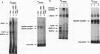Abstract
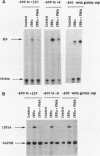
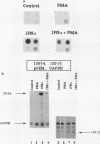
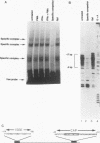
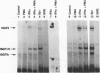
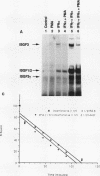
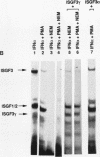
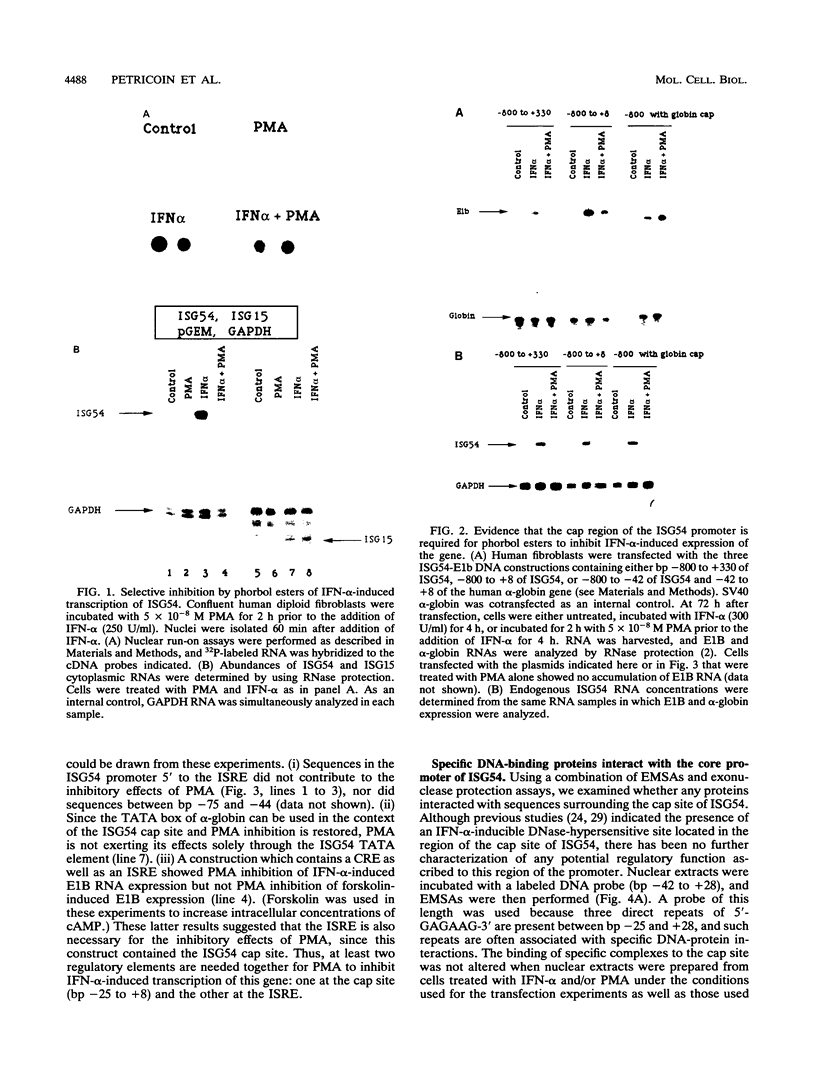
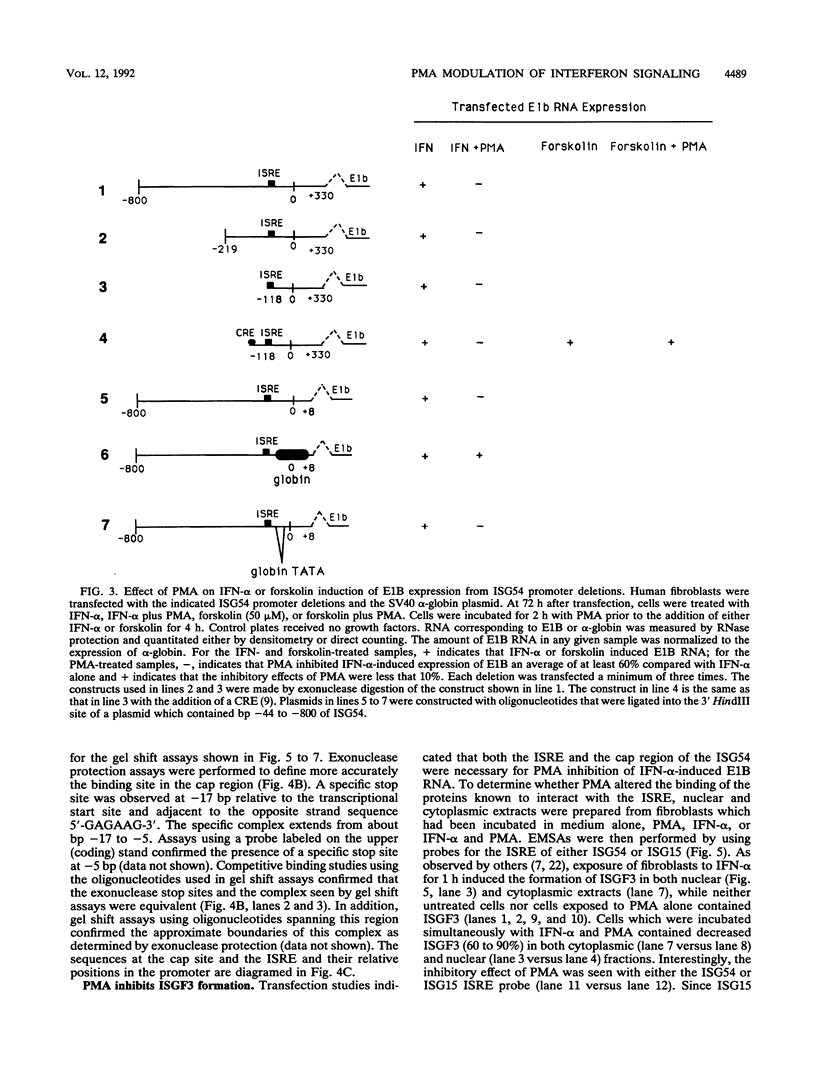
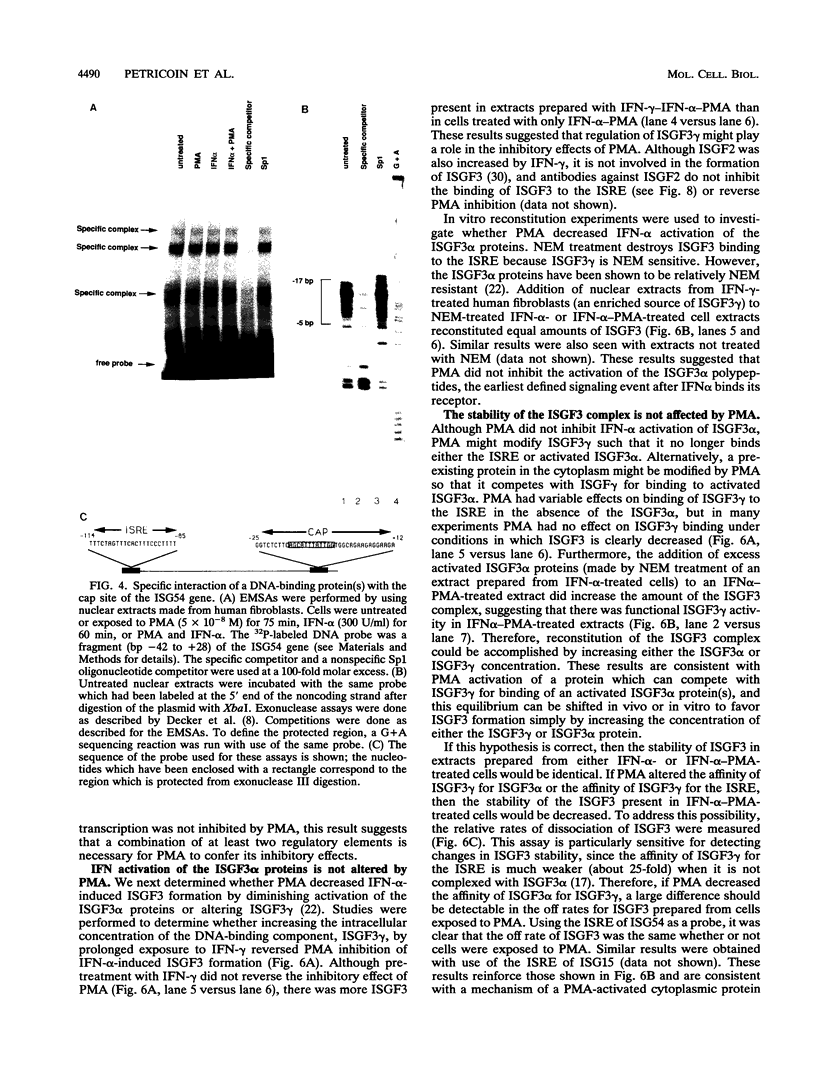
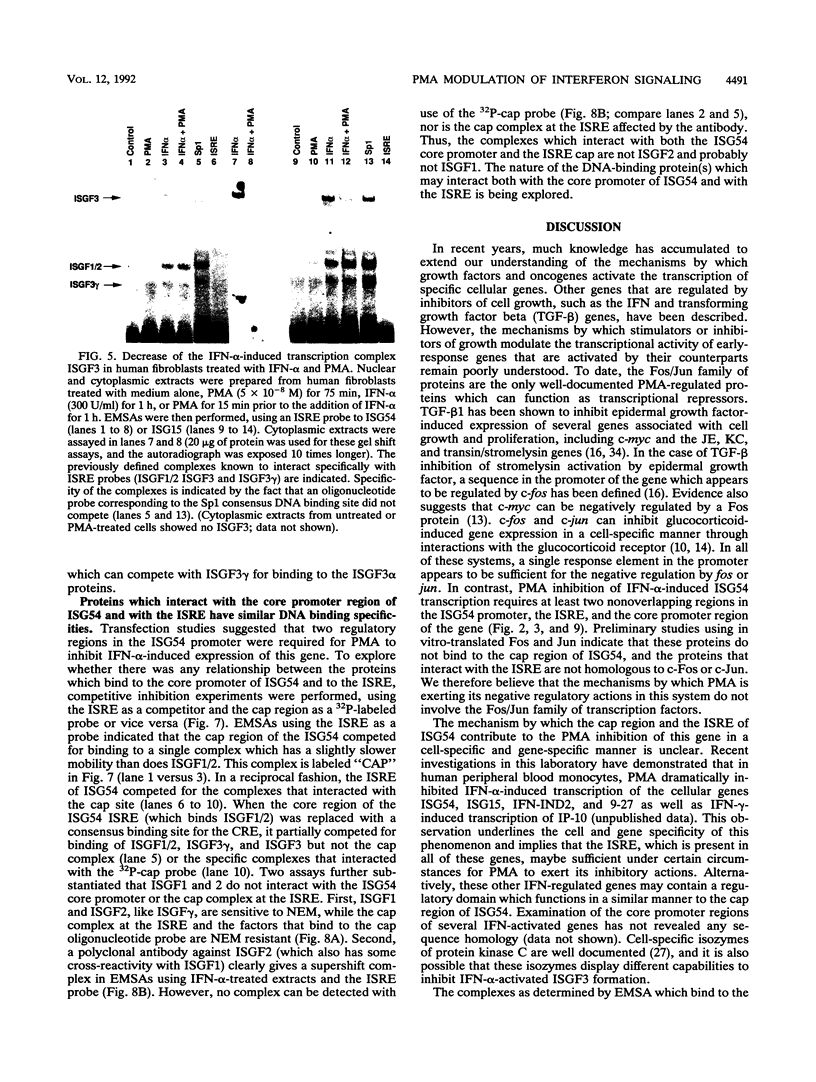
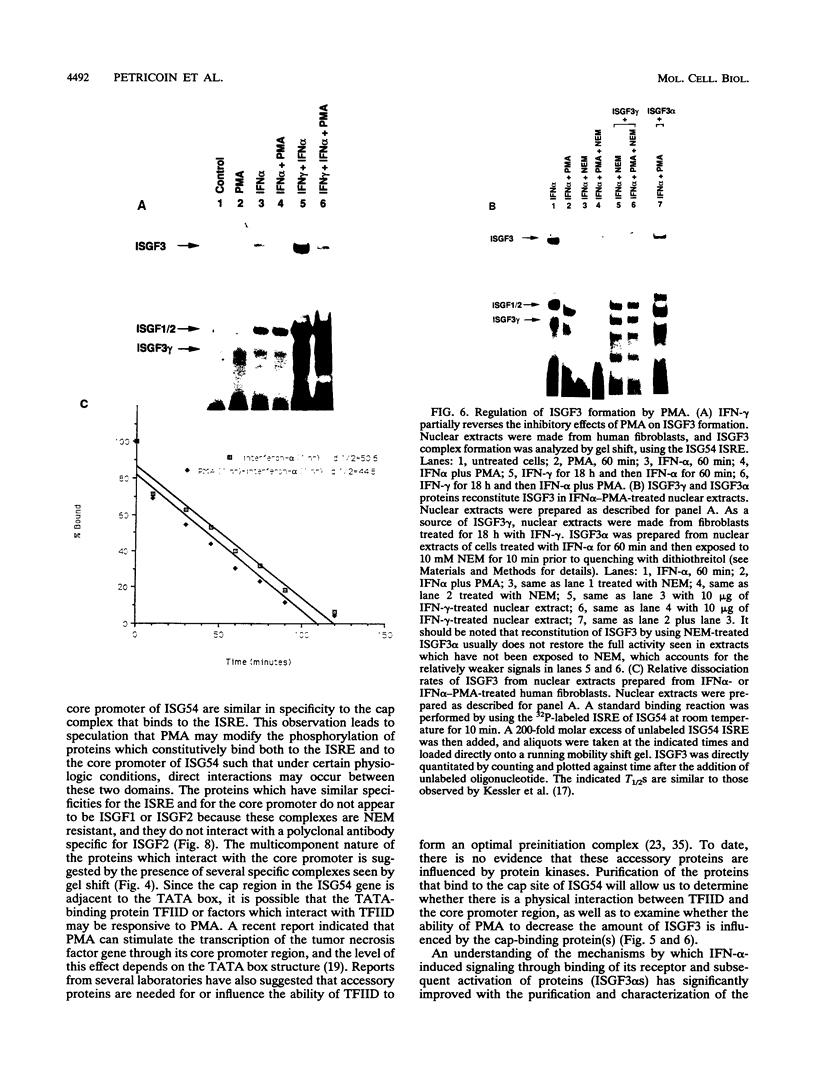
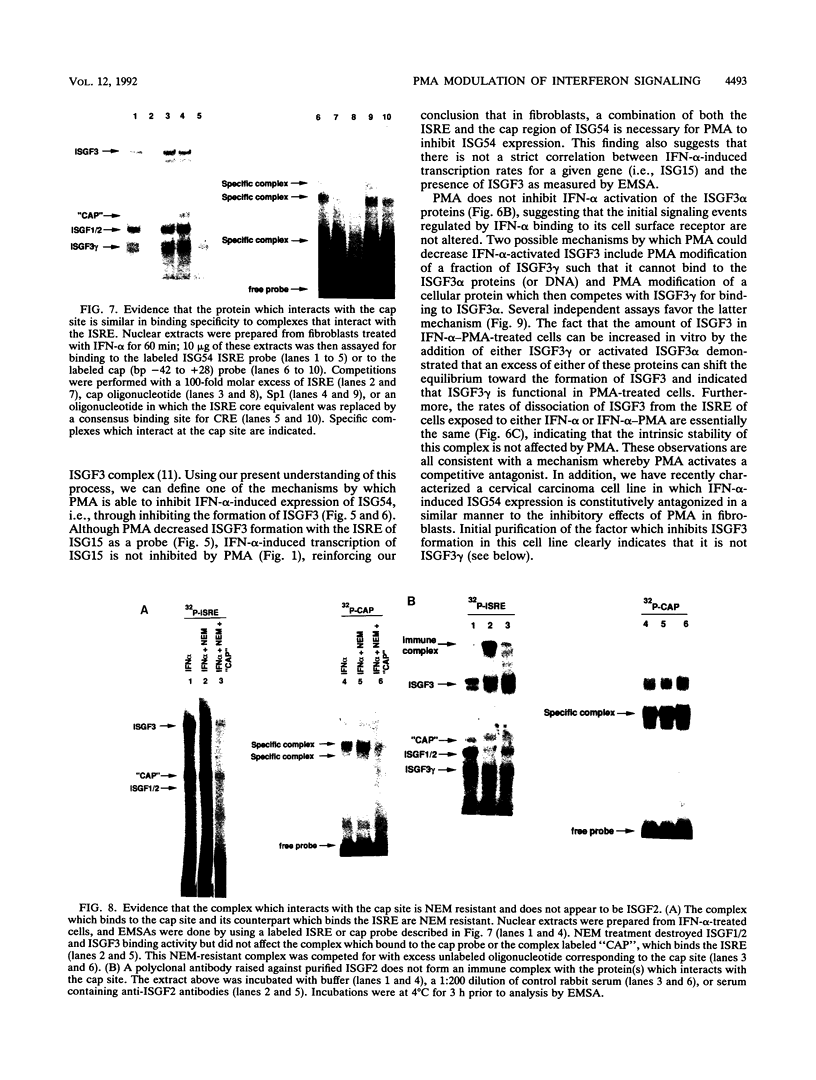
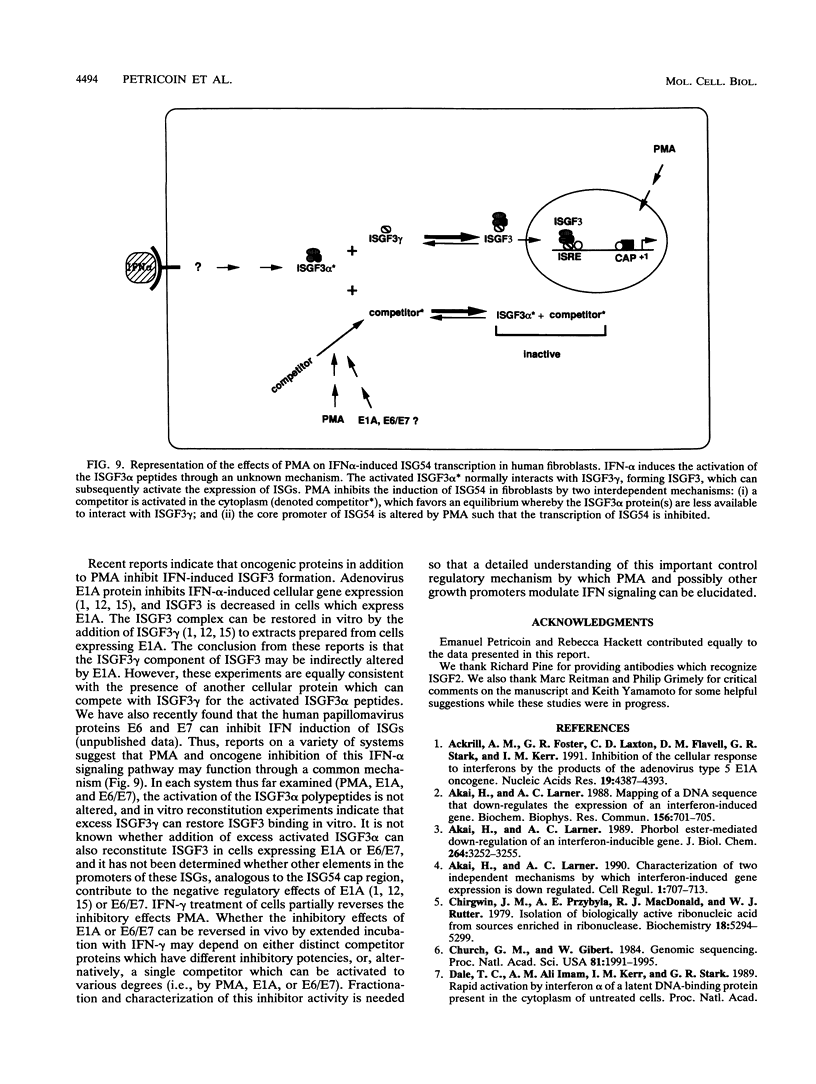
Phorbol esters activate the expression of a variety of early-response genes through protein kinase C-dependent pathways. In addition, phorbol esters may promote cell growth by the inhibition of expression of cellular gene products regulated by antiproliferative agents such as interferons (IFN)s. In human diploid fibroblasts, phorbol 12-myristate 13-acetate (PMA) selectively inhibits the IFN-alpha-induced cellular gene ISG54. Using transient transfection assays, we have delineated two elements in the promoter of this gene that are necessary for the inhibitory actions of PMA. These elements include (i) the IFN-stimulated response element (ISRE) which is necessary for IFN-alpha-induced cellular gene expression, and (ii) an element located near the site