Abstract
Background
Studies from the balloon angioplasty and bare metal stent eras have demonstrated that CABG is cost-effective compared with PCI for patients undergoing multivessel coronary revascularization—particularly among patients with complex CAD or diabetes. Whether these results apply in the drug-eluting stent (DES) era is unknown.
Methods and Results
Between 2005 and 2010, 1900 patients with diabetes and multivessel CAD were randomized to PCI with DES (DES-PCI; n=953) or CABG (n=947). Costs were assessed from the perspective of the U.S. health care system. Health state utilities were assessed using the EuroQOL. A patient-level microsimulation model based on U.S. life-tables and in-trial results was used to estimate lifetime cost-effectiveness. Although initial procedural costs were lower for CABG, total costs for the index hospitalization were $8,622/patient higher. Over the next 5 years, follow-up costs were higher with PCI, owing to more frequent repeat revascularization and higher outpatient medication costs. Nonetheless, cumulative 5-year costs remained $3,641/patient higher with CABG. Although there were only modest gains in survival with CABG during the trial period, when the in-trial results were extended to a lifetime horizon, CABG was projected to be economically attractive relative to DES-PCI, with substantial gains in both life expectancy and quality-adjusted life expectancy and incremental cost-effectiveness ratios <$10,000 per life-year or quality-adjusted life-year gained across a broad range of assumptions regarding the effect of CABG on post-trial survival and costs.
Conclusions
Despite higher initial costs, CABG is a highly cost-effective revascularization strategy compared with DES-PCI for patients with diabetes and multivessel CAD.
Keywords: CABG, cost effectiveness, diabetes mellitus, drug eluting stent, percutaneous coronary intervention
The optimal approach to revascularization in patients with diabetes and multivessel coronary artery disease (CAD) has been widely debated since the Balloon Angioplasty Revascularization Investigation (BARI) trial first reported improved survival for diabetic patients treated with bypass surgery (CABG) as compared with balloon angioplasty.1 In addition to their relative clinical benefits, the long-term economic impact of alternative revascularization strategies is an important consideration for both clinical guidelines and health policy. A cost-effectiveness analysis based on data from the BARI trial demonstrated that for the average diabetic patient, CABG was a cost-effective strategy compared with conventional balloon angioplasty.2,3 Since BARI, however, improvements in both surgical and percutaneous revascularization techniques, as well as medical therapy, have had an impact on the comparative clinical outcomes of the two revascularization strategies in both the short- and long-term. Nonetheless, most recent comparisons of revascularization outcomes in patients with diabetes in the bare metal and drug-eluting stent (DES) era have shown a trend toward more frequent major adverse cardiac and cerebrovascular events (MACCE) with PCI relative to CABG.4–8 While the rate of repeat revascularization procedures has been reduced with stenting and DES, rates have remained higher than for CABG.5,9 Moreover, economic analyses based on the available data (generally based on subset analyses of trials or observational studies) have suggested that CABG remains a cost-effective strategy.3,10–14
The Future REvascularization Evaluation in patients with Diabetes mellitus: Optimal management of Multivessel disease (FREEDOM) trial is the largest prospective randomized trial to compare the outcomes of multivessel coronary artery revascularization with drug-eluting stents vs. CABG among diabetic patients.15 Recently, 5-year results from FREEDOM showed that CABG was associated with significantly lower rates of the primary endpoint of death, MI or stroke, compared with DES-PCI, with the benefit driven by reductions in both death and MI.16 To provide additional insight into the relative value of these alternative revascularization strategies, we performed a prospective health economic evaluation alongside the FREEDOM trial, the results of which are the focus of this report.
METHODS
The design and methods of the FREEDOM trial have been described previously.15 Between April 2005 and April 2010, 1900 patients with type 1 or 2 diabetes and angiographically confirmed multivessel CAD with a clinical indication for revascularization and who were deemed suitable for both DES-PCI and CABG were randomly assigned on a 1:1 basis to either technique. PCI procedures were performed using standard techniques. Given the timeframe of patient recruitment, both sirolimus-eluting stents (SES) and paclitaxel-eluting stents (PES) were the predominant stents used in the trial. The trial protocol recommended that patients randomized to PCI receive only one type of DES. Newer generation DES were allowed during the later phases of the trial, provided there was regulatory approval for use within the respective region. The use of abciximab was recommended for patients undergoing PCI and was provided free of charge; at least 12 months of dual antiplatelet therapy with aspirin and clopidogrel was recommended after any DES procedure. Patients randomized to CABG underwent treatment according to standard techniques. Following revascularization, optimal medical therapy including high dose statin therapy, aggressive blood pressure control, and normalization of hemoglobin-A1c was recommended for both groups.
In-person assessments were performed at 1 month post-procedure, and 6 and 12 months post- randomization, and annually thereafter. In addition, telephone follow-up assessments were performed at 18 months and all subsequent semiannual time points. All sites obtained Institutional Review Board approval of the protocol, and all patients provided informed consent. The trial is registered at the National Institutes of Health website (www.clinical-trials.gov) as identifier NCT00086450.
Estimation of Medical Care Costs
Medical care costs for the index hospitalization and in-trial follow-up period were assessed using a combination of resource-based and event-based methods as described below. All costs were assessed from the perspective of the U.S. healthcare system and are reported in 2010 U.S. dollars.
PCI and CABG Procedure Costs
Detailed resource use was recorded for each revascularization procedure, and the cost for each item was estimated on the basis of the mean hospital acquisition cost for the item at 3 surveyed U.S. hospitals. Each DES was assigned a cost of $1500. Costs of antithrombotic therapy were based on the most current wholesale acquisition cost obtained from Micromedex Red Book.17 Costs of additional disposable equipment, overhead and depreciation for the cardiac catheterization laboratory and the operating room, and non-physician personnel were estimated using data from the micro-cost accounting systems of Saint Luke’s Mid America Heart Institute and adjusted for actual procedure duration. Resource utilization and cost data for the initial PCI procedure and any planned staged PCI procedures were combined in the reporting of results for the index procedure.
Post-Procedure Hospitalization Costs
Post-procedure costs for index hospitalizations were estimated using regression models based on FREEDOM-eligible Medicare patients who underwent either PCI (n=113,921) or CABG (n=43,866) and whose hospitalization data were included in the 2010 Medicare Provider and Review (MEDPAR) database. Total costs for these hospitalizations were estimated by multiplying hospital charges by the hospital and cost-center-specific cost-to-charge ratio.18,19 Linear regression models were then developed, using total Medicare hospitalization costs as the outcome, and socioeconomic factors, comorbidities and inhospital complications (identified on the basis of ICD-9 codes) as predictors (see Supplemental Table 1). Because of substantial variability in length of stay for revascularization procedures across the enrolling countries in FREEDOM, length of stay was not included as a predictor in these models. The final models for PCI and CABG were then used to predict non-procedural costs for each index hospitalization as well as any subsequent hospitalizations that involved a coronary revascularization procedure. To avoid double-counting procedural costs, the intercept for each model was adjusted to remove the costs directly related to the revascularization procedures, themselves, based on national averages for procedure duration and resource use.11
For follow-up hospitalizations that did not involve a revascularization procedure, MS-DRGs were assigned based on the primary indication for hospitalization and procedures performed during the hospitalization. Costs were then assigned based upon mean 2010 Medicare reimbursement rates for the DRG obtained from the Medicare Part A data files.20
Other Costs
Physician fees for PCI procedures and CABG procedures (including those for the primary surgeon, surgical assistant, and anesthesiologist) were based on the 2010 national Medicare fee schedule. Non-procedure related physician fees for revascularization-related hospitalizations were estimated for U.S. patients on the basis of post-procedure ICU and non-ICU length of stay and Medicare payment rates; for non-U.S. patients, post-procedure length of stay following CABG and PCI was estimated from regression models developed using 2010 MedPAR data and the same covariates as used in the cost models (see Supplemental Tables 1 and 2), and PCI and CABG-specific ratios of ICU vs. total post-procedure length of stay estimated from the trial data for U.S. patients. Physician costs for all other hospitalizations were estimated as a percentage of hospital costs according to DRG.21,22 Costs for outpatient visits, tests and procedures, and inpatient rehabilitation and skilled nursing facility days were estimated using national average 2010 Medicare reimbursement rates. Because the length of stay in cardiac rehabilitation varied considerably at the country level, the average number of rehab days according to treatment group and follow-up year for U.S. patients, was used to estimate the cost of rehabilitation stays for patients enrolled at non-U.S. sites. Outpatient medication use was assessed at each follow-up visit, and costs were assigned using the most current average wholesale prices from Micromedex Red Book.17 To account for expected reductions in the cost of generic clopidogrel in the very near future, it was assigned a cost of $30/month.
