Abstract
Pulmonary venous developmental anomalies have been evaluated conventionally with echocardiography and catheter angiography, multidetector CT angiography (MDCTA) and MR angiography are playing increasing roles in their characterisation. Here, we report a rare case of a 15-year-old boy, who presented with cyanosis and dyspnoea which he had had since childhood. Cardiac type of total anomalous pulmonary venous connection (TAPVC) was diagnosed and demonstrated using MDCTA in this case. Only a few case reports describing the MDCTA findings in cardiac TAPVC are available in the published literature.
Background
Total anomalous pulmonary venous connection (TAPVC), an uncommon congenital cardiac anomaly, is characterised by abnormal diversion of oxygenated blood into systemic venous circulation, where mixed blood flows to systemic circulation through an atrial septal defect (ASD), a patent foramen ovale (PFO) or a patent ductus arteriosus (PDA). The anomalous venous communication can be cardiac, supracardiac, infracardiac or mixed, with the supracardiac entity being the commonest. In cardiac type of TAPVC, pulmonary veins connect directly to the coronary sinus (CS) or right atrium (RA) and have no connection with the left atrium (LA). With the advent of multidetector CT (MDCT), it has become possible to conduct a non-invasive evaluation of this condition with high precision.
Case presentation
A 15-year-old boy presented with complaints of worsening cyanosis and dyspnoea. The patient had a history of breathlessness, recurrent chest infections, cyanosis and fatigability since childhood. Physical examination revealed tachypnoea, tachycardia and cyanosis of lips. Prominent right ventricular impulse was detected with a loud single S2 and a high-frequency early diastolic Graham Steel murmur. ECG showed prolongation of PR interval, peaked right atrial P waves, right axis deviation, tall right precordial R waves, inverted T waves and deep left precordial S wave of right ventricular enlargement.
Investigations
Chest roentgenogram showed cardiomegaly with right ventricular hypertrophy, prominent pulmonary conus and dilated pulmonary arteries (figure 1). Echocardiography revealed situs solitus with normal atrioventricular and ventriculoarterial concordance. Right-sided cardiac chambers were enlarged with a large osteum secundum type of ASD showing bidirectional flow. A chamber was seen posterior to the LA in which pulmonary veins were draining and it was communicating with dilated coronary sinus. Rest of the two pulmonary veins could not be interrogated properly due to a poor acoustic window. Contrast bubble echocardiography demonstrated flow of microbubbles from right to left atrium suggesting a functional right to left shunt. Based on these echo findings, the possibility of cardiac type of TAPVC was kept and patient was referred for 64 slice CT angiography for a definitive diagnosis and to rule out associated coronary anomalies as preoperative workup.
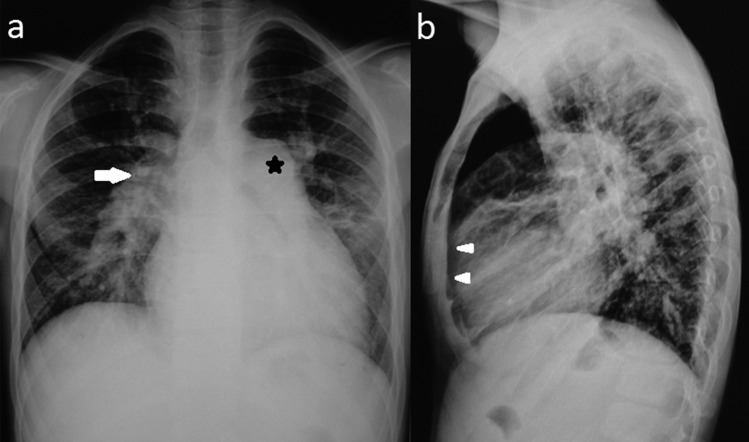
Figure 1.
Chest x-ray Posterior Anterior (A) and lateral (B) view show cardiomegaly with grossly dilated pulmonary conus (*) and pulmonary artery (→) with obliteration of retrosternal space (▸).
Retrospective ECG-gated cardiac CTA was performed using 70 ml of non-ionic contrast (iohexol 350 mg I/ml) with a 30 ml saline chase. ECG-gated tube current modulation was applied to reduce the radiation dose to the patient. The images were reconstructed in the diastolic phase.
Three-dimensional (3D) views in various projections along with multiplanar reformations (MPR) and volume rendering (VR) demonstrated all the four pulmonary veins confluencing into a common channel posterior to the LA, which was joining the dilated CS that ultimately opened in a dilated RA. A large ASD was clearly demonstrated with right heart enlargement and dilation of pulmonary trunk and bilateral pulmonary arteries suggesting development of pulmonary arterial hypertension (figures 2 and 3). No evidence of anomaly of origin, number or course of coronary arteries was noted. The patient was referred to the cardiac surgery unit for further management.