of transcription initiation. IFN-alpha treatment resulted in the rapid induction of ISGF3, a multisubunit transcription factor which binds to the ISRE. PMA caused a substantial reduction in IFN alpha-induced ISGF3 in both nuclear and cytoplasmic extracts, as determined by electrophoretic mobility shift assays with the ISRE as a probe. In vitro reconstitution experiments revealed that IFN-alpha activation of the ISGF3 alpha component of ISGF3 was not affected by PMA. Further experiments were consistent with the possibility that PMA regulated the activity of a cellular factor which competed with ISGF3 gamma for binding of the activated ISGF3 alpha polypeptides. Electrophoretic mobility shift assays using the cap site of ISG54 as a probe demonstrated the formation of a specific complex whose DNA binding activity was not affected by treatment of cells with PMA or IFN-alpha. Competitive inhibition studies were consistent with the DNA-protein complex at the cap site of ISG54 containing proteins with DNA binding sites in common with those which also interact with the ISRE. These data suggest a unique regulatory mechanism by which phorbol esters can modulate IFN signaling.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackrill A. M., Foster G. R., Laxton C. D., Flavell D. M., Stark G. R., Kerr I. M. Inhibition of the cellular response to interferons by products of the adenovirus type 5 E1A oncogene. Nucleic Acids Res. 1991 Aug 25;19(16):4387–4393. doi: 10.1093/nar/19.16.4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akai H., Larner A. C. Characterization of two independent mechanisms by which interferon-induced gene expression is down regulated. Cell Regul. 1990 Aug;1(9):707–713. doi: 10.1091/mbc.1.9.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akai H., Larner A. C. Mapping of a DNA sequence that down-regulates the expression of an interferon-induced gene. Biochem Biophys Res Commun. 1988 Oct 31;156(2):701–705. doi: 10.1016/s0006-291x(88)80899-4. [DOI] [PubMed] [Google Scholar]
- Akai H., Larner A. C. Phorbol ester-mediated down-regulation of an interferon-inducible gene. J Biol Chem. 1989 Feb 25;264(6):3252–3255. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker T., Lew D. J., Mirkovitch J., Darnell J. E., Jr Cytoplasmic activation of GAF, an IFN-gamma-regulated DNA-binding factor. EMBO J. 1991 Apr;10(4):927–932. doi: 10.1002/j.1460-2075.1991.tb08026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch P. J., Hoeffler J. P., Jameson J. L., Lin J. C., Habener J. F. Structural determinants for transcriptional activation by cAMP-responsive DNA elements. J Biol Chem. 1988 Dec 5;263(34):18466–18472. [PubMed] [Google Scholar]
