Abstract
Background
African catarrhine primates differ in bacterial disease susceptibility.
Methods
Human, chimpanzee, and baboon blood was stimulated with TLR-detected bacterial agonists and cytokine/chemokine induction assessed by real-time pcr.
Results
Humans and chimpanzees shared similar cytokine/chemokine responses, while baboon cytokine/chemokine induction differed. Generally, responses were agonist-independent.
Conclusions
These primates tend to generate species rather than agonist–specific responses to bacterial agonists.
Keywords: primate immunity, innate immunity, chemokines, cytokines, bacteria, Toll-like receptors, TLR, Esherichia coli, Mycobacterium
As African catarrhines, the common chimpanzee (Pan troglodytes) and baboon (Papio sp.) share 98.6% and 94% of their genomes, respectively, with humans and are considered important models of human infectious disease [4, 6, 20]. However, these species exhibit very different susceptibility to infectious bacterial pathogens that are associated, in humans, with marked dysregulation of early inflammatory responses [5, 7, 10, 12, 25]. For example, humans and chimpanzees require only small doses (i.e. 2-5 ng/kg) of Gram-negative bacteria or cell wall component lipopolysaccharide (LPS) to initiate severe bacterial sepsis, while baboons and other old world monkey species require much higher doses (0.1 mg/kg) [7, 18, 22, 24]. Similarly, humans and chimpanzees are very susceptible to Neisseria gonorrhea infections, while baboons and most other mammals are resistant [12]. Though less well understood, mycobacterial infections have been noted to rapidly progress in baboons and other old world monkeys [9, 16, 26]. The host factors responsible for disparate bacterial infection susceptibility in catarrhine primates [humans, apes, and old world monkeys (i.e. baboons, macaques)] are not well understood, though blood leukocyte reactivity to immune stimuli during early infection appears to differ between such species [2, 19, 24]. One possible explanation for disparate bacterial infection susceptibility between human, chimpanzees and baboons is inter-species differences in the initiation of the early innate immune responses.
The first two hours of infection in mammalian hosts is marked by an immediate induction of a core set of innate immune genes (i.e. cytokines and chemokines) that appear to exert control over the initial infection course and may affect disease susceptibility [8, 15]. Toll-like receptors (TLRs) are innate immune system receptors that assist in initiating this highly organized early innate immune response by recognizing pathogen-associated molecular patterns (PAMPs) and triggering immune gene induction. TLR2 recognizes lipoproteins and lipopeptides from Gram-positive bacteria, mycobacteria, fungi and parasites while TLR4 interacts with lipopolysaccharide LPS from Gram-negative bacteria [3, 13, 21]. Given the importance of chimpanzee and baboon models in understanding human disease course, comparative data on early TLR-mediated responses to bacterial PAMPs may contribute to a better understanding of the host factors responsible for inter-species differences in bacterial disease susceptibility. This study compares and contrasts the induction profile of chemokines and cytokines associated with the early innate immune response in humans, chimpanzees and baboons after stimulation with TLR2 and 4-detected PAMPs.
To investigate catarrhine early innate immune responses to TLR-detected bacterial PAMPs fresh blood from unrelated, healthy, adult humans (City College of New York IRB # 09-0073C), chimpanzees (Yerkes National Primate Research Center, Atlanta, Georgia) and baboons (Texas Biomedical Research Institute, San Antonio, Texas) was stimulated at 37°C for 90 minutes with ten fold serial dilutions of 10 ug/ml – 0.01 ug/ml of TLR2 and TLR4-detected PAMPs [Pam3CSK4, lipomannan from Mycobacterium smegmatis (LMMS), Ultrapure lipopolysaccharide from Escherichia coli 0111:B4 (LPS), Invivogen, San Diego, CA]. Chimpanzee and baboon blood samples were humanely collected in accordance with individual institutional IACUC requirements. After 90 minutes, red blood cells were lysed by hypotonic shock and total blood leukocyte total RNA isolated to synthesize cDNA (Qiagen RNEasy mini-kit, Quantitect Reverse Transcriptase kit, Qiagen San Diego, CA). Real-time pcr was performed using 0.1 uM of primers corresponding to conserved regions of cytokine/chemokine genes of all three species (Table 1). Reaction specificity was confirmed by DNA sequencing of the amplicons. Relative gene induction was calculated using the Pfaffl equation corrected for primer efficiency, and three reference genes (GAPDH, ACTB, B2M) [17] [23].
Table 1.
