Abstract
Rationale: The role of reactive oxygen species (ROS) signaling in the O2 sensing mechanism underlying acute hypoxic pulmonary vasoconstriction (HPV) has been controversial. Although mitochondria are important sources of ROS, studies using chemical inhibitors have yielded conflicting results, whereas cellular models using genetic suppression have precluded in vivo confirmation. Hence, genetic animal models are required to test mechanistic hypotheses.
Objectives: We tested whether mitochondrial Complex III is required for the ROS signaling and vasoconstriction responses to acute hypoxia in pulmonary arteries (PA).
Methods: A mouse permitting Cre-mediated conditional deletion of the Rieske iron-sulfur protein (RISP) of Complex III was generated. Adenoviral Cre recombinase was used to delete RISP from isolated PA vessels or smooth muscle cells (PASMC).
Measurements and Main Results: In PASMC, RISP depletion abolished hypoxia-induced increases in ROS signaling in the mitochondrial intermembrane space and cytosol, and it abrogated hypoxia-induced increases in [Ca2+]i. In isolated PA vessels, RISP depletion abolished hypoxia-induced ROS signaling in the cytosol. Breeding the RISP mice with transgenic mice expressing tamoxifen-activated Cre in smooth muscle permitted the depletion of RISP in PASMC in vivo. Precision-cut lung slices from those mice revealed that RISP depletion abolished hypoxia-induced increases in [Ca2+]i of the PA. In vivo RISP depletion in smooth muscle attenuated the acute hypoxia-induced increase in right ventricular systolic pressure in anesthetized mice.
Conclusions: Acute hypoxia induces superoxide release from Complex III of smooth muscle cells. These oxidant signals diffuse into the cytosol and trigger increases in [Ca2+]i that cause acute hypoxic pulmonary vasoconstriction.
Keywords: oxygen sensing, Rieske iron-sulfur protein, reactive oxygen species, roGFP, hypoxic pulmonary vasoconstriction
At a Glance Commentary
Scientific Knowledge on the Subject
The molecular mechanisms of hypoxia-induced pulmonary arterial (PA) vasoconstriction remain unclear. Our previous data suggest that mitochondrial reactive oxygen species (ROS) play a central role in activating hypoxic pulmonary vascular smooth muscle constriction. Previous studies attempting to clarify the role of the mitochondrial electron transport chain (ETC) using pharmacological inhibitors have yielded conflicting results. Studies using cell culture alone are not sufficient to establish the role of specific genes for the in vivo response.
What This Study Adds to the Field
A mouse was developed to permit conditional deletion of the Rieske iron-sulfur protein (RISP), which is required for superoxide generation at Complex III. Depletion of the RISP gene from cultured PA smooth muscle cells (SMC) abolished hypoxia-induced ROS signaling in the mitochondrial intermembrane space and the cytosol. Abrogation of hypoxia-induced ROS signaling was also observed in systemic vascular SMC. In PASMC, RISP depletion abolished the hypoxia-induced increases in cytosolic calcium, but responses to exogenous ROS were maintained. In precision-cut lung slices, depletion of RISP in smooth muscle abolished hypoxia-induced increases in cytosolic calcium. Deletion of the RISP gene in smooth muscle of intact mice attenuated the acute increase in right ventricular systolic pressure during hypoxia. These results demonstrate that hypoxia causes acute pulmonary vasoconstriction by stimulating the release of ROS from mitochondrial Complex III, which triggers calcium increases that cause smooth muscle contraction.
In the lung, alveolar hypoxia triggers acute constriction of small pulmonary arteries (PA), a response termed hypoxic pulmonary vasoconstriction (HPV). This response is recapitulated in cultured PA smooth muscle cells (PASMC), indicating that the oxygen-sensing mechanism underlying HPV is intrinsic to the PASMC (1–12). Our previous work has implicated increases in reactive oxygen species (ROS) signaling during hypoxia (9, 10, 12). Previous studies using mitochondrial inhibitors and mitochondria-deficient (ρ0) cells suggested that the electron transport chain (ETC) is required for hypoxia-induced ROS signaling in the pulmonary circulation (9, 11–18). We subsequently assessed ROS signaling in hypoxic PASMC using roGFP, a thiol-containing, redox-sensitive reporter (19–23) targeted to compartments within mitochondria or the cytosol (10). Unlike other methods (24–26), this targeted approach permitted the differentiation of hypoxia-induced ROS changes between mitochondrial subcompartments. During hypoxia, increased oxidation was detected in the mitochondrial intermembrane space (IMS) and the cytosol, whereas oxidation in the mitochondrial matrix decreased. Overexpression of catalase attenuated the hypoxia-induced roGFP oxidation and the associated increase in cytosolic calcium ([Ca2+]i), suggesting that increased mitochondrial ROS signaling is required for the hypoxia-induced increase in [Ca2+]i.
In tumor cell lines, we and others used RNA interference to suppress the mitochondrial Complex III Rieske iron-sulfur protein (RISP) and found that RISP suppression attenuates hypoxic ROS signaling and stabilization of hypoxia-inducible factor (HIF-1α), suggesting that RISP function is integral to the transduction of hypoxia necessary for the regulation of HIF-1α stability (16, 27, 28). Those findings suggested that a similar O2 sensing mechanism might function in pulmonary vascular cells. Based on that hypothesis, Korde and colleagues (29) used siRNA to suppress RISP in PASMC and found that hypoxic ROS responses were attenuated in PASMC, mitochondria isolated from PASMC, and Complex III isolated from PASMC mitochondria. These results similarly identify mitochondria as a source of increased ROS signaling during hypoxia. However, lack of a genetic model of RISP suppression has prevented testing this hypothesis in vivo. We have therefore generated a mouse with loxP sites flanking exon 2 of the RISP gene to perform these studies.
The present study examined the role of RISP depletion in ROS responses to acute hypoxia within the cytosol and mitochondrial subcompartments and the consequences of RISP depletion on hypoxia-induced [Ca2+]i signaling. For these studies, we used cultured pulmonary and systemic arterial smooth muscle cells (SASMC) and PA segments from mice. In vivo depletion of RISP in the smooth muscle cells of living mice permitted studies of the importance of smooth muscle RISP in the in vivo pulmonary vascular response to hypoxia.
Some of the results of these studies have been previously reported in the form of abstracts (30, 31).
Methods
Expanded methods are contained in the online supplement.
Pulmonary Microvessel SMC Isolation
The Northwestern University Institutional Animal Care and Use Committee approved all animal studies. PASMC were isolated from mouse lungs as described previously (9) using a modification of the method of Marshall and colleagues (6) (see online supplement).
Isolated PA Vessel Segments
Mouse lungs were perfused with an agarose–iron particle mixture, which is trapped in arteries because the particles are too large to transit pulmonary capillaries. Using a magnet, the PA vessel segments were separated from surrounding lung tissue as the connected lung tissue was digested with collagenase (type IV) (see online supplement).
Mouse Precision-Cut Lung Slices
Precision lung slices (200-μm thick) were obtained from mice as previously described (32) (see online supplement).
Conditional RISP Knockout Mouse
A mouse carrying loxP-flanked exon 2 of the mouse RISP gene (RISPflox/flox) was generated by Ozgene Pty Ltd. See online supplement for expansion of this method.
Conditional Smooth Muscle RISP Knockout Mouse
A smooth muscle–specific deletion of RISP was generated by crossing a tamoxifen-inducible Cre recombinase driven by the SMC myosin heavy chain promoter (SMC-MHC-Cre) (33) with an RISPflox/flox mouse to generate an SMC-MHC-Cre/RISPflox/flox mouse in a mixed genetic background. See online supplement for expansion of this method.
Statistics
Changes in roGFP oxidation, YC2.3 Förster resonance energy transfer (FRET), and Fura-2 were analyzed by using a two-way analysis of variance with repeated measures. A Newman-Keuls multiple-range test was used to evaluate significant differences between groups and times. Changes in RSVP and protein expression were analyzed by using a two-way analysis of variance. A Newman-Keuls multiple-range test was used to evaluate significant differences between groups. To control for experimental differences in the hypoxic responses, experimental studies and control experiments were always performed on the same day. Statistical significance was set at P < 0.05 (34).
