Abstract
Anaphylaxis is a rare condition in pregnancy. Drugs are the aetiological agents most often implicated. Maternal anaphylaxis can lead to significant fetal morbidity and even mortality if uterine perfusion and maternal oxygenation are compromised. Significant risk of neonatal neurological damage or death can occur even when the maternal clinical outcome is favourable. The authors present the case of a newborn, born at gestational age of 29 weeks, who died at 11 days of life with hypoxic–ischaemic cerebral injuries as a consequence of maternal anaphylaxis following the administration of amoxicillin in the community setting.
Background
Anaphylaxis in pregnancy is considered a rare condition with an estimated prevalence of 2.7 cases/1 00 000 deliveries.1 Antibiotics are the most common trigger.2–5 There is no evidence that anaphylaxis occurs in the fetus but maternal anaphylaxis can lead to significant fetal morbidity and mortality if uterine perfusion and maternal oxygenation are compromised.6 There is a significant risk of fetal/neonatal neurological damage or death even when the maternal outcome is favourable.6 All previous reports of maternal anaphylaxis occurred in a hospital setting.2–5 7–18 The authors report a case of maternal anaphylaxis in a community setting. In the era of chemoprophylaxis of Group B streptococcal (GBS) infections, accurate identification among predisposed pregnant women is mandatory.
Case presentation
The authors report the case of a neonate, first daughter of unrelated parents. The mother was aged 21 years (gravida 1, para 1) with a medical history of asthma but without previous episodes of anaphylaxis or drug allergies. She reported that she had been treated with amoxicillin earlier without any symptoms. The pregnancy had been uneventful with first and second trimester serology and the fetal sonograms were reported as normal.
Forty-eight hours before labour, the mother, at a gestational age of 28 weeks and 5 days, was admitted to another hospital's emergency service complaining of itching rash, oedema of the lips, vomiting and dizziness. She was under treatment with amoxicillin for an acute otitis media and reported the symptoms started soon after the second dose. Fluids, steroids and antihistamines were promptly administered parenterally. The obstetric evaluation was unremarkable with infection screening negative and fetal sonographic examination reported as normal. Owing to uterine contractility on cardiotocography (CTG) she was also medicated with indomethacin, magnesium and erythromycin. Hospital discharge took place a few hours later.
In the next 24 h the mother was admitted to another emergency service with a complaint of decreased fetal movements. Obstetric evaluation showed an immobile fetus, fetal tachycardia (170–180 bpm) with absence of variability. Based on these clinical findings the mother was transferred to our maternity hospital.
On admission, the mother's vital signs were normal and general physical examination was unremarkable. A single intrauterine pregnancy with intact membranes was confirmed. Maternal infection screening was negative. Maternal serum β-tryptase and urinary N-methylhystamine (NMH) were unavailable. Ultrasound examination confirmed an immobile fetus and the Doppler study revealed a high resistance in middle cerebral (1.9) and umbilical arteries (3.5). No more antenatal corticosteroids had been administered. An emergency caesarean section was performed. At birth the female newborn was pale, hypotonic and bradycardic, without spontaneous breathing or reflexes. The APGAR scores were 1 6 and 6 at the 1st, 5th and 10th min, respectively. The newborn was intubated, resuscitated and transferred to the intensive care nursery for further care. The birth weight was 1350 g (50th percentile). The blood gases showed metabolic acidosis (pH 7.18; BE: −7 mEq/l) which was quickly buffered.
