Abstract
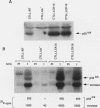
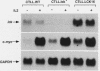
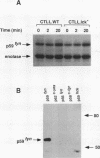
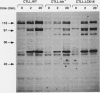
The growth, differentiation, and functional activities of antigen-stimulated T lymphocytes are regulated by the interaction of the T-cell-derived cytokine, interleukin-2 (IL-2), with the high-affinity IL-2 receptor (IL-2R). IL-2R occupancy initiates a rapid increase in intracellular protein tyrosine phosphorylation, suggesting that a receptor-coupled protein tyrosine kinase (PTK) serves as a proximal signaling element for the IL-2R. Previous studies implicated the src-family kinase, p56lck, as a potential IL-2R-linked signal transducer. In this study, we have characterized a spontaneous variant of the IL-2-dependent cytotoxic T-cell line, CTLL-2, which contains no detectable lck-derived mRNA transcripts, protein, or PTK activity. The p56lck-deficient CTLL-2 cells retained strict dependence on IL-2 for both viability and growth, indicating that p56lck activity was not required for the transduction of IL-2-mediated mitogenic signals. However, the p56lck-deficient cells exhibited a moderate decrease in their rate of IL-2-dependent proliferation. In contrast to this relatively modest proliferative defect, the p56lck-deficient cell line displayed a profound reduction in T-cell antigen receptor-dependent cytolytic effector functions. Both the proliferative and the cytolytic defects observed in the p56lck-deficient cells were completely reversed by transfection of these cells with a wild-type lck expression vector. These results indicate that p56lck expression is not obligatory for IL-2-mediated T-cell growth stimulation; however, this PTK plays a central role in the generation T-cell-mediated cytotoxic responses.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham N., Miceli M. C., Parnes J. R., Veillette A. Enhancement of T-cell responsiveness by the lymphocyte-specific tyrosine protein kinase p56lck. Nature. 1991 Mar 7;350(6313):62–66. doi: 10.1038/350062a0. [DOI] [PubMed] [Google Scholar]
- Amrein K. E., Sefton B. M. Mutation of a site of tyrosine phosphorylation in the lymphocyte-specific tyrosine protein kinase, p56lck, reveals its oncogenic potential in fibroblasts. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4247–4251. doi: 10.1073/pnas.85.12.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine J. A., Schlager J. W., Abraham R. T. Differential effects of interleukin-2 and interleukin-4 on protein tyrosine phosphorylation in factor-dependent murine T cells. Biochim Biophys Acta. 1990 May 2;1052(2):313–322. doi: 10.1016/0167-4889(90)90227-5. [DOI] [PubMed] [Google Scholar]
- Augustine J. A., Sutor S. L., Abraham R. T. Interleukin 2- and polyomavirus middle T antigen-induced modification of phosphatidylinositol 3-kinase activity in activated T lymphocytes. Mol Cell Biol. 1991 Sep;11(9):4431–4440. doi: 10.1128/mcb.11.9.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber E. K., Dasgupta J. D., Schlossman S. F., Trevillyan J. M., Rudd C. E. The CD4 and CD8 antigens are coupled to a protein-tyrosine kinase (p56lck) that phosphorylates the CD3 complex. Proc Natl Acad Sci U S A. 1989 May;86(9):3277–3281. doi: 10.1073/pnas.86.9.3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt A. L., Brunswick M., Bolen J. B., Mond J. J. Anti-immunoglobulin stimulation of B lymphocytes activates src-related protein-tyrosine kinases. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7410–7414. doi: 10.1073/pnas.88.16.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cooke M. P., Perlmutter R. M. Expression of a novel form of the fyn proto-oncogene in hematopoietic cells. New Biol. 1989 Oct;1(1):66–74. [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Changes in protein phosphorylation in Rous sarcoma virus-transformed chicken embryo cells. Mol Cell Biol. 1981 Feb;1(2):165–178. doi: 10.1128/mcb.1.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domzig W., Stadler B. M., Herberman R. B. Interleukin 2 dependence of human natural killer (NK) cell activity. J Immunol. 1983 Apr;130(4):1970–1973. [PubMed] [Google Scholar]
- Einspahr K. J., Abraham R. T., Dick C. J., Leibson P. J. Protein tyrosine phosphorylation and p56lck modification in IL-2 or phorbol ester-activated human natural killer cells. J Immunol. 1990 Sep 1;145(5):1490–1497. [PubMed] [Google Scholar]