Results
Hypoxia Increases ROS in the IMS and Cytosol and Decreases ROS in the Mitochondrial Matrix
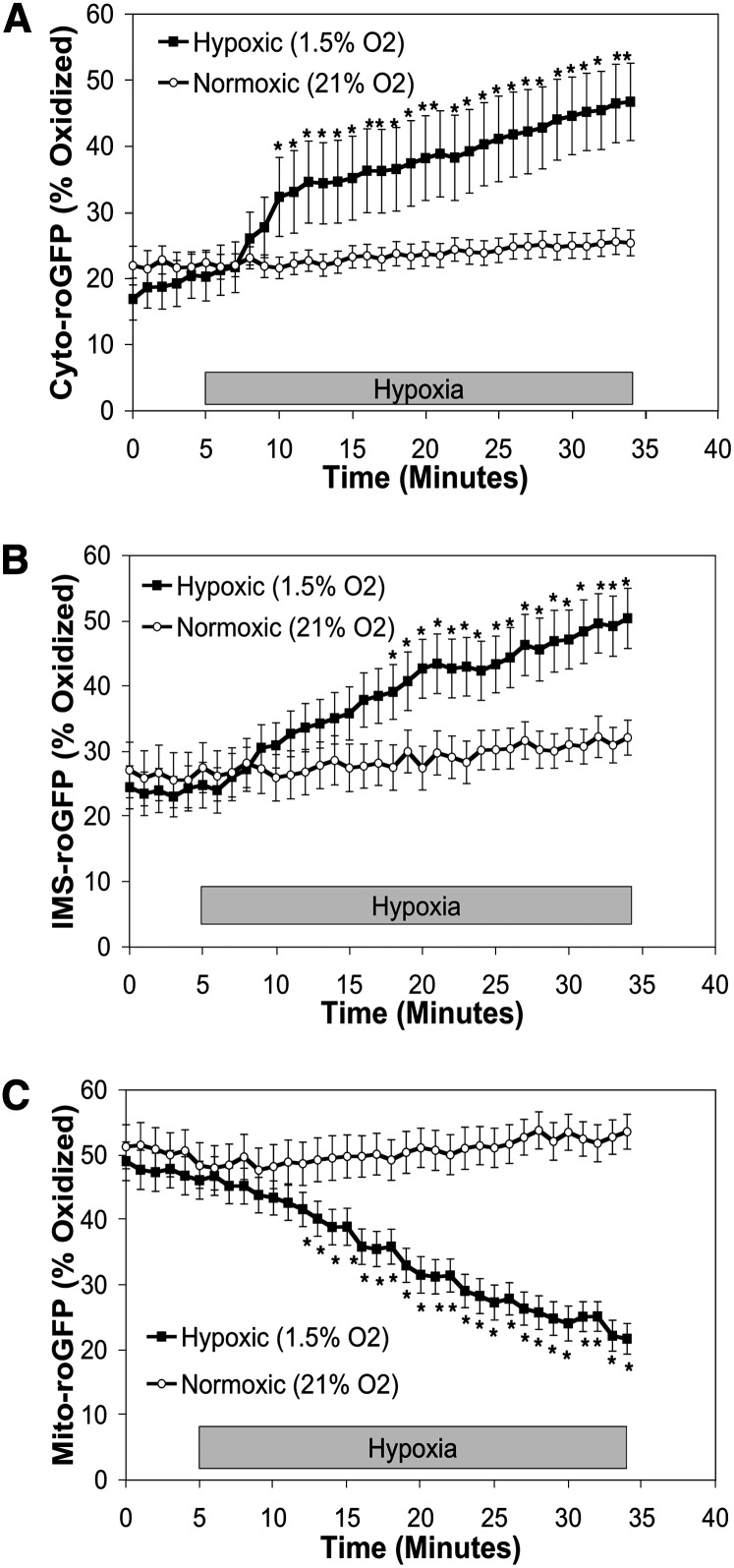
To assess ROS signaling in subcellular compartments of cultured wild-type mouse PASMC during hypoxia, we infected cells with adenoviruses harboring DNA to express roGFP targeted to the cytosol, mitochondrial matrix, or mitochondrial intermembrane space (IMS-roGFP) as we have done previously (10). We used roGFP to assess protein thiol oxidation; to our knowledge this reporter does not participate in signal transduction. Under normoxic conditions, the cytosolic roGFP oxidation averaged 20.3 ± 3.7% (Figure 1A). With the onset of hypoxia, cytosolic roGFP steadily oxidized, reaching 46.7 ± 5.8% within 30 minutes (Figure 1A). Oxidation of roGFP in the IMS also increased during hypoxia, from 24.8 ± 3.5% under normoxia to 50.4 ± 4.6% by 30 minutes (Figure 1B). In contrast, oxidation in the mitochondrial matrix decreased from 46.1 ± 2.9% during normoxia to 21.7 ± 2.3% after 30 minutes of hypoxia (Figure 1C). These results in mouse PASMC are consistent with previous results obtained in rat PASMC (10).
Figure 1.
Effects of hypoxia on roGFP oxidation in the cytosol, intermembrane space (IMS), and mitochondrial matrix in wild-type mouse pulmonary arterial smooth muscle cells (PASMC). Averaged results from multiple experiments in wild-type mouse PASMC expressing Cyto-roGFP (A), IMS-roGFP (B), or Mito-roGFP (C) superfused with normoxic (21% O2) or hypoxic (1.5% O2) media. Values are means ± SEM, n = 6 cover slips, 4 to 10 cells/cover slip. *P < 0.05 compared with normoxia.
Decreasing RISP Expression Attenuates Hypoxia-induced ROS Signaling in PASMC
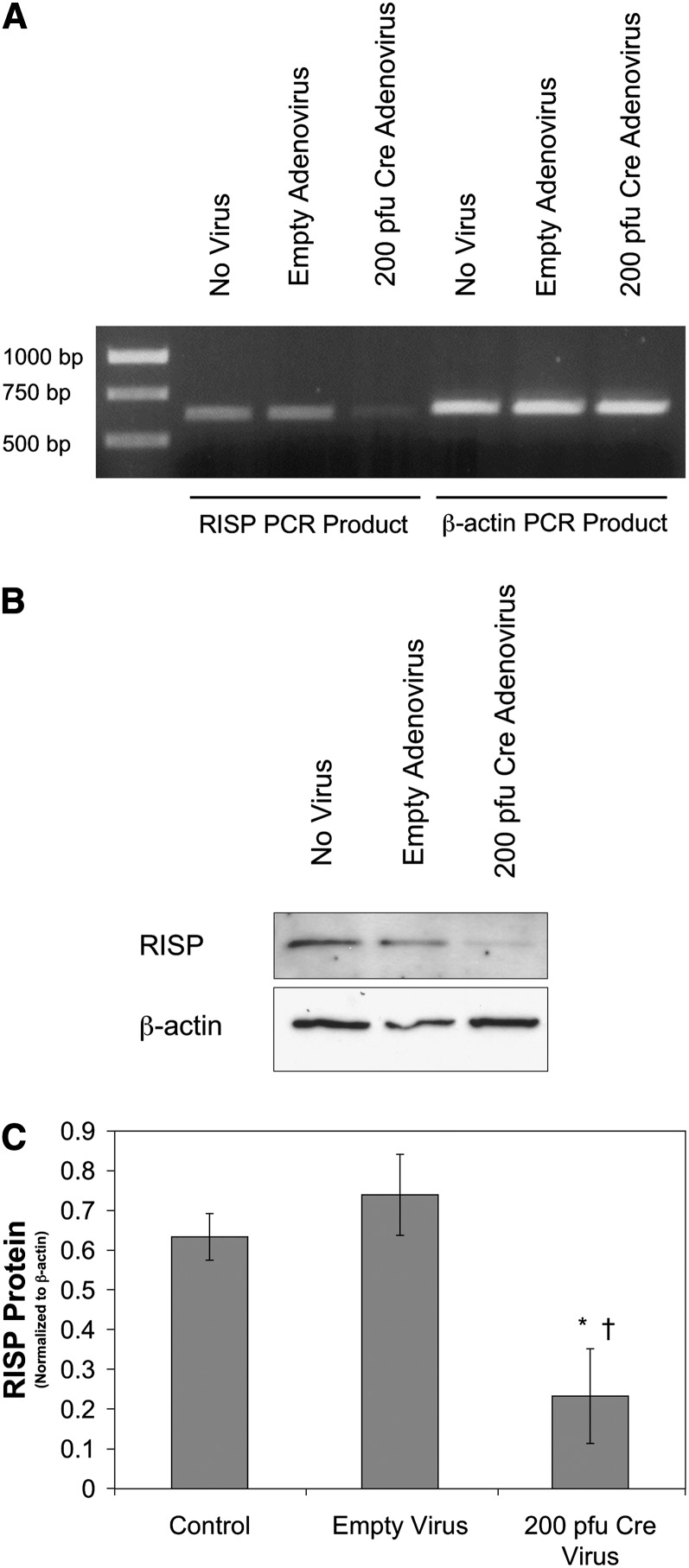
To determine whether intact Complex III function is required for the increase in ROS signaling during hypoxia, PASMC from mice carrying a conditional deletion of the ubiquinol-cytochrome c reductase, Uqcrfs1, (NM_025710) (RISPflox/flox) were transduced with a Cre-expressing adenovirus (200 pfu) to delete the RISP gene (RISP-KO). Reverse transcriptase–polymerase chain reaction demonstrated that RISP mRNA extracted from RISP-KO PASMC was decreased compared with untreated PASMC or PASMC treated with an empty adenovirus (Figure 2A). A significant decrease in RISP protein expression in RISP-KO PASMC was also confirmed by Western blot analysis (Figures 2B and 2C). Decreasing RISP expression did not affect the expression of other mitochondrial proteins, such as the cytochrome c oxidase subunit IV (COX IV) or the mitochondrial heme protein cytochrome c (see Figure E9 in the online supplement). However, it did render the mitochondrial membrane potential dependent on glycolytic ATP, which drives the ATP synthase in reverse to maintain the potential when electron transport is inhibited (Figure E4).