Genes, primer sequences and annealing sites
| Symbol | Forward Primer | Reverse Primer | NCBI accession numbers | Gene Category |
|---|---|---|---|---|
| GAPDH Homo sapiens Pan troglodytes Papio anubis |
5′-GAGTCAACGGATTTGGTCGT -3′ .......................................... .......................................... |
5′- TTGATTTTGGAGGGATCTCG- 3′ .......................................... .......................................... |
NM_002046.3 XM_001162023.1/XM_001162057.1/XM_001162096.1/XM_508955.2 AY179885.1 |
Reference |
| ACTB Homo sapiens Pan troglodytes Papio hamadryas |
5′- GGCATCCACGAAACTACCTT -3′ .......................................... .......................................... |
5′- CTTGCTGATCCACATCTGCT -3′ .......................................... .......................................... |
NM_001101.3 NM_001009945.1 Papio hamadryas genome Nov. 2008 Pham_1.0 * |
Reference |
| B2M Homo sapiens Pan troglodytes Papio hamadryas |
5′- GCTATCCAGCGTACTCCAAA- 3′ .......................................... .......................................... |
5′- AAGACAAGTCTGAATGCTCC -3′ .......................................... .......................................... |
NM_004048.2 NM_001009066.1 Papio hamadryas genome Nov. 2008 Pham_1.0 * |
Reference |
| IFNγ Homo sapiens Pan trogolodytes Papio anubis |
5′- ACTGCCAGGACCCATATGTA - 3′ .......................................... .......................................... |
5′- CCTTGATGGTCTCCACACTC -3′ .......................................... .......................................... |
NM_000619.2 XM_001151968.1 AY234217.1 |
Interferon |
| IL-1RN Homo sapiens Pan troglodytes Papio hamadryas |
5′- AAGATGTGCCTGTCCTGTGT -3′ .......................................... .......................................... |
5′- GCTCAGGTCAGTGATGTTAA -3′ .......................................... .......................................... |
NM_173842.1/NM_173841.1/ NM_000577.3/ NM_173843.1 XM_001147673.1/XM_001147825.1/ XM_001147954.1/ XM_515698.2 Papio hamadryas genome Nov. 2008 Pham_1.0 * |
Cytokine |
| TNFα Homo sapiens Pan troglodytes Papio ursinus |
5′- CAGACCAAGGTCAACCTCCT-3′ .......................................... .......................................... |
5′- AGACTCGGCAAAGTCGAGAT-3′ .......................................... .......................................... |
NM_000594.2 XM_001152827.1 AF019963.1 |
Cytokine |
| IL-1β Homo sapiens Pan troglodytes Papio hamadryas |
5′- GCTTGGTGATGTCTGGTCCA -3′ .......................................... .......................................... |
5′- GAGGCCCAAGGCCACAGGTA-3′ ........................................... ........................................... |
NM_000576.2 XM_001146864.1/XM_001146939.1/XM_001147075.1/ XM_515697.2 Papio hamadryas genome Nov. 2008 Pham_1.0 * |
Cytokine |
| IL-6 Homo sapiens Pan troglodytes Papio hamadryas |
5′- CTGGCAGAAAACAACCTGAA - 3′ .......................................... .......................................... |
5′- GCAGGAACTGGATCAGGACT – 3′ .......................................... .......................................... |
NM_000600.3 XM_001154396.1/XM_001154511.1/XM_518992.2 Papio hamadryas genome Nov. 2008 Pham_1.0 * |
Cytokine |
| IL-10 Homo sapiens Pan troglodytes Papio hamadryas |
5′- CCAAGCCTTGTCTGAGATGA - 3′ .......................................... .......................................... |
5′- GCCTTGCTCTTGTTTTCACA- 3′ .......................................... .......................................... |
NM_000572.2 XM_525040.2 AY796417.1 |
Cytokine |
| IL-12A Homo sapiens Pan trogolodytes Papio anubis |