- Diamond M. I., Miner J. N., Yoshinaga S. K., Yamamoto K. R. Transcription factor interactions: selectors of positive or negative regulation from a single DNA element. Science. 1990 Sep 14;249(4974):1266–1272. doi: 10.1126/science.2119054. [DOI] [PubMed] [Google Scholar]
- Fu X. Y., Kessler D. S., Veals S. A., Levy D. E., Darnell J. E., Jr ISGF3, the transcriptional activator induced by interferon alpha, consists of multiple interacting polypeptide chains. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8555–8559. doi: 10.1073/pnas.87.21.8555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutch M. J., Reich N. C. Repression of the interferon signal transduction pathway by the adenovirus E1A oncogene. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):7913–7917. doi: 10.1073/pnas.88.18.7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay N., Takimoto M., Bishop J. M. A FOS protein is present in a complex that binds a negative regulator of MYC. Genes Dev. 1989 Mar;3(3):293–303. doi: 10.1101/gad.3.3.293. [DOI] [PubMed] [Google Scholar]
- Jonat C., Rahmsdorf H. J., Park K. K., Cato A. C., Gebel S., Ponta H., Herrlich P. Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell. 1990 Sep 21;62(6):1189–1204. doi: 10.1016/0092-8674(90)90395-u. [DOI] [PubMed] [Google Scholar]
