Abstract
Rocky Mountain spotted fever (RMSF) is a serious tick-borne illness caused by Rickettsia rickettsii that is endemic in southeastern USA. Although RMSF has been described as causing the classic clinical triad of fever, headache and a characteristic rash, serious and potentially life-threatening manifestations can occur. Cardiopulmonary involvement, although infrequent, may occur with severe cases of RMSF. Rickettsial myocarditis is an uncommon occurrence. We present a case of a previously healthy 26-year-old man, who was hitch-hiking across the southeastern USA, with serologically proven RMSF causing adult respiratory distress syndrome, septic shock and myocarditis manifested by elevated cardiac enzymes and decrease in myocardial function. After treatment with antibiotics, the myocarditis resolved. Therefore, although unusual, clinicians should be aware of possible myocardial involvement in patients with appropriate tick-exposure histories or other clinical signs of RMSF.
Background
Rocky Mountain spotted fever (RMSF) is the most common tick-borne rickettsial disease in the USA and has the potential to be fatal even in previously healthy young persons. The diagnosis of RMSF presents a medical challenge for healthcare professionals because of the non-specific, wide-ranging presentations of the disease, especially early in its course—ranging from a mild, febrile illness to overwhelming, fulminant disease. Prompt empirical antimicrobial therapy and aggressive supportive care are essential to avoid life-threatening complications, and treatment should not be delayed awaiting diagnostic confirmation.
Case presentation
A previously healthy 26-year-old Caucasian man was brought to a Florida emergency department (ED) in late January from an outside facility with fever, hypotension and generalised malaise for the past 48 h. His medical history was otherwise unremarkable; psychiatric history was notable for bipolar disorder and chronic narcotic dependence. He was a former smoker, former alcoholic and occasionally used marijuana. He denied any allergies.
The history of present illness went back approximately 12–14 days prior when he decided to hitch-hike from his previous location in Tennessee to a southwest Florida facility to undergo detoxification from narcotics. His hitch-hiking journey took almost 2 weeks. The patient was at the facility for approximately 2 days when he began developing fever, hypotension, a dry cough and generalised malaise. He was empirically started on oseltamivir for treatment of presumed influenza. The patient's status did not improve and he continued to be febrile and hypotensive, and was subsequently sent to the ED for further evaluation.
Vital signs in the ED at presentation were temperature 39.3°C; pulse 92 beats/min; blood pressure (BP) 77/62 mm Hg; respiratory rate of 16 breaths/min with 90% oxygen saturation on room air. Physical examination revealed a pale, ill-appearing, obese Caucasian man with bilateral, coarse crackles over the lung fields and mild right upper quadrant tenderness to deep palpation. The rest of the physical examination was unremarkable. A complete blood count revealed a white blood cell count of 13.6 cells/mm3, haemoglobin of 13.6 mg/dl and platelet count of 225 000/mm3. Comprehensive metabolic panel was notable for sodium of 131 mmol/l, creatine of 1.9 mg/dl, blood urea nitrogen of 38 mg/dl, aspartate aminotransferase 717 U/l, alanine aminotransferase 759 U/l and direct bilirubin 1.32 mg/dl. Urine toxicology screen was unremarkable. ECG demonstrated normal sinus rhythm at 98 beats/min, with no acute ST-T wave abnormalities. Initial chest radiograph revealed perihilar opacities suggestive of bilateral infiltrates. CT of the abdomen and pelvis was unremarkable. Ultrasound of the abdomen revealed gallbladder wall thickening suggestive of cholecystitis. Blood cultures were drawn. The patient was started on intravenous normal saline, parenteral pipercillin-tazobactam was begun and he was admitted for presumed biliary sepsis (figures 1–3).