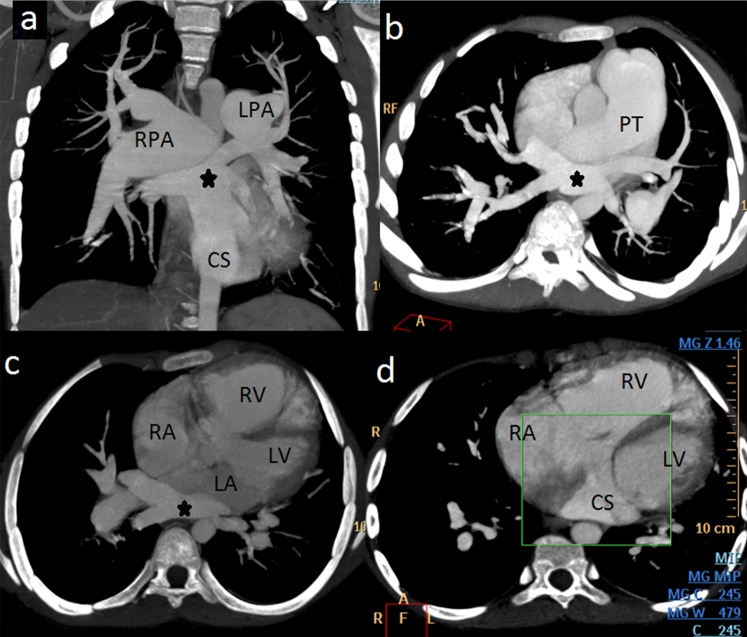
Figure 2.
Multidetector CT angiography (A) oblique coronal, (B) oblique axial and (C and D) axial maximum intensity projection (MIP) images show confluence of all four pulmonary veins (*) posterior to left atrium (LA), connected with dilated coronary sinus (CS) that ultimately opens into right atrium (RA). Right-sided cardiomegaly with grossly dilated pulmonary trunk (PT), right and left pulmonary arteries (RPA and LPA) are also visible.
Figure 3.
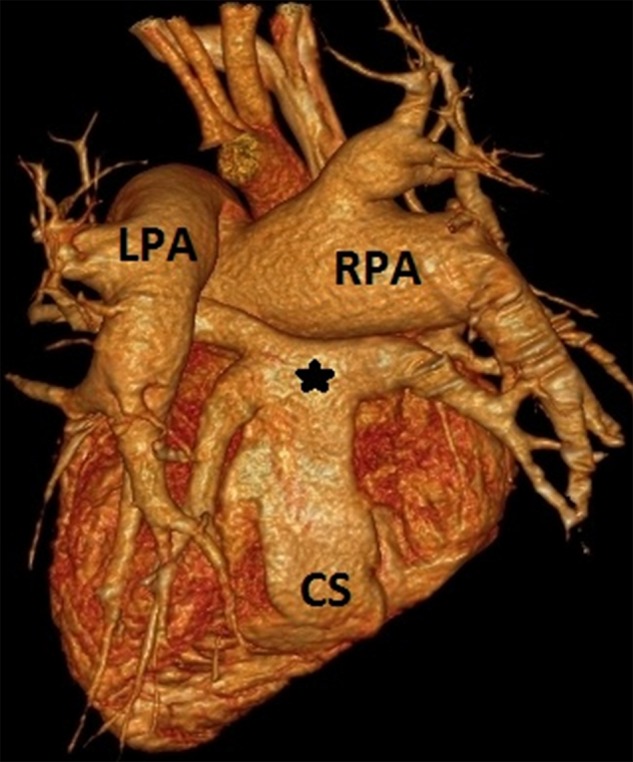
Multidetector CT angiography volume rendered (VR) image shows posterior aspect of heart demonstrating pulmonary venous confluence (*) connecting to dilated coronary sinus (CS). Right and left pulmonary arteries (RPA and LPA) are also visible.
Discussion
TAPVC is a relatively uncommon congenital defect, seen in nearly 1.5% of all patients with cardiovascular malformation, its reported incidence being 6.8/100 000 live-births.1 TAPVC encompasses a group of anomalies in which all the four pulmonary veins connect directly to the systemic venous circulation via persistent splanchnic connections. This abnormality results due to an error in the normal developmental sequence which results in failure in the transfer of pulmonary venous drainage from the splanchnic plexus to the left atrium.2 Darling and coworkers classified TAPVC into four types: supracardiac, cardiac, infracardiac and mixed varieties.3 Karamlou et al4 analysed 377 children with TAPVC and reported that these types account for 44%, 21%, 26% and 9%, respectively. In the supracardiac type, the pulmonary veins drain via an ascending vertical vein into the innominate vein, superior vena cava or azygous vein. In cardiac variety, the pulmonary venous confluence drains into the coronary sinus or, on rare occasions, individual pulmonary veins drain directly into the RA. In the infracardiac variety, pulmonary veins drain via a descending vertical vein into the portal vein or ductus venosus. The mixed type can involve any or all components of the three types.
