Abstract
Background
The use of chemotherapy has been proposed to increase the effectiveness of best supportive care (BSC) in patients with non-small cell lung cancer (NSCLC). Previous trials reported inconsistent findings regarding the efficacy and safety of chemotherapy on overall survival (OS) and treatment-related mortality. We performed a systematic review and meta-analysis to evaluate the effects of chemotherapy plus BSC versus BSC alone on survival of patients with NSCLC.
Methodology and Principal Findings
We systematically searched PubMed, EmBase, and the Cochrane Central Register of Controlled Trials for relevant literature. All eligible studies included patients with NSCLC who had received chemotherapy and BSC or BSC alone. All eligible studies measured at least 1 of the following outcomes: OS or treatment-related mortality. Overall, patients that received chemotherapy plus BSC had significant longer OS than those that received BSC alone (HR, 0.76; 95%CI, 0.69–0.84; P<0.001). Additionally, chemotherapy plus BSC as compared to BSC alone resulted in a 28% RR reduction (95%CI: 12–40; P = 0.001) in 6-month mortality, 11% RR reduction (95%CI: 8–15; P<0.001) in 12-month mortality, and 5% RR reduction (95%CI: 1–8; P = 0.02) in 2-year mortality. Toxicity was greater in patients that received chemotherapy plus BSC.
Conclusion/Significance
Chemotherapy plus BSC increased the OS and reduced the 6-month, 12-month, and 2-year mortality of NSCLC patients.
Introduction
Lung cancer is the leading cause of cancer death worldwide for both men and women. Around one and a half million new cases are diagnosed each year. NSCLC accounts for approximately 80–85% of all lung cancer cases and is the most common cause of cancer death in industrialized countries [1]–[2]. Surgery is generally regarded as the best treatment option; however, only approximately 30% of lung cancers are suitable for potentially curative resection. A further 20% of patients with locally advanced disease undergo radical thoracic radiotherapy or concurrent chemo-radiotherapy. The remaining 50% of patients with metastatic disease received BSC, and it has been suggested that addition of chemotherapy to BSC may offer further benefits [3].
Historically, standard chemotherapy has provided modest improvements to OS, and chemotherapy-treated patients scored better on the quality of life functioning scale than patients receiving BSC alone [4]–[6]. Previous trials [7] reported fewer lung cancer-related symptoms but worse toxicity-related symptoms, and the median survival time and 1-year survival rate with chemotherapy were prolonged to 8–10 months and 30–35%, respectively. Another study demonstrated that chemotherapy has improved progression-free survival, but that the effectiveness was limited or negated by its toxicity [8].
Here, we determined the effectiveness of chemotherapy in patients with NSCLC as compared to BSC in terms of OS, treatment-related mortality, and drug-related adverse events. We carried out a systematic review and meta-analysis of pooled data from randomized controlled trials to evaluate the effects of chemotherapy plus BSC on survival of patients with NSCLC.
Methods
Data sources, search strategy, and selection criteria
We adapted the Cochrane Central Register of Controlled Trials, Medline, and EmBase, with relevant text words and medical subject headings that included all spellings of chemotherapy agents, “chemotherapy,” “non-small cell lung cancer,” “NSCLC,” “randomized controlled trials,” “human,” and “English.” Reference lists from identified trials and review articles were manually scanned to identify any other relevant studies. Furthermore, we also searched http://www.ClinicalTrials.gov for information on registered randomized controlled trials to identify trials that were registered as completed, but whose results had not yet been published. This review was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) Statement issued in 2009 (Table S1) [9].
References identified by the search strategy were screened independently by 2 authors (CZ and HL) to evaluate their eligibility for inclusion in our meta-analysis. Any disagreement between these 2 reviewers was settled by the third reviewer (FY) until a consensus was reached. All completed randomized controlled trials that evaluated the effects of chemotherapy on survival of patients with NSCLC were eligible for inclusion. The analyzed outcomes were OS, treatment-related mortality, and any possible drug-related adverse events.
Data collection and quality assessment
For each of the included trials, details of the treatment given and outcomes were recorded independently by 2 authors (JL and WZ) and any disparities were resolved by a group discussion. Data were extracted from the included trials in terms of patient characteristics, intervention, and methodological characteristics of the included trials. The primary reported outcomes were summarized in tables. One author (JL) entered the data into the computer and another author (FY) checked it. The outcomes investigated included OS, treatment-related mortality, and any possible drug-related adverse events. The quality of the study was assessed using the Jadad score [10] (CZ) on randomization, concealment of the treatment allocation, blinding, completeness of follow-up, and the use of intention-to-treat analysis.
