.42 |
| Half-n- half | 18.78 | 10.76 |
Procedure
Sensory Methods
Participants were asked to refrain from eating and the use of chemesthetic agents (i.e. toothpaste, mouthwash, spicy food) for at least two hours prior to their session. Before beginning the test, participants were oriented to a gLMS [18] using a list of 15 imagined or remembered sensations that included both oral and non-oral items (Hayes, Allen, and Bennett, Under Review). Scale instructions and orientation encouraged participants to make ratings in a generalized context by indicating that the top of the scale should reflect their ‘strongest sensation of any kind.’ Both scale orientation questions and test questions were presented to the participant in the Plus module of Compusense five, version 5.2 (Guelph, ONT).
To evaluate the samples, participants were asked to make a single rating of ‘overall irritation in the throat’ and ‘bitterness’ on a gLMS immediately after swallowing the sample. Participants were instructed to place the 10 mL sample in their mouth and then tilt their head back to allow the sample to reach the throat. They were then instructed to allow the sample to sit at the back of the throat for 5 seconds before swallowing in two stages (swallowing, then immediately swallowing again). Swallowing in two stages purportedly ensures that the stimulus is distributed to the whole surface of the throat. This method has been used previously (e.g. [17, 19]) and is designed specifically to help localize the stimulus exposure to the throat. The participant’s first rating was made immediately after the second swallow. After rating, participants were allowed to rinse with 4 C RO (reverse osmosis) water ad libitum. A minimum inter-stimulus interval (ISI) of an additional 180 seconds was enforced between each sample. If a participant needed more time to recover at this point, they were given more water and asked to indicate when they felt ready to continue. Total session time was approximately 20 minutes.
Ibuprofen Analysis by HPLC
Ibuprofen sodium (2.50 % w/v) was introduced into dairy samples (skim milk, whole milk, and half-and-half) and allowed to equilibrate for 24 h at 4°C. Ibuprofen in the continuous phase was separated by filtration using Amicon Ultra 0.5 mL centrifugal filters with 10 kDa cutoff from EMD Millipore (Billerica, MA). The size of this filter would be expected to preclude both the fat phase and many proteins. The filtrate was diluted 1:2000 using methanol and then filtered over 0.45 μm PTFE syringe filters prior to HPLC analysis. Samples were introduced using a Shimadzu 20ADvp temperature-controlled autosampler (4 ºC) and separation achieved using a reverse phase Supelcosil LC-18 (4.6 × 150 mm, 5 μm; Supelco Inc., Bellefonte, PA). Ibuprofen was eluted using an isocratic method with a mobile phase of 0.1% v/v formic acid in a 74% v/v methanol in water solution. The injection volume was 20 μL and the flow rate held at 1 mL/min. Ibuprofen was detected at 220 nm using a Shimadzu SPD-20AV UV-Vis detector and quantitation based on an external standard curve.
Statistical Analysis
Data were analyzed using SAS 9.2 (Cary, NC). For sensory data, repeated measures main effects ANOVA were performed via proc mixed, with participants as a random effect, assuming compound symmetry for the covariance structure. Planned comparisons across individual samples were tested via unadjusted t-tests. The quinine ‘warm-up’ sample was excluded from primary analysis a priori. HPLC data were analyzed via 1 – way ANOVA in proc mixed, assuming compound symmetry for the covariance structure.
Results and Discussion
Sensory Data
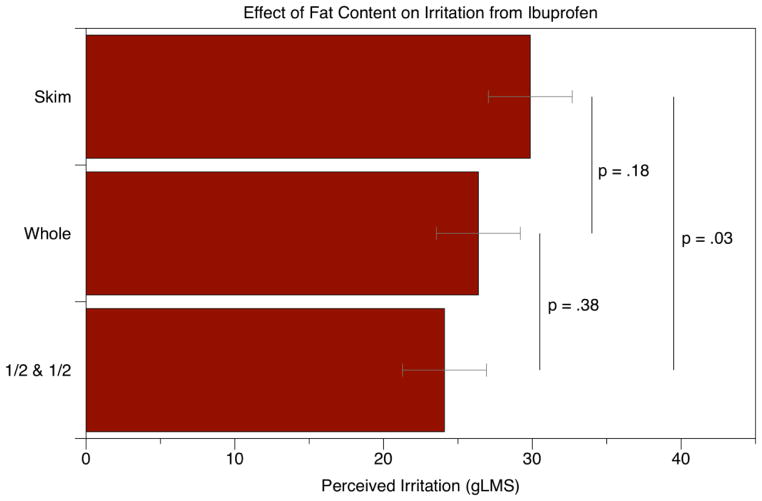
For the irritation ratings, we performed repeated-measures ANOVA with fat level and sample position as factors: the main effect of fat level was marginal [F(2,96)=2.56; p=0.083] while sample position showed no effect [F(2.96)=2.32; p=0.104]. Planned comparisons via t-tests indicated that the half-and-half samples were significantly less irritating [t96 = −2.24; p = 0.027] that skim milk samples. Irritancy in whole milk was intermediate between the low and high fat samples, although the differences with skim [t96 = −1.36; p = 0.18] and half-and-half [t96 = −0.89; p = 0.38] were not significant. As shown in Figure 1A, the mean irritation ratings in half-and-half samples were nearly 6 points lower than the skim milk sample on a gLMS, although both were still in the “moderate” to “strong” range of the scale. In a separate analysis, all ibuprofen samples were significantly more irritating than the quinine ‘warm-up’ sample, indicating the participants could successfully distinguish between bitterness and irritancy.
