Abstract
Bilateral facial palsy (BFP) is a very uncommon entity, particularly in the paediatric age group. Despite its several aetiologies, neuroborreliosis should be suspected, especially in children from endemic areas presenting with acute neurological disease of unknown cause. We present two cases of BFPs as the presenting forms of neuroborreliosis.
Background
Bilateral facial palsy (BFP) is defined as the involvement of the opposite side within 30 days of the onset of the first side.1 It has an incidence of 1:5 000 000 cases/year,2 corresponding to 0.3–2% of all cases of facial palsy.1
Although rare, this entity has several aetiologies. However, the leading cause in children is neuroborreliosis, corresponding to 50% of all cases, and BFP may be the only manifestation of the infection.3 In adults, the major manifestation is Bannwarth syndrome (facial palsy, lymphocytic meningitis and radiculitis), but this is rare in children.
The physiopathology is, in most of the cases, unknown. Ischaemia, oedema and infiltration of facial nerves by inflammatory cells and direct invasion of the nerves by microorganisms are the proposed mechanisms.4
This case highlights the relevance of the clinical signs, even when the diagnostic exams have conflicting results and possible differential diagnoses that have to be considered in this entity.
Case presentation
Patient 1: A 13-year-old girl, previously healthy, resident in a rural area, presented with BFP with Bell's phenomenon (figure 1) and no other neurological abnormal findings, fever or other symptoms. She had no history of tick bite or cutaneous rash.
Figure 1.
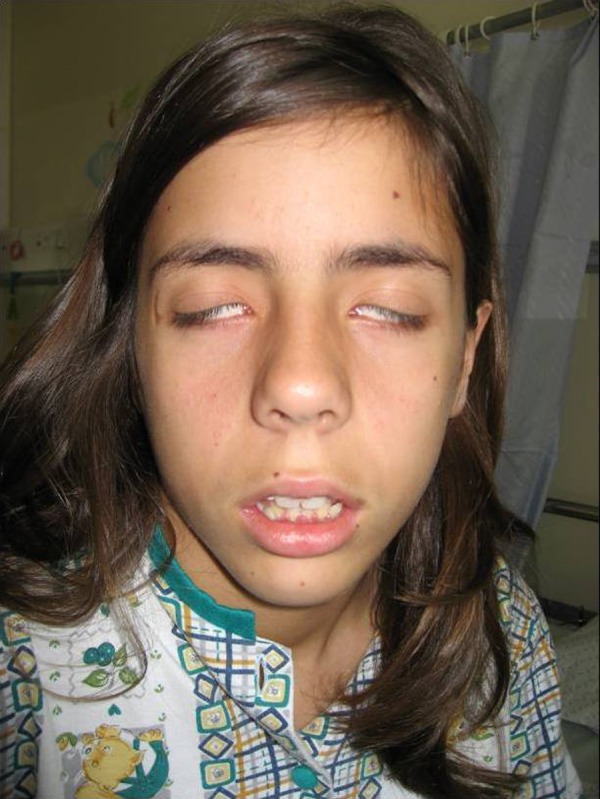
Patient 1, showing bilateral facial palsy and Bell's sign.
Patient 2: This 12-year-old boy, previously healthy, complained of pain in the right forearm that progressively extended to the arm, shoulder and neck. A week later, he developed vespertine low-grade fever lasting 2 days and a BFP, complete on the right side, and involving predominantly the left lower face with bilateral Bell's sign (figure 2), with no other neurological signs. He had travelled to Morocco and the Portuguese rural areas 1 month ago. Like patient 1, he had no history of tick bite or cutaneous rash.
Figure 2.