Figure 2.
Effect of Cre expression on Rieske iron-sulfur protein (RISP) expression in pulmonary arterial smooth muscle cells (PASMC) from conditional knockout mice. (A) Reverse transcriptase–polymerase chain reaction (RT-PCR) blot of mRNA extracted from uninfected (no virus), empty adenovirus–treated (200 pfu), and Cre-expressing adenovirus (200 pfu) PASMC isolated from RISP conditional knockout mice. The RT-PCR product for RISP is approximately 600 bp, whereas that for β-actin (as an internal control) is approximately 540 bp. (B) Western blot of lysates from untransfected (no virus), empty adenovirus–transfected (200 pfu), and Cre-expressing adenovirus–transfected (200 pfu) PASMC isolated from conditional knockout mice probed with antibodies against the RISP subunit or β-actin. (C) Quantified analysis of Western blots with RISP expression normalized to β-actin. Values are means ± SEM, n = 3 Western blots. *P < 0.05 compared with no virus, †P < 0.05 compared with empty adenovirus.
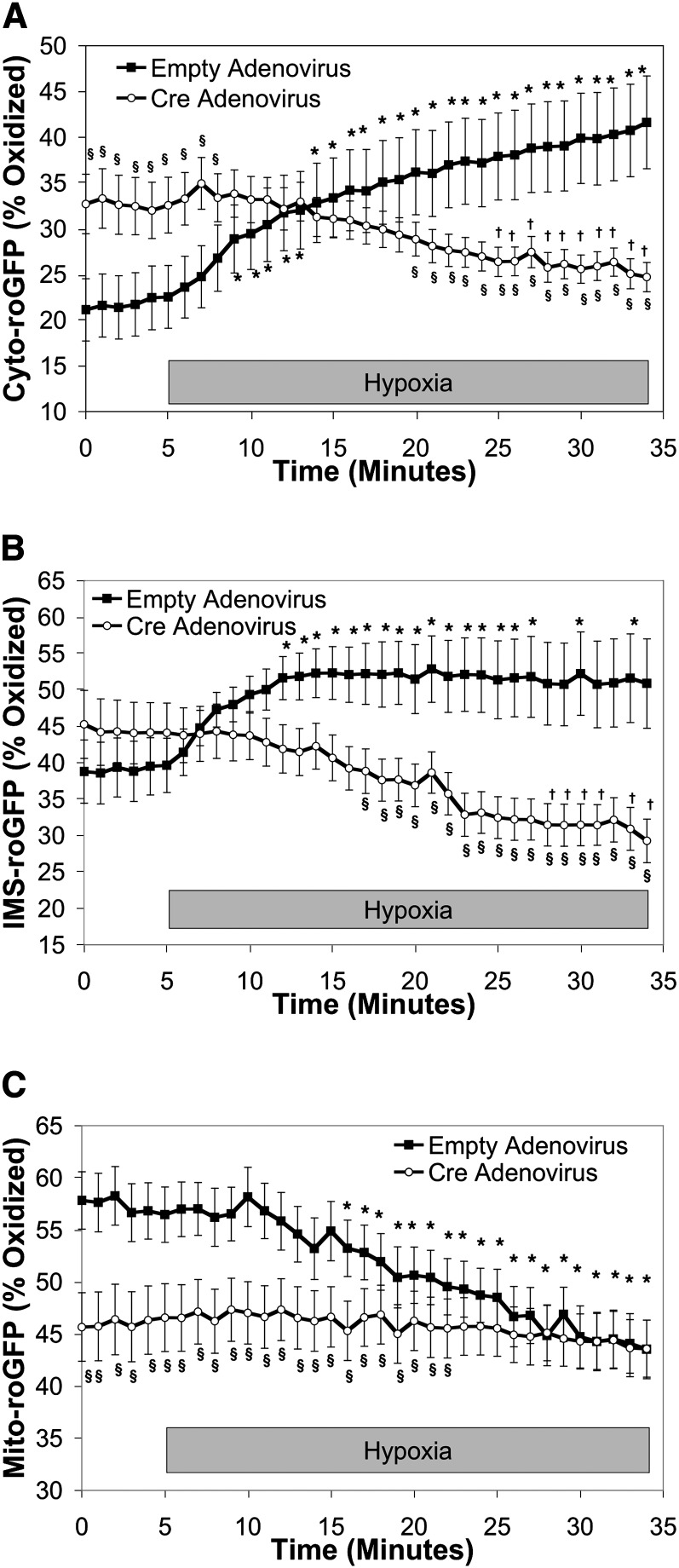
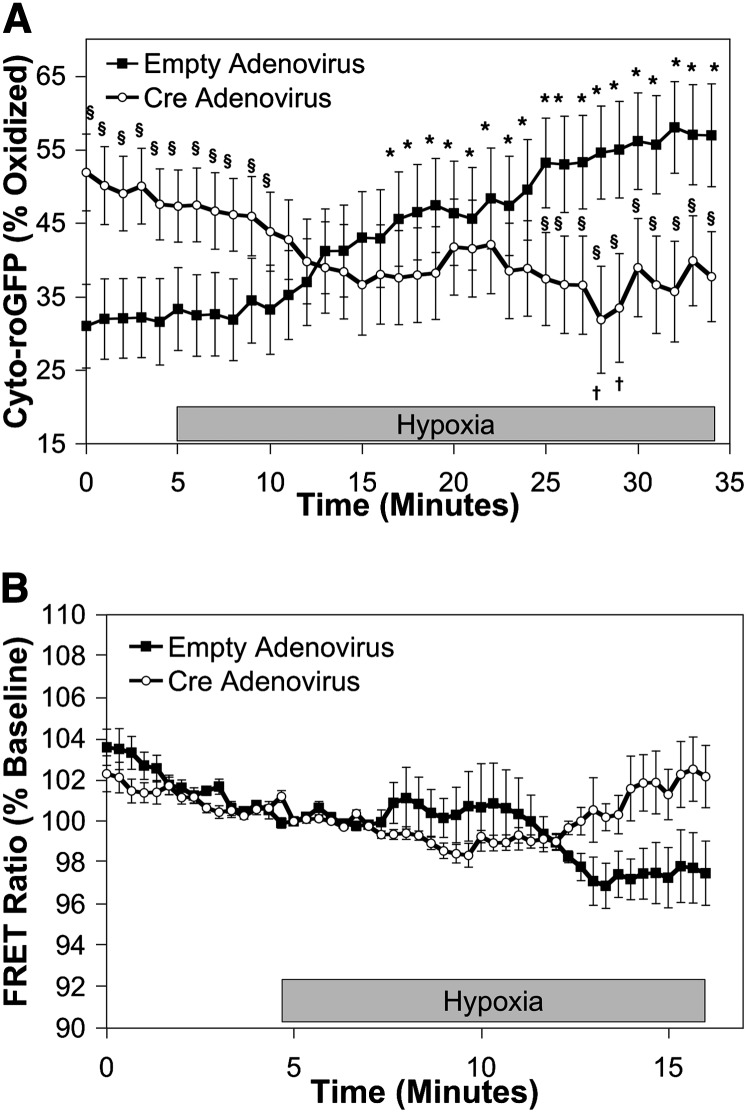
Cells in which RISP expression was decreased with adenoviral Cre exhibited increased baseline normoxic oxidation of Cyto-roGFP to 32.6 ± 3.0% (Figure 3A). Remarkably, however, hypoxia-induced oxidation of Cyto-roGFP was abolished. In fact, oxidation of cytosolic roGFP in RISP-KO PASMC decreased to 24.7 ± 1.6% during 30 minutes of hypoxia (Figure 3A). In contrast, decreased RISP expression had no effect on the level of baseline oxidation of IMS-roGFP (44.1 ± 4.1%). However, as with Cyto-roGFP, hypoxia-induced oxidation of IMS-roGFP oxidation in RISP-KO cells was abrogated. Furthermore, oxidation of IMS-roGFP tended to decrease during hypoxia (29.3 ± 3.0%) (Figure 3B). Finally, in RISP-KO PASMC expressing Mito-roGFP, oxidation averaged 46.6 ± 3.2% under normoxia and did not change during 30 minutes of hypoxia (Figure 3C). Thus, RISP is required for the acute increases in ROS observed in PASMC during hypoxia.
Figure 3.