Figure 1.
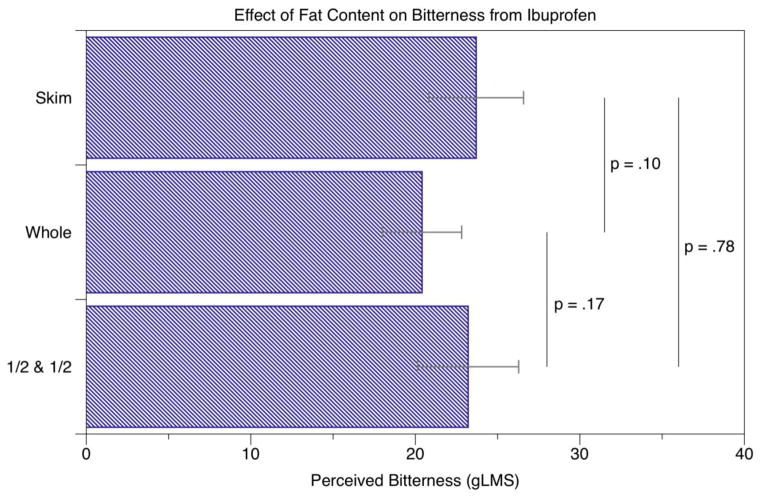
The effect of milk fat on irritation (A) and bitterness (B) from ibuprofen. A significant effect of fat was seen for irritation between the skim and half-and-half samples, but no significant effects were seen for bitterness.
Bitterness ratings are shown in Figure 1B. Repeated-measures ANOVA with fat level and sample position as factors indicated no effect of fat level [F(2,96)=1.58; p=0.21] while sample position was significant [F(2,96)=3.71; p=0.028]. The sample received last was less bitter than the second to last sample [t96 = 2.72; p = 0.007]; no other effects of position were observed (p’s <0.15).
Previous work suggests untrained participants are capable of pulling apart bitterness and pungency from capsaicin and other oral irritants [20]. Here, the lack of a hedonic response option for the samples may have caused affective responses be dumped [21] into both the bitterness and irritation ratings. Based on our experience with these stimuli, we believe dumping may have reduced the apparent effect size. Traditionally, it would be considered inappropriate to change the cognitive task and ask for both affective and intensity ratings in untrained participants [15], but due to the unfamiliar and unpleasant nature of the stimuli, the added cognitive load may be more than offset by the avoidance of dumping. Additional work is needed to clarify this trade-off. Alternatively, a trained panel approach could be used, although the lack of perceptually clean reference compounds would complicate the training process [17, 22, 23].
Analysis of continuous phase ibuprofen
After an addition of 2.50% w/v ibuprofen, ibuprofen concentrations remaining in the fraction not bound to protein or fat were 1.66 0.04%, 1.49 0.05%, and 1.66 0.01% w/v ibuprofen in skim milk, whole milk, and half-and-half, respectively. In one-way ANOVA, the amount of ibuprofen remaining in the aqueous phase differed across fat level [F(2,6)=5.0; p=0.05], but not in the manner we anticipated. The amount of ibuprofen was significantly lower in whole milk than either skim milk [t6=2.74; p=0.03] or half-and-half [t6=2.74; p=0.03]. Although unexpected, this finding does not change the overall interpretation: the irritancy reduction observed in the human sensory data could not be easily explained by partitioning into the lipid phase.