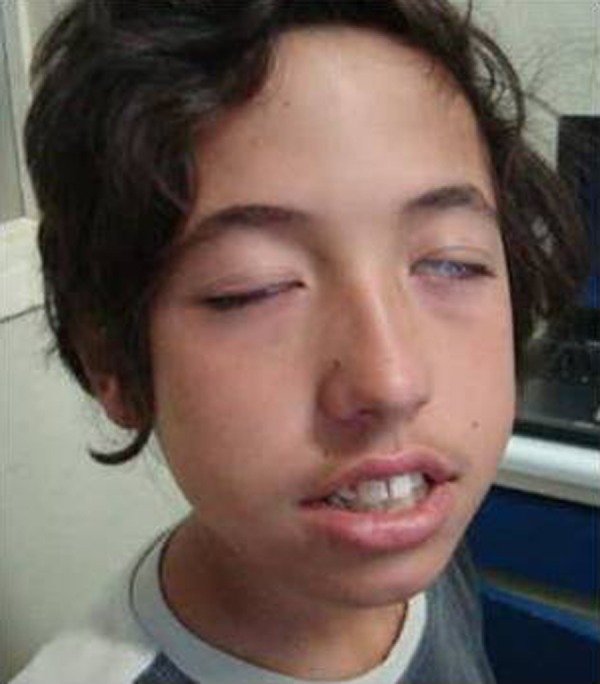
Patient 2, showing bilateral facial palsy, complete on the right side, and involving predominantly the left lower face with bilateral Bell's sign.
Investigations
Patient 1: Full blood count and C reactive protein were within normal limits. Cerebrospinal fluid (CSF) showed 0.8 cells/mm3 lymphocytes and normal glucose and protein levels. Cranioencephalic CT and positron emission tomography (PET) scans did not show any lesions. MRI was not performed due to the presence of orthodontic braces. Borrelia burgdorferi (Bb) antibodies were not detected by ELISA in serum and CSF on day 5 of the disease. Serum ELISA was repeated after 1 month and confirmed by Western blot; these showed a negative IgM with positive IgG.
Patient 2: Laboratory evaluation showed leukocytosis (20 680×103/μl), neutrophilia (87.5%) and a sedimentation rate of 8 mm/h. CSF analysis showed 500 cells/mm3, mostly lymphocytes (85%), a protein level of 108.3 mg/dl, without oligoclonal bands and a glucose level of 101.00 mg/dl. Cranial and spinal MRI revealed enlargement and inflammation of the spinal cord from the C2 level to the T1 level (figure 3) and signs of spinal cord, meningeal and radicular inflammation. Serum antibodies detected by ELISA and Western blot, and serum and CSF Bb PCR performed on day 16 of the disease were negative. However, CSF Bb IgM and IgG were positive, confirming the diagnosis. The CSF PCR was then repeated and was also positive. Serum antibodies to Bb were repeated after 1 month, showing seroconversion.
Figure 3.
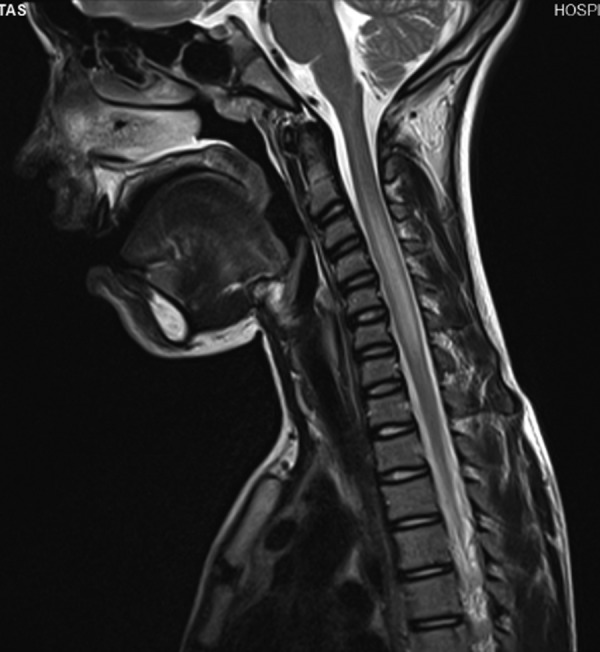
Cranial and spinal MRI of patient 2, showing inflammation of the spinal cord from C2 to T1.