Statistical analysis
The effect of treatment was estimated using hazard ratios (HRs) and risk ratios (RRs) with their corresponding confidence intervals (CIs). For time-to-event data, the log HRs and their variances were estimated using the methods proposed by Parmar [11] when CIs of HRs were reported. The summary HRs and their 95% CIs were estimated using a general variance-based method. RRs were computed for dichotomous variables, estimates of the treatment effects were obtained from the number of events reported in each arm and combined using the methods reported by Mantel and Haenszel [12]. The drug-related adverse events were analyzed as WHO grades 3 or more. We explored potential heterogeneity in estimates of treatment effect with univariate meta-regression for baseline characteristic of patients with NSCLC. After this, we performed subgroup analysis to explore potential effect on OS, the 12-month and 2-year mortality based on the number of patients, mean age, proportion of male patients, type of chemotherapy, and the Jadad score. All estimates of effects were derived using a random-effects model [13]–[14]. Heterogeneity of the treatment effects between studies was investigated visually by a scatter plot and statistically by the heterogeneity I2 statistic [15]. Egger test was used to check for potential publication bias [16]. All the reported p values are 2-sided, and p values of <0.05 were regarded as statistically significant for all included studies. All analyses were calculated using the software STATA (version 10.0).
Results
We identified 984 potential articles from our initial electronic search, of which 961 were excluded during an initial review of titles and abstracts. We retrieved full texts of the remaining 23 studies and 16 randomized controlled trials [8], [17]–[32] met the inclusion criteria (Figure 1 and Figure S1). Nine out of the included trials [19], [22], [26]–[32] were evaluating platinum-based chemotherapy plus BSC compared with BSC alone, 2 [17]–[18] were assessing gemcitabine or vinorelbine therapy plus BSC compared with BSC alone, 3 [20], [21], [25] were evaluating taxel therapy compared with BSC alone, and the remaining 2 [23], [24] assessed the effects of pemetrexed therapy compared with BSC alone. Most of other studies identified by our search did not examine the effects of chemotherapy, or did not compare different treatments. Furthermore, some trials were not original investigations, or were duplicates of reports that had already been published [33], [34]. Our final analysis of 16 trials included a total of 4,135 patients with NSCLC. Table 1 summarizes characteristics of patients included in the trials. The trials had a sample size that ranged from 48–725 patients, with a mean of 258 patients. Data for OS were available in 13 trials [17]–[19], [21], [22], [24], [26]–[32], for 6-month mortality in 4 trials [18], [23], [27], [29], for 12-month mortality in 11 trials [17], [18], [20]–[22], [25]–[27], [29], [31], [32], and for 2-year mortality in 7 trials [17], [22], [25]–[27], [31], [32]. Reporting of key indicators of trial quality was scarce, with earlier studies providing few details about the process of randomization, concealment of allocation, and the use of intention-to-treat analysis. The quality of the trials was also assessed by pre-defined criteria using Jadad score [10]. Overall, out of the 16 trials, 1 scored 5 [24], 4 scored 4 points [18], [20], [22], [23], 4 scored 3 points [17], [21], [26], [28], 6 scored 2 points [19], [25], [27], [29], [30], [32] and 1 trial scored 1 point [31] on the Jaded score.
Figure 1. Identification process for eligible studies.
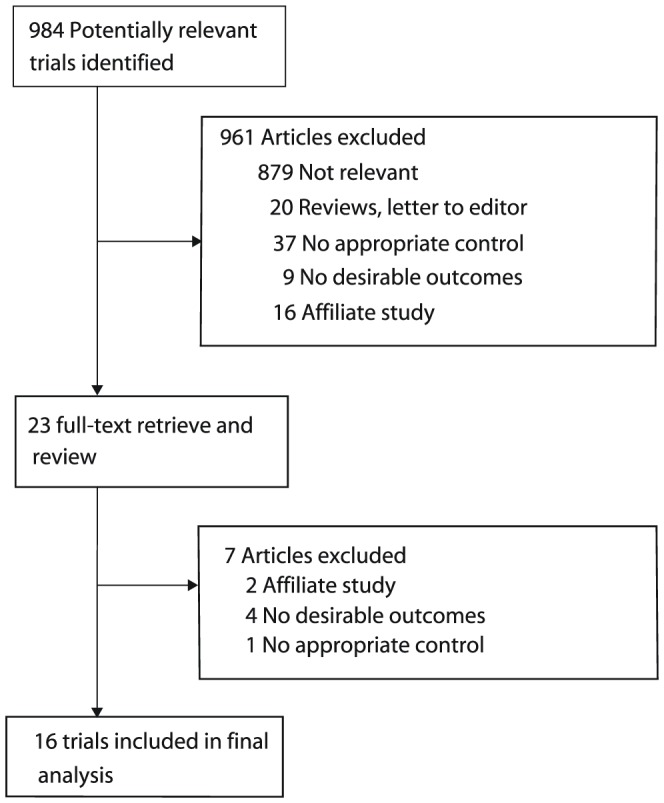
Table 1. Design and characteristic of trials included in our meta-analysis.