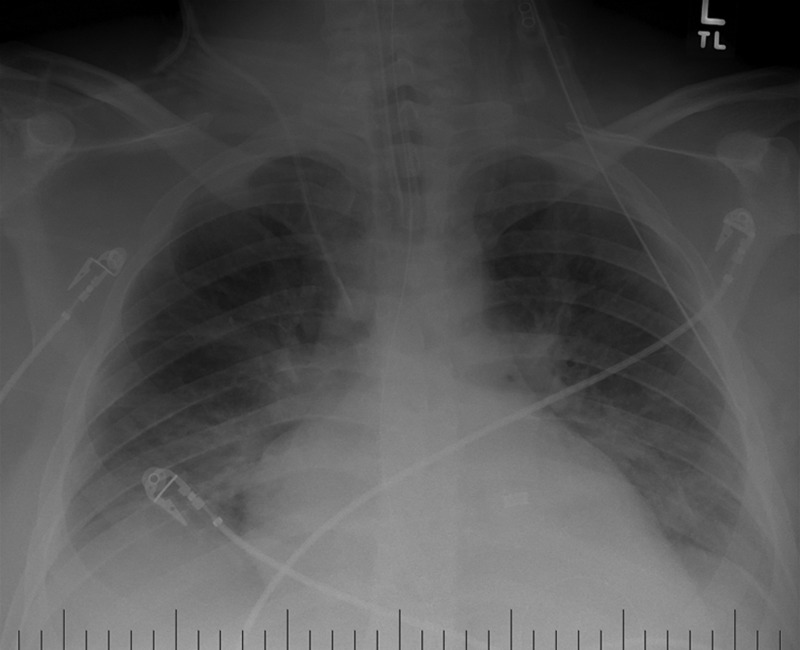
Figure 1.
Chest radiograph on hospital day 1 after emergent intubation showing scattered bilateral infiltrates consistent with adult respiratory distress syndrome.
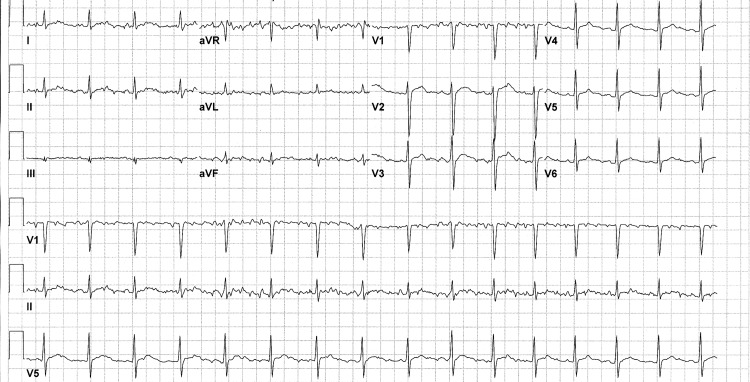
Figure 2.
ECG on hospital day 4 indicating prolonged QT and non-specific ST abnormality. Ventricular rate of 95 beats/min. Cardiac conduction abnormalities have been described in cases of rickettsial myocarditis.
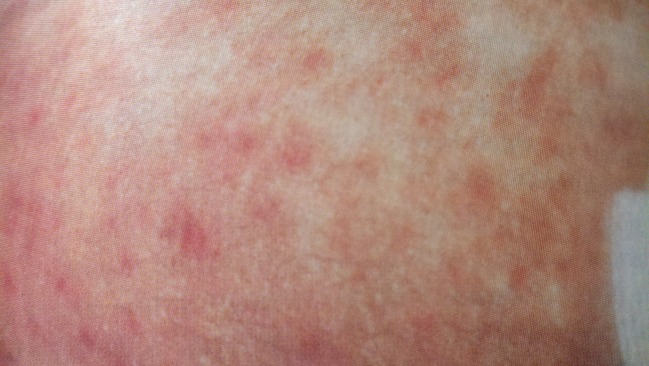
Figure 3.
Characteristic maculopapular rash of Rocky Mountain spotted fever on the arm of the patient, hospital day 2.
Seven hours into the hospitalisation, the patient began having diaphoresis and tachypnoea. He complained of worsening shortness of breath. His BP began to drop to below 80 mm Hg systolic, requiring massive fluid resuscitation. A repeat chest radiograph showed worsening pulmonary oedema and an albumin infusion was started. The patient was emergently intubated and a central line was inserted. Given the patient's lab abnormalities (including transaminitis and elevated bilirubin) and radiological evidence of cholecystitis, surgical consultation was initiated and the patient was taken to the operating room for an exploratory laparotomy. The procedure revealed no evidence of inflamed gallbladder or any acute abdominal pathology causing the patient's symptoms.