Differential diagnosis
Facial palsy may be caused by several other diseases, including infections (tuberculosis, Mycoplasma pneumoniae, leptospirosis, cytomegalovirus, herpes zoster, postinfluenza, Epstein-Barr virus, HIV, coxsackie, arbovirus, parotiditis, Cryptococcus), Guillain-Barré syndrome, fracture of the skull base, parotid or mastoid surgery, Moebius syndrome, leukaemia, lymphoma, ependymoma, vestibular schwannoma, multiple sclerosis, temporal arteritis, polyarteritis nodosa, Bell palsy, systemic lupus erythematosus, amyloidosis and Wegener granulomatosis.1 2
In our patients, all these other infectious diseases and autoimmune conditions were excluded.
Outcome and follow-up
Patient 1: Although initially Bb antibodies were negative, as the clinical suspicion of neuroborreliosis persisted, ceftriaxone was started. On day 12 of treatment, the patient developed a non-pruriginous maculopapular erythematous rash and fever (38.5 °C), leading to the withdrawal of ceftriaxone; the rash disappeared 3 days later. Prick test and intradermal testing were performed to evaluate the possibility of drug allergy, but these were negative. Four months later, the patient had recovered completely.
Patient 2: In this patient, Bb serum antibodies and serum and CSF Bb PCR were initially negative. However, the presence of Bannwarth syndrome, highly suggestive of neuroborreliosis, led to treatment with ceftriaxone. After 19 days of antibiotic treatment, the patient developed a maculopapular rash, without fever. Intradermal testing and ceftriaxone provocation test were negative. The patient recovered completely after 1 month.
Discussion
In these patients, peripheral BFP occurred in the context of neuroborreliosis. Given the rarity of this entity, these cases are particularly interesting, especially in Portugal, where borreliosis prevalence is one of the lowest in Europe, about 0.04 : 100 000.5
Neurological manifestations of borreliosis are more common in children. They appear in 10–15% of untreated patients, usually 2–3 months after infection, but a 9-month interval is still possible.6 Headache, acute facial palsy (sometimes bilateral) and aseptic meningitis are the most common manifestations, although peripheral neuropathy, focal neurological deficits, ataxia, myelitis, vertigo, progressive encephalomyelitis or intracranial hypertension may also occur.6 7 Facial palsy is present in 11%, being bilateral in 30–40% of these.1 Fever may be present, usually low grade.6 In adults, the major manifestation is Bannwarth syndrome, which is rare in children6 but was present in case 2. However, even in children, the combination of aseptic meningitis and peripheral facial nerve palsy is highly suggestive of Bb infection.8
None of our patients had erythema migrans or tick bite history, which would have facilitated the diagnosis, but these are found in neuroborreliosis only in 20–30% and 40–50% of cases, respectively.6 7
For definite neuroborreliosis, the following three criteria must be fulfilled7: (1) neurological symptoms suggestive of neuroborreliosis, with no other obvious reason; (2) CSF pleocytosis; (3) intrathecal Bb antibodies production. If the third criterion is lacking, Bb antibodies should be found in serum, after a period of 6 weeks. Our second patient fulfilled all criteria, while patient 1 showed seroconversion.
CSF analysis usually shows pleocytosis, lymphocytic predominance, high protein level and oligoclonal IgG bands.3 7 These bands are found in 55–63% of patients,9 although its absence does not rule out neuroborreliosis, as seen in patient 2.
The initial absence of serum Bb antibodies in both the patients did not exclude borreliosis because these were probably tested very early in the course of the disease. IgM appears within 2–4 weeks and peaks within 6–8 weeks, while IgG appears within 4–6 weeks and remains detectable for many years.3 Antibodies detected by ELISA have a sensibility of 70–90%.7 Borderline or positive serologies should be confirmed by Western blot to eliminate false-positive results.7 In our two patients, IgG became positive late in the course of the disease, but a children's series presenting facial palsy caused by borreliosis showed that IgM is usually negative and IgG is rarely positive.10
The diagnostic gold standard for neuroborreliosis is the presence of Bb antibodies in the CSF with evidence of intrathecal production, based on the determination of the antibody index. Bb IgM in CSF has a specificity of 96%.6 However, this technique has low sensitivity in the early phase of the disease (80% in the first 6 weeks).7 In patient 1, CSF Bb antibodies were not determined because serum antibodies were initially negative and there was no evidence of meningitis. In patient 2, even though the antibody index could not be determined, since serum IgM and IgG were initially absent, we can conclude that the CSF antibodies were originated from intrathecal production.