| Source | No. of patients | Sex (male, %) | Mean age, y | Stage of disease | Intervention | Jadad score |
| H Anderson [17] | 300 | 63.3 | 64.5 | Locally advanced and metastatic NSCLC | Gemcitabine plus BSC; BSC | 3 |
| The ELCVIS Group [18] | 154 | 87.0 | 74.0 | IIIB or IV NSCLC | Vinorelbine; BSC | 4 |
| RL Woods [19] | 188 | 81.9 | 61.0 | Advanced NSCLC | Cisplatin and vindesine; BSC | 2 |
| By Frances A [8], [20] | 204 | 67.2 | 61.0 | IIIA, IIIB or IV NSCLC | Docetaxel; BSC | 4 |
| M Ranson [21] | 157 | 75.0 | 64.0 | IIIB or IV NSCLC | Paclitaxel Plus BSC; BSC | 3 |
| SG Spiro [22] | 725 | 65.5 | 74.0 | Advanced NSCLC | cisplatin-based chemotherapy plus BSC; BSC | 4 |
| L Paz-Ares [23] | 539 | 58.1 | 61.3 | IIIB or IV NSCLC | Pemetrexed plus BSC; BSC | 4 |
| T Ciuleanu [24] | 663 | 73.0 | 60.5 | IIIB or IV NSCLC | Pemetrexed plus BSC; placebo plus BSC | 5 |
| K Roszkowski [25] | 207 | 81.6 | 59.3 | metastatic or non- resectable localized NSCLC | Docetaxel plus BSC; BSC | 2 |
| M Helsing [26] | 150 | 59.0 | 64.0 | Advanced NSCLC | Carboplatin, Etoposide plus BSC; BSC | 3 |
| G Cartel [27] | 102 | 73.0 | 56.6 | Stage IV NSCLC | Cisplatin, cyclophosphamide, mitomycin plus BSC; BSC | 2 |
| S Kaasa [28] | 87 | 79.3 | 62.0 | Inoperable, extensive NSCLC | Cisplatin, etoposide; symptomatic treatment | 3 |
| BR Cellerino [29] | 123 | 96.7 | 60.5 | Advanced NSCLC | Cyclophosphamide, epirubicin, cisplatin, methotrexate, etoposide, and lomustine; BSC | 2 |
| PA Ganz [30] | 48 | 89.6 | NG | advanced metastatic NSCLC | Cisplatin, vinblastine plus BSC; BSC | 2 |
| BE Rapp [31] | 137 | 74.5 | NG | Advanced NSCLC | vindesine and cisplatin/cyclophosphamide, doxorubicin, and cisplatin; BSC | 1 |
| MH Cullen [32] | 351 | 72.4 | 63 | Unresectable NSCLC | Mitomycin, ifosfamide, cisplatin plus palliative care; palliative care | 2 |
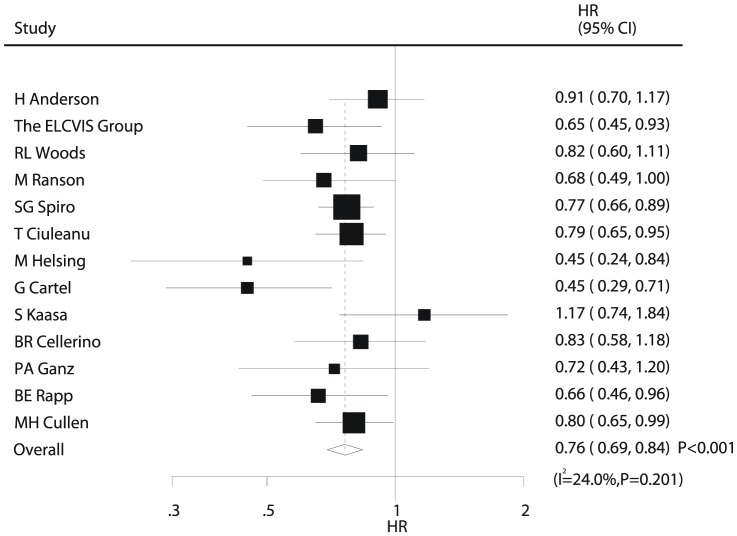
Data for OS were available from 13 trials [17]–[19], [21], [22], [24], [26]–[32]. Data from trials by Frances A [8], L Paz-Ares [22], and K Roszkowski [24] were excluded from the analysis of OS in our study because the authors did not provide this information. Overall, we concluded that chemotherapy plus BSC yielded a clinically and statistically significant 24% improvement in OS compared with BSC alone (HR, 0.76; 95%CI, 0.69–0.84; P<0.001, Figure 2). Although there was some evidence of heterogeneity across the trials included (I2 = 24%, P = 0.201), a sensitivity analysis indicated that the results were not affected by sequential exclusion of a particular trial from the pooled analysis.