5′- GAGTTCAAGACCATGAATGC -3′ .......................................... .......................................... |
5′- TGGCACAGTCTCACTGTTGA -3′ .......................................... .......................................... |
NM_000882.2 XM_001156599.1/ XM_516846.2 NM_001112637.1 |
Cytokine |
| CCL2 Homo sapiens Pan troglodytes Papio hamadryas |
5′- GCTCATAGCAGCCACCTTCA -3′ .......................................... .......................................... |
5′ - GGAATCCTGAACCCACTTCT -3′ .......................................... .......................................... |
NM_002982.3 XM_001174545.1/XM_001174551.1 Papio hamadryas genome Nov. 2008 Pham_1.0 * |
Chemokine |
| CCL3 Homo sapiens Pan troglodytes Papio hamadryas |
5′-TTGCTGTCCTCCTCTGCACC -3′ .......................................... .......................................... |
5′-GCTATGAAATTCTGTGGAAT -3′ .......................................... .......................................... |
NM_002983.2 NM_001034082.1 Papio hamadryas genome Nov. 2008 Pham_1.0 * |
Chemokine |
| IL-8/CXCL8 Homo sapiens Pan troglodytes Papio hamadryas |
5′- TGATAAATTTGGGGTGGAAA -3′ .......................................... .......................................... |
5′- GTTTTGCCAAGGAGTGCTAA -3′ .......................................... .......................................... |
NM_000584.2 XM_001156375.1/ XM_001156432.1/ XM_526587.2 Papio hamadryas genome Nov. 2008 Pham_1.0 * |
Chemokine |
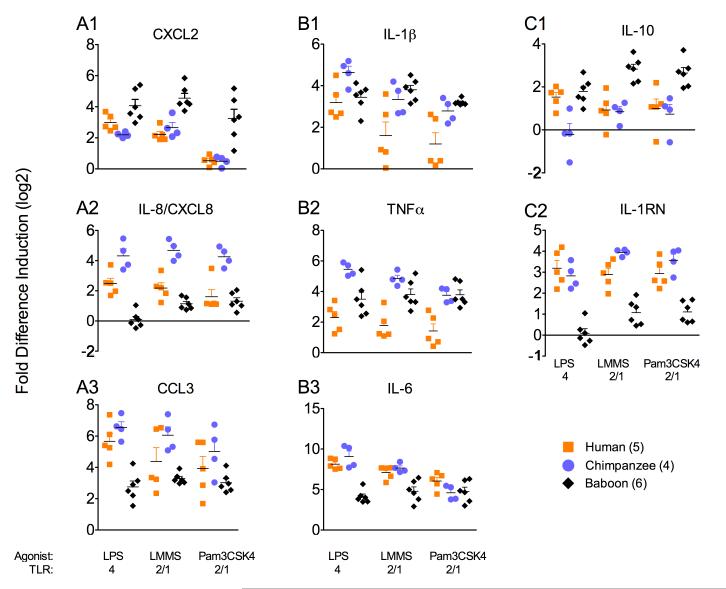
Of 14 genes tested by this method eight showed at least two-fold induction and notable differences between species, even though there were no obvious differences in the proportions of neutrophils, lymphocytes, monocytes and basophils/eosinophils between the species (Wright-Giemsa method, data not shown). The most striking differences were among the three chemokines CXCL2, IL-8/CXCL8 and CCL3. Whereas baboons expressed high levels of CXCL2 and low levels of IL-8/CXCL8 and CCL3, this pattern was reversed for humans and chimpanzees where most humans and all chimpanzees expressed significantly higher levels of IL-8/CXCL8 and CCL3 than CXCL2 (A panels). This pattern of chemokine induction was observed for all three PAMPs and was seen even at low doses of PAMP (0.01 ug/ml, data not shown). Though a subset of chemokines, species-specific induction of these cellular chemoattractants suggests that baboon and hominoid cellular responses to bacterial infection may differ, as CXCL2 and IL-8 attract mainly neutrophils and CCL3 is a chemoattractant for a broader range of cell subtypes.
A comparison of the pro-inflammatory cytokine responses, IL-1β, TNFα and IL-6, revealed that humans expressed appreciably lower levels of IL-1β and TNFα than did chimpanzees and baboons with the exception of the TNFα response to LPS which was similar for all three species (B panels). The IL-6 response, which was strikingly high for all three species, tended to be lower for baboons than for humans and chimpanzees, especially in response to LPS. These relationships were observed even at low doses of PAMPs (0.01 ug/ml, data not shown).
An analysis of the anti-inflammatory cytokine responses, IL-10 and IL-1RN, also revealed striking differences between the three species. Baboons expressed very liitle IL-1RN compared to humans and chimpanzees, irrespective of the PAMPs employed (C panels). In contrast, the IL-10 response was similar for all three species with the baboon response being somewhat greater than the responses of humans and chimpanzees. Again, these relationships were conserved even at low doses of PAMPs (data not shown).