- Kalvakolanu D. V., Bandyopadhyay S. K., Harter M. L., Sen G. C. Inhibition of interferon-inducible gene expression by adenovirus E1A proteins: block in transcriptional complex formation. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7459–7463. doi: 10.1073/pnas.88.17.7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr L. D., Miller D. B., Matrisian L. M. TGF-beta 1 inhibition of transin/stromelysin gene expression is mediated through a Fos binding sequence. Cell. 1990 Apr 20;61(2):267–278. doi: 10.1016/0092-8674(90)90807-q. [DOI] [PubMed] [Google Scholar]
- Kessler D. S., Veals S. A., Fu X. Y., Levy D. E. Interferon-alpha regulates nuclear translocation and DNA-binding affinity of ISGF3, a multimeric transcriptional activator. Genes Dev. 1990 Oct;4(10):1753–1765. doi: 10.1101/gad.4.10.1753. [DOI] [PubMed] [Google Scholar]
- Lauer J., Shen C. K., Maniatis T. The chromosomal arrangement of human alpha-like globin genes: sequence homology and alpha-globin gene deletions. Cell. 1980 May;20(1):119–130. doi: 10.1016/0092-8674(80)90240-8. [DOI] [PubMed] [Google Scholar]
- Leitman D. C., Mackow E. R., Williams T., Baxter J. D., West B. L. The core promoter region of the tumor necrosis factor alpha gene confers phorbol ester responsiveness to upstream transcriptional activators. Mol Cell Biol. 1992 Mar;12(3):1352–1356. doi: 10.1128/mcb.12.3.