Abstract
Rationale: Lung transplantation offers great promise for otherwise terminal lung diseases, but the development of bronchiolitis obliterans syndrome (BOS) continues to limit survival. Although acute rejection and lymphocytic bronchiolitis have been identified as risk factors for the development of BOS, it is unclear whether large-airway lymphocytic inflammation conveys the same risk.
Objectives: We evaluated lymphocytic bronchitis on endobronchial biopsies as a risk factor for BOS and mortality.
Methods: Endobronchial biopsies were collected and graded during surveillance after lung transplantation. We assessed samples with negative cultures collected in the first 90 days from 298 subjects and compared large-airway lymphocytic bronchitis assessed by a 0–2 “E-score” and with standard A and BR pathology scores for acute rejection and small-airway lymphocytic bronchiolitis, respectively.
Measurements and Main Results: We found surprisingly little association between large- and small-airway lymphocytic inflammation scores from a given bronchoscopy. Endobronchial lymphocytic bronchitis was more prevalent in subjects in BOS stage 0p and BOS stages 1–3 at the time of biopsy. Within 90 days after transplantation, increasing maximum E-score was associated with greater risk of BOS (adjusted hazard ratio, 1.76; 95% confidence interval, 1.11–2.78; P = 0.02) and in this analysis 90-day maximum E-scores were the only score type predictive of BOS (P < 0.01).
Conclusions: These results support a multicenter study to evaluate endoscopic biopsies for the identification of patients at increased risk for BOS. The association of endobronchial lymphocytic inflammation and BOS may have mechanistic implications.
Keywords: bronchiolitis obliterans, graft rejection, bronchoscopy, lung transplantation
At a Glance Commentary
Scientific Knowledge on the Subject
Bronchiolitis obliterans syndrome (BOS) remains a major cause of morbidity and mortality after lung transplantation. Lung allograft recipients typically undergo surveillance transbronchial biopsies to assess for small-airway lymphocytic bronchiolitis and acute rejection, which are risk factors for BOS. Before the present study, it was not known whether large-airway lymphocytic bronchitis identified by endobronchial biopsy is similarly predictive.
What This Study Adds to the Field
This study suggests that the presence of lymphocytic bronchitis on endobronchial biopsy obtained during the first 90 days of surveillance bronchoscopy may indicate increased risk for BOS. Subjects with more severe large-airway lymphocytic bronchitis suffered disproportionately from BOS and may represent a subpopulation of lung allograft recipients to target for measures to prevent graft dysfunction.
Lung transplantation can offer improved quality of life and survival from otherwise terminal lung diseases. However, the development of chronic rejection, manifested as bronchiolitis obliterans syndrome (BOS), is a major barrier to long-term patient survival (1). BOS can cause death from graft failure and can occur despite potent immunosuppression (2, 3). One risk factor for BOS is symptomatic acute rejection (4). When confirmed by transbronchial biopsy, acute rejection generally is treated with additional immunosuppressive medications (5). Most lung transplantation centers also use surveillance bronchoscopy to identify otherwise silent episodes of acute rejection (6), presuming that untreated acute rejection increases the risk of BOS (7, 8).
Previous studies revealed that small-airways lymphocytic bronchiolitis in transbronchial biopsy specimens also is associated with increased risk of BOS (9). Endobronchial biopsies have been evaluated in lung transplant recipients, in whom acute rejection and current BOS were associated with large-airway lymphocytosis (10). This lymphocytosis is predominantly CD4+ and CD45+ (10). Work from the present laboratory showed differential expression of gene transcripts in patients with large-airway, but not small-airway, lymphocytic bronchitis (11). However, the ability of pathologic changes in large airways to predict future development of BOS has been less clear (12).
The present study sought to determine whether lymphocytic bronchitis detected in endobronchial biopsies identifies patients at high risk for BOS. Secondarily, the study assessed whether this procedure could replace traditional transbronchial biopsy in post-transplant surveillance. We developed a novel severity scale (E-score) for large-airway lymphocytic bronchitis, and found that E score predicts increased risk of BOS. Endobronchial biopsy identified patients at risk for poor long-term outcomes that would not have been identified using transbronchial biopsy alone. Some preliminary findings from this study were previously reported in the form of an abstract (13).
Methods
Human Subjects
Written informed consent for chart review was obtained from all participants in this study, which was approved by the Institutional Committee on Human Research. Between 1997 and 2011, endobronchial and transbronchial biopsies were collected and scored as standard of care during surveillance bronchoscopy, which was performed 0.5, 1, 2, 3, 6, and 12 months after transplantation, with additional biopsies as dictated by symptom changes or fall in FEV1. For endobronchial biopsies, two or three biopsies were performed with cupped forceps in third- or fourth-generation carinae, typically in the lower lobes. Patients typically were treated for all but minimal grades of rejection as identified by transbronchial biopsy. Treating physicians had access to endobronchial biopsy results. Our primary analysis included all subjects alive and BOS-free 90 days after transplantation that had had at least one endobronchial or transbronchial biopsy in the first 90 days. We excluded biopsies from patients with concurrent infection, defined as positive bacterial, fungal, or viral studies from bronchoalveolar lavage fluid taken at the time of biopsy, as discussed in the online supplement.
Definition of BOS
BOS was defined per International Society for Heart and Lung Transplantation (ISHLT) guidelines (14) as greater than or equal to 20% decrease in FEV1 from baseline. Baseline FEV1 was defined as the average of the two best values measured after transplantation, at least 21 days apart (14). In addition to ISHLT guidelines, we required the decline in FEV1 to persist at least 21 days and not resolve by the end of the study to exclude reversible conditions that might be misdiagnosed as BOS.
