Abstract
An elderly woman presented febrile 5 days after stenting of multiple coronary arteries. Echocardiography showed a thickening of the aortic root, raising the possibility of stent infection. Four of four blood culture bottles grew Staphylococcus lugdunensis and repeat echo showed an aortic root abscess. Despite appropriate antibiotic treatment, the patient died. A 24-year-old man with a ventricular septal defect presented febrile 4 weeks after stenting of an aortic coarctation. Initial transoesophageal echo found no vegetations around the stent or elsewhere. Four of six blood culture bottles grew S lugdunensis. Following an episode of hypoxia, the imaging was repeated and a new large vegetation was seen on the pulmonary valve with two thin-walled cavities in the lungs on a CT pulmonary angiogram. The patient was treated with a long course of appropriate antibiotic therapy and discharged from hospital 6 weeks later.
Background
These are the first two cases in the medical literature of endovascular stent-associated infection with Staphylococcus lugdunensis, and therefore they represent a novel clinical finding. In addition, they illustrate an important concept, which is that although blood cultures growing ‘coagulase-negative staphylococci (CoNS)’ may often, correctly, be disregarded as contaminated, it is exceedingly important in this clinical setting that that does not happen. S lugdunensis is coagulase-negative, but, unlike most of the other organisms in the group, it is capable of producing fulminating infection similar to that caused by Staphylococcus aureus. Full identification to species level in the context of repeat blood cultures is key to enable diagnosis, treatment and prognosis, and therefore these cases will raise an awareness of the complication of endovascular stenting, particularly when undertaken via the femoral approach.
Case presentation
Case 1
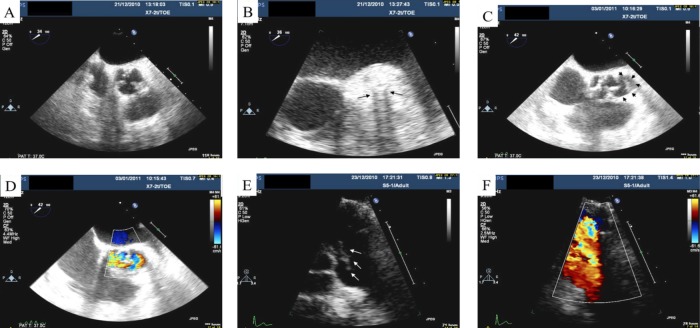
An elderly woman with a history of ischaemic heart disease presented with a 5-day history of fever after complex left main stem, proximal circumflex and left anterior descending artery stenting via the femoral approach. A Staphylococcus was isolated from two sets of blood cultures, which was coagulase negative but Staphaurex positive (Remel, Thermo Fisher Scientific). It was sensitive to penicillin, oxacillin, rifampicin and gentamicin and identified using phenotypic tests (API Staph profile 6716150, bioMérieux Clinical Diagnostics) as S lugdunensis. Treatment was started with intravenous vancomycin and gentamicin (she was allergic to penicillin). Transoesophageal echocardiography (TOE) findings suggested a thickening of the aortic root, raising the suspicion of stent infection (figure 1A,B). Subsequent echocardiographic follow-up detailed the development of a pericardial effusion and aortic root abscess (figure 1C,D). Despite supportive therapy, the patient died soon after.
Figure 1.
(A) Transoesophageal echocardiography showing a thickening of the aortic root, most prominent laterally around the left main stem stent. (B) Zoomed image focusing on the area of thickening. Note the tramline dropout artefact relating to the stent (black arrows). (C) Black arrowheads indicate the aortic root abscess. (D) With the addition of Doppler, colour flow is seen within the abscess. (E) Transthoracic echocardiogram with white arrows indicating the vegetation on the pulmonary valve. (F) A view of the pulmonary valve with colour Doppler.
Case 2
A 24-year-old man with a history of hypertension and subarterial ventricular septal defect (VSD) who had undergone stenting of an aortic coarctation via the right groin a month earlier re-presented with infective symptoms. He had also undergone catheterisation via the left groin for investigation of chest pain immediately post-stent insertion. A Staphylococcus was isolated from four of six blood culture bottles and identified (as above) as S. lugdunensis. This organism was again sensitive to penicillin, oxacillin, rifampicin and gentamicin. The same bacterium had also been isolated from an earlier blood culture taken by the patient's general practitioner, which had only been reported as ‘CoNS’. He was treated with intravenous benzylpenicillin and gentamicin plus oral rifampicin. A TOE showed a small sub-valvular VSD, no vegetations and no obvious infection surrounding the aortic stent. The patient then developed an episode of hypoxia. A CT pulmonary angiogram demonstrated two small, thin-walled cavities within the lungs. Subsequent transthoracic echocardiography demonstrated a new large vegetation on the pulmonary valve (PV) (with and without colour, figure 1E,F). His therapy was changed to intravenous vancomycin after he developed a drug reaction to penicillin. He was discharged from hospital after 6 weeks of appropriate antibiotic therapy.
Outcome and follow-up
Patient 1 unfortunately died as described in the case report. Patient 2 currently remains well after a year and a half of close follow-up.
Discussion
Most of the risk factors identified for endovascular stent-associated infection correlate either with maintenance of the arterial entry for the procedure (ie duration of procedure or arterial sheath in place for >1 day) or increased vascular injury. Patients typically present with fever, with or without chest pain, within a few days to weeks after their procedure.1 Infection may arise by direct contamination of the stent by femoral skin flora during insertion, or by transient bacteraemia from the same source. The first of our cases had classical echocardiographic findings and the second classical risk factors (in the context of a credible organism) to support diagnoses of endovascular stent-associated infection with S. lugdunensis.2 3 In the second case, infection of the coarct stent was difficult to demonstrate with imaging due to a dropout artefact; however, the development of vegetation on the PV some time after stent deployment points to an indwelling prosthetic device as the reservoir of infection.
Learning points.
Be reminded of the importance of insertion site preparation and aseptic technique when undertaking procedures relating to cardiovascular stenting, particularly via the femoral approach.
Be aware of the possible relevance of isolating ‘coagulase-negative staphylococci (CoNS)’ from blood cultures of patients who have recently undergone procedures involving endovascular stenting.
- Take further cultures and contact the microbiology/infectious diseases department without delay for:
- identification of CoNS to species level with full antimicrobial susceptibilities
- initiation of appropriate antibiotic therapy
- specialist consultation to oversee the progress of such cases.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Kaufmann BA, Kaiser C, Pfisterer ME, et al. Coronary stent infection: a rare but severe complication of percutaneous coronary intervention. Swiss Med Wkly 2005;135:483–7 [DOI] [PubMed] [Google Scholar]
- 2.Samore MH, Wessolossky MA, Lewis SM, et al. Frequency, risk factors, and outcome for bacteraemia after percutaneous translumial coronary angioplasty. Am J Cardiol 1997;79:873–7 [DOI] [PubMed] [Google Scholar]
- 3.Lessing MP, Crook DW, Bowler ICet al. Native-valve endocarditis caused by Staphylococcus lugdunensis. QJM 1996;89:855–8 [DOI] [PubMed] [Google Scholar]