Abstract
The Histidine Triad Proteins (HTPs), also known as Pht proteins in Streptococcus pneumoniae, constitute a family of surface-exposed proteins that exist in many pathogenic streptococcal species. Although many studies have revealed the importance of HTPs in streptococcal physiology and pathogenicity, little is known about their origin and evolution. In this study, after identifying all htp homologs from 105 streptococcal genomes representing 38 different species/subspecies, we analyzed their domain structures, positions in genome, and most importantly, their evolutionary histories. By further projecting this information onto the streptococcal phylogeny, we made several major findings. First, htp genes originated earlier than the Streptococcus genus and gene-loss events have occurred among three streptococcal groups, resulting in the absence of the htp gene in the Bovis, Mutans and Salivarius groups. Second, the copy number of htp genes in other groups of Streptococcus is variable, ranging from one to four functional copies. Third, both phylogenetic evidence and domain structure analyses support the division of two htp subfamilies, designated as htp I and htp II. Although present mainly in the pyogenic group and in Streptococcus suis, htp II members are distinct from htp I due to the presence of an additional leucine-rich-repeat domain at the C-terminus. Finally, htp genes exhibit a faster nucleotide substitution rate than do housekeeping genes. Specifically, the regions outside the HTP domains are under strong positive selection. This distinct evolutionary pattern likely helped Streptococcus to easily escape from recognition by host immunity.
Introduction
The genus Streptococcus comprises a wide variety of pathogenic and commensal gram-positive bacteria [1]. Among them, Streptococcus pyogenes, Streptococcus agalactiae and Streptococcus pneumoniae are the three most important streptococcal human pathogens [2]–[5]. Despite sharing the same hosts and showing similar pathogenicity, the genomes of these three species showed only a modicum of similarity. With dramatic gene gains and losses, chromosomal rearrangements and horizontal gene transfers having frequently occurred since their separation, the core-genome of these three streptococci only contains about 50% of the genes of each species [4]–[12]. As a result, it is difficult to identify virulence factors that are shared by all three streptococci; and many currently defined virulence-related proteins are species-specific [13]–[15].
However, several recent studies revealed that a family of proteins characterized by repeated histidine triad motifs (His-x-x-His-x-His) is present in all three human-pathogenic streptococci, and these proteins play roles in pathogenesis [16]–[18]. Proteins belonging to this family were first discovered in S. pneumoniae and were given different names by independent research groups, such as Pht (for pneumococcal histidine triad) [19], Php (for pneumococcal histidine protein) [20] and BVH [21]. Adamou et al. [19] found that although the sequence identity among four pht genes in S. pneumoniae varied from only 32% to 87%, a typical character of 4–6 duplicated histidine triad motifs was consistently shared by each protein. Searching for this signature, researchers subsequently identified genes homologous to pht genes in two other human pathogens, S. pyogenes [18], [22] and S. agalactiae [23]; and also in the zoonotic pathogen S. suis [24], [25]. The identified pht homologs were named, htpA and slr in S. pyogenes; sht and blr in S. agalactiae; and htpS in S. suis. For convenience, we hereafter refer to these genes as histidine triad protein (htp) genes, and the conserved histidine triad motifs as the HTP domain.
HTPs are a family of cell-surface exposed proteins that are involved in a diverse range of important biological functions [26]. The crystal structure of a PhtA protein fragment has revealed that the HTP domain forms a zinc-binding fold [27], [28]. The Zn2+ binding ability of HTP proteins was indeed confirmed for HtpA and PhtD by independent groups [18], [29]. Bioinformatic analysis further revealed that a cis-element recognized by AdcR, a zinc uptake regulator, often exists in the promoter region of these htp genes [30]. Recent experiments in both S. pneumoniae and S. suis confirmed that htp genes were regulated by AdcR [16], [25]. Furthermore, parallel studies in S. pneumoniae and S. agalactiae both support the concept that HTP proteins are involved in bacterial evasion of complement-mediated host immune responses by binding to complement factor H [16], [17]. The fusion of the HTP domain with a leucine-rich-repeat (LRR) domain was also identified [22], [23]. This new type of chimeric protein in S. pyogenes was recently reported as an adhesion protein binding to human Type I Collagen [31]. In addition, investigators have tried to introduce HTP as a subunit of multivalent vaccines against streptococcal infection. As a family of surface-exposed proteins that are expressed during the bacterial infection process, HTP homologs have been proven to possess strong immunogenicity and shown to induce specific humoral immunity in the host [24], [32]–[34]. Several studies have suggested that immunization with recombinant proteins of HTP homologs from different streptococcal species can prevent bacterial infection in mice [18]–[21], [24]. More importantly, immunization with PhtD also successfully confers protection against S. pneumoniae in rhesus monkeys, indicating its potential application to humans [35].