Outcome and follow-up
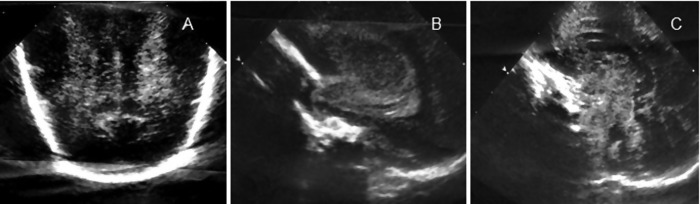
The mother's hospital course was uneventful and she was discharged home 4 days later. The baby, however, was not so fortunate. From day 1 of life she remained mechanically ventilated. Echocardiography showed global contractile dysfunction, without structural heart disease, stabilised with inotropic drugs. Chest radiograph showed grade III hyaline membrane disease. Infection and metabolic screens were negative along with blood cultures obtained following admission to the neonatal intensive care unit and maternal serologic studies (parvovirus, enterovirus and TORCH). She received empirical ampicillin and gentamicin, exogenous surfactant and standard care for the gestational age. Nevertheless, the neonate's neurological status remained severely compromised, without an obvious cause related to prematurity. She maintained an abnormal state of consciousness (comatose), hypotonic, without spontaneous movements, fixed mydriasis and absence of reflexes. Cranial ultrasonography from the first days (figure 1) and MRI (figure 2) at 10 days of age exhibited changes consistent with hypoxic–ischaemic cerebral lesions. On the 11th day of life the neonate died.
Figure 1.
Cranial ultrasound. Coronal planes (A) and sagittal planes (B and C): symmetric, diffuse periventricular white matter echogenicity (A), hyperechoic aspect of cerebellum, thalami and basal ganglia (B and C).
Figure 2.
MRI performed at 10 days of life: axial T1-weighted MRI showing frontoparietal, pericentral and temporal necrosis. Hypersignal of thalami and lentiform nuclei. Cavitation necrosis in brain stem—lesions compatible with diffuse hypoxic encephalomalacia.
Investigations
The mother was evaluated by an allergist. Positive skin test results and serum-specific IgE levels testing to ascertain the causative agent were undertaken and confirmed sensitisation to amoxicillin. She started a desensitisation therapy.
Morphological examination of the placenta showed normal maturation with severe vasoconstriction of umbilical arteries (figure 3). Placental mast cells colouration was negative suggesting previous degranulation of mast cells (figure 4).
Figure 3.

Umbilical cord pathology. Presence of three umbilical vessels with severe vasoconstriction of umbilical arteries (arrow).
Figure 4.

Placenta pathology. Placenta normal maturation. Mastocytes colouration was negative.
Neonatal autopsy was performed. The brain external examination was unremarkable. The internal examination revealed brain hypoxic–ischaemic lesions with diffuse subcortical leukomalacia (figure 5), thalami infarction (figure 6), white matter lesions with microvacuolation and gliosis in the frontal lobe and selective neuronal ischaemic degenerescence of brain stem, thalami and basal ganglia (figure 7) with reactive astrocytes. The histology of all other organs was unremarkable.
Figure 5.

Newborn autopsy. Coronal plane of brain with the presence of subcortical leukomalacia (arrow).
Figure 6.

Newborn autopsy. Thalami with an infarct lesion.
Figure 7.

Newborn autopsy. Signs of neuronal necrosis at medulla oblongata—eosinophilic cytoplasm and pycnotic nuclei (arrow).
Discussion
This case represents a life-threatening complication to both mother and her fetus following the standard practice of maternal infection treatment in an ambulatory setting.
Anaphylaxis is currently defined as a serious allergic reaction that is rapid in onset and might cause death.19 The lifetime prevalence of anaphylaxis in the general population is estimated at 0.05–2%.19–24 Data regarding prevalence among pregnant women is limited1 6 with an estimated prevalence near or at the time of delivery reported as 2.7 cases of anaphylaxis per 100 000 deliveries.1 Anaphylaxis in pregnancy places both mother and fetus at an increased risk of fatality or fetal hypoxic/ischaemic encephalopathy.25 Any agent that can trigger anaphylaxis in a non-pregnant state can potentially trigger it in susceptible pregnant women.6 26 During the first, second and third trimesters, potential triggers are similar to those in non-pregnant women. During labour and delivery, anaphylaxis is usually triggered by iatrogenic interventions such as oxytocin, or more commonly, an antimicrobial source such as penicillin or cephalosporin administered to the mother for prophylaxis of neonatal group B haemolytic streptococcal (GBS) infection.25
Although pregnant women are exposed to antibiotics in the community setting, all previously reported cases of antibiotic anaphylaxis during pregnancy have occurred in hospitalised women (table 1) even though this may represent publication bias.2–4 7–18 The cases have been reported in association with surgical prophylaxis prior to caesarean delivery, during prophylaxis of neonatal GBS infection or during treatment of maternal pyrexia. The authors report the first case of drug-induced maternal anaphylaxis in a community setting.