Quality of Life
The EuroQOL (EQ-5D) health status instrument was used to assess quality of life for each study patient at baseline, 1 month post-procedure, 6 and 12 months after randomization, and annually thereafter. Health state utility weights (range 0–1, higher=better health) were obtained from the EuroQOL data using an algorithm developed from the U.S. population.23
Statistical Analysis
Of the 1900 patients randomized in the FREEDOM trial, 36 patients assigned to CABG, and 9 patients assigned to PCI did not undergo any index procedure. All of these patients either died (n=3) or withdrew from the study (n=42) within 7 days of randomization. To avoid bias due to higher rates of withdrawal prior to CABG, the primary analytic population for the economic study consisted of all randomized patients who underwent at least one initial revascularization procedure, with patients categorized according to their assigned treatment (modified intention-to-treat [mITT] population, n=1855). A secondary analytic population included only those patients who underwent the revascularization procedure assigned (per protocol [PP] population, n=1832) and was used solely to examine initial treatment costs among those patients who actually underwent the specified procedures.
Categorical data are reported as frequencies, and continuous data are reported as mean ± standard deviation. Discrete variables were compared using Fisher’s exact test. Normally distributed continuous variables were compared using Student’s t-test, and non-normally distributed data were compared using the Wilcoxon rank-sum test. Treatment effects from Poisson regression models were used for the comparison of hospitalization rates. Kaplan Meier survival curves and logrank tests were used for the comparison of 5-year clinical events. Cost data are reported as both mean and median values, and confidence intervals for the differences in costs between treatment groups were obtained via bootstrapping.24
Quality-adjusted life expectancy during the trial period was estimated for each patient as the time-weighted average of his or her utility value, using the midpoint between assessments as the transition between health states, starting at the 30-day visit. The baseline utility was applied to the time from randomization to the index procedure, and the 30-day utility value was applied to the period from the procedure through the midpoint between the 30 day and 6-month follow-up. Missing utility values were estimated using multiple imputation, with baseline patient characteristics, previous utility values, and previous in-trial clinical events informing the imputation.
In-Trial Analysis of Costs, Life-Years and QALYs
The prolonged recruitment period for the FREEDOM trial together with the fixed stopping point yielded a wide range of follow-up durations for enrolled patients. To accommodate this wide range of administrative censoring times, methods for the analysis of censored data were used to obtain estimates of cumulative costs and QALYs over time. An inverse probability-weighted estimator was applied, whereby the time axis was divided into 3 month intervals, and costs for each interval were estimated as the observed costs during the interval for patients with complete data divided by the probability of not being censored within the interval.25 Similar methods were applied to estimation of quality-adjusted life expectancy. Life years gained at annual time points were estimated as the difference in the area between the Kaplan Meier survival curves for the two treatment groups. Confidence limits for the mean cumulative cost, life year and QALY estimates for each treatment group, as well as the difference between groups, were calculated using the bootstrap method.24
Cost-Effectiveness
The cost-effectiveness of CABG vs. PCI was assessed over a lifetime horizon using both life-years and QALYs as measures of health benefit; the study protocol specified that the analysis based on QALYs would be considered the primary analysis, consistent with current US guidelines.26 This analysis was based on a combination of (1) observed data up to the time of last follow-up for each patient, from which in-trial costs, life-years, and QALYs were estimated, and (2) projections of post-trial costs, life expectancy, and quality-adjusted life expectancy obtained from a Markov disease-simulation model. In this model, each surviving patient was assumed to face a monthly risk of death, with estimates of this risk based upon age-, sex- and race-matched risks of death obtained from U.S. life tables, calibrated to the observed 5-year mortality for the trial population.
For the PCI group, the comparison of the observed 5-year mortality for the trial population with that of an age, sex, and race-matched U.S. population yielded a mortality multiplier of 1.88. For the CABG group an additional multiplicative factor was applied in order to capture the prognostic benefit of CABG vs. DES-PCI. This multiplier was based on the hazard ratio derived from a landmark analysis of all-cause mortality from the FREEDOM patients, starting at 1 year after randomization. Three sets of analyses were performed based upon different assumptions regarding the duration of the survival benefit of CABG. The base case analysis assumed that the mortality hazard ratio for CABG vs. DES-PCI increased in a linear fashion from year 5 to year 10, and that there was no survival benefit of CABG beyond year 10 (i.e. hazard ratio=1). In sensitivity analyses, the survival benefit of CABG was assumed either (1) to remain constant through 10 years, with no further benefit beyond 10 years; or (2) to be in effect through 5 years only (i.e., no further benefit beyond the observed trial period).
Patient-level costs and utility weights applied to each projected year of life beyond the trial observation period were derived from regression models developed from the in-trial data (see Supplemental Tables 3 and 4). All projected life years, QALYs, and costs were discounted 3% annually based on time from randomization.
Uncertainty in the joint distribution of lifetime costs, life-years, and QALYs for each treatment group was estimated by the bootstrap method. To maintain consistency of the within-trial and post-trial CABG effect within each bootstrap sample, the effect of CABG on mortality, from the 1-year post-baseline landmark analysis, was re-estimated for each bootstrap replicate.
All analyses of data from the trial period, and the analyses of cost-effectiveness based on the combined trial data and long-term projections, were performed using SAS 9.3 (SAS Institute, Cary, NC). The Markov model used for projection of life expectancy beyond the trial was developed using TreeAge Pro 2012 (TreeAge Software, Inc., Williamstown, MA).
RESULTS
Patient Population
A total of 1900 patients with diabetes and multivessel CAD were randomized to either CABG (n=947) or DES-PCI (n=953). Of these, 45 patients did not undergo any form of revascularization (36 CABG, 9 PCI) and were excluded from the primary population for the economic analysis (Figure 1). Baseline characteristics for patients in the economic study population (mITT) and the 45 excluded patients (non-mITT) are summarized in Table 1. Patients who withdrew from the trial and had no index procedure tended to be older and have higher SYNTAX scores than the mITT population; otherwise there were no significant differences. Among the mITT population, there were no significant differences in any observed characteristics between the CABG and PCI groups. Nineteen percent of the mITT patients were enrolled in the U.S., more than 84% had 3-vessel CAD, and the median follow-up duration was 47 months.
Figure 1.
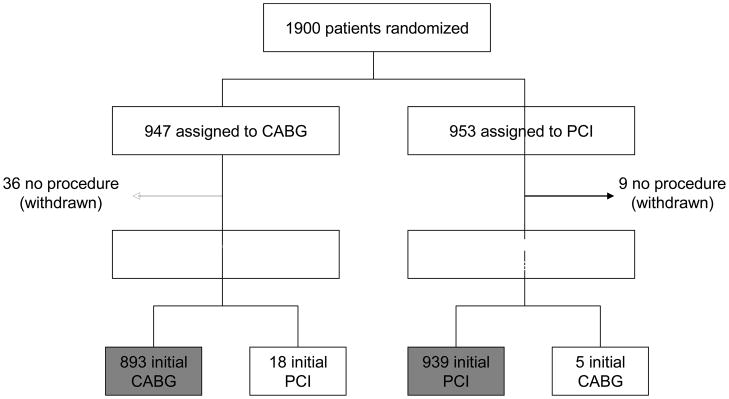
CONSORT Diagram. Black boxes represent the modified intention to treat (mITT) population that was the primary analytic population for the economic study. The grey boxes represent the per protocol (PP) population.
Table 1.