Large-Airway Lymphocytic Bronchitis Grading System
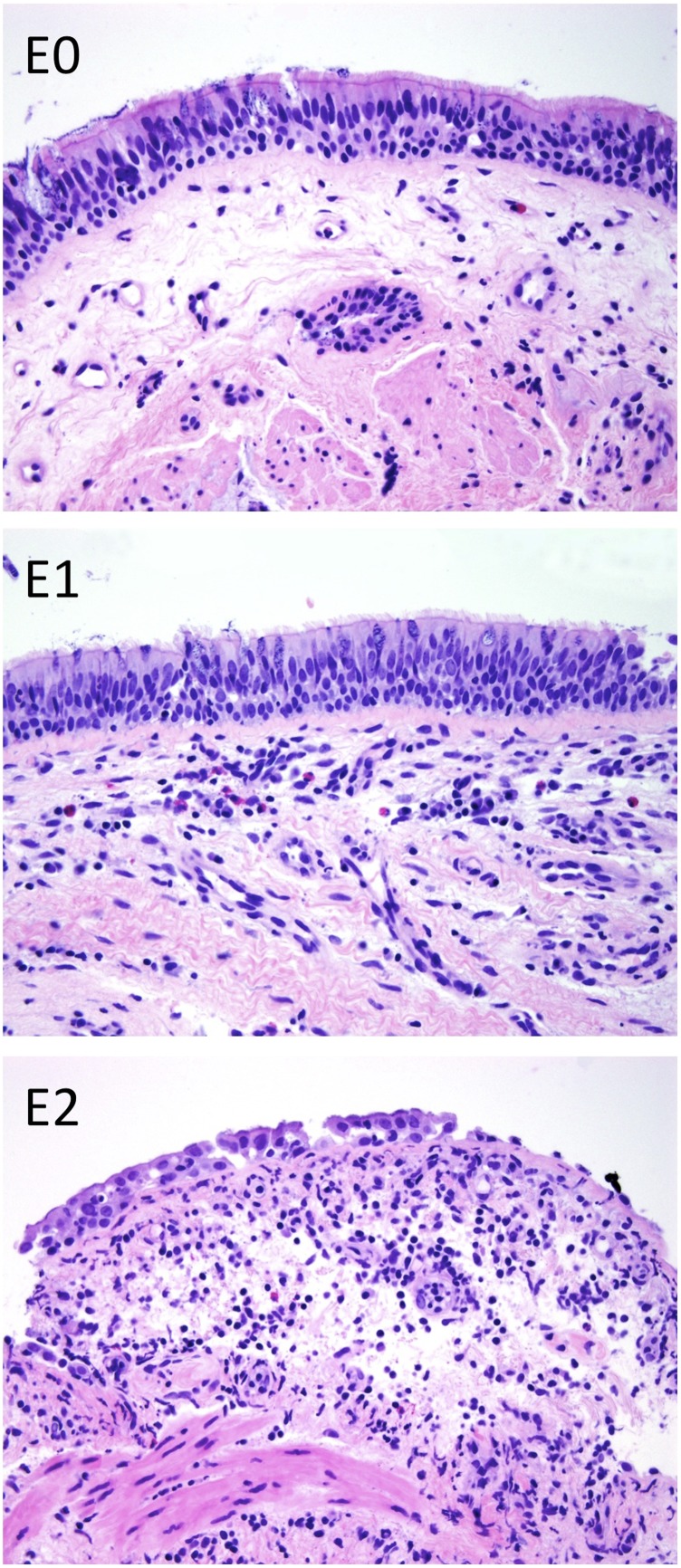
The presence of large-airway inflammation in endobronchial biopsies was evaluated at the time of biopsy based on the degree of cellular infiltration and reported as no, minimal, mild, moderate, or severe acute (neutrophilic) or chronic (lymphocytic) airway inflammation. We translated the results for lymphocytic bronchitis to a three-tiered E scoring system (Figure 1), where E0 denotes no inflammation; E1 denotes mild chronic inflammation with a thin band of lymphocytes in the subepithelium and occasional intraepithelial lymphocytes; and E2 denotes moderate to severe chronic inflammation with a prominent subepithelial band of lymphocytes, scattered intraepithelial lymphocytes, and epithelial cell necrosis. This scoring system is analogous to the BR classification used to grade lymphocytic bronchiolitis in transbronchial biopsy specimens (15).
Figure 1.
Large-airway lymphocytic bronchitis was graded according to the following criteria: E0, no chronic inflammation; E1, minimal to mild chronic inflammation with a band of subepithelial lymphocytes and up to occasional intraepithelial lymphocytes; and E2, moderate to severe chronic inflammation with a prominent subepithelial band of lymphocytes, scattered intraepithelial lymphocytes, and at least rare epithelial cell necrosis.
Data Collection and Statistical Analysis
Pathology and pulmonary function test reports were collected and processed with regular expression text recognition as described in the online supplement. Manual review of 200 randomly selected records, representing 5% of the total sample, revealed no errors.
For our primary analysis of the predictive use of pathology results in the first 90 days, we used a multivariate-adjusted Cox proportional hazards model with follow-up. Statistical analysis was performed in R (version 2.14.1, R Foundation for Statistical Computing, Vienna, Austria) using the “gee,” “irr,” and “survival” libraries and in GraphPad Prism (version 5.0a, GraphPad Software, San Diego, CA) and is described in greater detail in the online supplement.