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D. E., Kessler D. S., Pine R., Darnell J. E., Jr Cytoplasmic activation of ISGF3, the positive regulator of interferon-alpha-stimulated transcription, reconstituted in vitro. Genes Dev. 1989 Sep;3(9):1362–1371. doi: 10.1101/gad.3.9.1362. [DOI] [PubMed] [Google Scholar]
- Levy D., Darnell J. E., Jr Interferon-dependent transcriptional activation: signal transduction without second messenger involvement? New Biol. 1990 Oct;2(10):923–928. [PubMed] [Google Scholar]
- Levy D., Larner A., Chaudhuri A., Babiss L. E., Darnell J. E., Jr Interferon-stimulated transcription: isolation of an inducible gene and identification of its regulatory region. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8929–8933. doi: 10.1073/pnas.83.23.8929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisterernst M., Roeder R. G. Family of proteins that interact with TFIID and regulate promoter activity. Cell. 1991 Nov 1;67(3):557–567. doi: 10.1016/0092-8674(91)90530-c. [DOI] [PubMed] [Google Scholar]
- Mirkovitch J., Decker T., Darnell J. E., Jr Interferon induction of gene transcription analyzed by in vivo footprinting. Mol Cell Biol. 1992 Jan;12(1):1–9. doi: 10.1128/mcb.