Baseline Characteristics
| Revascularized Patients (mITT) | Non-Revascularized Patients (non-mITT) | mITT vs non-mITT P value* | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| CABG (n=911) | PCI (n=944) | P value | CABG (n=36) | PCI (n=9) | P value | ||
| Sociodemographic Characteristics | |||||||
| Age, years | 62.9 ± 9.2 | 63.1 ± 8.9 | 0.67 | 66.2 ± 9.7 | 69.2 ± 8.5 | 0.399 | 0.006 |
| Male, % | 69.9 | 73.2 | 0.12 | 58.3 | 77.8 | 0.447 | 0.183 |
| Body mass index (kg/m2) | 29.8 ± 5.3 | 29.6 ± 5.4 | 0.52 | 29.8 ± 5.1 | 31.2 ± 4.3 | 0.491 | 0.686 |
| Enrolling country, % | |||||||
| United States | 19.1 | 18.7 | 0.86 | 16.7 | 33.3 | 0.354 | 0.848 |
| Rest of world | 80.9 | 81.2 | 83.3 | 66.7 | |||
| Clinical Characteristics | |||||||
| Current smoker, % | 16.7 | 14.8 | 0.28 | 13.9 | 11.1 | 1.000 | 0.836 |
| Previous myocardial infarction, % | 25.1 | 26.3 | 0.58 | 22.2 | 22.2 | 1.000 | 0.730 |
| Peripheral Vascular Disease, % | 10.5 | 10.1 | 0.76 | 8.3 | 22.2 | 0.258 | 0.804 |
| COPD, % | 5.4 | 3.2 | 0.02 | 5.6 | 22.2 | 0.173 | 0.130 |
| Prior stroke, % | 2.7 | 3.8 | 0.16 | 8.3 | 0.0 | 1.000 | 0.197 |
| History of CHF, % | 28.3 | 25.5 | 0.18 | 19.4 | 33.3 | 0.393 | 0.610 |
| Angiographic Characteristics | |||||||
| LAD involvement, % | 93.0 | 90.7 | 0.07 | 97.1 | 100.0 | 1.000 | 0.252 |
| Three-vessel disease, % | 84.3 | 82.1 | 0.21 | 88.2 | 100.0 | 0.564 | 0.297 |
| SYNTAX score | 26.0 ± 8.8 | 26.2 ± 8.4 | 0.71 | 28.2 ± 8.6 | 31.0 ± 9.8 | 0.411 | 0.040 |
| SYNTAX score tertile | |||||||
| ≤22 | 36.6 | 34.7 | 0.375 | 26.5 | 33.3 | 0.181 | 0.058 |
| 23–32 | 43.3 | 46.5 | 44.1 | 11.1 | |||
| ≥33 | 20.1 | 18.8 | 29.4 | 55.6 | |||
| Initial treatment received, % | |||||||
| PCI | 2.0 | 99.5 | <0.001 | 0 | 0 | -- | -- |
| CABG | 98.0 | 0.5 | 0 | 0 | |||
| None | -- | -- | 100 | 100 | |||
COPD = chronic obstructive pulmonary disease; TIA = transient ischemic attack; CHF = congestive heart failure
comparison of combined treatment groups for mITT vs. non-mITT
Initial Treatment Costs
Among patients in the mITT cohort assigned to PCI, 99.5% underwent initial PCI and 0.5% underwent CABG. Among patients assigned to initial CABG, 98% underwent CABG and 2% underwent PCI. Resource utilization and costs for the initial revascularization procedures (including any staged procedures) are summarized in Table 2. Thirty-three percent of index PCI procedures were staged, with 31% involving 2 procedures, and 2% involving 3 or 4. On average, the initial PCI procedures required 2.3 guiding catheters, 3.1 guidewires, 3.4 angioplasty balloons, and 4.1 drug-eluting stents (range 0–13). While PCI procedure duration was considerably shorter than that for CABG, index procedure costs were significantly lower for CABG owing to the higher costs associated with stents and other consumable devices in the PCI group ($9,739 vs. $13,014 for the per protocol population, p<0.001). For the mITT population, the difference was slightly smaller ($9,776 vs. $12,998, p<0.001), since a small proportion of patients crossed over to the alternate treatment prior to revascularization.
Table 2.
Index Procedural Resource Utilization and Cost (Per Protocol Population)
| CABG (N=893) | PCI (N=939) | P value | |
|---|---|---|---|
| Number of PCI Procedures, % | |||
| 1 | --- | 66.6 (625/939) | |
| 2 | --- | 30.9 (290/939) | |
| 3 | --- | 2.3 (22/939) | |
| 4 | --- | 0.2 (2/939) | |
| Procedure duration, minutes | 248 ± 78 | 107 ± 67 | <0.001 |
| Drug-eluting stents | --- | 4.1 ± 1.9 | |
| Paclitaxel-eluting | --- | 1.9 ± 2.4 | |
| Sirolimus-eluting | --- | 2.1 ± 2.4 | |
| Other drug-eluting stents | --- | 0.1 ± 0.6 | |
| Bare metal stents | --- | 0.0 ± 0.3 | |
| Guiding catheters | --- | 2.3 ± 1.3 | |
| Guidewires | --- | 3.1 ± 2.3 | |
| Angioplasty balloons | --- | 3.4 ± 2.6 | |
| IABP | --- | 0.0 ± 0.2 | |
| Intravascular ultrasound catheters | --- | 0.1 ± 0.4 | |
| Atherectomy devices | --- | 0.1 ± 0.4 | |
| Contrast volume, ml | --- | 378 ± 186 | |
| Antithrombotic agents used, % | |||
| Unfractionated Heparin | --- | 87.2% (819/939) | |
| Bivalirudin | --- | 16.0% (150/939) | |
| Low molecular weight heparin | --- | 1.1% (10/939) | |
| Abciximab | --- | 53.9% (506/939) | |
| Other GP IIb/IIIa | --- | 28.6% (269/939) | |
| Index Procedure Costs, $ | 9,739 ± 2,453 [9,477] | 13,014 ± 5,173 [11,967] | <0. 001 |
Values in brackets represent medians
Clinical events and resource use during the index hospitalization for the mITT population are summarized in Table 3. Costs associated with the post-procedure hospital stay were significantly greater in the CABG group ($19,521 vs. $9,880, p<0.001) as were physician costs ($5,170 vs. $2,967, p<0.001). As a result, total index hospitalization costs were significantly higher for CABG than for DES-PCI ($34,467 vs. $25,845, p<0.001).
Table 3.
Index Hospitalization Events, Resource Utilization, and Costs (mITT population)
| CABG (N=911) | PCI (N=944) | Difference* (95% CI) | P value | |
|---|---|---|---|---|
| Death, % | 1.6% (15) | 0.7% (7) | 0.9% (−0.1%, 1.9%) | 0.072 |
| MI, % | 1.3% (12) | 1.6% (15) | −0.3% (−1.4%, 0.8%) | 0.625 |
| Stroke, % | 1.6% (15) | 0.2% (2) | 1.4% (0.6%, 2.3%) | 0.001 |
| Unplanned CABG, % | 0.2% (2) | 1.3% (12) | −1.1% (−1.8%, −0.3%) | 0.009 |
| Complications, % | ||||
| Major bleeding | 3.4% (31) | 2.8% (26) | 0.6% (−0.9%, 2.2%) | 0.124 |
| Transfusion | 32.8% (299) | 3.8% (36) | 29.0% (25.7%, 32.3%) | 0.009 |
| Atrial arrhythmia | 18.0% (164) | 1.7% (16) | 16.3% (13.7%, 18.9%) | 0.009 |
| Ventricular arrhythmia | 2.2% (20) | 0.5% (5) | 1.7% (0.6%, 2.7%) | 0.42 |
| Post-op infection | 5.5% (50) | 0.2% (2) | 5.3% (3.8%, 6.8%) | < 0.001 |
| Renal Failure | 4.8% (44) | 0.7% (7) | 4.1% (2.6%, 5.6%) | < 0.001 |
| Respiratory Failure | 2.4% (22) | 0.0% (0) | 2.4% (1.4%, 3.4%) | 0.002 |
| Cardiogenic shock | 1.1% (10) | 0.4% (4) | 0.7% (−0.1%, 1.5%) | < 0.001 |
| Major vascular | 4.3% (39) | 3.5% (33) | 0.8% (−1.0%, 2.5%) | 0.25 |
| Reoperation for bleeding | 3.0% (27) | 0.0% (0) | 3.0% (1.9%, 4.1%) | 0.42 |
| Other procedures, % | ||||
| Permanent pacemaker | 1.1% (10) | 0.7% (7) | 0.4% (−0.5%, 1.2%) | 0.38 |
| ICD implantation | 0.4% (4) | 0.1% (1) | 0.3% (−0.1%, 0.8%) | 0.55 |
| Initial hospitalization costs, $ | ||||
| Total procedural costs | 9,776 ± 2,558 [9,447] | 12,998 ± 5,165 [11,957] | −3,222 (−3,584, −2,859) | <0.001 |
| Hospital stay + ancillary services | 19,521 ± 7,912 [17,058] | 9,880 ± 5,718 [6,227] | 9,641 (9,019, 10,268) | <0.001 |
| Physician fees | 5,170 ± 768 [5,060] | 2,967 ± 1,060 [2,571] | 2,203 (2,114, 2,292) | <0.001 |
| Total | 34,467 ± 8,934 [31,910] | 25,845 ± 9,861 [22,479] | 8,622 (7,710, 9,483) | <0.001 |
Difference between CABG and PCI groups
Values in brackets represent medians
Follow-up Resource Utilization and Costs
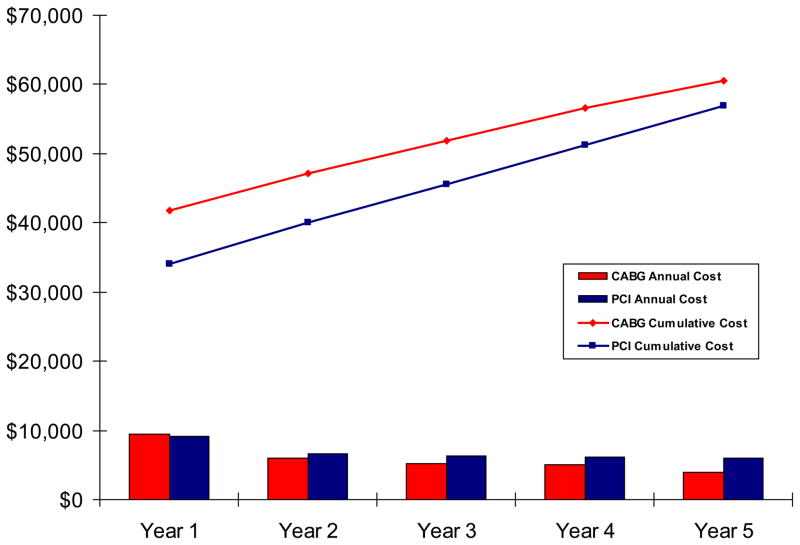
Follow-up clinical outcomes, resource utilization and costs are summarized in Table 4. During the first year of follow-up, rates of repeat revascularization by either PCI or CABG were higher among patients assigned to initial PCI. Although costs for cardiovascular hospitalizations were thus greater for the PCI group, these higher costs were offset by higher costs for non-cardiovascular hospitalizations, outpatient visits, and rehabilitation services for the CABG group such that total 1-year follow-up costs were similar for the 2 groups. During each subsequent year of follow-up, patients assigned to PCI continued to experience higher rates of cardiovascular-related hospitalizations, driven mainly by differences in rates of MI and revascularization procedures. Costs for outpatient medications after PCI remained higher than those following CABG for the first 3 years (related mainly to higher rates of use of dual antiplatelet therapy) and then equalized. Annual medical care costs were thus substantially lower for the CABG group for each subsequent year with annual differences ranging from $793 in year 2 to $1,594 in year 5. As a result, the difference in cumulative medical care costs between the CABG and PCI groups narrowed progressively from $8,622/patient after the completion of the index revascularization procedures to $3,641/patient by the end of the 5-year follow-up period (Table 5 and Figure 2).