The pH of the delivery media selected may have influenced this lack of partitioning. Ibuprofen is a propionic acid derivative with a pKa of 5.2 leading to pH-dependent increases in aqueous solubility [24] due to ionization which would also occur at the pH common to milk (pH 6.7). Different results may have been achieved in low pH milk products, like yogurt or kefir, where ibuprofen would remain protonated and thus more hydrophobic. Ionic and non-ionic surfactants have also been shown to increase ibuprofen aqueous solubility [25] with proteins and phospholipids in dairy products, potentially causing similar effects. Our results suggest that the ionized ibuprofen may be interacting with continuous phase solutes, such as individual whey proteins or casein micelles, but dialysis and additional HPLC analysis would be required to confirm this. Additionally, it may be that n-octanol/water partition coefficients (those used to generate the hypotheses of this experiment) are not good predictors of partitioning behavior in milk fat [26].
Limitations and Conclusions
Ibuprofen was used as a model compound for this work because it is safe, commonly used, easy to obtain, and known to cause both irritation and bitterness. However, a compound with a readily ionizable group may not be ideal for a partitioning study. Additionally, there are drawbacks to using milk as the delivery vehicle instead of a model emulsion system, but our goal here was a one of translational utility – to determine if there was an easy, at-home method caregivers could use to increase the palatability of oral pharmaceuticals. The 20% reduction observed here might be due to viscosity changes, as the viscosity of the skim and whole milk samples were not matched to that of half-and-half. Increasing viscosity has been shown to correlate with decreased perception of sweetness [27], bitterness [28], and capsaicin pungency [29]. In any case, the reduction observed is not easily explained by a partitioning of the compound in to the fat phase. Follow-up analyses to more completely characterize the distribution of ibuprofen within the food matrix may provide more insight to the mechanism by which this reduction occurs.
Acknowledgments
This manuscript was completed in partial fulfillment of the requirements for a Master of Science degree at the Pennsylvania State University by S.M.B. The authors thank Bonnie C. Ford for assistance with total solids and fat determination of our milk samples and our study participants for their time and participation.
Funding
This work was funded by funds from the Pennsylvania State University and a grant from the National Institutes of Health Institute of Deafness and Communication Disorders to the corresponding author [grant DC010904].
References
- 1.Mennella JA, Beauchamp GK. Optimizing oral medications for children. Clin Ther. 2008;30(11):2120–32. doi: 10.1016/j.clinthera.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawless HT. Evidence for neural inhibition in bittersweet taste mixtures. J Comp Physiol Psychol. 1979;93(3):538–47. doi: 10.1037/h0077582. [DOI] [PubMed] [Google Scholar]
- 3.Breslin PAS, Beauchamp GK. Suppression of Bitterness by Sodium: Variation Among Bitter Taste Stimuli. Chemical Senses. 1995;20(6):609–623. doi: 10.1093/chemse/20.6.609. [DOI] [PubMed] [Google Scholar]
- 4.Keast RSJ. The Effect of Zinc on Human Taste Perception. Journal of Food Science. 2003;68(5):1871–1877. [Google Scholar]
- 5.Greene TA, et al. Probenecid Inhibits the Human Bitter Taste Receptor TAS2R16 and Suppresses Bitter Perception of Salicin. PLoS One. 2011;6(5):e20123. doi: 10.1371/journal.pone.0020123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ley J. Masking Bitter Taste by Molecules. Chemosensory Perception. 2008;1(1):58–77. [Google Scholar]
- 7.Keast R, Breslin P. Bitterness Suppression with Zinc Sulfate and Na-Cyclamate: A Model of Combined Peripheral and Central Neural Approaches to Flavor Modification. Pharmaceutical Research. 2005;22(11):1970–1977. doi: 10.1007/s11095-005-6136-0. [DOI] [PubMed] [Google Scholar]