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto M., Fujita T., Kimura Y., Maruyama M., Harada H., Sudo Y., Miyata T., Taniguchi T. Regulated expression of a gene encoding a nuclear factor, IRF-1, that specifically binds to IFN-beta gene regulatory elements. Cell. 1988 Sep 9;54(6):903–913. doi: 10.1016/s0092-8674(88)91307-4. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y., Rivera V., Larner A. C. A role for the Na/K-ATPase in the control of human c-fos and c-jun transcription. J Biol Chem. 1992 May 5;267(13):8785–8788. [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Pestka S., Langer J. A., Zoon K. C., Samuel C. E. Interferons and their actions. Annu Rev Biochem. 1987;56:727–777. doi: 10.1146/annurev.bi.56.070187.003455. [DOI] [PubMed] [Google Scholar]
- Pine R., Darnell J. E., Jr In vivo evidence of interaction between interferon-stimulated gene factors and the interferon-stimulated response element. Mol Cell Biol. 1989 Aug;9(8):3533–3537. doi: 10.1128/mcb.9.8.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine R., Decker T., Kessler D. S., Levy D. E., Darnell J. E., Jr Purification and cloning of interferon-stimulated gene factor 2 (ISGF2): ISGF2 (IRF-1) can bind to the promoters of both beta interferon- and interferon-stimulated genes but is not a primary transcriptional activator of either. Mol Cell Biol. 1990 Jun;10(6):2448–2457. doi: 10.1128/mcb.10.6.