Table 4.
Follow-up Events, Resource Utilization, and Costs (mITT population)
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | 5-Year Cumulative* | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| CABG N=911 | PCI N=944 | CABG N=897 | PCI N=845 | CABG N=824 | PCI N=784 | CABG N=628 | PCI N=659 | CABG N=428 | PCI N=419 | CABG N=911 | PCI N=944 | p-value* | |
| Clinical Outcomes | |||||||||||||
| Death, % | 4.1 | 3.4 | 2.1 | 3.0 | 1.3 | 2.4 | 1.8 | 2.3 | 1.0 | 4.0 | 10.7 | 16.0 | 0.06 |
| MI, % | 3.7 | 5.8 | 0.8 | 1.0 | 0.4 | 1.9 | 0.3 | 2.1 | 0.5 | 3.0 | 6.0 | 13.9 | <0.001 |
| Stroke, % | 1.9 | 0.8 | 0.7 | 0.7 | 0.8 | 0.5 | 0.8 | 0.3 | 0.7 | 0.2 | 5.1 | 2.4 | 0.04 |
| Resource Utilization (events/100 pts) | |||||||||||||
| Repeat Revascularization | |||||||||||||
| PCI procedures | 4.6 | 8.4 | 3.8 | 6.0 | 2.3 | 5.3 | 0.8 | 4.6 | 2.4 | 5.1 | 3.3 | 6.8 | <0. 001 |
| CABG procedures | 0.0 | 2.4 | 0.0 | 1.4 | 0.0 | 1.1 | 0.0 | 1.2 | 0.0 | 1.4 | 0.0 | 1.7 | 0.99 |
| Total procedures | 4.6 | 10.8 | 3.8 | 7.5 | 2.3 | 6.4 | 0.8 | 5.8 | 2.4 | 6.5 | 3.3 | 8.5 | <0.001 |
| Diagnostic catheterization | 1.6 | 2.9 | 2.4 | 2.8 | 1.4 | 3.0 | 1.3 | 2.3 | 1.0 | 1.9 | |||
| Re-hospitalization | |||||||||||||
| Cardiovascular | 18.8 | 22.2 | 10.3 | 14.6 | 6.0 | 12.5 | 3.3 | 14.1 | 5.3 | 10.7 | 10.8 | 17.2 | <0.001 |
| Non-cardiovascular | 19.1 | 13.9 | 12.0 | 11.6 | 11.1 | 11.5 | 11.6 | 9.6 | 7.9 | 9.3 | 14.6 | 12.8 | 0.052 |
|
| |||||||||||||
| Costs per patient, $ | |||||||||||||
| Cardiovascular hospitalizations | 2377 | 2991 | 1208 | 1749 | 581 | 1587 | 211 | 1782 | 461 | 1598 | --- | --- | --- |
| Non-cardiovascular hospitalizations | 2380 | 1567 | 1356 | 1224 | 1050 | 1413 | 1437 | 1022 | 697 | 1104 | --- | --- | --- |
| Outpatient services | 1200 | 1041 | 1061 | 831 | 954 | 848 | 856 | 885 | 839 | 754 | --- | --- | --- |
| Rehab/skilled nursing stays | 1606 | 1229 | 339 | 486 | 714 | 240 | 529 | 454 | 264 | 787 | --- | --- | --- |
| Medications | 1928 | 2275 | 1988 | 2323 | 1846 | 2172 | 2018 | 2053 | 1660 | 1719 | --- | --- | --- |
| Total | 9491 | 9102 | 5952 | 6613 | 5145 | 6259 | 5051 | 6196 | 3921 | 5963 | --- | --- | --- |
Kaplan-Meier rates for clinical outcomes with p-values from logrank test; rates per person-year for resource utilization, with p-values obtained from Poisson regression.
Table 5.
Cumulative Costs, QALYs and Life Years for Years 1–5, after adjusting for censoring
| Time Since Randomization (Years) | Cumulative Costs ($)* | Cumulative QALYs* | Cumulative Life Years | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| CABG | PCI | Δ | CABG | PCI | Δ | CABG | PCI | Δ | |
| 1 | 41,855 | 33,976 | 7878 | 0.8163 | 0.8497 | −0.0333 | 0.9713 | 0.9796 | −0.0083 |
| 2 | 47,111 | 40,025 | 7086 | 1.6197 | 1.6542 | −0.0345 | 1.9209 | 1.9307 | −0.0098 |
| 3 | 51,848 | 45,596 | 6251 | 2.3729 | 2.4015 | −0.0286 | 2.8512 | 2.8518 | −0.0006 |
| 4 | 56,551 | 51,316 | 5235 | 3.0773 | 3.0815 | −0.0043 | 3.7676 | 3.7528 | 0.0148 |
| 5 | 60,501 | 56,860 | 3641 | 3.7191 | 3.6878 | 0.0312 | 4.6659 | 4.6130 | 0.0528 |
cumulative costs, QALYs, and life-years adjusted for censoring (see Methods for details)
Δ = difference between CABG and PCI groups
Figure 2.
Mean cumulative medical costs (lines) and mean annual follow-up costs (bars) in 2010 dollars, for the PCI and CABG groups.
Utility Weights and QALYs
Utility weights as assessed by the EQ-5D are summarized in Table 6. Overall, utility weights improved substantially for both treatment groups over the course of the trial. Not surprisingly, utility weights at 1 month follow-up were substantially lower after CABG than PCI (0.81 vs. 0.89, p<0.001), reflecting the more invasive nature and prolonged recovery of the former procedure. These differences were no longer apparent at 6 months, however, and utility weights remained similar for the 2 groups through the remainder of the 5-year follow-up period. As a result of these early differences, quality-adjusted life expectancy was lower with CABG than with PCI after the first year and remained lower through the first 4 years of follow-up (Table 5). However, owing to progressive differences in all-cause mortality, by the end of the 5-year follow-up period, life expectancy (4.665 vs. 4.613 years) and quality-adjusted life expectancy (3.719 vs. 3.688 QALYs) were actually greater with CABG than with PCI, although these differences were not statistically significant.
Table 6.
EQ-5D Utility Scores by Treatment (mITT population)
| Time Point | CABG | PCI |
|---|---|---|
| Baseline | 0.779 ± 0.185 [0.810] | 0.782 ± 0.188 [0.810] |
| 1 month | 0.810 ± 0.180 [0.827] | 0.894 ± 0.143 [1.000] |
| 6 months | 0.810 ± 0.180 [0.827] | 0.895 ± 0.143 [1.000] |
| 1 years | 0.810 ± 0.180 [0.827] | 0.881 ± 0.160 [1.000] |
| 2 years | 0.889 ± 0.164 [1.000] | 0.895 ± 0.143 [1.000] |
| 3 years | 0.895 ± 0.162 [1.000] | 0.891 ± 0.161 [1.000] |
| 4 years | 0.894 ± 0.148 [1.000] | 0.868 ± 0.177 [0.854] |
| 5 years | 0.876 ± 0.175 [1.000] | 0.884 ± 0.132 [0.854] |
Values in brackets represent medians
Cost-Effectiveness
Results from the lifetime cost-effectiveness analyses are summarized in Table 7. Based on the landmark analysis of outcomes from year 1 to 5, the estimated mortality hazard ratio for CABG vs. PCI was 0.60 (95% CI, 0.42 to 0.86); CABG was associated with a reduction in follow-up costs of $1,672/year (95% CI, $942 to $2,403); and there was no significant difference in follow-up utility weights (see Supplementary Appendix, Tables 3 and 4, for details). When these results were used to project clinical and economic outcomes beyond the trial period (Figure 3), we estimated that CABG would be associated with lifetime incremental costs of $5,392 (95% CI, $399 to $10,320) together with an increase in overall quality-adjusted life expectancy of 0.663 QALYs (95% CI, 0.177 to 1.132). The resulting ICER for CABG vs. PCI was $8,132/QALY gained, with 99.2% of bootstrap replicates below a societal willingness to pay threshold of $50,000/QALY (Figures 4 and 5, and Table 7, row 1). When outcomes were assessed in life years, CABG was associated with a gain in life expectancy of 0.794 years and an associated ICER of $6,791/life year gained (Figure 5 and Table 7, row 2).