Figure 2. Comparison of overall survival between chemotherapy plus best supportive care and best supportive care alone.
Data for the effect of chemotherapy on the 6-month mortality were available in 4 trials [18], [23], [27], [29], including 918 patients and 320 events of death. Overall, we noted that chemotherapy plus BSC resulted in a 28% reduction in the risk of 6-month mortality compared with BSC alone (RR, 0.72; 95%CI, 0.60–0.88; P = 0.001, Figure 3A). Additionally, we noted some evidence of heterogeneity in the magnitude of the effect across the included trials (I2 = 22%, P = 0.279); however, after sequential exclusion of trials from the pooled analysis, the results were not affected by exclusion of a specific trial.
Figure 3. Comparison of 6-month mortality (A), 12-month mortality (B), and 2-year mortality (C) between chemotherapy plus best supportive care and best supportive care alone.
The risk of 12-month mortality was reported in 11 trials [17], [18], [20]–[22], [25]–[27], [29], [31], [32], which included 2520 patients and recorded 1932 events of death. Overall, chemotherapy plus BSC reduced the risk of 12-month mortality by 11% without evidence of heterogeneity (RR, 0.89; 95%CI: 0.85–0.92, P<0.001, Figure 3B).
Seven trials [17], [22], [25]–[27], [31], [32], which provided data for the 2-year mortality, included 1883 patients and 1764 events of death. Overall, chemotherapy plus BSC reduced the risk of 2-year mortality by 5% (RR, 0.95; 95%CI, 0.92–0.99; P = 0.02, Figure 3C). However, because we noted a substantial heterogeneity in the RRs for the 2-year mortality from the individual trials (I2 = 63.5%, P = 0.012), we performed a subgroup analysis based on the number of patients, mean age, proportion of male, interventions, and Jadad score, to explore potential contributing factors.
We noted several adverse events reported by a few trials. Overall, we noted that treatment with chemotherapy plus BSC were associated with significant increase in the risks of neutropenia, leukopenia, anemia, infection, nausea/vomiting, alopecia, and ankle swelling (Table 2). No other significant differences were identified between the effects of chemotherapy plus BSC and BSC alone.
Table 2. Summary of the relative risks of grade 3 or worse toxicity assessed.
| Outcomes | Included studies | RR and 95% CI | P value | Heterogeneity (%) | P value for heterogeneity |
| Neutropenia | 7 | 31.01 (10.71–89.75) | <0.001 | 0 | 0.73 |
| Leukopenia | 6 | 11.49 (3.50–37.69) | <0.001 | 14 | 0.32 |
| Thrombocytopenia | 4 | 4.10 (0.91–18.51) | 0.07 | 0 | 0.97 |
| Anemia | 6 | 3.85 (1.58–9.38) | 0.003 | 12 | 0.34 |
| Infection | 7 | 2.10 (1.04–4.25) | 0.04 | 10 | 0.36 |
| Nausea and vomiting | 9 | 3.82 (1.31–11.14) | 0.01 | 47 | 0.06 |
| Asthenia | 6 | 1.97 (0.74–5.25) | 0.18 | 81 | <0.001 |
| Rash | 2 | 0.85 (0.49–1.46) | 0.55 | 0 | 0.72 |
| Pulmonary toxicity | 4 | 0.64 (0.29–1.42) | 0.27 | 58 | 0.07 |
| Alopecia | 3 | 15.84 (1.05–239.49) | 0.05 | 80 | 0.007 |
| Ankle swelling | 2 | 2.64 (1.61–4.33) | <0.001 | 0 | 0.96 |
| Constipation | 2 | 7.38 (0.95–57.05) | 0.06 | 0 | 0.78 |
| Cardiac toxicity | 3 | 1.14 (0.22–6.01) | 0.88 | 0 | 0.95 |
| Neuromotor | 3 | 3.76 (0.23–61.10) | 0.35 | 71 | 0.03 |
| Diarrhea | 3 | 4.15 (0.72–23.97) | 0.11 | 0 | 0.81 |
| Stomatitis/Mucositis | 5 | 3.12 (0.79–12.32) | 0.10 | 0 | 0.98 |
| Neurosensory | 4 | 1.33 (0.25–7.01) | 0.74 | 35 | 0.20 |
| Anorexia | 2 | 3.97 (0.47–33.31) | 0.20 | 0 | 0.41 |
| Peripheral neuropathy | 2 | 7.36 (0.92–59.00) | 0.06 | 0 | 0.70 |
In an exploratory attempt to identify sources of the residual differences between trials, we performed meta-regression analysis of the mean age, proportion of men, and interventions for OS. However, these variables did not appear to be important contributing factors to the effect of chemotherapy plus BSC for OS (Figure 4).