- 8.Challa R, et al. Cyclodextrins in drug delivery: An updated review. AAPS PharmSciTech. 2005;6(2):E329–E357. doi: 10.1208/pt060243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keast RSJ. Modification of the bitterness of caffeine. Food Quality and Preference. 2008;19(5):465–472. [Google Scholar]
- 10.Metcalf KL, Vickers ZM. Taste Intensities of Oil-in-Water Emulsions with Varying Fat Content. Journal of Sensory Studies. 2002;17(5):379–390. [Google Scholar]
- 11.Biagi GL, et al. Study of the lipophilic character of xanthine and adenosine derivatives : I. RM and log P values. Journal of Chromatography A. 1990;498(0):179–190. [Google Scholar]
- 12.Eros D, et al. Reliability of logP predictions based on calculated molecular descriptors: a critical review. Curr Med Chem. 2002;9(20):1819–29. doi: 10.2174/0929867023369042. [DOI] [PubMed] [Google Scholar]
- 13.Thurgood JE, Martini S. Effects of Three Emulsion Compositions on Taste Thresholds and Intensity Ratings of Five Taste Compounds. Journal of Sensory Studies. 2010;25(6):861–875. [Google Scholar]
- 14.Lawless HT, Hartono C, Hernandez S. Thresholds and Suprathreshold Intensity Functions for Capsaicin in Oil and Aqueous Based Carriers. Journal of Sensory Studies. 2000;15(4):437–477. [Google Scholar]
- 15.Lawless HT, Heymann H. Context Effects and Biases in Sensory Judgment Sensory Evaluation of Food. Springer; New York: 2010. pp. 203–225. [Google Scholar]
- 16.Hayes JE, V, Duffy B. Revisiting Sugar-Fat Mixtures: Sweetness and Creaminess Vary with Phenotypic Markers of Oral Sensation. Chemical Senses. 2007;32(3):225–236. doi: 10.1093/chemse/bjl050. [DOI] [PubMed] [Google Scholar]
- 17.Bennett SM, Hayes JE. Chemical Senses. 2012. Differences in the Chemesthetic Subqualities of Capsaicin, Ibuprofen, and Olive Oil. Epub 2012 Jan 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snyder D, Fast K, Bartoshuk L. Valid Comparisons of Suprathreshold Sensations. Journal of Consciousness Studies. 2004;11:96–112. [Google Scholar]
- 19.Cicerale S, et al. Sensory Characterization of the Irritant Properties of Oleocanthal, a Natural Anti-Inflammatory Agent in Extra Virgin Olive Oils. Chemical Senses. 2009;34(4):333–339. doi: 10.1093/chemse/bjp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green BG, Hayes JE. Capsaicin as a probe of the relationship between bitter taste and chemesthesis. Physiology & Behavior. 2003;79(4–5):811–821. doi: 10.1016/s0031-9384(03)00213-0. [DOI] [PubMed] [Google Scholar]
- 21.Clark CC, Lawless HT. Limiting response alternatives in time-intensity scaling: an examination of the halo-dumping effect. Chemical Senses. 1994;19(6):583–594. doi: 10.1093/chemse/19.6.583. [DOI] [PubMed] [Google Scholar]
- 22.Cliff M, Heymann H. Descriptive Analysis of Oral Pungency. Journal of Sensory Studies. 1992;7(4):279–290. [Google Scholar]
- 23.Bennett SM, Hayes, John E. The World of Food Ingredients. CNS Media BV; Singapore: 2012. Chemesthesis and Flavor; pp. 44–46. [Google Scholar]
- 24.Hansen NT, et al. Prediction of pH-Dependent Aqueous Solubility of Druglike Molecules. Journal of Chemical Information and Modeling. 2006;46(6):2601–2609. doi: 10.1021/ci600292q. [DOI] [PubMed] [Google Scholar]
- 25.Stephenson BC, et al. Experimental and theoretical investigation of the micellar-assisted solubilization of ibuprofen in aqueous media. Langmuir. 2006;22(4):1514–25. doi: 10.1021/la052530k. [DOI] [PubMed] [Google Scholar]
- 26.Scheytt T, et al. 1-Octanol/Water Partition Coefficients of 5 Pharmaceuticals from Human Medical Care: Carbamazepine, Clofibric Acid, Diclofenac, Ibuprofen, and Propyphenazone. Water, Air, & Soil Pollution. 2005;165(1):3–11. [Google Scholar]
- 27.Arabie P, Moskowitz H. The effects of viscosity upon perceived sweetness. Attention, Perception, & Psychophysics. 1971;9(5):410–412. [Google Scholar]
- 28.Moskowitz HR, Arabie P. Taste Intensity as a Function of Stimulus Concentration and Solvent Viscosity. Journal of Texture Studies. 1970;1(4):502–510. doi: 10.1111/j.1745-4603.1970.tb00748.x. [DOI] [PubMed] [Google Scholar]
- 29.Baron RF, Penfield MP. Capsaicin Heat Intensity - Concentration, Carrier, Fat Level, and Serving Temperature Effects. Journal of Sensory Studies. 1996;11(4):295–316. [Google Scholar]