- Farrar W. L., Ferris D. K. Two-dimensional analysis of interleukin 2-regulated tyrosine kinase activation mediated by the p70-75 beta subunit of the interleukin 2 receptor. J Biol Chem. 1989 Jul 25;264(21):12562–12567. [PubMed] [Google Scholar]
- Ferris D. K., Willette-Brown J., Ortaldo J. R., Farrar W. L. IL-2 regulation of tyrosine kinase activity is mediated through the p70-75 beta-subunit of the IL-2 receptor. J Immunol. 1989 Aug 1;143(3):870–876. [PubMed] [Google Scholar]
- Gillis S., Smith K. A. Long term culture of tumour-specific cytotoxic T cells. Nature. 1977 Jul 14;268(5616):154–156. doi: 10.1038/268154a0. [DOI] [PubMed] [Google Scholar]
- Grimm E. A., Robb R. J., Roth J. A., Neckers L. M., Lachman L. B., Wilson D. J., Rosenberg S. A. Lymphokine-activated killer cell phenomenon. III. Evidence that IL-2 is sufficient for direct activation of peripheral blood lymphocytes into lymphokine-activated killer cells. J Exp Med. 1983 Oct 1;158(4):1356–1361. doi: 10.1084/jem.158.4.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama M., Kono T., Kobayashi N., Kawahara A., Levin S. D., Perlmutter R. M., Taniguchi T. Interaction of the IL-2 receptor with the src-family kinase p56lck: identification of novel intermolecular association. Science. 1991 Jun 14;252(5012):1523–1528. doi: 10.1126/science.2047859. [DOI] [PubMed] [Google Scholar]
- Hatakeyama M., Mori H., Doi T., Taniguchi T. A restricted cytoplasmic region of IL-2 receptor beta chain is essential for growth signal transduction but not for ligand binding and internalization. Cell. 1989 Dec 1;59(5):837–845. doi: 10.1016/0092-8674(89)90607-7. [DOI] [PubMed] [Google Scholar]
- Hatakeyama M., Tsudo M., Minamoto S., Kono T., Doi T., Miyata T., Miyasaka M., Taniguchi T. Interleukin-2 receptor beta chain gene: generation of three receptor forms by cloned human alpha and beta chain cDNA's. Science. 1989 May 5;244(4904):551–556. doi: 10.1126/science.2785715. [DOI] [PubMed] [Google Scholar]
- Hefeneider S. H., Conlon P. J., Henney C. S., Gillis S. In vivo interleukin 2 administration augments the generation of alloreactive cytolytic T lymphocytes and resident natural killer cells. J Immunol. 1983 Jan;130(1):222–227. [PubMed] [Google Scholar]
- Horak I. D., Gress R. E., Lucas P. J., Horak E. M., Waldmann T. A., Bolen J. B. T-lymphocyte interleukin 2-dependent tyrosine protein kinase signal transduction involves the activation of p56lck. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1996–2000. doi: 10.1073/pnas.88.5.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T., Takeshita T., Numata N., Sugamura K. Protein phosphorylation mediated by IL-2/IL-2 receptor beta-chain interaction. J Immunol. 1988 Jul 1;141(1):174–179. [PubMed] [Google Scholar]
- Kamps M. P., Sefton B. M. Acid and base hydrolysis of phosphoproteins bound to immobilon facilitates analysis of phosphoamino acids in gel-fractionated proteins. Anal Biochem. 1989 Jan;176(1):22–27. doi: 10.1016/0003-2697(89)90266-2. [DOI] [PubMed] [Google Scholar]
- Kolber M. A., Quinones R. R., Henkart P. A. Target cell lysis by cytotoxic T lymphocytes redirected by antibody-coated polystyrene beads. J Immunol. 1989 Sep 1;143(5):1461–1466. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Benike C. J., Phillips J. H., Engleman E. G. Recombinant interleukin 2 enhanced natural killer cell-mediated cytotoxicity in human lymphocyte subpopulations expressing the Leu 7 and Leu 11 antigens. J Immunol. 1985 Feb;134(2):794–801. [PubMed] [Google Scholar]
- Leo O., Foo M., Sachs D. H., Samelson L. E., Bluestone J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo K. X., Sefton B. M. Cross-linking of T-cell surface molecules CD4 and CD8 stimulates phosphorylation of the lck tyrosine protein kinase at the autophosphorylation site. Mol Cell Biol. 1990 Oct;10(10):5305–5313. doi: 10.1128/mcb.10.10.5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marth J. D., Cooper J. A., King C. S., Ziegler S. F., Tinker D. A., Overell R. W., Krebs E. G., Perlmutter R. M. Neoplastic transformation induced by an activated lymphocyte-specific protein tyrosine kinase (pp56lck). Mol Cell Biol. 1988 Feb;8(2):540–550. doi: 10.1128/mcb.8.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marth J. D., Peet R., Krebs E. G., Perlmutter R. M. A lymphocyte-specific protein-tyrosine kinase gene is rearranged and overexpressed in the murine T cell lymphoma LSTRA. Cell. 1985 Dec;43(2 Pt 1):393–404. doi: 10.1016/0092-8674(85)90169-2. [DOI] [PubMed] [Google Scholar]