Figure 4. Meta-regression of (A) percentage male, (B) mean age, and (C) interventions for overall survival.
Subgroup analyses were done for OS, 12-month mortality, and 2-year mortality. Overall, we noted that chemotherapy (except gemcitabine/vinorelbine treatment) was associated with clinically and statistically significant improvement in OS as compared to BSC (Table 3). Similarly, we also observed that chemotherapy, except gemcitabine/vinorelbine, had a clear effect on the 12-month mortality(Table 3). Finally, we noted that chemotherapy was associated with reduced risk of 2-year mortality, when in percentage male greater than 80% and with platinum-based chemotherapy and taxel, and in trials with the Jadad score of 4 or 5 points (Table 3). No other significant differences were identified between the effects of chemotherapy and BSC, when based on additional subset factors (Table 3).
Table 3. Subgroup analysis of overall survival, 12-month mortality, and 2-year mortality after treatment with chemotherapy and best supportive care.
| Group | HR/RR and 95%CI | P value | Heterogeneity (%) | P value for heterogeneity | |
| Overall survival | Number of patients | ||||
| >200 | 0.80 (0.73–0.88) | <0.001 | 0 | 0.745 | |
| <200 | 0.71 (0.60–0.84) | <0.001 | 36.8 | 0.124 | |
| Mean age | |||||
| >64 | 0.75 (0.64–0.87) | <0.001 | 29.7 | 0.223 | |
| <64 | 0.79 (0.67–0.93) | 0.005 | 44.9 | 0.106 | |
| Gender (male, %) | |||||
| >80 | 0.76 (0.64–0.92) | 0.004 | 0 | 0.747 | |
| <80 | 0.76 (0.66–0.86) | <0.001 | 45.1 | 0.068 | |
| Intervention | |||||
| Platinum-based chemotherapy | 0.75 (0.65–0.86) | <0.001 | 37.2 | 0.121 | |
| Gemcitabine/vinorelbine | 0.79 (0.57–1.09) | 0.154 | 54.5 | 0.138 | |
| Taxel | 0.68 (0.48–0.97) | 0.034 | - | - | |
| Pemetrexed | 0.79 (0.65–0.96) | 0.015 | - | - | |
| Jadad score | |||||
| >4 | 0.77 (0.68–0.86) | <0.001 | 0 | 0.640 | |
| <4 | 0.76 (0.65–0.87) | <0.001 | 39.5 | 0.094 | |
| 12-monthmortality | Number of patients | ||||
| >200 | 0.90 (0.86–0.95) | <0.001 | 0 | 0.872 | |
| <200 | 0.85 (0.78–0.93) | <0.001 | 17.4 | 0.301 | |
| Mean age | |||||
| >64 | 0.89 (0.84–0.95) | <0.001 | 5.4 | 0.376 | |
| <64 | 0.88 (0.82–0.95) | 0.001 | 9.2 | 0.354 | |
| Gender (male, %) | |||||
| >80 | 0.87 (0.79–0.96) | 0.006 | 0 | 0.439 | |
| <80 | 0.89 (0.85–0.93) | <0.001 | 0.5 | 0.425 | |
| Intervention | |||||
| Platinum-based chemotherapy | 0.88 (0.83–0.93) | <0.001 | 0.6 | 0.412 | |
| Gemcitabine/vinorelbine | 0.88 (0.74–1.06) | 0.172 | 63.5 | 0.098 | |
| Taxel | 0.90 (0.82–0.99) | 0.028 | 0 | 0.713 | |
| Pemetrexed | - | - | - | - | |
| Jadad score | |||||
| >4 | 0.87 (0.81–0.93) | <0.001 | 0 | 0.570 | |
| <4 | 0.90 (0.85–0.95) | <0.001 | 2.7 | 0.409 | |
| 2-year mortality | Number of patients | ||||
| >200 | 0.96 (0.91–1.01) | 0.125 | 74.8 | 0.008 | |
| <200 | 0.93 (0.84–1.02) | 0.115 | 54.9 | 0.109 | |
| Mean age | |||||
| >64 | 0.96 (0.89–1.03) | 0.282 | 65.1 | 0.057 | |
| <64 | 0.93 (0.86–1.01) | 0.098 | 80.3 | 0.006 | |
| Gender (male, %) | |||||
| >80 | 0.89 (0.83–0.95) | <0.001 | - | - | |
| <80 | 0.97 (0.93–1.01) | 0.095 | 47.9 | 0.087 | |
| Intervention | |||||
| Platinum-based chemotherapy | 0.96 (0.92–1.00) | 0.046 | 48.1 | 0.103 | |
| Gemcitabine/vinorelbine | 1.01 (0.96–1.08) | 0.644 | - | - | |
| Taxel | 0.89 (0.83–0.95) | <0.001 | - | - | |
| Pemetrexed | - | - | - | - | |
| Jadad score | |||||
| >4 | 0.95 (0.91–0.99) | 0.012 | - | - | |
| <4 | 0.95 (0.90–1.00) | 0.068 | 68.2 | 0.008 |
We used Egger test [16] to check for potential publication bias, which showed no evidence of publication bias for the outcomes of OS (p value, 0.226), 12-month mortality (p value, 0.093) and 2-year mortality (p value, 0.217).
