Abstract
Cytomegalovirus (CMV) encephalitis is a rare infection that immunodeficient patients, mainly where HIV-positive, may suffer from. Several cases were described when complications with the treatment with monoclonal antibodies, like rituximab, for malignant lymphomas. We will describe here the case of a patient, who has developed CMV gastritis, then CMV encephalitis after the treatment of a CLL with a chemotherapy and maintenance therapy with rituximab.
Background
This case describes a rare infection, combining cytomegalovirus (CMV) gastritis and encephalitis. More and more patients are immunodeficient because of their pathology or treatment. They suffer from profound B-cell depletion or hypogammaglobulinaemia, further viral infection. These ones can mimic many symptoms and it is important to think about them.
Case presentation
A 75-year-old man was first seen for hyperlymphocytosis and the presence of Gumprecht shadows (or smudge cells: artefact that result from the rupture of fragile lymphocytes secondary to the process of making the blood film, common in chronic lymphocytic leukaemia). The immunophenotype of circulating lymphocytes showed positivity for CD5, CD19, CD20 and CD23 and negativity for CD38, FMC-7, CD22. A surface immunoglobulin of lambda type with high density was found. The Matutes score was calculated at 3. Conventional cytogenetic analysis and fluorescence in situ hybridisation (FISH) showed the presence of a trisomy 12, which is compatible with the diagnosis of atypical chronic lymphocytic leukaemia. The patient was treated with an association of chlorambucil and prednisone resulting in stable disease. Then, the patient received six cycles of fludarabine, cyclophosphamide and rituximab with complete remission. Three years after this treatment the reappearance of lymphocytes with identical phenotype was noticed (first diagnostic). They were associated to palpable splenomegaly. The patient received six cycles of fludarabine mitoxantrone and dexamethasone, and then consolidation therapy with rituximab 375 mg/m2, every 2 months for 1 year.
Six months after the end of the consolidation therapy, the patient was seen: he had lost 10 kg of weight, and suffered from epigastric pain, anorexia, (especially for solids) and dyspepsia. No hypertrophy of the lymphatic organs was noted. The symptoms were resistant to classic proton pump inhibitors.
Investigations
The patient underwent fibroscopy and that one showed multiple stenosing ulcers. Histopathological analysis of biopsies showed the presence of an infiltrate made by small lymphocytes CD3 and CD20 negatives (polyclonal reactive cells). The presence of irregular, cyanophilic ‘bird's eye’ inclusions was noticed. Gastric cells were also found positive for CMV antigens (Chemicon Abcys Valbyotech MAB 815–500 μg, dilution 1/60, immunohistochemistry). Blood tests showed deep hypogammaglobulinaemia associated to moderate lymphopenia composed of less than 0.2% of B lymphocyte and 96% of T lymphocyte. The level of CD4 cells was at 680/µl.
Differential diagnosis
In blood, PCR CMV were negative. All others PCR were negative too: herpes simplex virus (HSV), Epstein-barr virus (EBV), human herpesvirus 8 (HHV8), human herpes virus 6 (HHV6), toxoplasmosis and Aspergillus.
Treatment
The final diagnosis was CMV gastritis, secondary to immunodepression and an antiviral treatment was started by CIDOFOVIR at 5 mg/kg at days 1 and 8, followed by VALGANCICLOVIR maintenance at 450 mg to be taken twice daily. Two weeks after the end of the treatment of CIDOFOVIR, the patient had aphasia of broca, right hemiparesis. The patient stopped the treatment by VALGANCICLOVIR. He was send to an emergency unit, where a CT showed the presence of nodular, fronto-temporo-parietal tumour, thus suspecting lymphoma localisation. The patient underwent stereotaxic biopsies which showed the presence of a reactive T-cell infiltrate with viral inclusions and a vascular hypertrophy, but no histological signs of the recurrence of lymphoma, no T monoclonality and no glial proliferation (figure 1).
Figure 1.
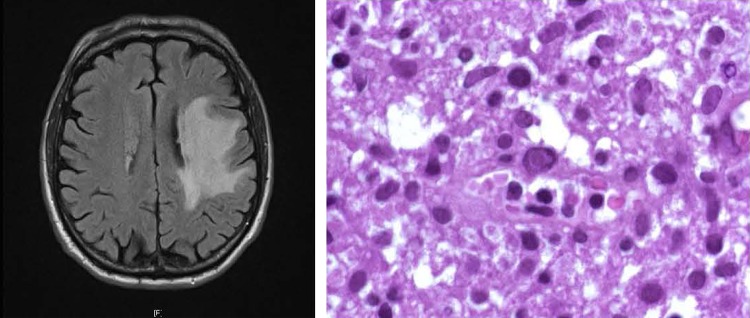
Image of tumoral lesion (left) and images of typical viral inclusions (right) in cytomegalovirus reactivation in patients treated with rituximab maintenance.
Outcome and follow-up
The patient restarted the VALGANCICLOVIR at an induction dose of 30 mg/kg daily for 3 weeks with partial recovery, and then maintenance therapy at 15 mg/kg daily. CT scan, performed at 3 months, did not show any evolution of the tumour.