Table 1.
Summary of case reports involving drug-induced anaphylaxis during pregnancy in the last 20 years
| Author (year) | Agent | Gestational age (weeks) at onset | Setting | Maternal clinical presentation | Maternal outcome | Neonatal outcome |
|---|---|---|---|---|---|---|
| Heim et al (1991)7 | Ampicillin | 36 | Hospital | Hypotension | Good | Neurological damage |
| Edmonson et al (1994)8 | Suxamethonium | 36 | Hospital | Hypotension | Good | Neurological damage |
| Suri et al (1999)9 | Suxamethonium | 23 | Hospital | Hypotension | Good | Neurological damage |
| Konno et al (1995)11 | Cefazolin | 36 | Hospital | Hypotension | Good | Good |
| Luciano et al (1997)10 | Iron | 27 | Hospital | Hypotension | Good | Neurological damage |
| Dunn et al (1999)12 | Penicillin | 35 | Hospital | Hypotension | Good | Good |
| Gei et al (2003)5 | Ampicillin | 40 | Hospital | Hypotension | Good | Good |
| Berardi et al (2004)13 | Ampicillin | 37 | Hospital | Hypotension | Good | Neurological damage |
| Jao et al (2006)14 | Cefazolin | 37 | Hospital | Hypotension | Good | Good |
| Sheikh et al (2007)15 | Penicillin | 37 | Hospital | Hypotension Respiratory |
Good | Death |
| Berthier et al (2007)16 | Amoxicillin | 39 | Hospital | Hypotension | Good | Good |
| Penicillin | 38 | Hospital | Hypotension | Good | Good | |
| Ceftriaxone | 37 | Hospital | Hypotension | Good | Neurological damage | |
| Amoxicillin | 40 | Hospital | Hypotension | Good | Death | |
| Amoxicillin | 36 | Hospital | Hypotension | Good | Neurological damage | |
| Sengupta et al (2008)3 | Cefotaxime | ? Term | Hospital | Hypotension | Good | Death |
| Chaudhuri et al (2009)2 | Penicillin | 40 | Hospital | Hypotension Rash |
Good | Neurological damage |
| Sleth et al (2009)17 | Amoxicillin | 38 s | Hospital | Hypotension Rash |
Good | Good |
The diagnosis of anaphylaxis during pregnancy is similar to non-pregnant patients and is based on a meticulous history and physical examination.6 26 It relies primarily on clinical criteria and is valid even if the results of laboratory tests are within normal limits, such as serum β-tryptase levels.6 26 Positive skin test results or increased serum specific IgE levels to potential triggering allergens confirm sensitisation but do not confirm the diagnosis of anaphylaxis because asymptomatic sensitisation is common in the general population.21 Clinical criteria have been defined and can be applied to pregnant women.6 19 26 It is considered to be highly likely when any one of three clinical criteria are fulfilled (box 1). The presence of reduced blood pressure or shock is not necessarily required. The terms anaphylactoid or pseudoanaphylaxis are no longer recommended for use.21 The mother of this neonate fulfilled the clinical criteria of anaphylaxis.
Box 1. Clinical criteria for diagnosing anaphylaxis.19.