Discussion
In 2007, as a result of a meta-analysis of the third-generation chemotherapeutic agents in the treatment of NSCLC [35], chemotherapy promised to become a significant advancement in the treatment of NSCLC. Another meta-analysis conducted by the NSCLC Collaborative Group concluded that treatment with chemotherapy plus BSC improved OS compared to best supportive care alone by 23% [36]. In this updated comprehensive quantitative review, we included more than 4135 patients with NSCLC with a broad range of baseline characteristics. We have used strict inclusion criteria to limit comparisons to the effect of chemotherapy in the treatment of NSCLC. Although this is not an individual data meta-analysis, and therefore, the comparison of survival is based on the number of deaths computed from a pooled risk difference, our current study suggests that chemotherapy plus BSC can prolong OS, and reduces the risks of 6-month mortality, 12-month mortality, and 2-year mortality. Since nearly all the trials in our study included patients with stage III/IV disease or advanced NSCLC, the conclusions should be applicable only to patients with advanced or metastatic NSCLC.
We found that the treatment with chemotherapy plus BSC yielded a statistically significant benefit in OS as compared with BSC alone. However, our subgroup analysis suggested that gemcitabine/vinorelbine therapy did not have an effect on OS as compared to BSC (HR, 0.79; 95%CI, 0.57–1.09). These conclusions may be unreliable because of a small number of trials (2 trials). Additionally, the trials by ELCVIS Group [18] suggested that treatment with vinorelbine plus BSC was associated with a statistically significant improvement in OS compared with BSC in patients with NSCLC (HR, 0.65; 95%CI, 0.45–0.93). However, the trial by H Anderson et al. [17] indicated that treatment with gemcitabine plus BSC did not have an effect on OS as compared with BSC alone (HR, 0.91; 95%CI, 0.70–1.17). The reasons for this might be as follows: (1) The efficacy of gemcitabine was greater in men than in women (2) Vinorelbine might have direct beneficial effects on OS in patients with NSCLC, these effects may be reduced or balanced by gemcitabine. (3) A few trials were included in such subsets and only 2 trials reported gemcitabine/vinorelbine therapy.
Previous meta-analysis [36] failed to demonstrate the efficacy of chemotherapy on the risk of short-term or long-term mortality. However, in our study chemotherapy plus BSC reduced 6-month, 12-month, and 2-year mortality risks as compared to BSC alone. Similarly, we noted that gemcitabine/vinorelbine therapy did not have an effect on 12-month mortality; the reasons for this have already been discussed above. However, it should be noted that the 2-year mortality were not reduced when percentage male less than 80% and the treatment was gemcitabine/vinorelbine therapy. The reason for this could be that the short follow-up in some trials made it difficult to comment on the effect of treatment on the 2-year mortality. Another potential explanation could be that the effects of chemotherapy on the risk of long-term mortality in men were superior to the effects observed in women.
As expected, the toxicity was significantly more severe in patients who received chemotherapy plus BSC. Symptomatic improvements due to the tumor shrinkage should be balanced with increased toxic effects of chemotherapy, and concerns remain regarding the impact of the increased toxicity of chemotherapy on patients' qualities of life. The increased risks of neutropenia, leukopenia, anemia, infection, nausea/vomiting, alopecia, and ankle swelling were detected in patients treated with chemotherapy plus BSC in our study. In addition, it should be noted that chemotherapy plus BSC significantly increased the risk of hematological toxicities.
The purpose of undertaking this review was to determine whether chemotherapy plus BSC would improve survival, and to present all available evidence in a systematic, quantitative and an unbiased fashion. The findings of this study demonstrated that treatment with chemotherapy plus BSC was associated with a statistically significant improvement in OS compared with BSC alone. Additionally, it had a clear effect on the 6-month, 12-month and 2-year mortality. Several technical limitations of this meta-analysis should be acknowledged. First, inherent assumptions were made for all meta-analyses, because the analyses used pooled data, either published or provided by the individual study; individual patient data or original data were unavailable, which did not allow us to perform more detailed analyses and to obtain more comprehensive results. Second, treatments given in those trials included second generation, third generation, and the fourth generation chemotherapy regiments, which prevented us from exploring the association between the type of chemotherapy and survival outcomes. Third, heterogeneity among the trials is another limitation of our study. We applied a random-effect model that took possible heterogeneity into consideration and performed subgroup analyses based on several important factors to further explore the source of heterogeneity. Fourth, data on progression-free survival were rarely available in these trials; therefore, no conclusions could be drawn.