The patient was subsequently brought to the intensive care unit for acute hypoxemic respiratory failure and septic shock of unknown aetiology. Arterial blood gas done on 100% oxygen with a rate of 12 breaths/min and tidal volume of 850 ml/breath showed pO2 of only 67 mm Hg, pCO2 of 50 mm Hg and pH of 7.31. The patient was started empirically on intravenous cefepime, vancomycin, levofloxacin and metronidazole; pipercillin-tazobactam was discontinued. Infusions of norepinephrine and vasopressin were also initiated to support BP and maintain mean arterial pressure above 60 mm Hg.
On hospital day 2, the patient began developing a faint, violet-coloured, maculopapular rash on the bilateral lower extremities and wrists. Serological testing for HIV, hepatitis, leptospirosis, ehrlichiosis, Q fever, syphilis and RMSF were performed. Parenteral doxycycline was added to the antimicrobial regimen. Urine legionella, urine pneumococcal antigens and influenza A/B nasopharyngeal swab were negative. His liver function tests and creatine continued to worsen. Because of anuria and volume overload, a dialysis catheter was inserted and intermittent haemodialysis was started.
Of note, the patient's pro-BNP (prohormone of B-type natriuretic peptide) was markedly elevated at over 46 000 ng/ml. The initial troponin level also returned elevated at 6.71 ng/ml. Transthoracic echocardiogram revealed severely decreased left ventricular systolic function with an ejection fraction of 20–25% and severe global hypokinesis. There was no evidence of any gross cardiac vegetation. The patient was subsequently started on intravenous dobutamine and bumetanide. Subsequent troponin testing trended lower.
Antibiotics and inotropes were continued for hospital days 2 and 3. Intubation was continued with stabilisation of oxygenation. Haemodialysis was continued. By now, the patient had developed a diffuse, circular, dark purple, petechial, blanching rash on his upper and lower extremities which had migrated proximally to also involve his abdomen. Serological studies for HIV, leptospirosis, ehrlichiosis and Q fever were negative. The hepatitis A, B and C virus panel was negative. The rapid plasma reagin was also negative. Blood cultures remained negative.
On hospital day 4, serological testing for Rickettsia rickettsii IgM antibody returned positive at over 1:128. Intravenous doxcycline was continued; other antimicrobial agents were stopped. The patient gradually improved. On hospital day 6, the patient was successfully extubated. Once off pressors, he was started on oral carvedilol, losartan and diuretics and was moved to the telemetry ward. Doxcycline was changed to oral form. His liver function tests and creatine normalised and haemodialysis was discontinued. The patient developed isolated areas of gangrene and skin necrosis of his toes where the rash once was, which required wound care and podiatric and vascular surgery treatments. He was sent to a physical rehabilitation facility after a 22-day total hospitalisation on oral doxycycline.
At a return visit several weeks later, the patient was asymptomatic. A repeat transthoracic echocardiogram done after completion of the course of oral doxycycline revealed improvement in ejection fraction (50–55%) and resolution of the global hypokinesis. Upon further questioning, although the patient denied remembering being bitten by a tick, he recalled that over the course of his hitch-hiking journey from Tennessee to Florida he would often sleep in high-grass, rural fields.
Discussion
RMSF is the most lethal and most frequently reported rickettsial disease in the USA.1 The geographic distributon of RMSF in the USA is determined by the activity of tick vectors that harbour R rickettsii, the causative bacterium of RMSF. The disease is found throughout North America, Central America and South America. In the USA, the disease has been reported in all states except for Vermont and Maine.2 Most cases of RMSF occur in the south Atlantic states (North and South Carolina, Virginia) and south central states (Tennessee, Oklahoma and Arkansas).
All rickettsial diseases have arthropods as their natural hosts and are thus, zoonoses. The three major tick species responsible for the spread of RMSF include the American dog tick (Dermacentor variabilis); the Rocky Mountain wood tick (Dermacentor andersoni) and the brown dog tick (Rhipicephalus sanguineus). D variabilis is found predominantly in the central USA and Atlantic coast; D andersoni is present in the Rocky Mountain region and Canada and R sanguineus is found in the southwestern USA and Central America.3 With these ticks functioning as both reservoirs and vectors for R rickettsii, humans are accidental and dead-end hosts. Ticks become infected by feeding on the blood of infected animals, through fertilisation or via trans-ovarial passage. R rickettsii is transmitted by the bite of an infected tick. When a tick is attached to and feeding on a human, a reactivation phenomenon occurs and the bacterium transforms from a once-dormant, harmless form to a disease-causing state, a process that requires a period of at least 4–6 h, although it may be as long as 24 h.4 The incubation period of RMSF is highly variable ranging from as little as 2 days to as long as 2 weeks.
