Abstract
Traffic-derived particulate matter (PM) is associated with cardiovascular morbidity and mortality, but the mechanism of this association is unclear. Prothrombotic processes have been linked to PM in epidemiological and animal models, but have not been consistently implicated in controlled human models. Diesel exhaust (DE) is a major contributor to PM. We conducted a controlled human exposure of DE in subjects with metabolic syndrome. The study objective was to evaluate DE exposure effects on prothrombotic markers in a population vulnerable to cardiovascular disease. A randomized, crossover, double-blinded design was used: 16 subjects with metabolic syndrome exposed on 3 different days (≥2 wk washout) to DE at 0 (filtered air, FA), 100 μg PM2.5/m3 (DE100) and 200 μg PM2.5/m3 (DE200). We assessed DE-associated changes in D-dimer, von Willebrand factor (VWF), and plasmin activator inhibitor-1 (PAI-1) at 3, 7, and 22 h after exposure initiation. A DE200-attributable decrease (1.17-fold; CI 1.04 to 1.34) in VWF was noted at 7 h. Significant changes did not occur in other primary endpoints. As previously noted with healthy subjects, strong diurnal patterns in PAI-1 were observed. Thus, in a novel study, we were unable to demonstrate a prothrombotic effect of moderate-dose diesel exhaust exposure in a population at risk for cardiovascular disease.
Proposed mechanisms for the association between particulate matter (PM) and cardiovascular disease (Miller et al., 2007) include endothelial dysfunction, alteration of autonomic control, systemic inflammation, and increase in coagulability (Brook et al., 2004). We previously reported a study showing little effect of diesel exhaust (DE) on markers of thrombosis in healthy subjects (Carlsten et al., 2007). Considering that a population particularly vulnerable to cardiovascular disease may most clearly demonstrate PM-related thrombotic phenomena (Palomo et al., 2006), we performed a similar investigation on subjects with the metabolic syndrome. We report findings regarding thrombotic markers in a controlled human experiment of exposure to DE, a major contributor to PM (U.S. EPA, 2000) in this population at higher risk of cardiovascular disease (Grundy, 2007).
Concern for a prothrombotic effect of PM is supported by limited epidemiologic evidence in both general (Baccarelli et al., 2007; Peters et al., 1997; Riediker et al., 2004) and susceptible populations (Peters et al., 1997; Rückerl et al., 2006, 2007a, 2007b). However, data from controlled human DE-exposure models are inconclusive, with studies of both susceptible populations (Blomberg et al., 2005) and a healthy population (Carlsten et al., 2007) unable to demonstrate increased prothrombotic factors, while another study of healthy subjects suggested impaired fibrinolysis associated with DE (Mills et al., 2005).
Cardiovascular morbidity and mortality has been associated with acute or subacute changes in D-dimer (Menown et al., 2003), von Willebrand factor (vWF) (Collet et al., 2003), and plasmin activator inhibitor-1 (PAI-1) (Sinkovic & Pogacar, 2004). Based on the inconsistent findings of prior controlled DE exposure studies regarding DE-induced hyperthrombosis and the strength of evidence suggesting that D-dimer, vWF, and PAI-1 are associated with acute myocardial infarction, we hypothesized that DE induces a subclinical hyperthrombotic state within several hours of exposure initiation, reflected by increases in these peripheral blood markers in those with the metabolic syndrome.
METHODS
Subject Recruitment and Inclusion Criteria
Subjects were eligible to participate in the study if they fulfilled the following inclusion criteria: age 18–49 yr; nonsmoking status at least 6 mo prior to recruitment; no history of ongoing medical care for heart disease or asthma; lack of arrhythmia or ischemia on electrocardiograph (ECG); normal spirometry (MicroDL, Micro Medical Ltd, Kent, UK); and having the metabolic syndrome (3 or more of the following 5 criteria [Grundy et al., 2005]: elevated waist circumference [men, equal to or greater than 40 inches; women, equal to or greater than 35 inches]; elevated triglycerides [equal to or greater than 150 mg/dl]; reduced HDL cholesterol [men, less than 40 mg/dl; women, less than 50 mg/dl]; elevated blood pressure [equal to or greater than 130/85 mm Hg]; elevated fasting glucose [equal to or greater than 100 mg/dl]). Subjects were required to be off blood thinning medications, statins, and antioxidants. Women of childbearing age underwent a urine pregnancy test at screening and before each exposure, and were instructed to practice effective contraception during the study. Power calculation was based on a prior study of effects of PM2.5 on vWF in a diabetic population (O’Neill et al., 2007), with 16 subjects thereby estimated to be needed for 80% power to detect a 25% increase in vWF. All subjects gave a written informed consent prior to the screening process. The consent form and study protocol were approved by the University of Washington Human Subjects Review Division.