In future trials, it will be important to focus on the effects of chemotherapy on the risk of progression-free survival and to explore the efficacy of chemotherapy plus BSC on the disease recurrence as compared to BSC alone. We suggest that the ongoing trials should be improved in the following ways. First, the adverse toxicity in clinical trials should be recorded and reported normatively, so that the side effects of any treatments can be evaluated in future trials. Second, the progression-free survival should contain more details. Third, the role of treatment duration, the type of chemotherapy, and the dosage should be investigated in more detail to establish the optimal dose and treatment approaches.
Supporting Information
PRISMA Checklist.
(DOC)
PRISMA Flowchart.
(DOC)
Funding Statement
The authors have no support or funding to report.
References
- 1. Jemal A, Siegel R, Ward E, Hao Y, Xu J, et al. (2009) Cancer statistics, 2009. CA Cancer J Clin 59: 225–249. [DOI] [PubMed] [Google Scholar]
- 2. Wingo PA, Cardinez CJ, Landis SH (2003) Long-term trends in cancer mortality in the United States, 1930–1998. Cancer 97: 3133–3275. [DOI] [PubMed] [Google Scholar]
- 3. Rudd R (1991) Chemotherapy in the treatment of non-small cell lung cancer. Respiratory Disease in Practice 7 6: 12–4. [Google Scholar]
- 4. Aisner J, Hansen HH (1981) Commentary: Current status of chemotherapy for non-small cell lung cancer. Cancer Treat Rep 65: 979–86. [PubMed] [Google Scholar]
- 5. Hoffman PC, Bitran JD, Golomb HM (1983) Chemotherapy of metastatic non-small cell bronchogenic carcinoma. Semin Oncol 10: 111–122. [PubMed] [Google Scholar]
- 6. Souhami R (1985) Chemotherapy in non-small cell bronchial carcinoma. Thorax 40: 641–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shepherd FA (1999) Chemotherapy for non-small cell lung cancer: Have we reached a new plateau? Semin Oncol 26: 3–11. [PubMed] [Google Scholar]
- 8. Shepherd BFA, Dancey J, Ramlau R, Mattson K, Gralla R, et al. (2000) Prospective randomized trials of docetaxel versus best supportive care in patients with non-small cell lung cancer previous treated with platinum-based chemotherapy. J Clin Oncol 18: 2095–2103. [DOI] [PubMed] [Google Scholar]
- 9. Moher D, Liberati A, Tetzlaff J, Altman DG, Grp P (2009) Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Plos Medicine [PMC free article] [PubMed] [Google Scholar]
- 10. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, et al. (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17: 1–12. [DOI] [PubMed] [Google Scholar]
- 11. Parmar MK, Torri V, Stewart L (1998) Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 17: 2815–2834. [DOI] [PubMed] [Google Scholar]
- 12.Deeks J, Higgins JPT (2011) Analysing data and undertaking meta-analyses.: Cochrane Handbook for Systematic Reviews of Interventions 5.0.0. Available: http://www.cochrane-handbook.org. Assessed July 15 2011.
- 13. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–88. [DOI] [PubMed] [Google Scholar]
- 14. Ades AE, Lu G, Higgins JP (2005) The interpretation of random-effects meta-analysis in decision models. Med Decis Making 25: 646–54. [DOI] [PubMed] [Google Scholar]
- 15. Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anderson H, Hopwood P, Stephens RJ, Thatcher N, Cottier B, et al. (2000) Gemcitabine plus best supportive care (BSC) vs BSC in inoperable non-small cell lung cancer- a randomized trial with quality of life as the primary outcome. Br J Cancer 83 4: 447–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. The Elderly Lung Cancer Vinorelbine Italian Study Group (1999) Effects of vinorelbine on quality of life and survival of elderly patients with advanced non-small cell lung cancer. J Natl Cancer Inst 91: 66–72. [DOI] [PubMed] [Google Scholar]
- 19. Woods RL, Williams CJ, Levi J, Page J, Bell D, et al. (1990) A randomised trial of cisplatin and vindesine versus supportive care only in advanced non-small cell lung cancer. Br J Cancer 61: 608–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dancey J, Shepherd FA, Gralla RJ, Kim YS (2004) Quality of life assessment of second-line docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy: results of a prospective, randomized phase III trial. Lung Cancer 43: 183–194. [DOI] [PubMed] [Google Scholar]