Anaphylaxis is highly likely when any one of the following three criteria is fulfilled:
1. Acute onset of an illness (minutes to several hours) with involvement of the skin, mucosal tissue or both (eg, generalised hives, pruritus or flushing and swollen lips-tongue-uvula) and at least one of the following:
A. Respiratory compromise (eg, dyspnoea, wheeze-bronchospasm, stridor, reduced PEF and hypoxemia)
B. Reduced blood pressure (BP) or associated symptoms of end-organ dysfunction (eg, hypotonia/collapse, syncope and incontinence)
2. Two or more of the following that occur rapidly after exposure to a likely allergen for that patient (minutes to several hours):
A. Involvement of the skin–mucosal tissue (eg, generalised hives, itch-flush and swollen lips-tongue-uvula)
B. Respiratory compromise (eg, dyspnoea, wheeze-bronchospasm, stridor, reduced PEF and hypoxemia)
C. Reduced BP or associated symptoms (eg, hypotonia/collapse, syncope and incontinence)
D. Persistent gastrointestinal symptoms (eg, cramping abdominal pain, vomiting)
3. Reduced BP after exposure to a known allergen for that patient (minutes to several hours):
A. Infants and children: low systolic BP (age-specific) or greater than 30% decrease in systolic BP
B. Adults: systolic BP of less than 90 mm Hg or greater than 30% decrease from that person’s baseline
Adapted from.19 BP, blood pressure; PEF, peak expiratory flow.
Maternal anaphylaxis constitutes a major concern for obstetricians and neonatologists. An alteration in immunological status due to increased progesterone level during pregnancy may predispose pregnant women to anaphylaxis even though the high levels of placental histaminase may act as a protective mechanism for the fetus.2 27 28
In the presented case, the mother had previously received penicillin-based antibiotics without any allergic reactions, suggesting that immunological changes in pregnancy may have triggered new-onset sensitisation. There is no solid evidence that anaphylaxis occurs in the fetus because specific maternal IgE antibodies are not transmitted across the placenta.29–32 The developing fetal central nervous system is often more affected.2 In the presented case, as reported in the literature, maternal hipovolemia, hypoxia, uterine hypoperfusion, umbilical vessels vasoconstriction and peripheral fetal vasodilation induced by histamine could lead to the impairment of fetal regulation of cerebral flow and induced severe neurological damage.6 26 As discussed in previous reports, the magnitude and duration of maternal hypotension probably determine the extent of injury while fetal maturity possibly dictates the site of injury.2 13 The primary sites affected in full term neonates are often the basal ganglia and thalamus. In contrast, in preterms, severe hypotension results in infarction of the deep grey matter, brainstem and cerebellum.2 13 33 Another issue that influences the management and outcome is the biphasic reaction of anaphylaxis that may occur in up to 20% of patients in the first 72 h.6 For this reason, continuous fetal monitoring for 24–72 h after maternal anaphylaxis is crucial in order to identify precocious signs of fetal distress.
Increased umbilical and cerebral arteries resistance index (RI) is another indicator of poor outcome. Sustained asphyxia with subsequent development of haemorrhaging or diffuse cerebral oedema, induce a loss of forward diastolic flow resulting in increased RI.33 The presence of gliosis and selective neuronal necrosis described in the pathological neonatal examination estimate the date of injury (10–15 days after hypoxic injury). The neonate died on the 11th day of life and the sentinel event took place 48 h before delivery. This suggests that central nervous system injuries were secondary to the described severe episode of maternal anaphylaxis.
Controversies exist regarding the best timing and mode of delivery of the neonate following anaphylaxis during pregnancy. In view of inadequate maternal resuscitation, immediate caesarean delivery may provide a better outcome for the neonate.2 Continuous vigilance, adequate knowledge and a high degree of suspicion are essential for prompt diagnosis and treatment. A consensus about the management of anaphylaxis in pregnancy is highly desirable. It is also advisable that all services develop and discuss a management protocol of anaphylaxis in pregnant women. In our case, the stabilisation of the mother in the emergency department with fluids, antihistamines and corticosteroids drugs, without adrenaline, was probably insufficient to prevent fetal brain damage and the reported final outcome.
Learning points.
Anaphylaxis is a rare event in pregnancy.
High degree of suspicion is essential for prompt diagnosis and treatment.
Permanent damage is observed in the neonates rather than in mothers.
Continuous vigilance of fetal well-being for 48–72 h after an episode of maternal anaphylaxis is crucial in order to identify precocious signs of fetal distress.
The development and implementation of a maternal anaphylaxis management protocol by a multidisciplinary team of obstetricians, neonatologists and anaesthesiologists is highly advisable.