Experimental Design
A crossover design was used, in which study participants were exposed on three different days to each of three different concentrations (separated by at least 2 wk), double-blinded, randomized, and counterbalanced to order. Exposure was calibrated at 0 (filtered air, FA), 100 μg/m3 (DE100), and 200 μg/m3 (DE200) of particulate DE, based on the mass of particles less than 2.5 microns in diameter (PM2.5). Exhaust was generated from a 2002 turbocharged direct injection 5.9-L Cummins B-series engine (6BT5.9G6, Cummins, Inc., Columbus, IN) via 100-kW generator distributed to a 116-m3 exposure room after a two-phase dilution of exhaust provided a total dilution of 400:1. Temperature and relative humidity were maintained at 18°C and 60%, respectively, and particle source composition (carbon and trace elements) was similar to that of the U.S. Environmental Protection Agency (EPA) light-duty diesel profile (Gould et al., 2008). Subjects, staff interacting with subjects, and staff conducting outcome assessments were blinded to exposure level.
Subjects fasted overnight and during the exposure. They ate a standardized meal, with the same food content and quantity, after each exposure. Exposure began consistently within 30 min of 9 a.m. on weekdays and lasted 2 h, during which time subjects were resting.
D-dimer, VWF, and PAI-1 were measured from a peripheral venous blood sample drawn from an indwelling iv catheter approximately 30 min prior to exposure (0 h) and at 3 h, 7 h, and 22 h after the start of each exposure.
D-dimer antigen and VWF antigen (Diagnostica Stago, Parsippany, NJ) and PAI-1 activity (Trinity Biotech, St. Louis, MO) were measured by enzyme-linked immunosorbent assay (ELISA).
Statistical Analysis
Our primary analyses evaluated DE-attributable changes (from baseline) in the following measures: D-dimer and VWF at 7 h and PAI-1 at 22 h. Secondary analyses evaluated DE-attributable changes in D-dimer and VWF at 3 h and 22 h and PAI-1 at 7 h.
To perform such analyses, raw outcome variable values were log-transformed in order to normalize their distributions. Subsequently, changes in each endpoint over the prescribed time intervals were assessed by paired t-tests for the FA and DE200 exposures. To directly assess DE-attributable changes (“DE effect”), these exposure-specific changes were compared to each other by paired t-tests. Post hoc sensitivity analyses previously described (Carlsten et al., 2007) were performed to assess the DE100 exposure. Finally, an analysis of variance (ANOVA) model inclusive of period and carryover effect terms was performed to assess possible such effects.
All analyses were performed with Stata version 8.0 (Stata Corp, College Station, TX).
RESULTS
Sixteen subjects with the metabolic syndrome provided data at both the FA and DE200 exposures. Demographics of the subjects are found in Table 1. The 16 subjects were relatively young, with a median age of 39 yr. Levels of gaseous copollutants (Table 2) were low, with nitrogen dioxide ranging from 15–30 ppb and carbon monoxide ranging between 0.2–0.65 ppm.
TABLE 1.
Demographics and metabolic syndrome data
| Age, median years (range) | 39 (25–48) |
| Gender, M:F | 10:6 |
| Race, Caucasian:other | 12:4 |
| BMI (kg/m2)a | 41.1 ± 7.2 |
| HDL cholesterol (mg/dl)a | 36.9 ± 4.7 |
| TG (mg/dl)a | 172.4 ± 111.2 |
| Glucose (mg/dl)a | 99.9 ± 10.7 |
| SBP (mm Hg)a | 123.7 ± 10.3 |
| DBP (mm Hg)a | 83.5 ± 7.9 |
Mean ± SD.
TABLE 2.