Results
Study Population
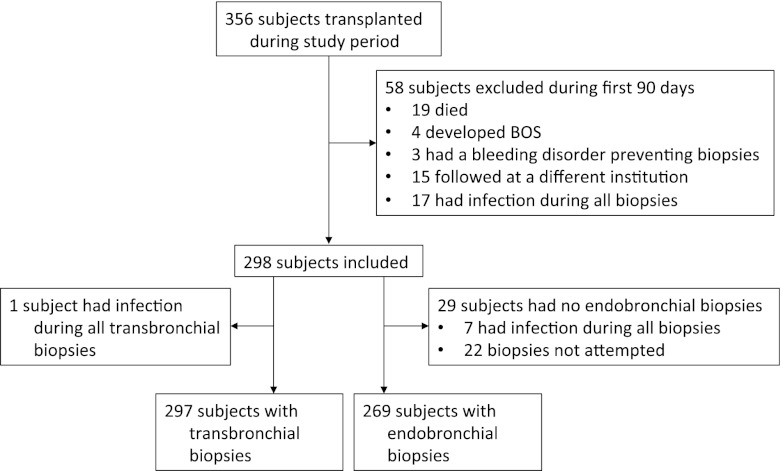
Of 356 subjects transplanted during the study period, 58 were excluded because they lacked adequate pathology results from the first 90 days (Figure 2). Specifically, 19 subjects died, 4 developed BOS, 3 had a bleeding disorder that prevented surveillance bronchoscopy, 15 had post-transplant surveillance at different institutions, and 17 had all biopsies in the setting of concomitant infection. Additionally, 29 subjects lacked endobronchial biopsy data, leaving 269 subjects with endobronchial biopsies for analysis. Seven of the 29 subjects had endobronchial and transbronchial biopsies, but all endobronchial biopsies were coincident with infection. The remaining 22 subjects did not have endobronchial biopsies attempted, reflecting the lead-in period when endobronchial biopsies were not consistently performed. In 1997, the first year that endobronchial biopsies were added to the standard surveillance protocol, they were obtained in only 16% of cases, whereas transbronchial biopsies were performed in 96% of cases. Over the course of the study, endobronchial biopsies and transbronchial biopsies were performed in 85% and 96% of cases, respectively. When biopsy tissue was obtained, it was uninterpretable (grade Ax, Bx, or Ex) in 2% of cases for both endobronchial and transbronchial biopsies. One subject had only endobronchial biopsy data available, leaving 297 subjects with transbronchial biopsies for analysis.
Figure 2.
Enrollment and exclusions for study subjects. Included subjects were transplanted between the date endobronchial biopsies were added as standard of care in March 1997 and November 2011, 90 days before the end of the data collection period. BOS = bronchiolitis obliterans syndrome.
Within the first 90 days, 1,151 biopsies were performed, of which 825 remained after samples with positive microbial cultures at the time of biopsy were excluded. Excluding biopsies done at the time of infection, subjects had a median of three biopsies in the first 90 days with an interquartile range (IQR) of 2. The frequency of biopsies was not different between subjects that at 5 years had either died (median, 3; IQR, 2; P = 0.20) or developed BOS (median, 3; IQR, 1.75; P = 0.91), as compared by Wilcoxon rank sum test. This lack of difference argues against a potential bias that sicker subjects received more biopsies.
The distributions of age, transplant type, cytomegalovirus (CMV) status, and transplant indication for subjects in this study were consistent with those for transplant recipients reported by the ISHLT (Table 1). In our subjects, the 5-year BOS and mortality rates were 48% and 44%, respectively, which compares with 5-year BOS and mortality rates of 49% and 47% in the ISHLT registry (16). By Cox proportional hazards modeling, the multivariate-adjusted 5-year BOS rates for subjects in the primary analysis were increased in subjects transplanted for bronchiectasis (hazard ratio [HR], 5.7; confidence interval [CI], 1.3–24.9; P = 0.02) and for pulmonary hypertension (HR, 3.7; CI, 1.2–11.1; P = 0.02) and decreased in double-lung allograft recipients (HR, 0.48; CI, 0.25–0.92; P = 0.03). Also, 5-year mortality was lower in double-lung allograft recipients (HR, 0.50; CI, 0.26–0.94; P = 0.03), consistent with ISHLT data (16). Otherwise, we did not observe statistically significant associations between subject characteristics and 5-year outcomes.
TABLE 1.
SUBJECT CHARACTERISTICS
| Overall | 298 (100) |
| Demographics | |
| Age at transplant | 55 |
| Male | 158 (53.3) |
| Female | 140 (46.7) |
| Transplant type | |
| Double Lung | 236 (79.2) |
| Single Lung | 51 (17.1) |
| Heart/Lung | 11 (2.9) |
| CMV status | |
| CMV D+/R+ | 86 (28.9) |
| CMV D+/R− | 41 (13.8) |
| CMV D−/R+ | 23 (7.7) |
| CMV D−/R− | 37 (12.4) |
| CMV Unknown | 111 (37.2) |
| Indication | |
| Pulmonary fibrosis | 119 (39.9) |
| COPD/emphysema | 54 (18.2) |
| Cystic fibrosis | 28 (9.6) |
| Pulmonary hypertension | 19 (5.5) |
| Bronchiectasis | 4 (1.4) |
| Other | 74 (25.4) |
Definition of abbreviations: CMV = cytomegalovirus; COPD = chronic obstructive pulmonary disease; D+ = donor positive; D− = donor negative; R+ = recipient positive; R− = recipient negative.
The frequency of each subject characteristic is shown as N (%). For age at transplant, the median value is shown.
Validation of the Endobronchial Biopsy Scoring System
To assess interobserver reliability, a subset of endobronchial biopsies was rescored by an independent pathologist in a blinded manner. To account for skew in the data, which might inappropriately elevate a Cohen kappa score, we randomly selected 10 biopsies of each score type to regrade. We calculated a weighted Cohen kappa of 0.35 (95% CI, 0.12–0.58; P < 0.01) for agreement between the scores. In the LARGO study of 845 subjects, interobserver agreement was 0.18 for A-scores and 0.04 for B-scores (17). Another study reported a kappa of 0.26 for B-scores and 0.65 for A-scores, although neither study is directly comparable because of differences in methodology (18). In summary, we found a fair strength of agreement for endobronchial pathology findings, which was comparable with the intraobserver reliability reported for transbronchial scores (19).