- Merida I., Gaulton G. N. Protein tyrosine phosphorylation associated with activation of the interleukin 2 receptor. J Biol Chem. 1990 Apr 5;265(10):5690–5694. [PubMed] [Google Scholar]
- Orosz C. G., Scott J. W., Gillis S., Finke J. H. Reversal of phorbol ester-mediated reduction of cloned T lymphocyte cytolysis by interleukin 2. J Immunol. 1985 Jan;134(1):324–329. [PubMed] [Google Scholar]
- Paya C. V., McKean D. J., Segal D. M., Schoon R. A., Showalter S. D., Leibson P. J. Heteroconjugate antibodies enhance cell-mediated anti-herpes simplex virus immunity. J Immunol. 1989 Jan 15;142(2):666–671. [PubMed] [Google Scholar]
- Perlmutter R. M., Marth J. D., Ziegler S. F., Garvin A. M., Pawar S., Cooke M. P., Abraham K. M. Specialized protein tyrosine kinase proto-oncogenes in hematopoietic cells. Biochim Biophys Acta. 1989 Feb;948(3):245–262. doi: 10.1016/0304-419x(89)90001-2. [DOI] [PubMed] [Google Scholar]
- Ruegemer J. J., Ho S. N., Augustine J. A., Schlager J. W., Bell M. P., McKean D. J., Abraham R. T. Regulatory effects of transforming growth factor-beta on IL-2- and IL-4-dependent T cell-cycle progression. J Immunol. 1990 Mar 1;144(5):1767–1776. [PubMed] [Google Scholar]
- Saltzman E. M., Thom R. R., Casnellie J. E. Activation of a tyrosine protein kinase is an early event in the stimulation of T lymphocytes by interleukin-2. J Biol Chem. 1988 May 25;263(15):6956–6959. [PubMed] [Google Scholar]
- Shaw A. S., Amrein K. E., Hammond C., Stern D. F., Sefton B. M., Rose J. K. The lck tyrosine protein kinase interacts with the cytoplasmic tail of the CD4 glycoprotein through its unique amino-terminal domain. Cell. 1989 Nov 17;59(4):627–636. doi: 10.1016/0092-8674(89)90008-1. [DOI] [PubMed] [Google Scholar]
- Shaw A. S., Chalupny J., Whitney J. A., Hammond C., Amrein K. E., Kavathas P., Sefton B. M., Rose J. K. Short related sequences in the cytoplasmic domains of CD4 and CD8 mediate binding to the amino-terminal domain of the p56lck tyrosine protein kinase. Mol Cell Biol. 1990 May;10(5):1853–1862. doi: 10.1128/mcb.10.5.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel J. P., Sharon M., Smith P. L., Leonard W. J. The IL-2 receptor beta chain (p70): role in mediating signals for LAK, NK, and proliferative activities. Science. 1987 Oct 2;238(4823):75–78. doi: 10.1126/science.3116668. [DOI] [PubMed] [Google Scholar]
- Teshigawara K., Wang H. M., Kato K., Smith K. A. Interleukin 2 high-affinity receptor expression requires two distinct binding proteins. J Exp Med. 1987 Jan 1;165(1):223–238. doi: 10.1084/jem.165.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G., Matsumoto-Kobayashi M., Clark S. C., Seehra J., London L., Perussia B. Response of resting human peripheral blood natural killer cells to interleukin 2. J Exp Med. 1984 Oct 1;160(4):1147–1169. doi: 10.1084/jem.160.4.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J. M., Brodsky M. H., Irving B. A., Levin S. D., Perlmutter R. M., Littman D. R. Interaction of the unique N-terminal region of tyrosine kinase p56lck with cytoplasmic domains of CD4 and CD8 is mediated by cysteine motifs. Cell. 1990 Mar 9;60(5):755–765. doi: 10.1016/0092-8674(90)90090-2. [DOI] [PubMed] [Google Scholar]
- Veillette A., Abraham N., Caron L., Davidson D. The lymphocyte-specific tyrosine protein kinase p56lck. Semin Immunol. 1991 May;3(3):143–152. [PubMed] [Google Scholar]
- Veillette A., Fournel M. The CD4 associated tyrosine protein kinase p56lck is positively regulated through its site of autophosphorylation. Oncogene. 1990 Oct;5(10):1455–1462. [PubMed] [Google Scholar]
- Vohr H. W., Hünig T. Induction of proliferative and cytotoxic responses in resting Lyt-2+ T cells with lectin and recombinant interleukin 2. Eur J Immunol. 1985 Apr;15(4):332–337. doi: 10.1002/eji.1830150405. [DOI] [PubMed] [Google Scholar]
- Zamoyska R., Derham P., Gorman S. D., von Hoegen P., Bolen J. B., Veillette A., Parnes J. R. Inability of CD8 alpha' polypeptides to associate with p56lck correlates with impaired function in vitro and lack of expression in vivo. Nature. 1989 Nov 16;342(6247):278–281. doi: 10.1038/342278a0. [DOI] [PubMed] [Google Scholar]