These anomalies can also be classified into obstructive and non-obstructive varieties. These obstructive symptoms may arise either due to extrinsic compression or inadequate calibre of the draining pulmonary vein(s), especially at the site of opening. Obstruction almost always occur in infracardiac type, may occur in supracardiac type and be uncommon in cardiac variety.
TAPVC is an important cause of neonatal cyanosis and may result in rapid death if a source of shunting of blood from right to left heart (pulmonary to systemic circulation) is not present. This shunting typically occurs through either an ASD/PFO or, less commonly, a PDA.
Accurate diagnosis and knowledge of the exact pattern of pulmonary venous drainage are crucial to allow appropriate preoperative planning. Chest radiography findings depend on the type of TAPVC and are variable. Echocardiography is a non-invasive imaging modality regarded as an initial screening tool in the evaluation of TAPVC. However, the diagnostic performance of echocardiography is heavily dependent on the operator's expertise and patient's acoustic window.5 Cardiac catheterisation may be performed for further evaluation, but it is an invasive and expensive method which requires general anaesthesia and fails to provide information regarding the 3D course of the vessel.6
Revolutions in the development of diagnostic imaging have enabled early and accurate diagnosis and markedly decreased postoperative mortality in TAPVC patients over the last several decades. Novel expansions in CT technology provide a non-invasive, faster and accurate diagnostic modality with better spatial and temporal resolution, in the form of MDCT, to visualise 3D display of vascular anatomy. By virtue of its ability to produce MPR, maximum intensity projection (MIP) and 3D VR images; it can excellently depict anomalous pulmonary venous structures with statistically similar detection rates that approach to 100%.7 The technique is superior to cardiac catheterisation, as it requires mild brief sedation and general anaesthesia is unnecessary with significantly reduced risk of radiation exposure.
MRI and gadolinium-enhanced MR angiography (MRA) have been used over the last few years as non-invasive modalities for the evaluation of pulmonary venous structures. Advantages include multiplanar capability, lack of ionising radiation and the ability to acquire dynamic imaging in different vascular (arterial and venous) phases using a single bolus of contrast material. Disadvantages include prolonged time for image acquisition, frequent need for patient sedation and incompatibility with metallic implants. MRA images have a large field of view, have high spatial resolution and may be acquired in either the sagittal or coronal plane. These images can also be reformatted in multiple planes as well as 3D reassembled as either MIP or VR images.8
Learning points.
In a young patient with progressive cyanosis, total anomalous pulmonary venous connection should always be suspected and patient should be thoroughly investigated with appropriate imaging techniques.
Multidetector CT angiography (MDCTA) is a non-invasive, rapid and accurate diagnostic modality for three-dimensional (3D) display of vascular anatomy in suspected cases of pulmonary venous connection anomalies.
The detailed 3D anatomical information provided by MDCTA may be helpful for selecting the surgical strategy.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Correa-Villasenor A, Ferencz C, Boughman JA, et al. Total anomalous pulmonary venous circulation: familial and environmental factors. Teratology 1991;44:415–28 [DOI] [PubMed] [Google Scholar]
- 2.Hirsch JC, Bove EL. Total anomalous pulmonary venous connection. Multimedia Manual of Cardiothoracic Surgery. 2007. Available from:http//mmcts.oxfordjournals.org/content/2007/0507/mmcts.2006.002253.full.pdf DOI:10.1510/mmcts.2006.002253. [DOI] [PubMed] [Google Scholar]
- 3.Craig JM, Darling RC, Rothney WB. Total pulmonary venous drainage into the right side of the heart; report of 17 autopsied cases not associated with other major cardiovascular anomalies. Lab Invest 1957;6:44–64 [PubMed] [Google Scholar]
- 4.Karamlou T, Gurofsky R, Al Sukhni E, et al. Factors associated with mortality and reoperation in 377 children with total anomalous pulmonary venous connection. Circulation 2007;115:1591–8 [DOI] [PubMed] [Google Scholar]
- 5.Masui T, Seelos KC, Kersting-Sommerhoff BA, et al. Abnormalities of the pulmonary veins: evaluation with MR imaging and comparison with cardiac angiography and echocardiography. Radiology 1991;181:645–9 [DOI] [PubMed] [Google Scholar]
- 6.Didier D, Higgins CB, Fisher MR, et al. Congenital heart disease: gated MR imaging in 72 patients. Radiology 1986;158:227–35 [DOI] [PubMed] [Google Scholar]
- 7.Kim TH, Kim YM, Suh CH, et al. Helical CT angiography and three-dimensional reconstruction of total anomalous pulmonary venous connections in neonates and infants. AJR 2000;175:1381–6 [DOI] [PubMed] [Google Scholar]
- 8.Valsangiacomo ER, Levasseur S, McCrindle BW, et al. Contrastenhanced MR Angiography of pulmonary venous abnormalities in children. Pediatr Radiol 2003;33:92–8 [DOI] [PubMed] [Google Scholar]