R rickettsii is a non-motile, weakly Gram-negative coccobacillus whose cell wall contains lipopolysaccharides. It is an obligate intracellular bacterium which possesses two major immunogenic outer membrane proteins: OmpA and OmpB.5 These proteins are not only used by the organism to attach to host cells but also play a vital role in the human antibody response in patients with RMSF.
Almost any body organ can be affected by the bacteria leading to a diverse range of systemic manifestations. Interestingly, R rickettsia has been shown to not only have a tropism for endothelial cells but also the smooth muscle layer of small blood vessels causing a severe vasculitis that affects the skin and microcirculation of various organs leading to numerous complications.6 Cell-to-cell spread of the bacteria and the resultant multiorgan vasculitis is the underlying pathological mechanism for most of the clinical features and laboratory aberrations seen with the disease. The major pathophysiological results of endothelial injury are oedema, hypovolaemia, hypotension and hypoalbuminaemia. In severe cases of RMSF, the extensive vasculitis can lead to small vessel occlusions.
The classic clinical triad of RMSF comprises fever (usually greater than 38.9°C (102°F)), headache and rash, which are rarely all present early in the disease. Rash is a major diagnostic clue and is present in most patients several days after the onset of the fever and progresses through stages. In the early phases of disease, the rash may be blanching, macular and non-pruritic. The rash may become petechial and, in rare cases, purpura, skin necrosis and gangrene may ensue. The characteristic centripetal progression of the maculopapular rash from wrists and ankles to the trunk actually only occurs in a minority of patients.
To date, RMSF is the only tick-borne disease that can directly cause congestive heart failure secondary to myocarditis.7 Rickettsial myocarditis is an uncommon complication of RMSF and studies to reveal the exact pathogenesis of myocardial damage in severe cases of the disease are limited. R rickettsii has been shown to infect myocardial tissue, as part of the underlying vasculitis, involving the venules, capillaries and arterioles of the cardiac muscle, especially in fatal cases. Inflammation of the myocardium consists of a lymphocytic infiltration.8 In an effort to elucidate the contribution of cardiac involvement to the morbidity of RMSF, Walker et al9 showed that increased heart weight and increased interstitial cardiac volume occurred in six of nine cases of fatal RMSF at necroscopy. This study also demonstrated particular immunofluorescent staining of R rickettsii in cardiac tissue in eight of the nine cases, which provided a possible pathogenic mechanism for endothelial cell injury-induced myocarditis. Cases of rickettsial myocarditis may go unrecognised. Paddock et al10 studied nine patients who died of serologically unconfirmed RMSF in whom diagnosis was established postmortem by the use of immunohistochemical staining. Four of eight patients with myocardial tissue available had evidence of localised myocarditis.10 Cardiac conduction abnormalities, including atrioventricular and bundle branch blocks have been described in cases of RMSF but their impact on morbidity and mortality remains uncertain.
Undifferentiated symptoms such as fever, malaise, chills and headache are present early in the disease process. Such generic symptoms rarely lead to an initial clinical suspicion of the actual diagnosis in patients with RMSF, unless the clinical history includes tick-exposure or a tick-bite. In the case of our patient, the initial working diagnosis was incorrectly thought to be biliary sepsis. Adding to the clinical diagnostic complexity, up to 40% of patients with documented RMSF are unaware of a recent history of a tick-bite.11 Similarly, our patient also had no recollection of a tick-bite. There are several reasons why patients with RMSF may not recognise that they may have been bitten by a tick. First, the tick-bites themselves are typically painless and form no eschar or scar at the site of the bite. In addition, the ticks often attach to sites of the body where they are challenging to identify (eg, scalp, axillae and genital areas). Although the rash is helpful in reaching the diagnosis, it may be absent at presentation. RMSF without a rash (ie, spotless RMSF) should be considered ehrlichiosis until proven otherwise.