Effect of Rieske iron-sulfur protein (RISP) depletion on hypoxia-induced reactive oxygen species (ROS) signaling in the cytosol, intermembrane space (IMS) and mitochondrial matrix in pulmonary arterial smooth muscle cells (PASMC) from conditional knockout mice. (A–C) PASMC isolated from RISP mice and treated with empty (control) or Cre-expressing adenovirus. Average results from multiple experiments in PASMC expressing Cyto-roGFP (A), IMS-roGFP (B), or Mito-roGFP (C) superfused with normoxic (21% O2) or hypoxic (1.5% O2) media. Values are means ± SEM, n = 6 cover slips, 4 to 10 cells/cover slip. *P < 0.05 compared with normoxic baseline of empty virus–treated PASMC. †P < 0.05 compared with normoxic baseline of Cre-expressing PASMC. §P < 0.05 compared with empty virus–treated PASMC.
RISP Depletion Attenuates the Hypoxia-induced Increase in [Ca2+]i in PASMC
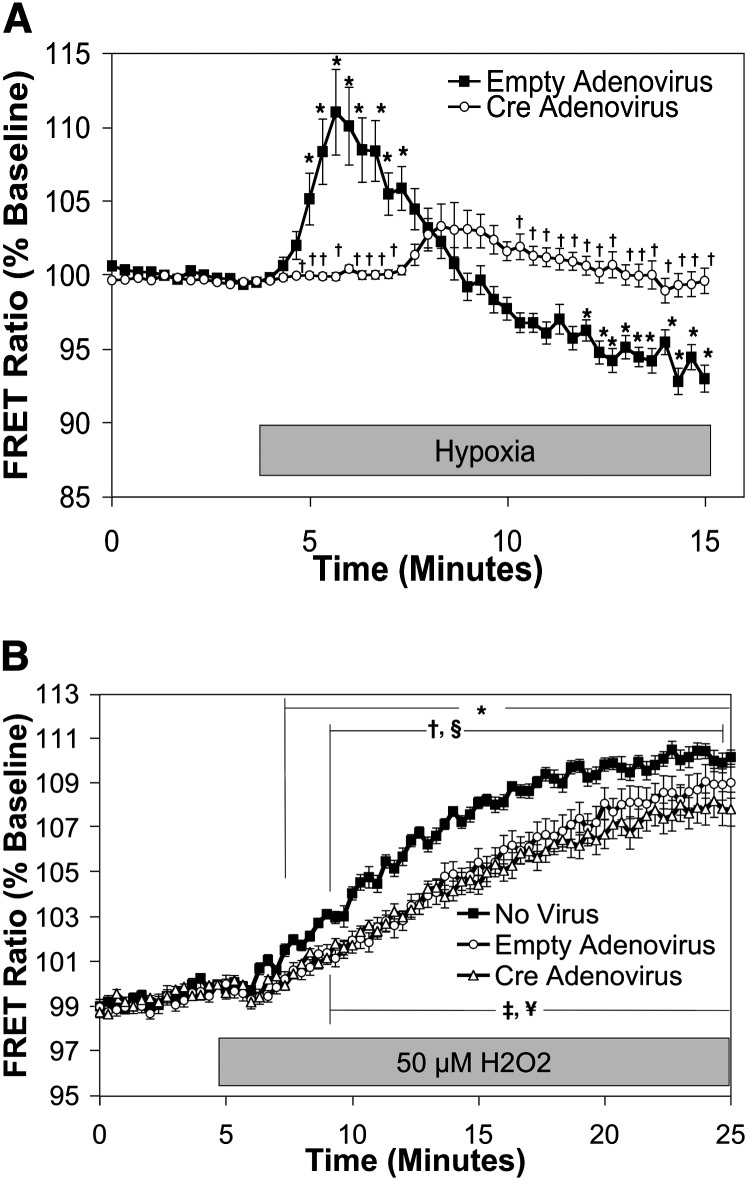
To examine the effect of RISP depletion on the hypoxia-induced increase in [Ca2+]i, cytosolic calcium was assessed using a FRET sensor, YC2.3 (35), expressed in mouse PASMC using a recombinant adenovirus (10, 12). Similar to the response observed previously in rat PASMC (10), the YC2.3 FRET ratio increased within 2 minutes after the start of hypoxia in cells transduced with empty adenovirus, demonstrating increases in [Ca2+]i (111.0 ± 2.9% of baseline; Figure 4A). Cells in which RISP expression was decreased with adenoviral Cre exhibited alterations in both the magnitude and time course of the hypoxia-induced [Ca2+]i increase. No increase in FRET ratio was observed during the initial 4 minutes, during which time hypoxia acutely increases and subsequently decreases [Ca2+]i in control cells. A delayed tendency to increase (P value not significant) was observed well after the increase seen in control cells (103.3 ± 1.5% of baseline at the 8-min mark; Figure 4A). We confirmed that RISP depletion did not alter calcium responses to exogenous H2O2 application, as similar responses to H2O2 were observed in untreated PASMC, in cells treated with empty adenovirus, and in cells treated with Cre adenovirus (110.2 ± 0.3, 109.0 ± 0.7, 107.9 ± 0.8% of baseline, respectively; Figure 4B). These results indicate that RISP is required for the acute increase in [Ca2+]i signaling in response to hypoxia.
Figure 4.
Effect of Rieske iron-sulfur protein (RISP) depletion on [Ca2+]i in pulmonary arterial smooth muscle cells (PASMC) during hypoxia. PASMC were treated with empty (control) or Cre-expressing adenovirus. (A) Effects of hypoxia on [Ca2+]i in PAMSC assessed by YC2.3. Values are means ± SEM, n = 6 cover slips, 4 to 10 cells/cover slip. *P < 0.05 compared with normoxic baseline of empty virus–treated PASMC. †P < 0.05 compared with empty virus–treated PASMC. (B) Effects of exogenous H2O2 (50 μmol/L) on [Ca2+]i in RISP-knockout mouse PASMC assessed by the calcium-sensitive, Förster resonance energy transfer (FRET) sensor YC2.3. Values are means ± SEM, n = 6 cover slips, 4 to 10 cells/cover slip. *P < 0.05 compared with baseline of untreated (no virus) PASMC. ¥P < 0.05 compared with baseline of empty virus–treated PASMC. ‡P < 0.05 compared with baseline of Cre-expressing PASMC. †P < 0.05 compared with empty virus–treated PASMC. §P < 0.05 compared with Cre-expressing PASMC.
RISP Depletion Attenuates Hypoxia-induced Increases in ROS Signaling in Systemic Arterial Smooth Muscle Cells
We previously reported that PASMC and systemic arterial smooth muscle cells (SASMC) share similar patterns of redox signaling in response to hypoxia, despite exhibiting differing downstream contractile responses (10). To determine whether the hypoxia-induced increase in cytosolic ROS signaling in SASMC also depends on Complex III, Cyto-roGFP responses were assessed in RISP-KO SASMC isolated from renal artery. In SASMC transduced with empty adenovirus, Cyto-roGFP oxidation increased during 30 minutes of hypoxia (33.3 ± 5.6 to 56.9 ± 7.0%; Figure 5A). RISP depletion increased the basal oxidation of Cyto-roGFP (47.3 ± 4.9%; Figure 5A), but oxidation decreased (37.7 ± 6.1%) during 30 minutes of hypoxia (Figure 5A). RISP depletion had no detectable effect on the minimal [Ca2+]i signaling response to hypoxia, as assessed by YC2.3 FRET in SASMC (Figure 5B). These results demonstrate that Complex III is required for the hypoxia-induced ROS signaling observed in SASMC.
Figure 5.
Effect of Rieske iron-sulfur protein (RISP) depletion on hypoxia-induced roGFP oxidation in the cytosol of mouse systemic arterial smooth muscle cells (SASMC) isolated from RISP conditional knockout mice. (A, B) SASMC isolated from RISP mice and treated with an empty (control) or Cre-expressing adenovirus. (A) Averaged results from multiple experiments in SASMC expressing Cyto-roGFP superfused with normoxic (21% O2) or hypoxic (1.5% O2) media. (B) Effects of hypoxia on [Ca2+]i in SAMSC assessed by the calcium-sensitive, Förster resonance energy transfer (FRET) sensor YC2.3. Values are means ± SEM, n = 6 cover slips, 4 to 10 cells/cover slip. *P < 0.05 compared with normoxic baseline of empty virus–treated pulmonary arterial smooth muscle cells (PASMC). †P < 0.05 compared with normoxic baseline of Cre-expressing PASMC. §P < 0.05 compared with empty adenovirus–infected PASMC.