In summary, this study suggests that the early innate immune responses of humans, chimpanzees, and baboons to TLR2 and TLR4-detected PAMPs differ, with the patterns, in general, being species-specific rather than agonist-specific. These species differences in cytokine/chemokine induction may affect immune cell activation and trafficking. Interestingly, the disparate baboon and hominoid cytokine/chemokine responses noted here agree with observations that cercopithecoid (old world monkey) and hominoid diverge in susceptibility to bacterial infections. It is interesting, for example, that baboon IL-8/CXCL8 and IL-6 induction tends to be minimal, as high levels of these proteins during early severe sepsis have been correlated with negative clinical outcomes [11, 14].
These studies are an early step in improving our understanding of African catarrhine innate immune responses to bacteria. As these non-human primates are important biomedical models for human medicine, it is very important to highlight inter-species differences in early innate immune function. The results of this study may help to explain inter-species differences in susceptibility to major human bacterial-mediated diseases.
FIGURE 1.
African catarrrhine early cytokine and chemokine responses to stimulation with TLR2 and TLR4 PAMPs. Human (orange squares), chimpanzee (blue circles) and baboon (black diamonds) blood was stimulated with LPS from E. coli 0111:B4, LMMS and Pam3CSK4 for 90 minutes and chemokine/cytokine induction was quantified by real-time PCR. 10 ug dose shown as Log base 2 here. Colored bars represent standard error of the mean (SEM), while black bars represent the mean. Dots in the scatter represent different individuals. Pairwise comparisons of significant different in gene induction were completed by unpaired t-tests [<0.05, unless otherwise noted. P value pairwise by PAMP was non significant for CXCL2 – LPS: H-B, LMMS: H-C, Pam3CSK4: H-C; for IL-8/CXCL8 - Pam3CSK4: H-B; for CCL3 – LPS: H-C, LMMS: H-C, H-B, Pam3CSK4: H-C, C-B, H-B; for IL-1β - LPS: H-B, Pam3CSK4: C-B; for IL-6 – LPS: H-C, LMMS: H-C, Pam3CSK4: H-C, C-B, H-B; TNFα -LPS: H-B, LMMS: H-C, C-B, Pam3CSK4: C-B; for IL-1RN – LPS: H-C, Pam3CSK4: H-C; for IL-10 – LPS: C-B, LMMS: H-C, Pam3CSK4: H-C; where human (H), chimpanzee (C ) and baboon (B)]
ACKNOWLEDGMENTS
This research was supported by National Science Foundation grant DDIG 0752297 (to J.F.B.); Wenner Gren Dissertation Fieldwork grant 7845 (to J.F.B); the National Institutes of Health /NAID grant R01AI023859-24 (to S.M.G.); core facilities support was provided by a grant to the RCMI program to The City College of New York from NIH/NCRR, G12RR03060. Chimpanzee blood samples were provided by Yerkes National Primate Research Center, supported by NIH Grant RR000165; baboon blood samples were provided by the Texas Biomedical Research Institute (previously known as Southwest National Primate Research Center), supported by NIH-NCRR grant P51 RR013986. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
REFERENCES
- 1.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barreiro LB, Marioni JC, Blekhman R, Stephens M, Gilad Y. Functional comparison of innate immune signaling pathways in primates. PLoS Genet. 2011;6:e1001249. doi: 10.1371/journal.pgen.1001249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beutler B. TLR4 as the mammalian endotoxin sensor. Curr Top Microbiol Immunol. 2002;270:109–120. doi: 10.1007/978-3-642-59430-4_7. [DOI] [PubMed] [Google Scholar]
- 4.CSAC Initial sequence of the chimpanzee genome and comparison with the human genome. Nature. 2005;437:69–87. doi: 10.1038/nature04072. [DOI] [PubMed] [Google Scholar]