Concordance between Large- and Small-Airway Biopsy Scores
Because endobronchial biopsies can be obtained with less procedural risk than transbronchial biopsies, we tested the hypothesis that the E-score could be a surrogate for the A- or B-scores (Table 2). This analysis was not limited to the first 90 days, but samples coincident with infection were excluded. Overall, we observed at least minimal perivascular lymphocytosis (A-score ≥ 1) in 18% of samples; small-airway lymphocytic bronchiolitis (BR-score ≥ 1) in 15% of samples; and large-airway lymphocytic bronchitis (E-score ≥ 1) in 25% of samples. Using a generalized estimating equation model, we found that the presence of inflammation of any one type was positively associated with inflammation of the other two types (P < 0.001 for all comparisons). BR- and E-scores had the greatest degree of concordance, which may not be surprising because they both measure airway inflammation. E-scores and A-scores had the least concordance. We conclude that these scores, although positively correlated, are not sufficiently concordant that one can substitute for the other.
TABLE 2.
CONCORDANCE BETWEEN BIOPSIES
| A-Score |
BR-Score |
E-Score |
||||||||
| Score Type | Overall Prevalence | Probability A-Score > 0 | Odds Ratio | 95% CI | Probability BR-Score > 0 | Odds Ratio | 95% CI | Probability E-Score > 0 | Odds Ratio | 95% CI |
| A-score > 0 | 18.2% | 32% | 3.82 | 2.90–5.02 | 30% | 2.19 | 1.62–2.95 | |||
| BR-score > 0 | 15.3% | 39% | 3.73 | 2.84–4.91 | 46% | 5.46 | 4.14–7.19 | |||
| E-score > 0 | 25.0% | 25% | 2.19 | 1.62–2.97 | 32% | 5.57 | 4.20–7.38 | |||
Definition of abbreviation: CI = confidence interval.
The conditional probability of inflammation for the score types listed in the columns is shown, given the presence of inflammation of the type listed in the row. Odds ratios and confidence intervals were calculated using a generalized estimating equation model. P value for each odds ratio < 0.001.
Association between Large-Airway Lymphocytic Bronchitis and BOS, Including Stage 0p
A small study including four patients with BOS suggested that patients with BOS are more likely to have lymphocytic bronchitis (10). At the same time, potential BOS stage (BOS-0p), as defined by FEV1 81–90% of baseline or forced expiratory flow, midexpiratory phase, less than or equal to 75% of baseline, has been proposed as a predictor of patients who go on to develop BOS and has been validated in single- and double-lung allograft recipients (20, 21). We were therefore interested to see if endobronchial lymphocytic bronchitis was associated with BOS stages 1–3 in our large cohort and whether there would be an association between BOS-0p and lymphocytic bronchitis (Table 3). We compared the prevalence of pathologic findings across subjects stratified by the BOS score at the time that the biopsy was obtained. We used a multivariate-adjusted generalized estimating equation model to account for repeat measures within subjects and potential confounding of patient characteristics.
TABLE 3.
LARGE-AIRWAY LYMPHOCYTIC BRONCHITIS IS MORE PREVALENT IN SUBJECTS WITH POTENTIAL (0P) AND STAGE 1–3 BOS
| BOS Stage | Pathology Prevalence | Odds Ratio | 95% CI | P Value |
| Large-airway lymphocytic bronchitis (E-score > 0) | ||||
| Stage 0 | 19.5% | 1.00 | ||
| Stage 0p | 25.5% | 1.43 | 1.02–2.00 | 0.04* |
| Stages 1–3 | 31.0% | 1.92 | 1.39–2.66 | 7.3 × 10−5† |
| Acute rejection (A-score > 0) | ||||
| Stage 0 | 17.3% | 1.00 | ||
| Stage 0p | 17.7% | 1.03 | 0.79–1.34 | 0.82 |
| Stages 1–3 | 20.4% | 1.29 | 0.95–1.74 | 0.10 |
| Small-airway lymphocytic bronchiolitis (BR-score > 0) | ||||
| Stage 0 | 12.0% | 1.00 | ||
| Stage 0p | 15.2% | 1.33 | 0.93–1.91 | 0.12 |
| Stages 1–3 | 18.9% | 1.75 | 1.27–2.41 | 6.8 × 10−4† |
Definition of abbreviations: BOS = bronchiolitis obliterans syndrome; CI = confidence interval.
The prevalence of the given pathology type is shown stratified by BOS stage at the time of biopsy. Odds ratios and P values are calculated from an adjusted generalized estimating equation model.
P < 0.05.
P < 0.001.
After exclusion of biopsies done at the time of infection, 81% of subjects had at least one biopsy done during BOS stage 0p. Compared with subjects in BOS stage 0 at the time of biopsy, we observed an increased prevalence of endobronchial lymphocytic bronchitis in subjects with BOS 0p (19.5% vs. 25.5% for BOS 0 and BOS 0p, respectively; P = 0.04) and with BOS stage 1–3 (19.5% vs. 31% for BOS 0 and BOS stage ≥ 1, respectively; P < 0.001). By contrast, we did not see an increase in the prevalence of acute rejection in patients with BOS 0p or stages 1–3 when compared with stage 0. Small-airway lymphocytic bronchiolitis was more frequent in subjects in BOS stages 1–3 than stage 0 (18.9% vs. 12%; P < 0.001). The finding that large-airway lymphocytic bronchitis was associated with a spirometric precursor of BOS suggests that it might also have use in predicting patients who go on to develop BOS.