Exposure characteristics, mean values (SEM)
| Filtered air | DEa | |
|---|---|---|
| PM2.5 (μg/m3) | 5.14 (0.60) | 206.43 (1.36) |
| NO (ppb) | 34.13 (5.76) | 1685.92 (72.42) |
| NO2 (ppb) | 15.02 (0.67) | 30.34 (1.46) |
| CO (ppm) | 0.20 (0.05) | 0.65 (0.08) |
200 μg PM2.5/m3 (nominal concentration).
No adverse effects were reported.
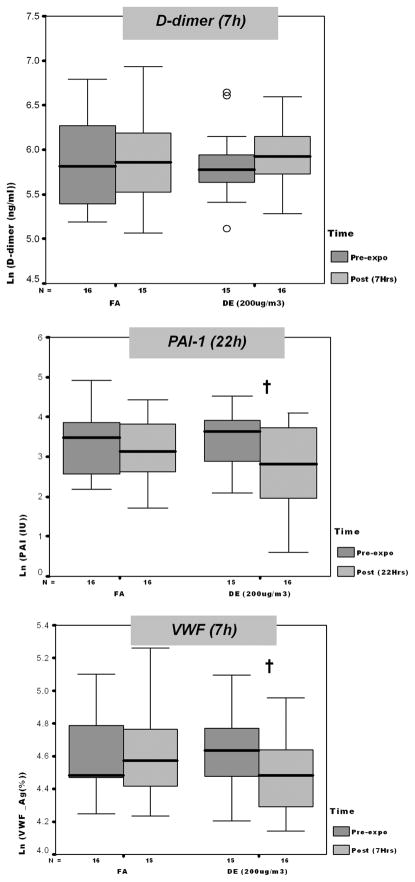
As shown in Figure 1 and Table 3, there were significant decreases in VWF associated with DE200 at 7 h and significant decreases in PAI-1 associated with DE200 at 22 h. However, DE200-attributable effects, after accounting for diurnal variability, were significant only for VWF. Changes in other primary analysis endpoints associated with FA were not significantly different from changes associated with DE200.
FIG. 1.
Coagulation marker changes from 0 to 7 h postexposure, by exposure level, except PAI-1 (22 h). Each panel depicts medians (bars) and interquartile ranges (whiskers) for each marker associated with given dose and time point. Open circles represent data points outside of interquartile range. FA, filtered air; DE, 200 μg/m3 (PM2.5) diesel exhaust. Dagger indicates 0 versus 7 h (or for PAI-1, 22 h) fold-change significant at p < .05 (paired t-test).
TABLE 3.
Coagulation markers, change by exposure (filtered air vs. diesel exhaust)
| Change from pre- to postexposure, folda (95% CI) | |||
|---|---|---|---|
| FA | DE (200 μg PM2.5/m3) | DE effectb | |
| D-dimer (ng/ml) | 1.04 (−1.16, 1.25) | 1.12 (−1.08, 1.35) | 1.05 (−1.16, 1.27) |
| VWF (antigen %) | 1.02 (−1.08, 1.14) | −1.13 (−1.22, −1.03)c | −1.17 (−1.34, −1.04)d |
| PAI-1 (IU) | −1.25 (−1.92, 1.25) | −2.19 (−3.22, −1.48)e | −1.70 (−3.35, 1.15) |
Fold change (using log-transformed data) between preexposure and 7 h postexposure initiation (D-dimer and VWF) or between preexposure and 22 h postexposure initiation (PAI-1), paired t-test.
Fold difference, with positive difference indicating increase in given marker over DE exposure interval relative to change over FA exposure interval (paired t-test).
Significant at p = .007.
Significant at p = .02.
Significant at p = .0007.
Significant among secondary analyses was the following: PAI-1 diurnal effects were noted at 7 h, with 5.7-fold decrease associated with FA, p < .0001, and 7.39-fold decrease associated with DE, p < .0001; however, there was no DE effect, p = .16. Changes at secondary time points were otherwise nonsignificant.
There were no carryover or period effects. Results were insensitive to removal of outliers, as there was a nonsignificant sharpening of trends noted in primary analyses. There was no significant change in primary endpoints using repeated measures ANOVA regression including DE100 data.