Bb PCR has a low sensitivity (less than 40%6 7), especially if the symptoms are present for less than 6 weeks,7 and its specificity is about 98–100%. Thus, despite constituting a helpful test for the diagnosis, it should not be used in isolation.
In 2001, Jhaveri et al11 reported a case of neuroborreliosis in which a rash resembling erythema migrans appeared after the initiation of treatment with ceftriaxone, named the Jarisch-Herxheimer-like reaction. This reaction can appear a few days after the start of antibiotic therapy.12 We cannot be sure that our patients presented this reaction, but the investigation for ceftriaxone allergy was negative in both.
Learning points.
Bilateral facial palsy (BFP) is a very uncommon condition.
Its most common cause is neuroborreliosis.
BFP associated with aseptic meningitis and radiculitis is highly suggestive of neuroborreliosis, even in children
The diagnosis of this infection is difficult and implies the accurate interpretation of results of diagnostic examination.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Jain V, Deshmukh A, Gollomp S. Bilateral facial paralysis case presentation and discussion of differential diagnosis. J Gen Intern Med 2006;21:C7–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Price T, Fife DG. Bilateral simultaneous facial nerve palsy. J Laryngol Otol 2002;116:46–8 [DOI] [PubMed] [Google Scholar]
- 3.Mlodzikowska-Albrecht J, Zarowski M, Steinborn B, et al. Bilateral facial nerve palsy in the course of neuroborreliosis in children—dynamics, laboratory tests and treatment. Rocz Akad Med Bialymst 2005;50:64–9 [PubMed] [Google Scholar]
- 4.Ronthal M. Bell's palsy: pathogenesis, clinical features, and diagnosis; (updated 17 Sep 2009). http://www.uptodate.com (accessed 25 April 2011).
- 5.Lopes de Carvalho I, Núncio MS. Laboratory diagnosis of Lyme borreliosis at the Portuguese National Institute of Health (1990–2004). Euro Surveill 2006;11:257–60 [PubMed] [Google Scholar]
- 6.López-Alberola RF. Neuroborreliosis and the pediatric population: a review. Rev Neurol 2006;42:91–6 [PubMed] [Google Scholar]
- 7.Mygland A, Ljøstad U, Fingerle V, et al. EFNS guidelines on the diagnosis and management of European Lyme neuroborreliosis. Eur J Neurol 2010;17:8–16 [DOI] [PubMed] [Google Scholar]
- 8.Waespe N, Steffen I, Heininger U. Etiology of aseptic meningitis, peripheral facial nerve palsy, and a combination of both in children. Pediatr Infect Dis J 2010;29:453–6 [DOI] [PubMed] [Google Scholar]
- 9.Bednarova J. Cerebrospinal-fluid profile in neuroborreliosis and its diagnostic significance. Folia Microbiol 2006;51:599–603 [DOI] [PubMed] [Google Scholar]
- 10.Porwancher R. Predictive model for Lyme meningitis. Pediatrics 2008;118:438–49 [DOI] [PubMed] [Google Scholar]
- 11.Jhaveri R, Cherry JD, Phillips S, et al. Erythema migrans after ceftriaxone treatment of aseptic meningitis caused by Borrelia burgdorferi. Pediatr Infect Dis J 2001;20:1010–2 [DOI] [PubMed] [Google Scholar]
- 12.Sigal LH. Misconceptions about Lyme disease: confusions hiding behind ill-chosen yerminology. Ann Intern Med 2002;136:413–19 [DOI] [PubMed] [Google Scholar]