- 21. Ranson M, Davidson N, Nicolson M, Falk S, Carmichael J, et al. (2000) Randomized Trial of Paclitaxel Plus Supportive Care Versus Supportive Care for Patients With Advanced Non-Small-Cell Lung Cancer. J Natl Cancer Inst 92: 1074–80. [DOI] [PubMed] [Google Scholar]
- 22. Spiro SG, Rudd RM, Souhami RL, Brown J, Fairlamb DJ, et al. (2004) Chemotherapy versus supportive care in advanced non-small cell lung cancer: improved survival without detriment to quality of life. Thorax 59: 828–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Paz-Ares L, Marinis F, Dediu M, Thomas M, Pujol JL, et al. (2012) Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol 13: 247–55. [DOI] [PubMed] [Google Scholar]
- 24. Ciuleanu T, Brodowicz T, Zielinski C, Kim JH, Krzakowski M, et al. (2009) Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small cell lung cancer: a randomised, double-blind, phase 3 study. Lancet 374: 1432–40. [DOI] [PubMed] [Google Scholar]
- 25. Roszkowski K, Pluzanska A, Krzakowski M, Smith AP, Saigi E, et al. (2000) A multicenter, randomized, phase III study of docetaxel plus best supportive care versus best supportive care in chemotherapy-naive patients with metastatic or non-resectable localized non-small cell lung cancer (NSCLC). Lung Cancer 27: 145–57. [DOI] [PubMed] [Google Scholar]
- 26. Helsing M, Bergman B, Thaning L, Hero U (1998) Quality of life and survival in patients with advanced non-small cell lung cancer receiving supportive care plus chemotherapy with carboplatin and etoposide or supportive care only. A multicentre randomised phase III trial. Eur J Cancer 34: 1036–44. [DOI] [PubMed] [Google Scholar]
- 27. Cartel G, Cartel F, Cantone A, Causarano D, Genco G, et al. (1993) Cisplatin- Cyclophosphamide-Mitomycin Combination Chemotherapy With Supportive Care Versus Supportive Care Alone for Treatment of Metastatic Non-Small-Cell Lung Cancer. J Natl Cancer Inst 85: 794–800. [DOI] [PubMed] [Google Scholar]
- 28. Kaasa S, Lund E, Torud E, Hatlevoll R, Hmt H (1991) Symptomatic Treatment Versus Combination Chemotherapy for Patients With Extensive Non-Small Cell Lung Cancer. Cancer 67: 2443–2447. [DOI] [PubMed] [Google Scholar]
- 29. Cellerino BR, Tummarello D, Guidi F, Isidori P, Raspugli M, et al. (1991) A Randomized Trial of Alternating Chemotherapy Versus Best Supportive Care in Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 9: 1453–1461. [DOI] [PubMed] [Google Scholar]
- 30. Ganz P, Figlin RA, Haskell CM, La Soto N, Siau J (1989) Supportive Care Versus Supportive Care and Combination Chemotherapy in Metastatic Non-Small Cell Lung Cancer: Does Chemotherapy Make a Difference? Cancer 63: 1271–78. [DOI] [PubMed] [Google Scholar]
- 31. Rapp BE, Pater JL, Willan A, Cormier Y, Murray N, et al. (1988) Chemotherapy can prolong survival in patients with advanced non-small cell lung cancer-report of a Canadian multicenter randomized trial. J Clin Oncol 6: 633–41. [DOI] [PubMed] [Google Scholar]
- 32. Cullen MH, Billingham LJ, Woodroffe CM, Chetiyawardana AD, Gower NH, et al. (1999) Mitomycin, ifosfamide, and cisplatin in unresectable non-small cell lung cancer: effects on survival and quality of life. J Clin Oncol 17: 3188–3194. [DOI] [PubMed] [Google Scholar]
- 33. Cohen MH, Cortazar P, Justice R, Pazdur R (2010) Approval Summary: Pemetrexed Maintenance Therapy of Advanced/Metastatic Nonsquamous, Non-Small Cell Lung Cancer (NSCLC). The Oncologist 15: 1352–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Belani CP, Wu YL, Chen YM, Kim JH, Yang SH, et al. (2012) Efficacy and Safety of Pemetrexed Maintenance Therapy versus Best Supportive Care in Patients from East Asia with Advanced, Nonsquamous Non-small Cell Lung Cancer: An Exploratory Subgroup Analysis of a Global, Randomized, Phase 3 Clinical Trial. J Thorac Oncol 7: 567–73. [DOI] [PubMed] [Google Scholar]
- 35. Baggstrom MQ, Stinchcombe TE, Fried DB, Poole C, Hensing TA, et al. (2007) Third-generation chemotherapy agents in the treatment of advanced non-small cell lung cancer: a meta-analysis. J Thorac Oncol 2: 845–53. [DOI] [PubMed] [Google Scholar]
- 36. Non-Small Cell Lung Cancer Collaborative Group (2010) Chemotherapy and supportive care versus supportive care alone for advanced non-small cell lung cancer. Cochrane Database of Systematic Reviews 5: CD007309. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA Checklist.
(DOC)
PRISMA Flowchart.
(DOC)