Table 7.
Lifetime Cost-Effectiveness Results for Base Case and Sensitivity Analyses
| Cost with CABG, $ | Cost with PCI, $ | Δ Cost ($) (95% CI) | QALYs with CABG | QALYs with PCI | ΔQALYs with CABG (95%CI) | ICER ($/QALY) | % Dominant | % Dominated | %< $50K | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tapered CABG Effect Between 5 and 10 Years | Base Case | 114,571 | 109,179 | 5,392 (399, 10,320) | 10.667 | 10.004 | 0.663 (0.177, 1.132) | 8,132 | 1.5 | 0.4 | 99.2 |
| Lifetime analysis, Cost per Life Year Gained* | 114,571 | 109,179 | 5,392 (399, 10,320) | 12.177* | 11.383* | 0.794* | 6,791* | 1.5 | 0.2 | 99.7 | |
| Lifetime analysis, Per Protocol population | 114,855 | 108,878 | 5,976 (1,207, 10,925) | 10.688 | 9.980 | 0.708 (0.202, 1.221) | 8,440 | 0.7 | 0.2 | 99.3 | |
| 10 year time frame | 81,710 | 80,295 | 1,416 (−2,061, 5,017) | 6.684 | 6.455 | 0.230 (−0.008, 0.457) | 6,156 | 22.8 | 1.7 | 95.8 | |
| Lifetime analysis, no CABG effect on long term costs | 121,244 | 109,179 | 12,045 (6,933, 17,103) | 10.667 | 10.004 | 0.663 (0.177, 1.132) | 18,167 | 0.0 | 0.5 | 97.4 | |
| Fixed CABG Effect Between 5 and 10 Years | Lifetime analysis | 116,147 | 109,179 | 6,968 (1,273, 12,327) | 10.867 | 10.004 | 0.864 (0.278, 1.426) | 8,064 | 0.7 | 0.2 | 99.7 |
| 10 year analysis | 81,846 | 80,295 | 1,551 (−1,822, 5,292) | 6.704 | 6.455 | 0.249 (0.016, 0.519) | 6,229 | 18.8 | 1.4 | 96.7 | |
| No Effect of CABG after 5 Years | Lifetime analysis | 118,664 | 109,179 | 9,485 (4,905, 13,995) | 10.355 | 10.004 | 0.351 (−0.033, 0.713) | 27,022 | 0.0 | 3.3 | 82.4 |
| 10 year analysis | 87,155 | 80,295 | 6,861 (3,408, 10,230) | 6.541 | 6.455 | 0.086 (−0.088, 0.254) | 79,779 | 0.0 | 17.8 | 27.0 |
Results presented in row 2 represent life years (columns 6–8) and cost per life year gained (column 9)
All costs, life-years, and QALYs discounted at 3% per year
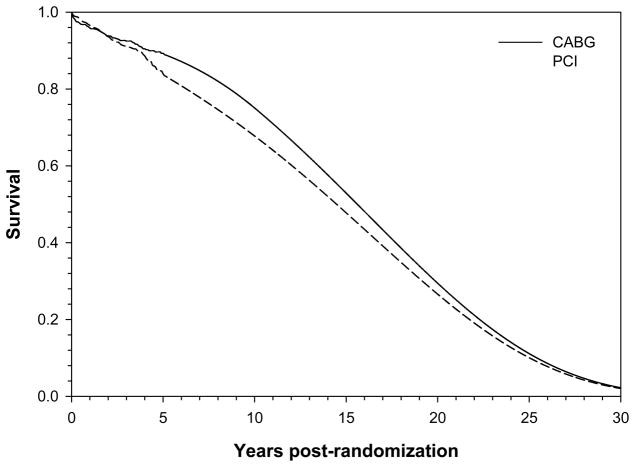
Figure 3.
Observed survival through 5 years and predicted survival beyond 5 years for the CABG (solid) and PCI (dashed) groups.
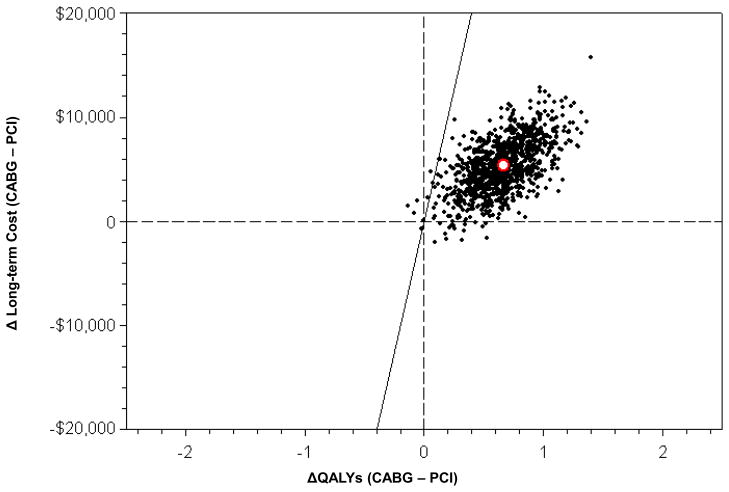
Figure 4.
Joint distribution of projected lifetime incremental costs and quality-adjusted life expectancy for CABG vs. PCI based on bootstrap replication of the FREEDOM trial population-- plotted on the cost-effectiveness plane. The white circle represents the estimated mean values (incremental cost = $5392, incremental QALYs=0.663)
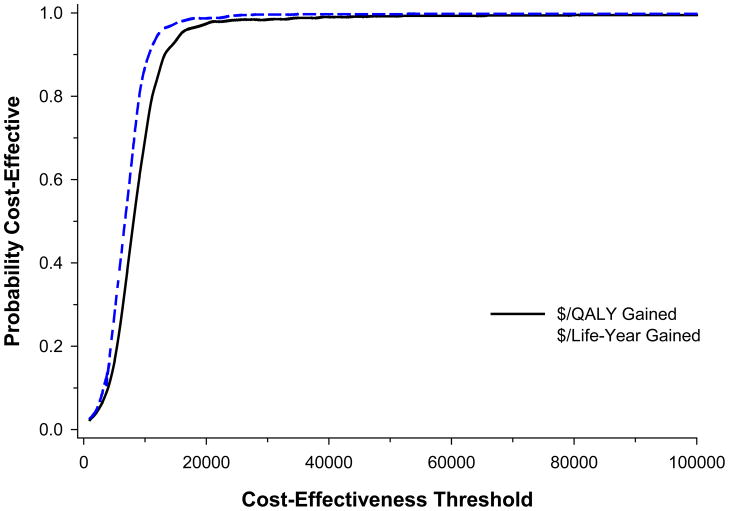
Figure 5.
Cost effectiveness acceptability curve of CABG vs. PCI, in $/QALY gained (black, solid line) and $/Life year gained (blue, dashed line). The probability that CABG is cost-effective is calculated as the proportion of bootstrap-derived estimates falling below a given cost-effectiveness threshold, is plotted across a range of possible cost-effectiveness thresholds.
These results were robust across a wide range of alternative assumptions regarding the duration and magnitude of the benefit of CABG over PCI on both survival and costs beyond the timeframe observed in the trial. When we assumed that the observed benefits of CABG would be sustained through 10 years after initial treatment, the estimated QALY gains with CABG increased to 0.864 QALYs with an associated ICER of $8,064/QALY gained-- similar to the base case results. Even under the highly conservative assumption of no further benefit of CABG on either survival or costs beyond 5 years, the ICER remained relatively favorable at $27,022/QALY gained and was <$50,000/QALY in 82.4% of bootstrap replicates. Similar results were seen when the analysis was based on life years rather than QALYs (see Supplemental Tables 5 and 6)
Subgroup Analyses
Results from prespecified subgroup analyses are presented in Table 8. Despite the greater uncertainty associated with reduced sample sizes, these results were generally consistent with our primary analysis. For all subgroups examined, with the exception of patients without LAD involvement, our analysis demonstrated that CABG was associated with greater quality adjusted life expectancy than PCI with mean differences ranging from 0.28 to 1.16 QALYs. For two subgroups, age 60–69 and HgbA1c <7%, CABG was an economically dominant strategy, with lower lifetime costs and greater quality-adjusted life expectancy than PCI. For patients without LAD involvement (n=150), PCI was associated with greater overall costs and quality-adjusted life expectancy with an ICER of $20,661/QALY gained relative to CABG. However, these results were relatively unstable owing to the small sample size of the subgroup. Estimated ICERs for all other subgroups were <$25,000/QALY gained with CABG, and most were <$10,000/QALY gained.