- 5.Gibbs RA, Rogers J, Katze MG, Bumgarner R, Weinstock GM, Mardis ER, Remington KA, Strausberg RL, Venter JC, Wilson RK, Batzer MA, Bustamante CD, Eichler EE, Hahn MW, Hardison RC, Makova KD, Miller W, Milosavljevic A, Palermo RE, Siepel A, Sikela JM, Attaway T, Bell S, Bernard KE, Buhay CJ, Chandrabose MN, Dao M, Davis C, Delehaunty KD, Ding Y, Dinh HH, Dugan-Rocha S, Fulton LA, Gabisi RA, Garner TT, Godfrey J, Hawes AC, Hernandez J, Hines S, Holder M, Hume J, Jhangiani SN, Joshi V, Khan ZM, Kirkness EF, Cree A, Fowler RG, Lee S, Lewis LR, Li Z, Liu Y-s, Moore SM, Muzny D, Nazareth LV, Ngo DN, Okwuonu GO, Pai G, Parker D, Paul HA, Pfannkoch C, Pohl CS, Rogers Y-H, Ruiz SJ, Sabo A, Santibanez J, Schneider BW, Smith SM, Sodergren E, Svatek AF, Utterback TR, Vattathil S, Warren W, White CS, Chinwalla AT, Feng Y, Halpern AL, Hillier LW, Huang X, Minx P, Nelson JO, Pepin KH, Qin X, Sutton GG, Venter E, Walenz BP, Wallis JW, Worley KC, Yang S-P, Jones SM, Marra MA, Rocchi M, Schein JE, Baertsch R, Clarke L, Cs√°r√∂s Ms, Glasscock J, Harris RA, Havlak P, Jackson AR, Jiang H, Liu Y, Messina DN, Shen Y, Song HX-Z, Wylie T, Zhang L, Birney E, Han K, Konkel MK, Lee J, Smit AFA, Ullmer B, Wang H, Xing J, Burhans R, Cheng Z, Karro JE, Ma J, Raney B, She X, Cox MJ, Demuth JP, Dumas LJ, Han S-G, Hopkins J, Karimpour-Fard A, Kim YH, Pollack JR, Vinar T, Addo-Quaye C, Degenhardt J, Denby A, Hubisz MJ, Indap A, Kosiol C, Lahn BT, Lawson HA, Marklein A, Nielsen R, Vallender EJ, Clark AG, Ferguson B, Hernandez RD, Hirani K, Kehrer-Sawatzki H, Kolb J, Patil S, Pu L-L, Ren Y, Smith DG, Wheeler DA, Schenck I, Ball EV, Chen R, Cooper DN, Giardine B, Hsu F, Kent WJ, Lesk A, Nelson DL, O’Brien WE, Pr√°fer K, Stenson PD, Wallace JC, Ke H, Liu X-M, Wang P, Xiang AP, Yang F, Barber GP, Haussler D, Karolchik D, Kern AD, Kuhn RM, Smith KE, Zwieg AS, Sequencing RMG, Consortium A Evolutionary and Biomedical Insights from the Rhesus Macaque Genome. Science. 2007;316:222–234. doi: 10.1126/science.1139247. [DOI] [PubMed] [Google Scholar]
- 6.Gomes I, Sharma TT, Mahmud N, Kapp JD, Edassery S, Fulton N, Liang J, Hoffman R, Westbrook CA. Highly abundant genes in the transcriptosome of human and baboon CD34 antigen-positive bone marrow cells. Blood. 2001;98:93–99. doi: 10.1182/blood.v98.1.93. [DOI] [PubMed] [Google Scholar]
- 7.Haudek SB, Natmessnig BE, Furst W, Bahrami S, Schlag G, Redl H. Lipopolysaccharide dose response in baboons. Shock. 2003;20:431–436. doi: 10.1097/01.shk.0000090843.66556.74. [DOI] [PubMed] [Google Scholar]
- 8.Jenner RG, Young RA. Insights into host responses against pathogens from transcriptional profiling. Nat Rev Microbiol. 2005;3:281–294. doi: 10.1038/nrmicro1126. [DOI] [PubMed] [Google Scholar]
- 9.Langermans JA, Andersen P, van Soolingen D, Vervenne RA, Frost PA, van der Laan T, van Pinxteren LA, van den Hombergh J, Kroon S, Peekel I, Florquin S, Thomas AW. Divergent effect of bacillus Calmette-Guerin (BCG) vaccination on Mycobacterium tuberculosis infection in highly related macaque species: implications for primate models in tuberculosis vaccine research. Proc Natl Acad Sci U S A. 2001;98:11497–11502. doi: 10.1073/pnas.201404898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin MJ, Rayner JC, Gagneux P, Barnwell JW, Varki A. Evolution of human-chimpanzee differences in malaria susceptibility: relationship to human genetic loss of N-glycolylneuraminic acid. Proc Natl Acad Sci U S A. 2005;102:12819–12824. doi: 10.1073/pnas.0503819102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marty C, Misset B, Tamion F, Fitting C, Carlet J, Cavaillon JM. Circulating interleukin-8 concentrations in patients with multiple organ failure of septic and nonseptic origin. Crit Care Med. 1994;22:673–679. doi: 10.1097/00003246-199404000-00025. [DOI] [PubMed] [Google Scholar]