RISP Depletion Attenuates Hypoxia-induced Increases in ROS Signaling in Isolated PA Vessels
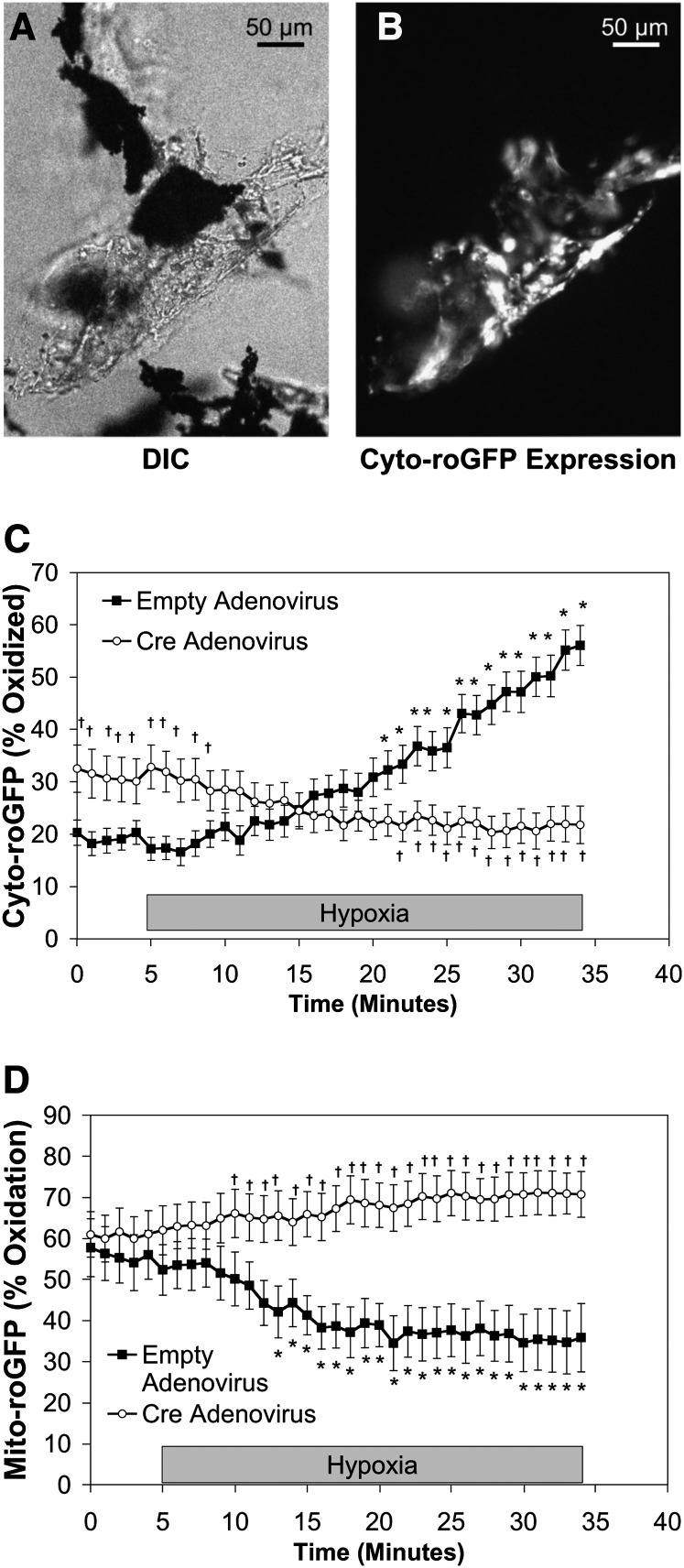
To determine whether hypoxia-induced ROS signaling occurs in intact vessels, ROS signaling was assessed in cells from PA vessels isolated from RISPflox/flox mice (Figure 6A). Freshly isolated PA vessels were transduced with a Cre-expressing adenovirus, and the resulting effect of decreased RISP expression (Figure E7) on hypoxia-induced subcellular compartmental ROS signaling was assessed using Cyto- (Figure 6B) and Mito-roGFP. As seen in isolated PASMC, 30 minutes of hypoxia increased Cyto-roGFP oxidation in isolated PA from 17.2 ± 2.2% to 56.1 ± 3.8%, whereas it decreased Mito-roGFP oxidation from 52.3 ± 6.1 to 35.8 ± 8.5% (Figures 6C and 6D, respectively). Decreasing RISP expression increased normoxic baseline oxidation of Cyto-roGFP to 32.6 ± 4.5% from 20.3 ± 2.4% (Figure 6C). However, hypoxia-induced oxidation of Cyto-roGFP was abolished in the cells of RISP-KO PA segments (Figure 6C). Although RISP depletion did not affect the oxidation of Mito-roGFP in RISP-KO PA vessels under normoxia, it did attenuate the progressive decrease in oxidation during acute hypoxia (Figure 6D). These results indicate that RISP is required for the changes in ROS observed in isolated PA vessels during hypoxia.
Figure 6.
Effect of Rieske iron-sulfur protein (RISP) depletion on hypoxia-induced roGFP oxidation in the cytosol and mitochondrial matrix of cells in pulmonary arterial (PA) vessels isolated from RISP conditional knockout mice. As part of the isolation procedure for PA smooth muscle cells (PASMC), PA microvessel segments are generated that contain iron particles in their lumen. These iron particles allow for the microvessel segments to be constrained in the flow-through chamber using a magnet, thus allowing for their study under both normoxic and hypoxic conditions. (A) DIC image of PA vessel with iron particles (black objects) in the lumen. (B) Same PA vessel as in (A) demonstrating Cyto-roGFP fluorescence in vascular cells. Average results from multiple experiments in PA vessels expressing Cyto-roGFP (C) and Mito-roGFP (D) superfused with normoxic (21% O2) or hypoxic (1.5% O2) media. Values are means ± SEM, n = 6 PA vessels. *P < 0.05 compared with normoxic baseline of empty virus–treated PA vessels. †P < 0.05 compared with empty virus–treated PA vessels. DIC = differential interference contrast.
RISP Depletion Attenuates the Hypoxia-induced Increase in [Ca2+]i in PASMC of Precision-Cut Lung Slices
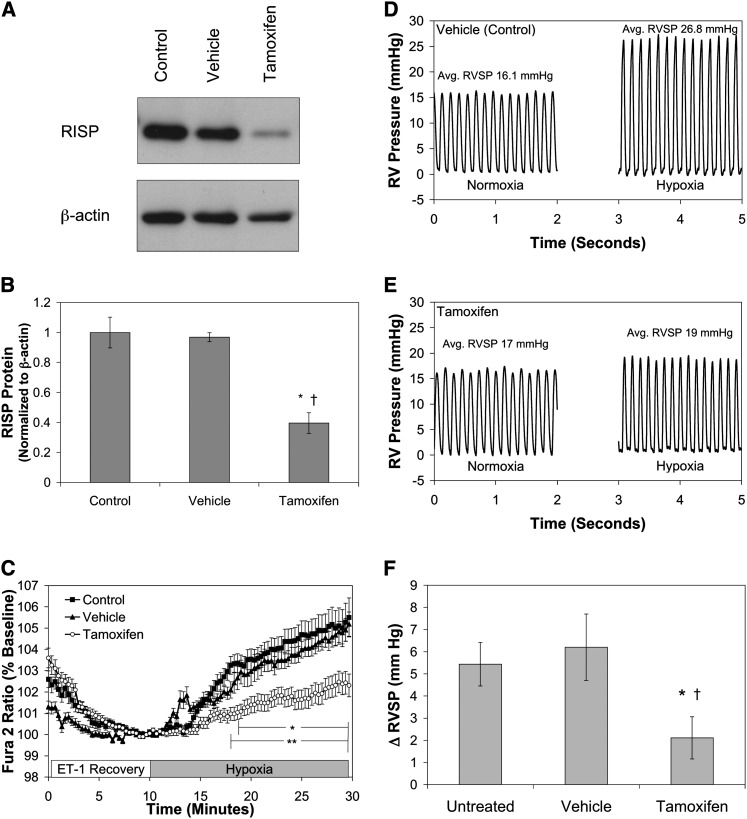
To further assess hypoxia-induced ROS signaling in vivo, RISP was deleted in smooth muscle of SMC-MHC-Cre/RISPflox/flox mice by tamoxifen injections (20 mg/kg body weight in corn oil for five consecutive days), vehicle treatment only, or untreated mice. Mice were then housed for 6 weeks to ensure complete depletion of existing protein in SMC of the tamoxifen-treated animals. At 6 weeks, a significant decrease in RISP protein in the abdominal aorta was confirmed, relative to Vehicle and Control mice (Figures 7A and 7B). The decreased SMC RISP expression had no effect on PA wall thickness, right ventricular mass as assessed by the Fulton index, or cardiac function (Figure E2). The mice remained normal in appearance and behavior despite the chronic loss of Complex III function in their smooth muscle cells.
Figure 7.