Table 8.
Lifetime Cost-Effectiveness Results for Subgroups
| Subgroup | Cost with CABG, $ | Cost with PCI, $ | ΔC-P Cost ($) (95% CI) | QALYs with CABG | QALYs with PCI | QALYs Gained with CABG (95% CI) | ICER: Cost ($) per QALY gained | % Dominant | % Dominated | % < $50K |
|---|---|---|---|---|---|---|---|---|---|---|
| Male (n=1,328) | 107,527 | 104,468 | 3,059 (−2,304, 8,406) | 10.856 | 10.078 | 0.778 (0.205, 1.358) | 3,932 | 10.3 | 0.0 | 99.8 |
| Female (n=527) | 131,295 | 122,046 | 9,249 (−729, 18,900) | 10.311 | 9.801 | 0.510 (−0.519, 1.427) | 18,135 | 0.6 | 11.0 | 77.3 |
| Age <60 (n=624) | 136,342 | 125,152 | 11,190 (3,656, 18,572) | 13.897 | 12.737 | 1.160 (0.526, 1.838) | 9,647 | 0.3 | 0.0 | 99.8 |
| Age 60–69 (n=621) | 106,301 | 108,066 | −1,765 (−9,533, 5,504) | 9.947 | 9.671 | 0.276 (−0.486, 0.938) | CABG Dominant | 45.7 | 1.1 | 80.5 |
| Age ≥ 70 (n=610) | 93,926 | 87,034 | 6,892 (−3,018, 15,804) | 6.853 | 6.459 | 0.349 (−0.516, 1.159) | 19,748 | 2.5 | 15.1 | 71.9 |
| SYNTAX Score <23 (n=657) | 113,201 | 104,417 | 8,784 (753, 16,272) | 10.883 | 10.477 | 0.407 (−0.479, 1.180) | 21,582 | 0.3 | 14.7 | 73.5 |
| SYNTAX Score 23–32 (n=828) | 115,602 | 111,441 | 4,160 (−3,670, 11,619) | 10.728 | 9.731 | 0.997 (0.225, 1.699) | 4,172 | 13.7 | 0.1 | 99.2 |
| SYNTAX Score >32 (n=359) | 114,220 | 113,247 | 973 (−10,177, 11,337) | 10.177 | 9.863 | 0.315 (−0.843, 1.373) | 3,088 | 22.9 | 7.3 | 72.4 |
| LAD (n=1,695) | 115,124 | 109,463 | 5,661 (505, 10,981) | 10.713 | 9.991 | 0.722 (0.203, 1.239) | 7,841 | 1.7 | 0.3 | 99.4 |
| No LAD (n=150) | 105,875 | 108,189 | −2,314 (−52,357, 10,225) | 10.126 | 10.239 | −0.112 (−6.933, 1.313) | Not estimable | 18.0 | 8.6 | 43.7 |
| 2 vessel disease (n=310) | 103.264 | 92,313 | 10,950 (−2,486, 21,181) | 11.029 | 10.312 | 0.718 (−1.343, 1.739) | 15,251 | 0.6 | 11.2 | 78.8 |
| 3 vessel disease (n=1,534) | 116,891 | 112,830 | 4,061 (−1,367, 9,483) | 10.634 | 9.937 | 0.697 (0.141, 1.248) | 5,826 | 6.6 | 0.3 | 99.2 |
| HgbA1c < 7 (n=617) | 103,579 | 103,852 | −273 (−6,879, 7,142) | 10.262 | 9.934 | 0.328 (−0.482, 1.132) | CABG Dominant | 34.6 | 2.0 | 80.8 |
| HgbA1c ≥ 7 (n=1,096) | 120,399 | 111,893 | 8,507 (1,618, 15,266) | 11.011 | 10.064 | 0.946 (0.297, 1.547) | 8,993 | 0.5 | 0.4 | 99.5 |
| US (n=351) | 126,113 | 121,412 | 4,701 (−7,997, 17,253) | 10.796 | 9.676 | 1.120 (0.084, 2.033) | 4,197 | 21.8 | 0.3 | 98.1 |
| Non-US (n=1,504) | 111,978 | 106,356 | 5,622 (506, 10,653) | 10.655 | 10.079 | 0.576 (0.006, 1.144) | 9,760 | 1.0 | 2.0 | 96.5 |
All subgroup analyses based on mITT population using base case assumptions for the relative effect of CABG on survival and costs
DISCUSSION
This study is the first direct comparison of economic outcomes of DES-PCI vs. CABG among patients with diabetes and multivessel CAD. Our results reveal that although CABG was associated with an increase in initial costs of ~$9,000/patient, these up-front costs were partially offset by lower costs in subsequent years due principally to a lower rate of repeat revascularization procedures (and, to a lesser extent, less use of cardiac medications). Over the first 5 years of follow-up, CABG improved life expectancy by ~0.05 years and quality-adjusted life expectancy by ~0.03 QALYs while increasing total costs by ~$3,600. When the observed in-trial results were extrapolated over a lifetime horizon, CABG was associated with much larger gains in quality-adjusted life expectancy relative to PCI (0.66 QALYs in the base case) while projected lifetime costs remained ~$5,400/patient higher with CABG. Thus, under our base case assumptions regarding the duration and magnitude of benefit of CABG over DES-PCI, we found that CABG was associated with a lifetime ICER of $8,132/QALY gained. Although there are no universally accepted standards for cost-effectiveness in the U.S. health care system, ICERs <$50,000/QALY gained are commonly considered to be reasonably cost-effective, and ICERs <$20,000/QALY are considered highly cost-effective.27 In our base case analysis, the probability that the ICER for CABG vs. DES-PCI would be <$50,000/QALY was >99%. These findings thus suggest that compared with DES-PCI, CABG is a highly attractive use of scarce societal healthcare resources.
These results were robust to a broad range of sensitivity analyses. Importantly, the ICER for CABG remained <$50,000/QALY gained (and in most cases <$10,000/QALY) in all analyses except those that were restricted to the first 10 years of follow-up. Although an “in-trial” cost-effectiveness analysis would have demonstrated an ICER for CABG of $68,958 per life-year gained and $116,699 per QALY gained, such an analysis would be misleading by failing to account for the significant 5-year survival advantage with CABG over DES-PCI (89.3 vs. 83.9%) and its impact on longer-term life expectancy. Indeed, our analysis demonstrates that the projected benefits of CABG on life expectancy and quality-adjusted life expectancy would be substantial even if there were no further prognostic benefit of CABG beyond the trial period. These findings underscore the importance of a long-term evaluation in understanding the clinical and economic benefits of procedures like CABG, where a higher initial cost must be balanced against clinical benefits that extend well into the future.
These results were also consistent across a wide range of subgroups defined by age, gender, angiographic extent of CAD (including SYNTAX score), and health care system (U.S. vs. other countries). The only possible exception to this finding was the subgroup of patients without significant LAD disease, for whom PCI was estimated to improve long-term survival at an ICER of ~$20,700/QALY gained relative to CABG. These findings were relatively uncertain, however, given the small sample size in this subgroup. Nonetheless, these results are broadly consistent with those of previous studies that have suggested that the prognostic benefit of CABG over PCI is restricted to patients who receive a LIMA graft to the LAD.28
Comparison with Previous Studies
Overall, the cost and cost-effectiveness results from FREEDOM are similar to those reported from the BARI trial, in which CABG was estimated to have a lifetime incremental cost-effectiveness ratio of $14,300/life year gained compared with conventional balloon angioplasty.3 There are important differences in the results of these 2 studies, however. In BARI, most of the cost offsets for CABG accrued during the first 1–2 years of follow-up, due to relatively high rates of repeat revascularization procedures necessary to treat restenosis after conventional balloon angioplasty. In contrast, cost offsets were minimal during the first year in FREEDOM and accrued at a relatively constant rate throughout the 5-year follow-up period thereafter. Although detailed angiographic data were not assessed at the time of repeat revascularization in FREEDOM, it is likely that this event pattern reflects the fact that drug-eluting stents have led to dramatic reductions in restenosis compared with either balloon angioplasty alone or bare metal stents.29,30 Nonetheless, diabetic patients remain at substantial risk of events related to progression of CAD31,32—events that are unrelated to restenosis and are unlikely to be prevented by inherently focal therapies such as stenting.