Elevated E-Scores for the Prediction of BOS
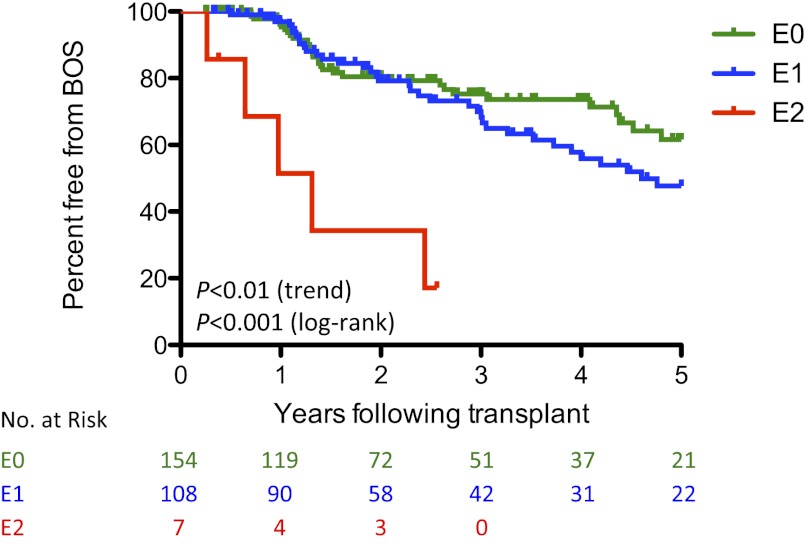
To evaluate the association between lymphocytic bronchitis and the future development of BOS, we performed a Kaplan-Meyer analysis assessing the development of BOS between 90 days and 5 years after transplant for subjects stratified by the maximum E-score within the first 90 days (Figure 3). We observed a significant difference in rates of BOS between the groups that was predicted by E-score (P < 0.001 by the Mantel-Cox log-rank test). Increasing E-score was associated with more rapid development of BOS (P < 0.01 by log-rank test for trend).
Figure 3.
Maximum E-score in 90 days predicts bronchiolitis obliterans syndrome (BOS). A Kaplan-Meier plot is shown for the development of BOS after transplantation stratified by maximum 90-day E-score. Data were left-truncated at 90 days. The curves are nonoverlapping (P < 0.001 by the Mantel-Cox log-rank test). Increasing E-score was associated with more rapid development of BOS (P < 0.01 by log-rank test for trend).
We used a Cox proportional hazards model to test the use of a maximum score for a given pathologic finding within the first 90 days to predict the outcomes of BOS and mortality after 90 days (Table 4). This model was adjusted for age, sex, transplant indication, transplant type, and CMV status. We observed an increase in the development of BOS in patients with increasing E-sores, with HR of BOS of 1.76 per unit increase in maximum E-score (95% CI, 1.11–2.78; P = 0.02). We did not observe a statistically significant effect of the maximum 90-day A- or BR- score on the rate of BOS or of any pathology score on mortality. When the analysis was performed using a cutoff of 1 year, only E2-grade pathology was associated with BOS (HR, 4.95; 95% CI, 1.66–14.7; P < 0.01) (see online supplement for complete results).
TABLE 4.
COMPARISON OF 90-DAY MAXIMUM PATHOLOGY SCORES FOR PREDICTING OUTCOMES
| BOS |
Mortality |
||||||
| Subjects | Hazard Ratio | 95% CI | P Value | Hazard Ratio | 95% CI | P Value | |
| E-score (ordinal) | 269 | 1.76 | 1.11– 2.78 | 0.02* | 0.97 | 0.64–1.45 | 0.87 |
| E0 | 154 | 1.00 | 1.00 | ||||
| E1 | 108 | 1.37 | 0.84–2.25 | 0.21 | 0.76 | 0.47–1.21 | 0.24 |
| E2 | 7 | 9.78 | 3.04–31.5 | 1.3 × 10−4† | 2.13 | 0.79–5.79 | 0.14 |
| A-score (ordinal) | 297 | 0.94 | 0.74–1.21 | 0.66 | 0.98 | 0.78–1.22 | 0.86 |
| A0 | 183 | 1.00 | 1.00 | ||||
| A1 | 59 | 0.59 | 0.32–1.10 | 0.10 | 0.55 | 0.31–0.98 | 0.04 |
| A2 | 45 | 0.91 | 0.51–1.65 | 0.76 | 0.89 | 0.54–1.48 | 0.65 |
| A3 | 10 | 1.12 | 0.41–3.36 | 0.78 | 1.47 | 0.61–3.58 | 0.39 |
| BR-score (ordinal) | 297 | 0.99 | 0.65–1.51 | 0.96 | 1.08 | 0.74–1.58 | 0.69 |
| BR0 | 209 | 1.00 | 1.00 | ||||
| BR1 | 84 | 0.91 | 0.56–1.46 | 0.69 | 1.01 | 0.66–1.52 | 0.98 |
| BR2 | 4 | 1.73 | 0.58–7.70 | 0.47 | 2.32 | 0.52–10.3 | 0.27 |
Definition of abbreviations: BOS = bronchiolitis obliterans syndrome; CI = confidence interval.
Hazard ratios are calculated using a Cox proportional hazards model adjusted for age, sex, transplant indication and type, and cytomegalovirus status.
P < 0.05.
P < 0.001.