Discussion
Nowadays, the consensus on the treatment of CLL associates chemotherapy to monoclonal antibodies.1 The treatment with anti-CD20 antibodies associates a deep and slow recovering B lymphocytes depletion with severe hypogammaglobulinemia.2 In this condition of severe immunodepression, several cases of viral infection/reactivation were described with viruses like: CMV, HBV, HCV, varicella, JC John Cunningham virus, enterovirus and parvovirus.3 These viral infections are mainly controlled either by cellular immunity (varicella zoster virus, CMV, JC virus) or by the humoral immune response (enterovirus, hepatitis B virus, parvovirus B19). CMV reactivation are very rare in an immunocompetent host, frequently associated to HIV evolution where the level of CD4 is very low (under 200/μl) and sometimes described after the treatment with monoclonal antibodies and chemotherapy:3 4 the B lymphopenia, and therefore, lack of B-cell regulation, interferes with activation of T cells,5 which are essential to neutralise microorganisms.
Severe CMV reactivation varies from asymptomatic to severe syndromes such as interstitial pneumonia, retinitis, hepatitis, encephalitis or disseminated forms and is a source of significant morbidity and mortality in immunocompromised hosts.6 Meningoencephalitic infections, a very rare complication, could happen in many ways:
Diffuse micronodular encephalitis with lesions of the white and grey matter and clinical presentations that varies from encephalitis to demence.
Ventriculitis touching ependyme and necrosis of the periventricular zones; the symptoms are multiple varying from confusion, autism, paralysis of cranial nerves, nystagmus or ataxia.
Polyradiculonevritis involving peripheral nerves with symptoms like paresthesia, pain, areflexia or diminution of muscle strength.7
The patient was treated for atypical chronic lymphocytic leukaemia with immunosuppressive chemotherapy associated to monoclonal antibody immunotherapy. Next, the patient underwent maintenance therapy with rituximab. Initially, the patient was diagnosed with CMV gastritis in the context of severe B depletion, due to the treatment with rituximab. After specific antiviral treatment the patient developed symptoms that made us in dilemma about a possible evolution of the lymphoma in central nervous system or a viral complication. The stereotaxic biopsies favoured the hypothesis of viral infection and T-cell reaction normal infiltrate. The diagnosis of CMV infection can be dissociated, with positivity of antibodies or antigens in cerebrospinal fluid or biopsies but negative in blood.8 After the treatment, the symptoms diminished and CT scan confirmed that positive evolution.
It is well established that T-cell response is the major effector mechanism for controlling CMV replication and dissemination in the organism. Rituximab maintenance therapy induces a deep and persistent B-cell depletion sometimes resulting in severe hypogammaglobulinemia. CMV reactivation can be explained by a difficult recovery of T-helper lymphocytes and an altered viral antigen presentation, due to the lack of regulatory B cells. Cases of severe, lethal CMV reactivation after treatment with rituximab have already been described.9
Learning points.
The evolution of treatment of lymphoma and the evolving concepts of maintenance therapy makes the use of monoclonal antibodies very abundant.
The deep and persistent B-cell depletion induced by this therapy may alter the viral antigen presenting T helper cells, thus favouring viral reactivations, sometimes with very grave issues.
Viral reactivation must be taken into consideration in the case of infectious complications or unusual meningitis or encephalitis symptoms.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Keating MJ, O'Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol 2005;23:4079–88 [DOI] [PubMed] [Google Scholar]
- 2.Gea-Banacloche JC. Rituximab-associated infections. Semin Hematol 2010;47:187–98 [DOI] [PubMed] [Google Scholar]
- 3.Kelesidis T, Daikos G, Boumpas D, et al. Does rituximab increase the incidence of infectious complications? A narrative review. Int J Infect Dis 2011;15:e2–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aksoy S, Harputluoglu H, Kilickap S, et al. Rituximab-related viral infections in lymphoma patients. Leuk Lymphoma 2007;48:1307–12 [DOI] [PubMed] [Google Scholar]
- 5.Berthelot J-M, Jamin C, Amrouche K, et al. Regulatory B cells play a key role in immune system balance. Joint Bone Spine (Internet) 2012. juill 31 (cité 2012 sept 3); http://www.ncbi.nlm.nih.gov/pubmed/22858147 [DOI] [PubMed] [Google Scholar]
- 6.Hubacek P, Keslova P, Formankova R, et al. Cytomegalovirus encephalitis/retinitis in allogeneic haematopoietic stem cell transplant recipient treated successfully with combination of cidofovir and foscarnet. Pediatr Transplant 2009;13:919–22 [DOI] [PubMed] [Google Scholar]
- 7.Miller GG, Boivin G, Dummer JS, et al. Cytomegalovirus ventriculoencephalitis in a peripheral blood stem cell transplant recipient. Clin Infect Dis 2006;42:e26–9 [DOI] [PubMed] [Google Scholar]
- 8.Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis 2002;34:1094–7 [DOI] [PubMed] [Google Scholar]
- 9.Suzan F, Ammor M, Ribrag V. Fatal reactivation of cytomegalovirus infection after use of rituximab for a post-transplantation lymphoproliferative disorder. N Engl J Med 2001;345:1000. [DOI] [PubMed] [Google Scholar]