Another difference between the FREEDOM and BARI results that impacts the cost-effectiveness of therapy is the timing of the mortality benefit. In BARI, improved survival with CABG was evident in the first year of follow-up, with increasing benefits over time. As a result, CABG was already an economically attractive revascularization strategy within the first several years after initial treatment. In contrast, in FREEDOM, mortality was similar after DES-PCI or CABG through the first 3 years of follow-up with divergence of the survival curves only in years 4 and 5. These findings may relate to (1) more complete and durable revascularization with DES-PCI in FREEDOM compared with “plain old” balloon angioplasty in BARI; (2) increased durability of left internal mammary artery grafts vs. saphenous vein grafts,33 the former of which were used more often in FREEDOM; or (3) greater rate of use of preventive measures (including high dose statins, ACE-inhibitors or ARBs, and aggressive dual antiplatelet therapy) in FREEDOM which may have protected PCI patients from the progression of atherosclerosis and the development of atherothrombotic events in the earlier phases of follow-up.34
Limitations
Our study should be considered in light of several important limitations. First, our economic analysis was carried out from a U.S. healthcare system perspective although FREEDOM enrolled patients from 18 countries. To address these issues, costs associated with the index procedures were estimated from detailed resource use data which would not be expected to vary significantly by geographic region. Since hospital length of stay varies considerably between countries, we used costing methodology that was independent of length of stay and depends only on the assumption that clinical outcomes and procedural complications are similar across healthcare systems. In addition, our study was limited by the need to extrapolate results from 5 years of follow-up data to a lifetime horizon in order to capture the full benefits of the alternative revascularization strategies. Our approach to extrapolation required several assumptions regarding the impact of CABG vs. PCI on long-term survival, health care costs, and quality of life that are not verifiable at the present time. Accordingly, we varied our assumptions of the magnitude and durability of the impact of CABG in a range of sensitivity analyses, the results of which demonstrated the robustness of the overall cost-effectiveness results to plausible variation in these key parameters.
Finally, most PCI patients in the FREEDOM trial received first generation DES, which were the only DES available for use at the time. Recently, second generation DES (primarily everolimus-eluting stents) have been shown to reduce rates of MI and stent thrombosis relative to first generation DES.35 As a result, the cost results from our study may not generalize to a setting characterized by exclusive use of second generation DES for PCI. No studies, however, have demonstrated a mortality reduction with second vs. first generation DES,36 and it is the mortality reduction obtained from CABG that primarily drives the favorable cost-effectiveness results in our study.
Conclusions
In FREEDOM, the largest randomized trial to compare CABG vs. DES-PCI for the treatment of diabetic patients with multivessel CAD, we found that CABG provides not only better long-term clinical outcomes than DES-PCI but that these benefits are achieved at an overall cost that represents an attractive use of societal health care resources. These findings suggest that existing guidelines that recommend CABG for diabetic patients with multivessel CAD37,38 remain appropriate in current practice and may provide additional support for strengthening those recommendations.
Supplementary Material
Acknowledgments
We thank Khaja Chinnakondepalli for his programming support for this analysis.
Funding Sources: The FREEDOM Trial was supported by U01 grants #01HL071988 and #01HL092989 from the National Heart, Lung and Blood Institute (NHLBI) with provision of stents from Cordis, Johnson and Johnson and Boston Scientific, provision of abciximab and an unrestricted research grant from Eli Lilly and provision of clopidogrel from Sanofi-Aventis and Bristol Myers Squibb. The views expressed are not necessarily the views of NHLBI, but of the individual authors.
Footnotes
Clinical Trial Registration Information: http://www.clinical-trials.gov; Identifier: NCT00086450
Conflict of Interest Disclosures: Dr. Magnuson has received grant support from Abbott Vascular, Astra Zeneca, Boston Scientific, Daiichi Sankyo, Edwards Lifesciences, Eli Lilly and Medtronic. Dr. Farkouh has received grant support from Eli Lilly and other research support from Boston Scientific, Bristol-Myers Squibb, Cordis, Eli Lilly and Sanofi-Aventis. Dr Cohen has received grant support from Abbott Vascular, Astra Zeneca, Biomet, Boston Scientific, Edwards Lifesciences, Eli Lilly, Jannsen Pharmaceuticals and Medtronic, and consulting fees from Abbott Vascular, Astra Zeneca, Eli Lilly and Medtronic.
References
- 1.The Bypass Angioplasty Revascularization Investigation (BARI) Investigators. Comparison of coronary bypass surgery with angioplasty in patients with multivessel disease. N Engl J Med. 1996;335:217–225. doi: 10.1056/NEJM199607253350401. [DOI] [PubMed] [Google Scholar]
- 2.Hlatky MA, Rogers WJ, Johnstone I, Boothroyd D, Brooks MM, Pitt B, Reeder G, Ryan T, Smith H, Whitlow P, Wiens R, Mark DB. Medical care costs and quality of life after randomization to coronary angioplasty or coronary bypass surgery. Bypass Angioplasty Revascularization Investigation (BARI) Investigators. N Engl J Med. 1997;336:92–99. doi: 10.1056/NEJM199701093360203. [DOI] [PubMed] [Google Scholar]
- 3.King SB, 3rd, Kosinski AS, Guyton RA, Lembo NJ, Weintraub WS. Eight-year mortality in the emory angioplasty versus surgery trial (east) J Am Coll Cardiol. 2000;35:1116–1121. doi: 10.1016/s0735-1097(00)00546-5. [DOI] [PubMed] [Google Scholar]
- 4.Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, Stahle E, Feldman TE, van den Brand M, Bass EJ, Van Dyck N, Leadley K, Dawkins KD, Mohr FW. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–972. doi: 10.1056/NEJMoa0804626. [DOI] [PubMed] [Google Scholar]
- 5.Serruys PW, Ong AT, van Herwerden LA, Sousa JE, Jatene A, Bonnier JJ, Schonberger JP, Buller N, Bonser R, Disco C, Backx B, Hugenholtz PG, Firth BG, Unger F. Five-year outcomes after coronary stenting versus bypass surgery for the treatment of multivessel disease: The final analysis of the arterial revascularization therapies study (ARTS) randomized trial. J Am Coll Cardiol. 2005;46:575–581. doi: 10.1016/j.jacc.2004.12.082. [DOI] [PubMed] [Google Scholar]
- 6.Serruys PW, Unger F, Sousa JE, Jatene A, Bonnier HJ, Schonberger JP, Buller N, Bonser R, van den Brand MJ, van Herwerden LA, Morel MA, van Hout BA. Comparison of coronary-artery bypass surgery and stenting for the treatment of multivessel disease. N Engl J Med. 2001;344:1117–1124. doi: 10.1056/NEJM200104123441502. [DOI] [PubMed] [Google Scholar]
- 7.SoS Investigators. Coronary artery bypass surgery versus percutaneous coronary intervention with stent implantation in patients with multivessel coronary artery disease (the stent or surgery trial): A randomised controlled trial. Lancet. 2002;360:965–970. doi: 10.1016/S0140-6736(02)11078-6. [DOI] [PubMed] [Google Scholar]
- 8.Kapur A, Hall RJ, Malik IS, Qureshi AC, Butts J, de Belder M, Baumbach A, Angelini G, de Belder A, Oldroyd KG, Flather M, Roughton M, Nihoyannopoulos P, Bagger JP, Morgan K, Beatt KJ. Randomized comparison of percutaneous coronary intervention with coronary artery bypass grafting in diabetic patients. 1-year results of the cardia (coronary artery revascularization in diabetes) trial. J Am Coll Cardiol. 2010;55:432–440. doi: 10.1016/j.jacc.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Abizaid A, Costa MA, Centemero M, Abizaid AS, Legrand VM, Limet RV, Schuler G, Mohr FW, Lindeboom W, Sousa AG, Sousa JE, van Hout B, Hugenholtz PG, Unger F, Serruys PW Arterial Revascularization Therapy Study G. Clinical and economic impact of diabetes mellitus on percutaneous and surgical treatment of multivessel coronary disease patients: Insights from the arterial revascularization therapy study (arts) trial. Circulation. 2001;104:533–538. doi: 10.1161/hc3101.093700. [DOI] [PubMed] [Google Scholar]
- 10.Cohen DJ, Lavelle TA, Van Hout B, Li H, Lei Y, Robertus K, Pinto D, Magnuson EA, McGarry TF, Lucas SK, Horwitz PA, Henry CA, Serruys PW, Mohr FW, Kappetein AP. Economic outcomes of percutaneous coronary intervention with drug-eluting stents versus bypass surgery for patients with left main or three-vessel coronary artery disease: One-year results from the syntax trial. Catheter Cardiovasc Interv. 2012;79:198–209. doi: 10.1002/ccd.23147. [DOI] [PubMed] [Google Scholar]