From this analysis it seems that subjects developing E2 pathology in the first 90 days were at dramatically increased risk for subsequently developing BOS, with HR of 11.2 compared with subjects with a maximum score of 0 (CI, 3.41–36.6; P < 0.001). Out of concern that this finding might be related to the relatively small number of subjects in this category, which was about 3% of the overall population, we extended the analysis by examining subjects between 90 days and 1 year, during which an additional four subjects developed E2. Of the 11 subjects with E2 scores in the first year after transplantation, nine developed BOS, with a median time from transplant to BOS of 1.3 years, and only one subject was alive and BOS-free at 5 years. Of note, the subject characteristics of the E2 subgroup were not different from the characteristics of the overall population. In summary, the maximum E-score within the first 90 days after transplantation was positively correlated with the subsequent development of BOS, and the subgroup with maximum E-score of 2 fared much worse with respect to the development of BOS.
Comparison of E-Score with A- and BR-Scoring Methods
Our data suggest that E-scores seem to be a stronger predictor of the development of BOS than the results from transbronchial biopsies. Given that there was some correlation between E-scores and BR-scores, we were interested to determine if data from endobronchial biopsies improved the ability to predict BOS above what was present from transbronchial biopsies. We performed a log-likelihood test on our Cox proportional hazards model, wherein we sequentially added score types to a base model for the prediction of BOS that included the patient characteristics of age, sex, transplant indication, transplant type, and CMV status.
As shown in Table 5, adding BR- and A- scores to the base model did not improve ability to predict BOS, whereas subsequent addition of E-scores did (P < 0.01). Alternatively, adding E-scores improved the base model (P < 0.01), but sequential addition of A- and BR-scores did not improve the model. We conclude that for this dataset, the maximum E-score in 90 days was necessary and sufficient for optimal prediction of BOS, to the extent that it can be predicted by pathologic findings on surveillance bronchoscopy.
TABLE 5.
ENDOBRONCHIAL DATA IMPROVE PREDICTION OF BOS
| Log-Likelihood Ratio | Chi-Square | Degrees of Freedom | P Value | |
| Base model | −366.45 | 14.5 | 17 | 0.63 |
| BR-score | −366.15 | 0.59 | 2 | 0.74 |
| A-score | −364.93 | 2.4 | 3 | 0.48 |
| E-score | −359.09 | 11.7 | 2 | 0.003* |
Definition of abbreviation: BOS = bronchiolitis obliterans syndrome.
Log-likelihood analysis was performed on the Cox proportional hazards model showing the improvement in predictive ability as terms are sequentially added. The base model was adjusted for age, sex, indication, transplant type, and cytomegalovirus status.
P < 0.01.
Neutrophilic Bronchitis
Because alloimmune T-cell reactivity is thought to play a critical role in the process of chronic rejection, we hypothesized that early lymphocytic bronchitis would more readily predict BOS than early neutrophilic bronchitis. To evaluate this, we assessed neutrophilic bronchitis using the same criteria as lymphocytic bronchitis. There was a limited correlation between these scores (R2 = 0.04; P = 0.001), the distribution of which is shown in the online supplement. Using the same adjusted Cox proportional hazards model, we observed increased rates of BOS with increasing degrees of inflammation, with HR of 1.48 for the 90-day maximum neutrophilic bronchitis score as an ordinal (95% CI, 1.02–2.16; P = 0.04). Applying log-likelihood analysis, we found that lymphocytic bronchitis scores could improve prediction when added to a base model subsequent to neutrophilic bronchitis scores (P = 0.03), but that a model including patient characteristics and lymphocytic bronchitis scores was not improved by adding neutrophilic bronchitis scores (P = 0.44). The observation that neutrophilic bronchitis was a weaker predictor of BOS is consistent with the notion that airway lymphocytes reflect a chronic donor-specific immune response that is responsible for BOS.
Discussion
We found increased risk of BOS with increasing maximum E-score in the first 90 days. Most of this effect reflected the 3% of subjects with a maximum E-score of 2, who had a 10-fold increased risk for BOS, whereas the association with a maximum score of 1 was not significant. In this single-center analysis, the maximum inflammation score derived from endobronchial biopsies at 90 days was more accurate than A- or BR-scores in predicting development of BOS.
A previous study reported an association between small-airway lymphocytic bronchiolitis and BOS that was not observed in the present work (9). However, it is difficult to compare these studies because of differences in statistical methodology. The observed lack of association in our study may be related to treatment of patients with small-airway lymphocytic bronchiolitis with increased immunosuppression (22). Variances between study metrics may also contribute to the observed differences. In particular, our study used the revised ISHLT pathology scoring system, which collapses five categories of inflammation into three. The two studies were of similar size. However, in contrast to the previous study, we observed few subjects with high-grade small-airway bronchiolitis, and many more with no inflammation. Although uninterpretable results represented only a small fraction of biopsies, they may have contributed to the observed lack of association. These discordant outcomes may reflect differences between pathologist’s ratings (18), institutional differences, or clinical practice changes over time.
An initial motivation for this study was to determine to what extent endobronchial biopsies could substitute for transbronchial biopsies. We observed that endobronchial biopsies did not correlate well enough with the A-score of acute rejection to substitute for transbronchial biopsies. This lack of correlation is perhaps not surprising, because the perivascular inflammation seen in transbronchial biopsies cannot be visualized on an endobronchial biopsy. For a patient with a clinical suspicion of rejection, transbronchial biopsy is preferred to confirm the diagnosis. However, in the context of data suggesting that detection of silent acute rejection may not affect long-term outcomes (22–24), the use of surveillance bronchoscopy in otherwise asymptomatic patients may be to identify those at increased risk for BOS (25). A strategy that avoids transbronchial biopsy in surveillance of asymptomatic patients could reduce risk of procedural complications (26, 27). Our finding that large-airway lymphocytic bronchitis more accurately identifies patients at risk for BOS than pathology results of transbronchial biopsies supports a strategy using only lower-risk endobronchial biopsies for surveillance. However, the merits of a strategy excluding transbronchial biopsy in surveillance should be addressed prospectively.