The chemoprophylaxis of SBS infections and treatment of pregnant infections should give warning for the accurate identification of predisposed women.
Acknowledgments
The authors thank Dra Teresa Tomé (Director of Department of Pediatrics, Dr Alfredo da Costa Maternity Hospital, Lisbon, Portugal), Dra Cristina Matos (Neonatologist of Dr Alfredo da Costa Maternity Hospital, Lisbon, Portugal) and Dr Carlos Barros (Obstetrician and Gynecologist of Department of Obstetrics and Gynaecology, Dr Alfredo da Costa Maternity Hospital, Lisbon, Portugal) for critical revision of manuscript. The authors would like to acknowledge Dr Kevin Carrigy for correcting the English and the anonymous reviewers for their comments that help improve the manuscript.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Mulla ZD, Ebrahim MS, Gonzalez JL. Anaphylaxis in the obstetric patient: analysis of a statewide hospital discharge database. Ann Allergy Asthma Immunol 2010;104:55–9 [DOI] [PubMed] [Google Scholar]
- 2.Chaudhuri K, Gonzales J, Jesurun CA, et al. Anaphylactic shock in pregnancy: a case study and review of the literature. Int J Obstet Anesth 2008;17:350–7 [DOI] [PubMed] [Google Scholar]
- 3.Sengupta A, Kohli JK. Antibiotic prophylaxis in cesarean section causing anaphylaxis and intrauterine fetal death. J Obstet Gynaecol Res 2008;34:252–4 [DOI] [PubMed] [Google Scholar]
- 4.Khan R, Anastasakis E, Kadir RA. Anaphylactic reaction to ceftriaxone in labour. An emerging complication. J Obstet Gynaecol 2008;28:751–3 [DOI] [PubMed] [Google Scholar]
- 5.Gei AF, Pacheco LD, Vanhook JW, et al. The use of a continuous infusion of epinephrine for anaphylactic shock during labor. Obstet Gynecol 2003;102:1332–5 [DOI] [PubMed] [Google Scholar]
- 6.Schatz M, Simons E, Dombrowski M. Anaphylaxis in pregnant and breastfeeding women. In: Basow DS, ed. UpToDate Waltham, MA, USA: UpToDate, 2012. [Google Scholar]
- 7.Heim K, Alge A, Marth C. Anaphylactic reaction to ampicillin and severe complication in the fetus. Lancet 1991;337:859–60 [DOI] [PubMed] [Google Scholar]
- 8.Edmondson WC, Skilton RWH. Anaphylaxis in pregnancy—the right treatment? Anaesthesia 1994;49:454–5 [DOI] [PubMed] [Google Scholar]
- 9.Suri S, Salfield S, Baxter P. Congenital paraplegia following maternal hypotension. Dev Med Child Neurol 1999;41:273–4 [DOI] [PubMed] [Google Scholar]
- 10.Luciano R, Zuppa AA, Maragliao G, et al. Fetal encephalopathy after maternal anaphylaxis. Case Report Biol Neonat 1997;71:190–3 [DOI] [PubMed] [Google Scholar]
- 11.Konno R, Nagase S. Anaphylactic reaction to cefazolin in pregnancy. Asia-Oceania J Obstet Gynaecol 1995;21:577–9 [DOI] [PubMed] [Google Scholar]
- 12.Dunn A, Blomquist J, Khouzami V. Anaphylaxis in labor secondary to prophylaxis against group B Streptococcus:a case report. J Reprod Med 1999;44:381–4 [PubMed] [Google Scholar]
- 13.Berardi A, Rossi K, Cavalleri F, et al. Maternal anaphylaxis and fetal brain damage after intrapartum chemoprophylaxis. J Perinat Med 2004;32:375–7 [DOI] [PubMed] [Google Scholar]
- 14.Jao M, Cheng P, Shaw S, et al. Anaphylaxis to cefazolin during labor secondary to prophylaxis for group B Streptococcus: a case report. J Reprod Med 2006;51:655–8 [PubMed] [Google Scholar]
- 15.Sheikh J. Intrapartum anaphylaxis to penicillin in a woman with rheumatoid arthritis who had no prior penicillin allergy. Ann Allergy Asthma Immunol 2007;99:287–9 [DOI] [PubMed] [Google Scholar]