- 11.Hlatky MA, Boothroyd DB, Melsop KA, Brooks MM, Mark DB, Pitt B, Reeder GS, Rogers WJ, Ryan TJ, Whitlow PL, Wiens RD. Medical costs and quality of life 10 to 12 years after randomization to angioplasty or bypass surgery for multivessel coronary artery disease. Circulation. 2004;110:1960–1966. doi: 10.1161/01.CIR.0000143379.26342.5C. [DOI] [PubMed] [Google Scholar]
- 12.Hlatky MA, Rogers WJ, Johnstone I, Boothroyd D, Brooks MM, Pitt B, Reeder G, Ryan T, Smith H, Whitlow P, Wiens R, Mark DB. Medical care costs and quality of life after randomization to coronary angioplasty or coronary bypass surgery. Bypass angioplasty revascularization investigation (BARI) investigators. N Engl J Med. 1997;336:92–99. doi: 10.1056/NEJM199701093360203. [DOI] [PubMed] [Google Scholar]
- 13.Weintraub WS, Mahoney EM, Zhang Z, Chu H, Hutton J, Buxton M, Booth J, Nugara F, Stables RH, Dooley P, Collinson J, Stuteville M, Delahunty N, Wright A, Flather MD, De Cock E. One year comparison of costs of coronary surgery versus percutaneous coronary intervention in the stent or surgery trial. Heart. 2004;90:782–788. doi: 10.1136/hrt.2003.015057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weintraub WS, Mauldin PD, Becker E, Kosinski AS, King SB., 3rd A comparison of the costs of and quality of life after coronary angioplasty or coronary surgery for multivessel coronary artery disease. Results from the Emory Angioplasty versus Surgery Trial (EAST) Circulation. 1995;92:2831–2840. doi: 10.1161/01.cir.92.10.2831. [DOI] [PubMed] [Google Scholar]
- 15.Farkouh ME, Dangas G, Leon MB, Smith C, Nesto R, Buse JB, Cohen DJ, Mahoney E, Sleeper L, King S, 3rd, Domanski M, McKinlay S, Fuster V. Design of the future revascularization evaluation in patients with diabetes mellitus: Optimal management of multivessel disease (freedom) trial. Am Heart J. 2008;155:215–223. doi: 10.1016/j.ahj.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 16.Farkouh M, Domaski M, Sleeper LA, Siami FS, Dangas G, Mack M, Yang M, Cohen DJ, Rosenberg Y, Solomon SD, Desai AS, Gersch BJ, Magnuson EA, Lasky A, Boineau R, Weinberger J, Ramanathan K, Sousa E, Rankin J, Buse J, Hueb W, Smith CR, Muratov V, Bansilal S, King S, Bertrand M, Fuster V for the FREEDOM Trial Investigators. Comparison of multivessel coronary revascularization strategies in diabetic patients. N Engl J Med. 2012 doi: 10.1056/NEJMoa1211585. [DOI] [Google Scholar]
- 17.Micromedex 2.0. Greenwood Village; Colorado: [Accessed 08/22/2012]. [Google Scholar]
- 18.Ashby J. The accuracy of cost measures derived from medicare cost report data. Hosp Cost Manag Account. 1992;3:1–8. [Google Scholar]
- 19.Taira DA, Seto TB, Siegrist R, Cosgrove R, Berezin R, Cohen DJ. Comparison of analytic approaches for the economic evaluation of new technologies alongside multicenter clinical trials. Am Heart J. 2003;145:452–458. doi: 10.1067/mhj.2003.3. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Medicare and Medicaid Services. 100% MEDPAR inpatient hospital national data for fiscal year 2010. [Accessed on July 1, 2012];Short stay inpatient diagnosis groups. Available at http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/MedicareFeeforSvcPartsAB/downloads/DRG10.pdf.
- 21.Mitchell J, Burge R, Lee A, McCall N, Katz L, Dittus R, Heck D, Kinney E, Parchman M, Iezzoni L. Per case prospective payment for episodes of hospital care. Health Economics Research, Inc; Needham, MA: 1995. [Google Scholar]
- 22.Weintraub WS, Mahoney EM, Lamy A, Culler S, Yuan Y, Caro J, Gabriel S, Yusuf S. Long-term cost-effectiveness of clopidogrel given for up to one year in patients with acute coronary syndromes without st-segment elevation. J Am Coll Cardiol. 2005;45:838–845. doi: 10.1016/j.jacc.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 23.Shaw JW, Johnson JA, Coons SJ. Us valuation of the EQ-5D health states: Development and testing of the d1 valuation model. Med Care. 2005;43:203–220. doi: 10.1097/00005650-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Efron B. Better bootstrap confidence intervals. JASA. 1987;82:171–200. [Google Scholar]
- 25.Bang H, Tsiatis AA. Estimating medical costs with censored data. Biometrika. 2000;87:329–343. [Google Scholar]
- 26.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-Effectiveness in Health and Medicine. New York, NY: Oxford University Press; 1996. [Google Scholar]
- 27.Hirth RA, Chernew ME, Miller E, Fendrick AM, Weissert WG. Willingness to pay for a quality-adjusted life year: In search of a standard. Med Decis Making. 2000;20:332–342. doi: 10.1177/0272989X0002000310. [DOI] [PubMed] [Google Scholar]
- 28.Detre KM, Guo P, Holubkov R, Califf RM, Sopko G, Bach R, Brooks MM, Bourassa MG, Shemin RJ, Rosen AD, Krone RJ, Frye RL, Feit F. Coronary revascularization in diabetic patients: A comparison of the randomized and observational components of the Bypass Angioplasty Revascularization Investigation (BARI) Circulation. 1999;99:633–640. doi: 10.1161/01.cir.99.5.633. [DOI] [PubMed] [Google Scholar]
- 29.Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O’Shaughnessy C, Caputo RP, Kereiakes DJ, Williams DO, Teirstein PS, Jaeger JL, Kuntz RE, Investigators S. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349:1315–1323. doi: 10.1056/NEJMoa035071. [DOI] [PubMed] [Google Scholar]
- 30.Stone GW, Ellis SG, Cox DA, Hermiller J, O’Shaughnessy C, Mann JT, Turco M, Caputo R, Bergin P, Greenberg J, Popma JJ, Russell ME, Investigators T-I. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004;350:221–231. doi: 10.1056/NEJMoa032441. [DOI] [PubMed] [Google Scholar]
- 31.Daemen J, Boersma E, Flather M, Booth J, Stables R, Rodriguez A, Rodriguez-Granillo G, Hueb WA, Lemos PA, Serruys PW. Long-term safety and efficacy of percutaneous coronary intervention with stenting and coronary artery bypass surgery for multivessel coronary artery disease: A meta-analysis with 5-year patient-level data from the ARTS, ERACI-II, MASS-II, and SOS trials. Circulation. 2008;118:1146–1154. doi: 10.1161/CIRCULATIONAHA.107.752147. [DOI] [PubMed] [Google Scholar]
- 32.Glaser R, Selzer F, Faxon DP, Laskey WK, Cohen HA, Slater J, Detre KM, Wilensky RL. Clinical progression of incidental, asymptomatic lesions discovered during culprit vessel coronary intervention. Circulation. 2005;111:143–149. doi: 10.1161/01.CIR.0000150335.01285.12. [DOI] [PubMed] [Google Scholar]
- 33.Loop FD, Lytle BW, Cosgrove DM, Stewart RW, Goormastic M, Williams GW, Golding LA, Gill CC, Taylor PC, Sheldon WC, Proudfit WL. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med. 1986;314:1–6. doi: 10.1056/NEJM198601023140101. [DOI] [PubMed] [Google Scholar]
- 34.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R Cholesterol Treatment Trialists. Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 35.Stone GW, Rizvi A, Newman W, Mastali K, Wang JC, Caputo R, Doostzadeh J, Cao S, Simonton CA, Sudhir K, Lansky AJ, Cutlip DE, Kereiakes DJ. Everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease. N Engl J Med. 2010;362:1663–1674. doi: 10.1056/NEJMoa0910496. [DOI] [PubMed] [Google Scholar]
- 36.Palmerini T, Biondi-Zoccai G, Della Riva D, Stettler C, Sangiorgi D, D’Ascenzo F, Kimura T, Briguori C, Sabate M, Kim HS, De Waha A, Kedhi E, Smits PC, Kaiser C, Sardella G, Marullo A, Kirtane AJ, Leon MB, Stone GW. Stent thrombosis with drug-eluting and bare-metal stents: Evidence from a comprehensive network meta-analysis. Lancet. 2012;379:1393–1402. doi: 10.1016/S0140-6736(12)60324-9. [DOI] [PubMed] [Google Scholar]
- 37.Hillis LD, Smith PK, Anderson JL, Bittl JA, Bridges CR, Byrne JG, Cigarroa JE, Disesa VJ, Hiratzka LF, Hutter AM, Jr, Jessen ME, Keeley EC, Lahey SJ, Lange RA, London MJ, Mack MJ, Patel MR, Puskas JD, Sabik JF, Selnes O, Shahian DM, Trost JC, Winniford MD. 2011 accf/aha guideline for coronary artery bypass graft surgery: A report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation. 2011;124:e652–735. doi: 10.1161/CIR.0b013e31823c074e. [DOI] [PubMed] [Google Scholar]
- 38.Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Nallamothu BK, Ting HH. 2011 accf/aha/scai guideline for percutaneous coronary intervention: A report of the american college of cardiology foundation/american heart association task force on practice guidelines and the society for cardiovascular angiography and interventions. Circulation. 2011;124:e574–651. doi: 10.1161/CIR.0b013e31823ba622. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.