Effect of Rieske iron-sulfur protein (RISP) depletion on hypoxia-induced increases in [Ca2+]i in smooth muscle cells (SMC) of precision-cut lung slices and right ventricular systolic pressure (RVSP) of SMC- myosin heavy chain (MHC)-Cre/RISPflox/flox mice. (A) Western blot of lysates from abdominal aorta of untreated (Control), tamoxifen vehicle (Vehicle), or tamoxifen (Tamoxifen) isolated from SMC-MHC-Cre/RISPflox/flox mice probed with antibodies against the RISP subunit or β-actin. (B) Quantified analysis of Western blots expressed RISP expression normalized to β-actin. Values are means ± SEM, n = 3 Western blots. *P < 0.05 compared with Control, †P < 0.05 compared with Vehicle. (C) Hypoxia (1.5% O2 for 20 min)-induced changes in [Ca2+]i as assessed by calcium-mediated changes in FURA-2 measured in cells within the wall of the pulmonary arteries in precision-cut lung slices. Values are means ± SEM, n = 6 lung slices. *P < 0.05 compared with normoxic baseline for Control and Vehicle, **P < 0.05 compared with Vehicle for Tamoxifen. (D) Hypoxia (5% O2 for 1 min) increases the RVSP in the RV pressure tracing from a Vehicle (Control) mouse. (E) RISP depletion suppresses the hypoxia (5% O2 for 1 min)-induced increase in RVSP as observed in the RV pressure tracing from a tamoxifen-treated mouse. (F) Hypoxia (5% O2 for 1 min)-induced changes in hypoxic pulmonary vasoconstriction as denoted by changes in the RVSP of mice. Normoxic baseline RVSP for the Untreated, Vehicle, and Tamoxifen mice were 17.6 ± 1.5, 17.3 ± 2.3, and 17.0 ± 1.1 mm Hg, respectively. Values are means ± SEM, n = 7–9 animals. *P < 0.05 compared with Untreated. †P < 0.05 compared with Vehicle.
To further evaluate the role of RISP in HPV, intracellular calcium responses in the media of PA were assessed using FURA-2 in fresh precision-cut lung slices from SMC-MHC-Cre/RISPflox/flox mice. Superfusion of lung slices from Control or Vehicle mice with hypoxic media (20 min; 1.5% O2) caused an increase in [Ca2+]i manifested by a change in the FURA-2 ratio from baseline to 106 ± 0.9 and 105 ± 0.6% of baseline, respectively (Figure 7C). Superfusion with hypoxia media also caused constriction of the PA (Figure E3). RISP-KO in the SMC significantly attenuated the [Ca2+]i response to hypoxia in lung slice preparations (102 ± 0.5% of baseline; Figure 7C). Thus, hypoxia-induced increases in [Ca2+]i in PA of lung slices requires a functional Complex III.
RISP Depletion Attenuates Hypoxia-induced Increases in Right Ventricular Systolic Pressure of an Intact Mouse
To determine the role of RISP in the HPV response in vivo, hypoxia-induced changes in right ventricular systolic pressure (RVSP) were assessed in SMC-MHC-Cre/RISPflox/flox mice ventilated under normoxic and hypoxic conditions using a Micro Tip pressure transducer catheter (Millar, Houston, TX). Changes in RVSP reflect changes in the PA pressure and thus provide an assessment of the HPV response (Figures 7D and 7E). Normoxic baseline RVSP for the Untreated, Vehicle, and Tamoxifen mice were 17.6 ± 1.5, 17.3 ± 2.3, and 17.0 ± 1.1 mm Hg, respectively (P = not significant). When untreated or vehicle-treated mice were switched from room air to hypoxic (5% O2/95% N2) ventilation, RVSP increased by 5.4 ± 1.0 and 6.2 ± 1.5 mm Hg, respectively (Figure 7F). In tamoxifen-treated mice, the hypoxia-induced change in RSVP was significantly smaller (2.1 ± 1.0 mm Hg; Figure 7F). These results demonstrate that RISP is required for the HPV response.
Discussion
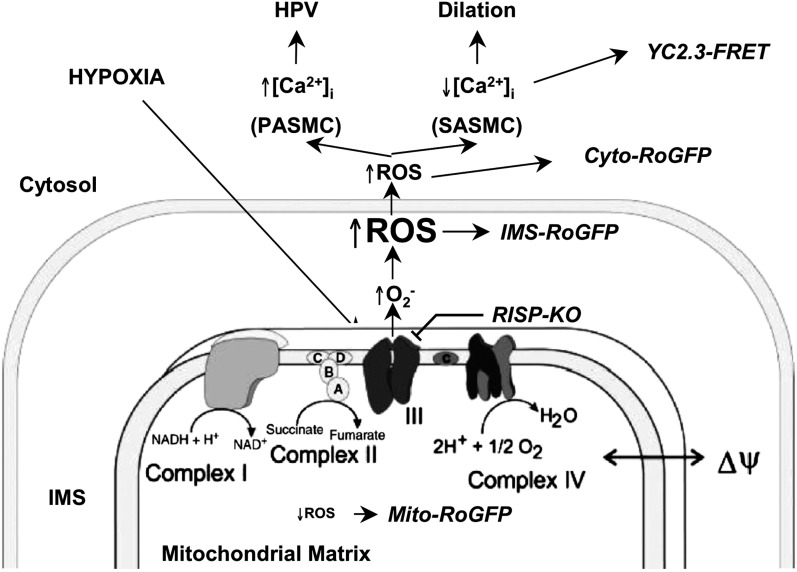
We developed a mouse model to permit conditional deletion of the nuclear-encoded RISP gene to assess its role in hypoxia-induced ROS and Ca2+ signaling in the pulmonary circulation. Using the roGFP sensor to assess subcellular oxidant signaling, we find that depletion of RISP abolishes the ROS response to hypoxia in isolated PASMC and in isolated PA segments. Depleting RISP also abolishes hypoxia-induced [Ca2+]i signaling in isolated PASMC as well as in PA within freshly cut lung slices. Although the response to acute hypoxia is ablated by RISP loss, cultured PASMC retain the ability to activate [Ca2+]i signaling in response to H2O2, indicating that the signaling pathway downstream from ROS is not affected by RISP depletion. Depletion of RISP in smooth muscle of the intact mouse also attenuates hypoxia-induced increases in PA pressure, as assessed by changes in RVSP. Collectively these studies indicate that Complex III is required for hypoxia-induced ROS signaling that triggers the acute increase in cytosolic [Ca2+]i and contraction in the pulmonary circulation (Figure 8). These findings implicate mitochondrial ROS in the oxygen-sensing response in acute HPV.
Figure 8.
Model depicting mitochondria functioning as the O2 sensor underlying the acute hypoxic pulmonary vasoconstriction (HPV) response. IMS = intermembrane space; KO = knockout; NAD+ = nicotinamide adenine dinucleotide; NADH = NAD+ reduced; PASMC = pulmonary arterial smooth muscle cells; ROS = reactive oxygen species; SASMC = systemic arterial smooth muscle cells; YC2.3-FRET = the calcium-sensitive, Förster resonance energy transfer sensor.
We previously showed that the cytosol and the IMS of rat PASMC and SASMC exhibit increases in oxidant signaling during hypoxia, whereas the opposite response occurs in the matrix (10). This appears to reflect an increase in the generation of superoxide at the outer surface of the inner mitochondrial membrane and a decrease in nonspecific superoxide generation in the matrix. Depletion of RISP abolished the hypoxia-induced increase in ROS in the IMS and the decrease in the matrix, which argues against the idea that ROS generation in the latter compartment is nonspecific and Po2 dependent. Alternatively, these results could also be explained by a shift the direction of ROS secretion from the inner mitochondrial membrane, away from the matrix compartment and toward the IMS. A hypoxia-induced shift in the conformation of Complex III would presumably be needed to explain such a change in direction of ROS release. In either case, the present study confirms and extends our previous work showing that pharmacological inhibition of Complex III with myxothiazol, which binds at the quinol oxidation (Qo) site of the bc1 complex, attenuated the hypoxia-induced increase in ROS and Ca2+ signaling in PASMC as well as the HPV response in the isolated perfused lung, whereas antimycin A, which binds to the Qi site, had no effect on the HPV response (9, 11, 12). RISP acts by transferring a single electron from ubiquinol bound at the Qo site to cytochrome c1, resulting in the formation of ubisemiquinone at the Qo site, which has the potential to transfer its electron to molecular oxygen, thus forming superoxide. By depleting RISP, the formation of ubisemiquinone at the Qo site is prevented, thus mimicking the response to myxothiazol. Taken together, these results are also consistent with a broader body of work indicating that hypoxia increases mitochondrial ROS signaling (10, 17, 18, 27–29, 36–39).