- 16.Berthier A, Sentilhes L, Hamou L, et al. Antibiotiques en fin de grossesse. À propos de cinq réactions allergiques sévères. Gynécologie Obstétrique Fertilité 2007;35:464–72 [DOI] [PubMed] [Google Scholar]
- 17.Sleth JC, Lafforgue E, Cherici O, et al. [Anaphylaxis in terminal pregnancy: two case studies and review of the literature]. Annales Françaises D'anesthèsie Et De Rèanimation 2009;28:790–4 [DOI] [PubMed] [Google Scholar]
- 18.Sleth JC, Lafforgue E, Cherici O, et al. Anaphylaxis in terminal pregnancy: two case studies and review of the literature. Ann Fr Anesth Reanim 2009;28:790–4 [DOI] [PubMed] [Google Scholar]
- 19.Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second Symposium on the Definition and Management of Anaphylaxis: Summary Report—Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network Symposium. Ann Emerg Med 2006;47:373–80 [DOI] [PubMed] [Google Scholar]
- 20.Simons FE, Sampson HA. Anaphylaxis epidemic: fact or fiction? J Allergy Clin Immunol 2008;122:1166–8 [DOI] [PubMed] [Google Scholar]
- 21.Simons FE. Anaphylaxis. J Allergy Clin Immunol 2010;125(Supplement 2):S161–81 [DOI] [PubMed] [Google Scholar]
- 22.Lieberman P, Camargo CA, Jr, Bohlke K, et al. Epidemiology of anaphylaxis: findings of the American College of Allergy, Asthma and Immunology Epidemiology of Anaphylaxis Working Group. Ann Allergy Asthma Immunol 2006;97:596–602 [DOI] [PubMed] [Google Scholar]
- 23.Decker WW, Campbell RL, Manivannan V, et al. The etiology and incidence of anaphylaxis in Rochester, Minnesota: a report from the Rochester Epidemiology Project. J Allergy Clin Immunol 2008;122:1161–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clark S, Camargo CA., Jr Epidemiology of anaphylaxis. Immunol Allergy Clin NA 2007;27:145–63 [DOI] [PubMed] [Google Scholar]
- 25.Simons F, Estelle R, Ardusso L, et al. World Allergy Organization Guidelines for the Assessment and Management of Anaphylaxis. WAO J 2011;4:13–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schatz M. Recognition and management of allergic disease during pregnancy. In: Basow DS, ed. UpToDate. Waltham, MA: UpToDate, 2012 [Google Scholar]
- 27.Maintz L, Schwarzer V, Bieber T, et al. Effects of histamine and diamine oxidase activities on pregnancy: a critical review. Hum Reprod Update 2008;14:485–95 [DOI] [PubMed] [Google Scholar]
- 28.Draisci G, Zanfini B, Nucera E, et al. Latex sensitization: a special risk for the obstetric population? Anesthesiology 2011;114:565–9 [DOI] [PubMed] [Google Scholar]
- 29.Smith P, Ownby D. Clinical significance if immunoglobulin E. In: Adkinson NF, Bochner BS, Busse WW, et al., eds. Middletońs allergy principles and practice. 7th edn Philadelphia: Mosby, 2009 [Google Scholar]
- 30.Bønnelykke K, Pipper CB, Bisgaard H. Sensitization does not develop in utero. J Allergy Clin Immunol 2008;121:646–51 [DOI] [PubMed] [Google Scholar]
- 31.MacGinnitie A. In utero anaphylaxis. Med Hypotheses 2011;76:70–2 [DOI] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention Prevention of perinatal group B streptococcal disease—revised guidelines from CDC. MMWR 2010;59:1–32 [PubMed] [Google Scholar]
- 33.Chao CP, Zaleski CG, Patton AC. Neonatal hypoxic-ischemic encephalopathy: multimodality imaging findings. Radiographics 2006;26(Suppl 1):S159–72 [DOI] [PubMed] [Google Scholar]