Diseases and conditions to consider in the differential diagnosis of RMSF include meningitis, pneumonia, ehrlichiosis, Q fever, other rickettsial diseases, thrombotic thrombocytopenic purpura, leptospirosis, dengue fever, infectious mononucleosis, bacterial sepsis, toxic shock syndrome, syphilis, enteroviral infections, viral hepatitis, Lyme disease and malaria. The characteristic rash of RMSF can also be mistaken for a drug reaction if RMSF is treated with antibiotics targeted for another microbe. Adding to the vastness of the differential diagnosis of RMSF is that it can encompass both tick-borne and non-tick-borne diseases.
The diagnosis of RMSF rests upon physical manifestations (fever, headache and rash) and epidemiological factors (possible tick-exposure history). However, clinical diagnosis is difficult because initial symptoms can be non-specific and may lead physicians to make an incorrect diagnosis. Lab abnormalities such as anaemia, thrombocytopenia, elevated liver enzymes, increased bilirubin and hyponatraemia have all been reported with RMSF.12 Antibodies to R rickettsii are not detectable until 7–10 days after the onset of illness and, therefore, provide inadequate diagnostic value. The indirect fluorescent antibody test, currently the gold standard for serological testing for rickettsial disease, is highly sensitive.11 A fourfold increase in titres in paired samples or a convalescent titre greater than 1:64 are also diagnostic.13 The latter was used to make the diagnosis in our patient's case.
The treatment of choice for RMSF is doxycycline for at least 7 days. Doxycycline is preferred because it is also active against other tick-borne infections, such as ehrlichiosis and other rickettsial diseases, which are also frequently on the differential diagnoses for patients with clinical signs and/or epidemiological exposure history to ticks. Chloramphenicol is reserved for situations when doxycycline is contraindicated, such as pregnancy. Treatment with chloramphenicol is also associated with higher percentage of fatal outcomes than those treated with tetracyclines.14
Our case report illustrates several important points. First, it emphasises the severity of RMSF and the multiplicity of organ systems that can potentially be involved with the infection. Our patient's recovery can be attributed to the appropriate management of numerous organ failures—cardiac (inotropes, afterload reduction), pulmonary (ventilatory support), renal (fluid management, haemodialysis) and early, accurate antimicrobial selection (ie, doxycycline). Our patient was started on intravenous doxycycline by hospital day 2 and failure to treat early on clinical suspicion would likely have led to a negative outcome. Depending on the patient's age and other underlying medical comorbidities, RMSF has a case–death ratio of 10–25%.15 Significantly increased morbidity and mortality are associated with delayed antirickettsial therapy after the day-5 mark.16 Like other tick-borne diseases, RMSF displays seasonality, with most cases occurring in late spring and early summer. However, in the southern USA, winter-time cases of RMSF are more common17 (our patient presented in Florida in late January). Therefore, the index of suspicion should be kept high year-round by the general clinician. Our patient's hitch-hiking excursion began in Tennessee highlighting the significance of geographic distribution of rickettsial disease, as a large number of RMSF cases occur in southeastern USA.
In conclusion, RMSF is a dangerous acute infectious disease of the western hemisphere that continues to occur at increasing incidence in various parts of the USA.18 It should be included in the differential diagnoses of any undifferentiated fever in a traveller. Absence of a tick-bite or tick-bite history, should not dissuade the clinician from considering the diagnosis. Substantial damage to the endothelium in the skin, lungs, heart, kidneys, gastrointestinal tract, brain, skeletal muscle and other sites by the bacterium results in the variable and often severe clinical manifestations associated with untreated disease. If not treated promptly, it can progress into deadly physiological derangements and