Are mitochondria the only source of ROS during hypoxia? Studies using a genetic knockout of p47phox suggest that cytosolic nicotinamide adenine dinucleotide phosphate reduced oxidase systems may also contribute to the HPV response during acute hypoxia (40). The blockade of hypoxia-induced ROS responses we observe with depletion of RISP suggests that the mitochondria may act as the initiators of ROS production, which could be amplified by engagement of nonphagocytic nicotinamide adenine dinucleotide phosphate reduced oxidase systems elsewhere in the cell. Such “ROS-induced ROS release” might permit small ROS signals generated by mitochondria to activate ROS signaling throughout the cell, thereby avoiding mitochondrial damage that might arise if the entire cellular oxidant signal originated from that organelle (15). Regardless of the source, ROS signals trigger diverse functional responses to hypoxia in PASMC, including calcium signaling and AMP-regulated kinase (AMPK) activation (12, 41).
Depletion of RISP also attenuated hypoxia-induced increases in Cyto-roGFP oxidation in SASMC (Figure 5A). This attenuation suggests that Complex III is required for hypoxic ROS signaling in diverse vascular cell types, and it is not consistent with the observation by Michelakis and colleagues (24) that SASMC mitochondria behave reciprocally to PASMC in terms of ROS generation during hypoxia. In PASMC, hypoxia elicits increases in [Ca2+]i to cause vasoconstriction (42–47), whereas SASMC decrease [Ca2+]i and undergo relaxation (24, 48, 49). Our observation that RISP depletion in SASMC has no detectable effect on calcium signaling (Figure 5B) is not surprising, as SASMC do not increase [Ca2+]i during hypoxia. Interestingly, although hypoxia increased [Ca2+]i in isolated PASMC, there was a significant decrease in [Ca2+]i during the second phase of the response to hypoxia (Figure 4A). This did not occur in the experiments in lung slices (Figure 7C), suggesting that other cell types may be required for a sustained increase in PASMC [Ca2+]i during more prolonged hypoxia.
Korde and colleagues used siRNA to decrease RISP in PASMC and observed a decrease in ROS generation in isolated mitochondria under normoxia (29). However, interpretation of their data is complicated because factors regulating ROS production in the intact cell, such as the membrane potential and respiratory rate, are dramatically altered when the organelle is removed from the cell. Other studies using genetic suppression of RISP in tumor cell lines show that ROS derived from Complex III are important for hypoxic signaling (16, 27, 28). However, one cannot assume that the O2 sensing and signaling pathways in tumor cells are identical to those in PASMC. The present study was therefore undertaken to provide a more complete assessment of its role in triggering acute responses in the pulmonary circulation.
To our knowledge, this is the first study to genetically target a subunit of the ETC to evaluate its role in the HPV response in an intact animal. Interestingly, depletion of RISP in smooth muscle of the adult mouse produced no overt phenotype (Figure E2), indicating that SMC can survive and function using glycolytic ATP, albeit with an impaired HPV response. The animals appeared healthy, and the depletion of RISP did not affect the ability of the cells to respond to exogenous H2O2, indicating that cells were not globally impaired. After depletion of RISP, the cultured PASMC continued to proliferate, indicating that loss of ETC function does not induce bioenergetic deficiency.
Although the present study demonstrates the importance of mitochondrial ROS for responses to acute hypoxia in the lung, chronic hypoxia in obstructive lung disease represents a major clinical problem associated with remodeling of pulmonary vessels and the development of pulmonary hypertension. It is not clear whether the O2 sensing and ROS signaling pathways we link to the acute responses in the present report are also involved in triggering the remodeling of the pulmonary circulation during chronic hypoxia. However, given the fact that chronic depletion of RISP is well tolerated by the mice, it will be possible to test this question in future studies involving prolonged hypoxic exposure.
Supplementary Material
Acknowledgments
The authors thank Dr. Christopher Rhodes (The University of Chicago, Chicago, IL) for producing the adenovirus expressing the YC2.3 FRET sensor and Dr. Stefan Offermanns of the University of Heidelberg for providing the SMC-MHC-Cre mouse. They also thank Dr. Robert W. Dettman (Northwestern University, Chicago, IL) for informative discussions.
Footnotes
Supported by National Institutes of Health grants HL66315, HL35440, and HL079650 (P.T.S.), American Heart Association grant 0235457Z (G.B.W.), and National Institutes of Health grant NS056313 (J.D.M.).
Author Contributions: Conception and design: G.B.W., J.D.M., R.D.G., P.T.M., P.T.S. Analysis and interpretation: G.B.W., J.D.M., R.D.G., P.T.M., J.M.S., D.D., M.K.B., P.T.S. Data acquisition: G.B.W., J.D.M., P.T.M., J.M.S., D.D., M.K.B. Manuscript preparation: G.B.W., J.D.M., P.T.M., P.T.S.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201207-1294OC on January 17, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Rounds S, McMurtry IF. Inhibitors of oxidative ATP production cause transient vasoconstriction and block subsequent pressor responses in rat lungs. Circ Res 1981;48:393–400 [DOI] [PubMed] [Google Scholar]
- 2.McMurtry IF. Angiotensin is not required for hypoxic constriction in salt solution-perfused rat lungs. J Appl Physiol 1984;56:375–380 [DOI] [PubMed] [Google Scholar]
- 3.Grimminger F, Weissmann N, Spriestersbach R, Becker E, Rosseau S, Seeger W. Effects of NADPH oxidase inhibitors on hypoxic vasoconstriction in buffer-perfused rabbit lungs. Am J Physiol 1995;268:L747–L752 [DOI] [PubMed] [Google Scholar]
- 4.Weissmann N, Grimminger F, Voswinckel R, Conzen J, Seeger W. Nitro blue tetrazolium inhibits but does not mimic hypoxic vasoconstriction in isolated rabbit lungs. Am J Physiol 1998;274:L721–L727 [DOI] [PubMed] [Google Scholar]
- 5.Weissmann N, Grimminger F, Walmrath D, Seeger W. Hypoxic vasoconstriction in buffer-perfused rabbit lungs. Respir Physiol 1995;100:159–169 [DOI] [PubMed] [Google Scholar]
- 6.Marshall C, Mamary AJ, Verhoeven AJ, Marshall BE. Pulmonary artery NADPH-oxidase is activated in hypoxic pulmonary vasoconstriction. Am J Respir Cell Mol Biol 1996;15:633–644 [DOI] [PubMed] [Google Scholar]
- 7.Zhao Y, Rhoades RA, Packer CS. Hypoxia-induced pulmonary arterial contraction appears to be dependent on myosin light chain phosphorylation. Am J Physiol 1996;271:L768–L774 [DOI] [PubMed] [Google Scholar]
- 8.Zhang F, Carson RC, Zhang H, Gibson G, Thomas HM., III Pulmonary artery smooth muscle cell [Ca2+]i and contraction: responses to diphenyleneiodonium and hypoxia. Am J Physiol 1997;273:L603–L611 [DOI] [PubMed] [Google Scholar]
- 9.Waypa GB, Chandel NS, Schumacker PT. Model for hypoxic pulmonary vasoconstriction involving mitochondrial oxygen sensing. Circ Res 2001;88:1259–1266 [DOI] [PubMed] [Google Scholar]
- 10.Waypa GB, Marks JD, Guzy R, Mungai PT, Schriewer J, Dokic D, Schumacker PT. Hypoxia triggers subcellular compartmental redox signaling in vascular smooth muscle cells. Circ Res 2010;106:526–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waypa GB, Marks JD, Mack MM, Boriboun C, Mungai PT, Schumacker PT. Mitochondrial reactive oxygen species trigger calcium increases during hypoxia in pulmonary arterial myocytes. Circ Res 2002;91:719–726 [DOI] [PubMed] [Google Scholar]
- 12.Waypa GB, Guzy R, Mungai PT, Mack MM, Marks JD, Roe MW, Schumacker PT. Increases in mitochondrial reactive oxygen species trigger hypoxia-induced calcium responses in pulmonary artery smooth muscle cells. Circ Res 2006;99:970–978 [DOI] [PubMed] [Google Scholar]
- 13.Weissmann N, Ebert N, Ahrens M, Ghofrani HA, Schermuly RT, Hanze J, Fink L, Rose F, Conzen J, Seeger W, et al. Effects of mitochondrial inhibitors and uncouplers on hypoxic vasoconstriction in rabbit lungs. Am J Respir Cell Mol Biol 2003;29:721–732 [DOI] [PubMed] [Google Scholar]