- 12.McGee ZA, Gregg CR, Johnson AP, Kalter SS, Taylor-Robinson D. The evolutionary watershed of susceptibility to gonococcal infection. Microb Pathog. 1990;9:131–139. doi: 10.1016/0882-4010(90)90087-7. [DOI] [PubMed] [Google Scholar]
- 13.Means TK, Jones BW, Schromm AB, Shurtleff BA, Smith JA, Keane J, Golenbock DT, Vogel SN, Fenton MJ. Differential effects of a Toll-like receptor antagonist on Mycobacterium tuberculosis-induced macrophage responses. J Immunol. 2001;166:4074–4082. doi: 10.4049/jimmunol.166.6.4074. [DOI] [PubMed] [Google Scholar]
- 14.Moscovitz H, Shofer F, Mignott H, Behrman A, Kilpatrick L. Plasma cytokine determinations in emergency department patients as a predictor of bacteremia and infectious disease severity. Crit Care Med. 1994;22:1102–1107. doi: 10.1097/00003246-199407000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Nau GJ, Richmond JF, Schlesinger A, Jennings EG, Lander ES, Young RA. Human macrophage activation programs induced by bacterial pathogens. Proc Natl Acad Sci U S A. 2002;99:1503–1508. doi: 10.1073/pnas.022649799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Payne KS, Novak JJ, Jongsakul K, Imerbsin R, Apisitsaowapa Y, Pavlin JA, Hinds SB. Mycobacterium tuberculosis infection in a closed colony of rhesus macaques (Macaca mulatta) J Am Assoc Lab Anim Sci. 2011;50:105–108. [PMC free article] [PubMed] [Google Scholar]
- 17.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Redl H, Bahrami S, Schlag G, Traber DL. Clinical detection of LPS and animal models of endotoxemia. Immunobiology. 1993;187:330–345. doi: 10.1016/S0171-2985(11)80348-7. [DOI] [PubMed] [Google Scholar]
- 19.Soto PC, Stein LL, Hurtado-Ziola N, Hedrick SM, Varki A. Relative over-reactivity of human versus chimpanzee lymphocytes: implications for the human diseases associated with immune activation. J Immunol. 2010;184:4185–4195. doi: 10.4049/jimmunol.0903420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spindel ER, Pauley MA, Jia Y, Gravett C, Thompson SL, Boyle NF, Ojeda SR, Norgren RB., Jr. Leveraging human genomic information to identify nonhuman primate sequences for expression array development. BMC Genomics. 2005;6:160. doi: 10.1186/1471-2164-6-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takeuchi O, Kaufmann A, Grote K, Kawai T, Hoshino K, Morr M, Muhlradt PF, Akira S. Cutting edge: preferentially the R-stereoisomer of the mycoplasmal lipopeptide macrophage-activating lipopeptide-2 activates immune cells through a toll-like receptor 2- and MyD88-dependent signaling pathway. J Immunol. 2000;164:554–557. doi: 10.4049/jimmunol.164.2.554. [DOI] [PubMed] [Google Scholar]
- 22.van Deventer SJ, Buller HR, ten Cate JW, Aarden LA, Hack CE, Sturk A. Experimental endotoxemia in humans: analysis of cytokine release and coagulation, fibrinolytic, and complement pathways. Blood. 1990;76:2520–2526. [PubMed] [Google Scholar]
- 23.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Bulow GU, Puren AJ, Savage N. Interleukin-1 from baboon peripheral blood monocytes: altered response to endotoxin (lipopolysaccharide) and Staphylococcus aureus stimulation compared with human monocytes. Eur J Cell Biol. 1992;59:458–463. [PubMed] [Google Scholar]
- 25.Walker CM. Comparative features of hepatitis C virus infection in humans and chimpanzees. Springer Semin Immunopathol. 1997;19:85–98. doi: 10.1007/BF00945027. [DOI] [PubMed] [Google Scholar]
- 26.Walsh DS, Dela Cruz EC, Abalos RM, Tan EV, Walsh GP, Portaels F, Meyers WM. Clinical and histologic features of skin lesions in a cynomolgus monkey experimentally infected with mycobacterium ulcerans (Buruli ulcer) by intradermal inoculation. Am J Trop Med Hyg. 2007;76:132–134. [PubMed] [Google Scholar]