in rare cases, myocarditis, septic shock and adult respiratory distress syndrome. Close haemodynamic monitoring is crucial in critically ill patients with fulminant cases of RMSF, as the vasculitis associated with the disease can sometimes affect the cardiac muscle and ultimately lead to myocarditis. RMSF is an important emerging human infection that infrequently causes myocardial disease. However, it is important to recognise the potential cardiovascular complications of this infection as early initiation of antimicrobial therapy can be life-preserving. Further studies are needed to better understand not only the exact pathogenesis of rickettsial myocarditis but also the effect of cardiopulmonary dynamics in cases of severe RMSF on overall morbidity.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Masters EJ, Olson GS, Weiner SJ, et al. ‘Rocky Mountain spotted fever: a clinician's dilemma’. Arch Intern Med 2003;163:769–74 [DOI] [PubMed] [Google Scholar]
- 2.Abrahamian FM. Consequences of delayed diagnosis of Rocky Mountain spotted fever in children—West Virginia, Michigan, Tennessee, and Oklahoma, May-July 2000. Ann Emerg Med 2001;37:537–40 [DOI] [PubMed] [Google Scholar]
- 3.Demma LJ, Traeger MS, Nicholson WL, et al. Rocky Mountain spotted fever from an unexpected tick vector in Arizona. N Engl J Med 2005;353:587–94 [DOI] [PubMed] [Google Scholar]
- 4.Moore JJ. Time relationships in the wood-tick transmission of Rocky Mountain spotted fever. J Infect Dis 1911;8:339–47 [Google Scholar]
- 5.Crocquet-Valdes PA, Diaz-Montero CM, Feng HM, et al. Immunization with a portion of rickettsial outer membrane protein A stimulates protective immunity against spotted fever rickettsiosis. Vaccine 2001;20:979–88 [DOI] [PubMed] [Google Scholar]
- 6.Walker DH. Rickettsiae and rickettsial infections: the current state of knowledge. Clin Infect Dis 2007;45(Suppl):S39–44 [DOI] [PubMed] [Google Scholar]
- 7.Lankford HV, Glauser FL. Cardiopulmonary dynamics in a severe case of Rocky Mountain spotted fever. Arch Intern Med 1980;140:1357–9 [PubMed] [Google Scholar]
- 8.Shah SS, McGowan JP. Rickettsial, Ehrlichial and Bartonella infections of the myocardium and pericardium. Front Biosci 2003;8:e197–201 [DOI] [PubMed] [Google Scholar]
- 9.Walker DH, Paletta CE, Cain BG. Pathogenesis of myocarditis in Rocky Mountain Spotted Fever. Arch Pathol Lab Med 1980;104:171–4 [PubMed] [Google Scholar]
- 10.Paddock CD, Greer PW, Ferebee TL, et al. Hidden mortality attributable to Rocky Mountain spotted fever: immunohistochemical detection of fatal, serologically unconfirmed disease. J Infect Dis 1999;179:1469–76 [DOI] [PubMed] [Google Scholar]
- 11.Sexton DJ, Kaye KS. Rocky Mountain spotted fever. Med Clin North Am 2002;86:351–60 [DOI] [PubMed] [Google Scholar]
- 12.Kirk JL, Fine IX, Sexton DJ, et al. Rocky Mountain spotted fever: a clinical review based on 48 confirmed cases, 1943–1986. Medicine (Baltimore) 1990;69:35–45 [PubMed] [Google Scholar]
- 13.Lacz NL, Schwartz RA, Kapila R. Rocky Mountain Spotted Fever. J Eur Acad Dermatol Venereol 2006;20:411–17 [DOI] [PubMed] [Google Scholar]
- 14.Holman RC, Paddock CD, Curns AT, et al. Analysis of risk fac tors for fatal Rocky Mountain spotted fever: evidence for superiority of tetracyclines for therapy. J Infect Dis. 2001;184:1437–44 [DOI] [PubMed] [Google Scholar]
- 15.Jones TF, Craig AS, Paddock CD, et al. Family cluster of Rocky Mountain spotted fever. Clin Infect Dis 1999;28:853–9 [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention Consequences of delayed diagnosis of Rocky Mountain spotted fever in children-West Virginia, Michigan, Tennessee, and Oklahoma, May-July 2000. MMWR Morb Mortal Wkly Rep 2000;49:885–8 [PubMed] [Google Scholar]
- 17.Dalton MJ, Clarke MJ, Holman RC, et al. National surveillance for Rocky Mountain spotted fever, 1981–1992: epidemiologic summary and evaluation of risk factors for fatal outcome. Am J Trop Med Hyg 1995;52: 405–13 [DOI] [PubMed] [Google Scholar]
- 18.Openshaw JJ, Swerdlow DL, Krebs JW, et al. Rocky Mountain spotted fever in the United States, 2000–2007: interpreting contemporary increases in incidence. Am J Trop Med Hyg 2010;83:174–82 [DOI] [PMC free article] [PubMed] [Google Scholar]