- 14.Wang QS, Zheng YM, Dong L, Ho YS, Guo Z, Wang YX. Role of mitochondrial reactive oxygen species in hypoxia-dependent increase in intracellular calcium in pulmonary artery myocytes. Free Radic Biol Med 2007;42:642–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rathore R, Zheng YM, Niu CF, Liu QH, Korde A, Ho YS, Wang YX. Hypoxia activates NADPH oxidase to increase [ROS]i and [Ca2+]i through the mitochondrial ROS-PKCepsilon signaling axis in pulmonary artery smooth muscle cells. Free Radic Biol Med 2008;45:1223–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab 2005;1:401–408 [DOI] [PubMed] [Google Scholar]
- 17.Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci USA 1998;95:11715–11720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leach RM, Hill HM, Snetkov VA, Robertson TP, Ward JP. Divergent roles of glycolysis and the mitochondrial electron transport chain in hypoxic pulmonary vasoconstriction of the rat: identity of the hypoxic sensor. J Physiol 2001;536:211–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dooley CT, Dore TM, Hanson GT, Jackson WC, Remington SJ, Tsien RY. Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. J Biol Chem 2004;279:22284–22293 [DOI] [PubMed] [Google Scholar]
- 20.Hanson GT, Aggeler R, Oglesbee D, Cannon M, Capaldi RA, Tsien RY, Remington SJ. Investigating mitochondrial redox potential with redox-sensitive green fluorescent protein indicators. J Biol Chem 2004;279:13044–13053 [DOI] [PubMed] [Google Scholar]
- 21.Cannon MB, Remington JS. Redox-sensitive green fluorescent protein: probes for dynamic intracellular redox responses. A review. Methods Mol Biol 2009;476:50–64 [DOI] [PubMed] [Google Scholar]
- 22.Lohman JR, Remington SJ. Development of a family of redox-sensitive green fluorescent protein indicators for use in relatively oxidizing subcellular environments. Biochemistry 2008;47:8678–8688 [DOI] [PubMed] [Google Scholar]
- 23.Jiang K, Schwarzer C, Lally E, Zhang S, Ruzin S, Machen T, Remington SJ, Feldman L. Expression and characterization of a redox-sensing green fluorescent protein (reduction-oxidation-sensitive green fluorescent protein) in Arabidopsis. Plant Physiol 2006;141:397–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michelakis ED, Hampl V, Nsair A, Wu X, Harry G, Haromy A, Gurtu R, Archer SL. Diversity in mitochondrial function explains differences in vascular oxygen sensing. Circ Res 2002;90:1307–1315 [DOI] [PubMed] [Google Scholar]
- 25.Archer SL, Michelakis ED, Thebaud B, Bonnet S, Moudgil R, Wu XC, Weir EK. A central role for oxygen-sensitive K+ channels and mitochondria in the specialized oxygen-sensing system. Novartis Found Symp 2006;272:157–171; discussion 171–155, 214–157 [PubMed] [Google Scholar]
- 26.Weir EK, Archer SL. The role of redox changes in oxygen sensing. Respir Physiol Neurobiol 2010;174:182–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brunelle JK, Bell EL, Quesada NM, Vercauteren K, Tiranti V, Zeviani M, Scarpulla RC, Chandel NS. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab 2005;1:409–414 [DOI] [PubMed] [Google Scholar]
- 28.Mansfield KD, Guzy RD, Pan Y, Young RM, Cash TP, Schumacker PT, Simon MC. Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-alpha activation. Cell Metab 2005;1:393–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Korde AS, Yadav VR, Zheng YM, Wang YX. Primary role of mitochondrial Rieske iron-sulfur protein in hypoxic ROS production in pulmonary artery myocytes. Free Radic Biol Med 2011;50:945–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waypa GB, Marks JD, Guzy R, Mungai PT, Schriewer J, Schumacker PT. Hypoxia triggers subcellular compartmental redox signaling in vascular smooth muscle cells [abstract]. Presented at the Keystone Symposia: Hypoxia: Molecular Mechanisms of Oxygen Sensing and Response Pathways. January 19–24, 2010, Keystone, CO. [DOI] [PMC free article] [PubMed]
- 31.Waypa GB, Marks JD, Guzy R, Mungai PT, Dokic D, Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ros signaling in vascular smooth muscle cells [abstract]. Presented at the American Thoracic Society 2011 International Conference. May 13–18, 2011, Denver, CO.
- 32.Desireddi JR, Farrow KN, Marks JD, Waypa GB, Schumacker PT. Hypoxia increases ROS signaling and cytosolic Ca(2+) in pulmonary artery smooth muscle cells of mouse lungs slices. Antioxid Redox Signal 2010;12:595–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wirth A, Benyo Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, Orsy P, Horvath B, Maser-Gluth C, Greiner E, et al. G12-g13-larg-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med 2008;14:64–68 [DOI] [PubMed] [Google Scholar]
- 34.Wallenstein S, Zucker CL, Fleiss JL. Some statistical methods useful in circulation research. Circ Res 1980;47:1–9 [DOI] [PubMed] [Google Scholar]
- 35.Griesbeck O, Baird GS, Campbell RE, Zacharias DA, Tsien RY. Reducing the environmental sensitivity of yellow fluorescent protein. Mechanism and applications. J Biol Chem 2001;276:29188–29194 [DOI] [PubMed] [Google Scholar]
- 36.Guzy RD, Sharma B, Bell E, Chandel NS, Schumacker PT. Loss of the SdhB, but not the SdhA, subunit of complex II triggers reactive oxygen species-dependent hypoxia-inducible factor activation and tumorigenesis. Mol Cell Biol 2008;28:718–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pastukh V, Ruchko M, Gorodnya O, Wilson GL, Gillespie MN. Sequence-specific oxidative base modifications in hypoxia-inducible genes. Free Radic Biol Med 2007;43:1616–1626 [DOI] [PubMed] [Google Scholar]
- 38.Lavani R, Chang WT, Anderson T, Shao ZH, Wojcik KR, Li CQ, Pietrowski R, Beiser DG, Idris AH, Hamann KJ, et al. Altering CO2 during reperfusion of ischemic cardiomyocytes modifies mitochondrial oxidant injury. Crit Care Med 2007;35:1709–1716 [DOI] [PubMed] [Google Scholar]
- 39.Gusarova GA, Dada LA, Kelly AM, Brodie C, Witters LA, Chandel NS, Sznajder JI. Alpha1-AMP-activated protein kinase regulates hypoxia-induced Na,K-Atpase endocytosis via direct phosphorylation of protein kinase C zeta. Mol Cell Biol 2009;29:3455–3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weissmann N, Zeller S, Schafer RU, Turowski C, Ay M, Quanz K, Ghofrani HA, Schermuly RT, Fink L, Seeger W, et al. Impact of mitochondria and NADPH oxidases on acute and sustained hypoxic pulmonary vasoconstriction. Am J Respir Cell Mol Biol 2006;34:505–513 [DOI] [PubMed] [Google Scholar]
- 41.Mungai PT, Waypa GB, Jairaman A, Prakriya M, Dokic D, Ball MK, Schumacker PT. Hypoxia triggers AMPK activation through reactive oxygen species-mediated activation of calcium release-activated calcium channels. Mol Cell Biol 2011;31:3531–3545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morio Y, McMurtry IF. Ca(2+) release from ryanodine-sensitive store contributes to mechanism of hypoxic vasoconstriction in rat lungs. J Appl Physiol 2002;92:527–534 [DOI] [PubMed] [Google Scholar]
- 43.Evans AM, Dipp M. Hypoxic pulmonary vasoconstriction: cyclic adenosine diphosphate-ribose, smooth muscle Ca(2+) stores and the endothelium. Respir Physiol Neurobiol 2002;132:3–15 [DOI] [PubMed] [Google Scholar]
- 44.Snetkov VA, Aaronson PI, Ward JP, Knock GA, Robertson TP. Capacitative calcium entry as a pulmonary specific vasoconstrictor mechanism in small muscular arteries of the rat. Br J Pharmacol 2003;140:97–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang J, Shimoda LA, Sylvester JT. Capacitative calcium entry and TRPC channel proteins are expressed in rat distal pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol 2004;286:L848–L858 [DOI] [PubMed] [Google Scholar]
- 46.Gonzalez-Pacheco FR, Caramelo C, Castilla MA, Deudero JJ, Arias J, Yague S, Jimenez S, Bragado R, Alvarez-Arroyo MV. Mechanism of vascular smooth muscle cells activation by hydrogen peroxide: role of phospholipase C gamma. Nephrol Dial Transplant 2002;17:392–398 [DOI] [PubMed] [Google Scholar]
- 47.Weissmann N, Dietrich A, Fuchs B, Kalwa H, Ay M, Dumitrascu R, Olschewski A, Storch U, Mederos y Schnitzler M, Ghofrani HA, et al. Classical transient receptor potential channel 6 (TRPC6) is essential for hypoxic pulmonary vasoconstriction and alveolar gas exchange. Proc Natl Acad Sci USA 2006;103:19093–19098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saitoh S, Zhang C, Tune JD, Potter B, Kiyooka T, Rogers PA, Knudson JD, Dick GM, Swafford A, Chilian WM. Hydrogen peroxide: a feed-forward dilator that couples myocardial metabolism to coronary blood flow. Arterioscler Thromb Vasc Biol 2006;26:2614–2621 [DOI] [PubMed] [Google Scholar]
- 49.Lopez-Barneo J, Pardal R, Montoro RJ, Smani T, García-Hirschfeld J, Ureña J. K+ and Ca2+ channel activity and cytosolic [Ca2+] in oxygen-sensing tissues. Respir Physiol 1999;115:215–227 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.