This study has limitations. The extent to which treatment targeting pathology identified on transbronchial or endobronchial biopsies, such as more aggressive use of immunosuppressive medications, prevented the eventual development of BOS is not known (22). At some level, availability of endobronchial biopsy results is likely to have influenced treatment decisions, including those based on interpretation of A- or B-grade biopsy results. Also, because treatments evolved over the course of the study and BOS takes time to develop, the study may be biased to some degree by an interaction between time of transplant and type of biopsies performed. Finally, as a single-center study, our results may not be generalizable to all settings in which lung transplantation occurs.
The finding that large-airway lymphocytic bronchitis is associated with BOS may have important mechanistic and clinical implications. Our finding that lymphocytic rather than neutrophilic bronchitis was most predictive of BOS implicates lymphocytes in this pathology. Although BOS is generally considered a small-airway disease, the target antigens of the allogeneic immune response also may be present in large airways. Large airway–specific lymphocytosis could also result from a nonalloimmune mechanism, such as a cryptic viral infection or gastroesophageal reflux, both of which may preferentially affect large airways and have been associated with chronic graft rejection (1). Finally, lymphocytic bronchitis may not be directly pathologic but rather reflect the degree of donor-specific immune response. Pathology similar to lymphocytic bronchitis has been reported in rat models of lung transplantation (28), where a chronic CD4+ immune response in the large airways was associated with persistent expression of allogenic major histocompatibility complex class II antigens. Because major histocompatibility complex class II antigens are highly expressed in bronchial epithelium (29, 30), the degree of lymphocytosis here may reflect the systemic donor-specific immune response. It is logical that patients with increased donor-specific immune responses would be at greater risk for chronic rejection. Further investigation into these potential mechanisms may help identify more specific subsets of allograft recipients at increased risk for BOS.
Evaluation of large airways for the presence of lymphocytic bronchitis over the first 90 days improved prognostication in lung allograft recipients in this single-center study. Nonetheless, it remains unclear whether low-grade (E1) inflammation is prognostic and whether treatment based on endobronchial biopsies improves outcomes. These results should be confirmed and expanded in prospective multicenter studies before serving as a basis for changing clinical practices.
Supplementary Material
Acknowledgments
The authors thank John Horton for statistical advice; James Frank for manuscript suggestions; and Jasleen Kukreja, the surgical director of the UCSF lung transplant program, for her support of lung transplantation research. They also thank Chiyo Uchida and her colleagues in the radiology department for assistance coordinating subject enrollment.
Footnotes
Supported by a Veterans Administration research fellowship (J.R.G.), the Nina Ireland Lung Diseases Program (G.H.C.), the NIH (P01 HL024136) (G.H.C.), and a Veterans Administration career development award (N.N.T.).
Author Contributions: J.R.G. wrote the manuscript, and acquired and analyzed the data. J.R.G., N.N.T., S.R.H., J.A.G., and G.H.C. conceived the ideas and participated in data analysis and manuscript revisions. A.U. reviewed pathology. N.P.J. supervised statistical analyses. K.D.J. constructed the scoring system, reviewed pathology, and provided images.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201206-1025OC on December 13, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Estenne M, Hertz MI. Bronchiolitis obliterans after human lung transplantation. Am J Respir Crit Care Med 2002;166:440–444 [DOI] [PubMed] [Google Scholar]
- 2.Hayes D., Jr A review of bronchiolitis obliterans syndrome and therapeutic strategies. J Cardiothorac Surg 2011;6:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hopkins PM, McNeil K. Evidence for immunosuppression in lung transplantation. Curr Opin Organ Transplant 2008;13:477–483 [DOI] [PubMed] [Google Scholar]
- 4.Heng D, Sharples LD, McNeil K, Stewart S, Wreghitt T, Wallwork J. Bronchiolitis obliterans syndrome: incidence, natural history, prognosis, and risk factors. J Heart Lung Transplant 1998;17:1255–1263 [PubMed] [Google Scholar]
- 5.Trulock EP. Management of lung transplant rejection. Chest 1993;103:1566–1576 [DOI] [PubMed] [Google Scholar]
- 6.Kukafka DS, O'Brien GM, Furukawa S, Criner GJ. Surveillance bronchoscopy in lung transplant recipients. Chest 1997;111:377–381 [DOI] [PubMed] [Google Scholar]
- 7.Hopkins PM, Aboyoun CL, Chhajed PN, Malouf MA, Plit ML, Rainer SP, Glanville AR. Association of minimal rejection in lung transplant recipients with obliterative bronchiolitis. Am J Respir Crit Care Med 2004;170:1022–1026 [DOI] [PubMed] [Google Scholar]
- 8.Hachem RR, Khalifah AP, Chakinala MM, Yusen RD, Aloush AA, Mohanakumar T, Patterson GA, Trulock EP, Walter MJ. The significance of a single episode of minimal acute rejection after lung transplantation. Transplantation 2005;80:1406–1413 [DOI] [PubMed] [Google Scholar]
- 9.Glanville AR, Aboyoun CL, Havryk A, Plit M, Rainer S, Malouf MA. Severity of lymphocytic bronchiolitis predicts long-term outcome after lung transplantation. Am J Respir Crit Care Med 2008;177:1033–1040 [DOI] [PubMed] [Google Scholar]
- 10.Irani S, Gaspert A, Vogt P, Russi EW, Weder W, Speich R, Boehler A. Inflammation patterns in allogeneic and autologous airway tissue of lung transplant recipients. Am J Transplant 2005;5:2456–2463 [DOI] [PubMed] [Google Scholar]
- 11.Xu X, Golden JA, Dolganov G, Jones KD, Donnelly S, Weaver T, Caughey GH. Transcript signatures of lymphocytic bronchitis in lung allograft biopsy specimens. J Heart Lung Transplant 2005;24:1055–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Law L, Zheng L, Orsida B, Levvey B, Oto T, Kotsimbos AT, Snell GI, Williams TJ. Early changes in basement membrane thickness in airway walls post-lung transplantation. J Heart Lung Transplant 2005;24:1571–1576 [DOI] [PubMed] [Google Scholar]
- 13.Trivedi NN, Caughey GH, Carillo E, Mattucci T, Golden JA, Hays SR. Endobronchial biopsy in lung allograft recipients is more sensitive than transbronchial biopsy in detecting lymphocytic bronchitis/bronchiolitis, a risk factor for bronchiolitis obliterans syndrome. Presented at the American Thoracic Society International Conference. May 16–21, 2008, Toronto, Canada.