DISCUSSION AND CONCLUSIONS
Using a controlled human exposure approach and a limited set of markers, we were not able to show a prothrombotic effect of diesel exhaust on select circulating markers in subjects with the metabolic syndrome. To our knowledge, the effect of traffic-derived particulate matter on such endpoints in those with metabolic syndrome has not previously been studied, despite evidence that those with atherosclerosis-prone syndromes may be at higher risk for PM-related outcomes than are those with diabetes alone (Goldberg et al., 2006). Unlike our null results, O’Neill et al. (2007) documented statistically significant increase in plasma VWF in association with PM exposure at longer moving averages, though VWF did not increase at most time points examined. They also document a consistent increase in plasma VCAM-1 in association with fine particles.
It is important to explore the reasons for which our hypothesis was not supported by the data from our study. There may in fact be no prothrombotic effect of these brief exposures, but there are several alternative explanations for our findings. One is our choice of markers; we limited our markers based on the rationale outlined in the introduction, but it is possible that other endpoints, including markers of platelet activation or VWF activity (as a marker of endothelial injury), would have been more sensitive. Furthermore, given the evidence from prior studies and this one, future mechanistic studies may be better focused on details of impaired fibrinolysis specifically (Mills et al., 2005), including release of tissue plasminogen activator (Mills et al., 2007) and/or increased generation of intravascular thrombin (Mutlu et al., 2007); it is possible that many circulating markers are simply not sensitive enough to capture physiology occurring at the level of the human thrombus. Furthermore, some changes may be best reflected in conjunction with cofactors, such as bradykinin (Mills et al., 2005). Another potential explanation for our null study is that individuals with metabolic syndrome may have increased expression of antioxidant enzymes or other protective mechanisms upregulated by a chronic inflammatory state, which overcomes any insult of the DE exposure in our model. However, existing laboratory evidence suggests that those with metabolic syndrome have depleted antioxidant capabilities (Grattagliano et al., 2007; Roberts et al., 2006). Another important possibility for our null results is that our exposure system and consequent exhaust was significantly different from the exposure environments in other studies. In particular, the results of Mills et al. (2005, 2007) were obtained via an exposure system that produced considerably more nitrogen dioxide in the exposure atmosphere. Because nitrogen dioxide has been associated with increased coagulability (Baccarelli et al., 2007), this difference in gaseous copollutants between the exhaust systems could lead to the disparate findings. Also, the studies of Mills et al. were at higher concentrations of PM (300 μg/m3), which could lead to more systemic effects that did our concentrations; notably, the concentrations in our study are closer to those commonly found in most current urban environments, with the exception of specific occupational scenarios and some ambient settings in the developing world. Finally, our study may have been limited by sample size, although it was powered appropriately for the primary endpoints and our confidence intervals exclude a major increase in the measured markers.
It is also important to explore the difference between our findings and epidemiological studies’ documentation of increases in some of the same markers we studied. We would anticipate that the experimental design we employed would be substantially more robust than epidemiological approaches to control the confounding factors of daily life, which inevitably impact the measured analytes. So even though the considerably larger sample sizes impart these epidemiological studies with increased statistical power to detect changes in such markers, there is reason to believe that confounding and exposure misclassification limit conclusions from those studies. Furthermore, in spite of attempts to control for copollutants and other potential confounders such as temperature and humidity, epidemiological approaches are fundamentally more susceptible to such confounding, hidden or incompletely controlled, than is a controlled exposure model such as we used. Ultimately, it may be that thrombotic (and/or antithrombolytic) mechanisms involving circulating mediators are not predominant in explaining the consistent evidence linking PM (or diesel exhaust specifically) exposure to cardiovascular phenomena (Miller et al., 2007; Mills et al., 2007), but more research is clearly needed before drawing such a conclusion.
In summary of this novel study, we were unable to demonstrate, using a limited set of markers, a prothrombotic effect of moderate-dose diesel exhaust exposure on a population vulnerable to cardiovascular disease. VWF decreased slightly with 200 μg/m3 DE, but other prothrombotic markers were unchanged in adults with the metabolic syndrome. As the biological plausibility of the decrease in VWF at 7 h is uncertain, this study should be replicated before assessing its insight into the mechanism of air pollution’s cardiovascular effects. We suggest that future studies should include measures of platelet activation, VWF activity (as a marker of endothelial injury), multiple measures of fibrinolysis, efforts to explore endothelial aspects of thrombosis, and possibly multiple levels of gaseous copollutants.