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli G., Orenstein J. M., Kinter A., Folks T. M., Fauci A. S. Interferon-alpha but not AZT suppresses HIV expression in chronically infected cell lines. Science. 1989 May 5;244(4904):575–577. doi: 10.1126/science.2470148. [DOI] [PubMed] [Google Scholar]
- Reich N., Evans B., Levy D., Fahey D., Knight E., Jr, Darnell J. E., Jr Interferon-induced transcription of a gene encoding a 15-kDa protein depends on an upstream enhancer element. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6394–6398. doi: 10.1073/pnas.84.18.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M., Dougan S. T., McFadden G., Greenberg M. E. Calcium and growth factor pathways of c-fos transcriptional activation require distinct upstream regulatory sequences. Mol Cell Biol. 1988 Jul;8(7):2787–2796. doi: 10.1128/mcb.8.7.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehara K., LeRoy E. C., Grotendorst G. R. TGF-beta inhibition of endothelial cell proliferation: alteration of EGF binding and EGF-induced growth-regulatory (competence) gene expression. Cell. 1987 May 8;49(3):415–422. doi: 10.1016/0092-8674(87)90294-7. [DOI] [PubMed] [Google Scholar]
- Tanese N., Pugh B. F., Tjian R. Coactivators for a proline-rich activator purified from the multisubunit human TFIID complex. Genes Dev. 1991 Dec;5(12A):2212–2224. doi: 10.1101/gad.5.12a.2212. [DOI] [PubMed] [Google Scholar]