- 14.Estenne M, Maurer JR, Boehler A, Egan JJ, Frost A, Hertz M, Mallory GB, Snell GI, Yousem S. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant 2002;21:297–310 [DOI] [PubMed] [Google Scholar]
- 15.Stewart S, Fishbein MC, Snell GI, Berry GJ, Boehler A, Burke MM, Glanville A, Gould FK, Magro C, Marboe CC, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant 2007;26:1229–1242 [DOI] [PubMed] [Google Scholar]
- 16.Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dobbels F, Kirk R, Rahmel AO, Stehlik J, Hertz MI. The Registry of the International Society for Heart and Lung Transplantation: Twenty-eighth Adult Lung and Heart-Lung Transplant Report–2011. J Heart Lung Transplant 2011;30:1104–1122 [DOI] [PubMed] [Google Scholar]
- 17.Arcasoy SM, Berry G, Marboe CC, Tazelaar HD, Zamora MR, Wolters HJ, Fang KC, Keshavjee S. Pathologic interpretation of transbronchial biopsy for acute rejection of lung allograft is highly variable. Am J Transplant 2011;11:320–328 [DOI] [PubMed] [Google Scholar]
- 18.Chakinala MM, Ritter J, Gage BF, Aloush AA, Hachem RH, Lynch JP, Patterson GA, Trulock EP. Reliability for grading acute rejection and airway inflammation after lung transplantation. J Heart Lung Transplant 2005;24:652–657 [DOI] [PubMed] [Google Scholar]
- 19.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–174 [PubMed] [Google Scholar]
- 20.Lama VN, Murray S, Mumford JA, Flaherty KR, Chang A, Toews GB, Peters-Golden M, Martinez FJ. Prognostic value of bronchiolitis obliterans syndrome stage 0-p in single-lung transplant recipients. Am J Respir Crit Care Med 2005;172:379–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hachem RR, Chakinala MM, Yusen RD, Lynch JP, Aloush AA, Patterson GA, Trulock EP. The predictive value of bronchiolitis obliterans syndrome stage 0-p. Am J Respir Crit Care Med 2004;169:468–472 [DOI] [PubMed] [Google Scholar]
- 22.Swanson SJ, Mentzer SJ, Reilly JJ, Bueno R, Lukanich JM, Jaklitsch MT, Kobzik L, Ingenito EP, Fuhlbrigge A, Donovan C, et al. Surveillance transbronchial lung biopsies: implication for survival after lung transplantation. J Thorac Cardiovasc Surg 2000;119:27–38 [DOI] [PubMed] [Google Scholar]
- 23.Valentine VG, Taylor DE, Dhillon GS, Knower MT, McFadden PM, Fuchs DM, Kantrow SP. Success of lung transplantation without surveillance bronchoscopy. J Heart Lung Transplant 2002;21:319–326 [DOI] [PubMed] [Google Scholar]
- 24.Valentine VG, Gupta MR, Weill D, Lombard GA, LaPlace SG, Seoane L, Taylor DE, Dhillon GS. Single-institution study evaluating the utility of surveillance bronchoscopy after lung transplantation. J Heart Lung Transplant 2009;28:14–20 [DOI] [PubMed] [Google Scholar]
- 25.Sandrini A, Glanville AR. The controversial role of surveillance bronchoscopy after lung transplantation. Curr Opin Organ Transplant 2009;14:494–498 [DOI] [PubMed] [Google Scholar]
- 26.McWilliams TJ, Williams TJ, Whitford HM, Snell GI. Surveillance bronchoscopy in lung transplant recipients: risk versus benefit. J Heart Lung Transplant 2008;27:1203–1209 [DOI] [PubMed] [Google Scholar]
- 27.Diette GB, Wiener CM, White P., Jr The higher risk of bleeding in lung transplant recipients from bronchoscopy is independent of traditional bleeding risks: results of a prospective cohort study. Chest 1999;115:397–402 [DOI] [PubMed] [Google Scholar]
- 28.Uyama T, Winter JB, Groen G, Wildevuur CR, Monden Y, Prop J. Late airway changes caused by chronic rejection in rat lung allografts. Transplantation 1992;54:809–812 [DOI] [PubMed] [Google Scholar]
- 29.Kalb TH, Chuang MT, Marom Z, Mayer L. Evidence for accessory cell function by class II MHC antigen-expressing airway epithelial cells. Am J Respir Cell Mol Biol 1991;4:320–329 [DOI] [PubMed] [Google Scholar]
- 30.Taylor PM, Rose ML, Yacoub MH. Expression of MHC antigens in normal human lungs and transplanted lungs with obliterative bronchiolitis. Transplantation 1989;48:506–510 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.