Acknowledgments
Support for this study was provided by grants R830954 and R827355 from the U.S. Environmental Protection Agency, M01RR-00037 from the National Institutes of Health (University of Washington–General Clinical Research Center), and ES013195, ES011139, and P30ES07033 (University of Washington–Center for Ecogenetics and Environmental Health) from the National Institute for Environmental Health Sciences. We thank Mary Aulet, Timothy Gould, Karen Jansen, Sara Jarvis, Jim Stewart, and the University of Washington’s General Clinical Research Center staff for invaluable assistance with the conduct of this study; Anne Ho for assistance with manuscript preparation; Wayne Chandler for reviewing the manuscript; and the study subjects for their willingness to contribute.
References
- Baccarelli A, Zanobetti A, Martinelli I, Grillo P, Hou L, Giacomini S, Bonzini M, Lanzani G, Mannucci PM, Bertazzi PA, Schwartz J. Effects of exposure to air pollution on blood coagulation. J Thromb Haemost. 2007;5(2):252–260. doi: 10.1111/j.1538-7836.2007.02300.x. [DOI] [PubMed] [Google Scholar]
- Blomberg A, Tornqvist H, Desmyter L, Deneys V, Hermans C. Exposure to diesel exhaust nanoparticles does not induce blood hypercoagulability in an at-risk population. J Thromb Haemost. 2005;3(9):2103–2105. doi: 10.1111/j.1538-7836.2005.01559.x. [DOI] [PubMed] [Google Scholar]
- Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, Luepker R, Mittleman M, Samet J, Smith SC, Jr, Tager I. Air pollution and cardiovascular disease: A statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109(21):2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- Carlsten C, Kaufman JD, Peretz A, Trenga CA, Sheppard L, Sullivan JH. Coagulation markers in healthy human subjects exposed to diesel exhaust. Thromb Res. 2007;120(6):849–855. doi: 10.1016/j.thromres.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collet JP, Montalescot G, Vicaut E, Ankri A, Walylo F, Lesty C, Choussat R, Beygui F, Borentain M, Vignolles N, Thomas D. Acute release of plasminogen activator inhibitor-1 in ST-segment elevation myocardial infarction predicts mortality. Circulation. 2003;108(4):391–394. doi: 10.1161/01.CIR.0000083471.33820.3C. [DOI] [PubMed] [Google Scholar]
- Goldberg MS, Burnett RT, Yale JF, Valois MF, Brook JR. Associations between ambient air pollution and daily mortality among persons with diabetes and cardiovascular disease. Environ Res. 2006;100(2):255–267. doi: 10.1016/j.envres.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Gould T, Larson T, Stewart J, Kaufman JD, Slater D, McEwen N. A controlled inhalation diesel exhaust exposure facility with dynamic feedback control of PM concentration. Inhal Toxicol. 2008;20(1):49–52. doi: 10.1080/08958370701758478. [DOI] [PubMed] [Google Scholar]
- Grattagliano I, Palmieri VO, Portincasa P, Moschetta A, Palasciano G. Oxidative stress-induced risk factors associated with the metabolic syndrome: A unifying hypothesis. J Nutr Biochem. 2007;19(8):491–504. doi: 10.1016/j.jnutbio.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Grundy SM. Metabolic syndrome: A multiplex cardiovascular risk factor. J Clin Endocrinol Metab. 2007;92(2):399–404. doi: 10.1210/jc.2006-0513. [DOI] [PubMed] [Google Scholar]
- Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- Menown IB, Mathew TP, Gracey HM, Nesbitt GS, Murray P, Young IS, Adgey AA. Prediction of recurrent events by D-dimer and inflammatory markers in patients with normal cardiac troponin I (PREDICT) study. Am Heart J. 2003;145(6):986–992. doi: 10.1016/S0002-8703(03)00169-8. [DOI] [PubMed] [Google Scholar]
- Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, Kaufman JD. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356(5):447–458. doi: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- Mills NL, Tornqvist H, Gonzalez MC, Vink E, Robinson SD, Soderberg S, Boon NA, Donaldson K, Sandstrom T, Blomberg A, Newby DE. Ischemic and thrombotic effects of dilute diesel-exhaust inhalation in men with coronary heart disease. N Engl J Med. 2007;357(11):1075–1082. doi: 10.1056/NEJMoa066314. [DOI] [PubMed] [Google Scholar]
- Mills NL, Tornqvist H, Robinson SD, Gonzalez M, Darnley K, MacNee W, Boon NA, Donaldson K, Blomberg A, Sandstrom T, Newby DE. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation. 2005;112(25):3930–3936. doi: 10.1161/CIRCULATIONAHA.105.588962. [DOI] [PubMed] [Google Scholar]
- Mutlu GM, Green D, Bellmeyer A, Baker CM, Burgess Z, Rajamannan N, Christman JW, Foiles N, Kamp DW, Ghio AJ, Chandel NS, Dean DA, Sznajder JI, Budinger GR. Ambient particulate matter accelerates coagulation via an IL-6-dependent pathway. J Clin Invest. 2007;117(10):2952–2961. doi: 10.1172/JCI30639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill MS, Veves A, Sarnat JA, Zanobetti A, Gold DR, Economides PA, Horton ES, Schwartz J. Air pollution and inflammation in type 2 diabetes: a mechanism for susceptibility. Occup Environ Med. 2007;64(6):373–379. doi: 10.1136/oem.2006.030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomo I, Alarcon M, Moore-Carrasco R, Argiles JM. Hemostasis alterations in metabolic syndrome (review) Int J Mol Med. 2006;18(5):969–974. [PubMed] [Google Scholar]
- Peters A, Doring A, Wichmann HE, Koenig W. Increased plasma viscosity during an air pollution episode: A link to mortality? Lancet. 1997;349 (9065):1582–1587. doi: 10.1016/S0140-6736(97)01211-7. [DOI] [PubMed] [Google Scholar]
- Riediker M, Cascio WE, Griggs TR, Herbst MC, Bromberg PA, Neas L, Williams RW, Devlin RB. Particulate matter exposure in cars is associated with cardiovascular effects in healthy young men. Am J Respir Crit Care Med. 2004;169(8):934–940. doi: 10.1164/rccm.200310-1463OC. [DOI] [PubMed] [Google Scholar]
- Roberts CK, Barnard RJ, Sindhu RK, Jurczak M, Ehdaie A, Vaziri ND. Oxidative stress and dysregulation of NAD(P)H oxidase and antioxidant enzymes in diet-induced metabolic syndrome. Metabolism. 2006;55(7):928–934. doi: 10.1016/j.metabol.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Rückerl R, Ibald-Mulli A, Koenig W, Schneider A, Woelke G, Cyrys J, Heinrich J, Marder V, Frampton M, Wichmann HE, Peters A. Air pollution and markers of inflammation and coagulation in patients with coronary heart disease. Am J Respir Crit Care Med. 2006;173(4):432–441. doi: 10.1164/rccm.200507-1123OC. [DOI] [PubMed] [Google Scholar]
- Rückerl R, Greven S, Ljungman P, Aalto P, Antoniades C, Bellander T, Berglind N, Chrysohoou C, Forastiere F, Jacquemin B, von Klot S, Koenig W, Küchenhoff H, Lanki T, Pekkanen J, Perucci CA, Schneider A, Sunyer J, Peters A. Air pollution and inflammation (interleukin-6, C-reactive protein, fibrinogen) in myocardial infarction survivors. Environ Health Perspect. 2007a;115(7):1072–1080. doi: 10.1289/ehp.10021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rückerl R, Phipps RP, Schneider A, Frampton M, Cyrys J, Oberdörster G, Wichmann HE, Peters A. Ultra-fine particles and platelet activation in patients with coronary heart disease—Results from a prospective panel study. Part Fibre Toxicol. 2007b;4:1. doi: 10.1186/1743-8977-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkovic A, Pogacar V. Risk stratification in patients with unstable angina and/or non-ST-elevation myocardial infarction by Troponin T and plasminogen-activator-inhibitor-1 (PAI-1) Thromb Res. 2004;114(4):251–257. doi: 10.1016/j.thromres.2004.06.040. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency. National air pollutant emission trends. Office of Air Quality Planning and Research; 2000. pp. 3–14. 1990–1998, http://www.epa.gov/ttn/chief/trends/trends98/trends98.pdf, Table 3–6. [Google Scholar]