In contrast to extensive studies on the biological functions and potential clinical applications of HTP proteins, little attention has been paid to the origin and evolution of this family. Interestingly, all currently investigated htp genes are encoded by pathogenic streptococcal species. Whether this family of proteins also exists in non-pathogenic Streptococcus or other bacteria is still unknown. With the development of sequencing technology, many streptococcal genomes have been fully sequenced [36]. Herein, we investigated the distribution of htp genes among 105 streptococcal genomes representing 38 different species/subspecies, explored the origin and evolutionary history of the htp gene family among different streptococcal groups, and discovered the distinct evolutionary patterns of this cell surface-exposed protein family.
Results
Reconstructing the Phylogenetic Tree of Streptococcus
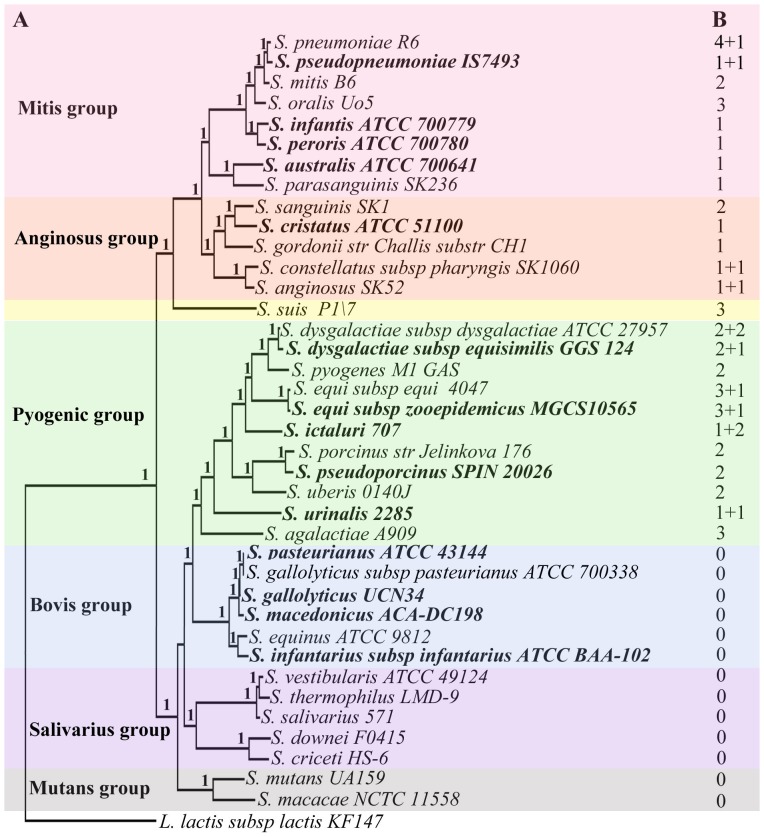
To elucidate the evolutionary history of htp genes, it is necessary to determine the phylogenetic relationships within the genus Streptococcus. Based solely on 16S rDNA sequences, a previous study [1] divided streptococcal species into six groups (Anginosus, Bovis, Mitis, Mutans, Pyogenic, and Salivarius). Herein, based on 530 single-copy orthologous gene sequences extracted from representative genomes of 38 streptococcal species/subspecies and also an out-group species Lactococcus lactis, we were able to generate a 192,452 long alignment matrix and to reconstruct a well-supported species tree for Streptococcus. The entire tree encompasses 38 taxa in total, with fourteen newly added. As shown in Figure 1A, by using Lactococcus lactis as an out-group species, two major clades were identified with strong supports. One clade comprises the Anginosus group, Mitis group and S. suis (the latter being a species that has heretofore not been assigned to any group). Another streptococcal clade consists of four other groups (Salivarius, Mutans, Bovis, and Pyogenic). Overall, the phylogenetic tree we obtained (Figure 1A) was consistent with the previous 16S tree [1], [4]. However, there were also two differences. In the previous 16S tree [1], the S. sanguinis and S. gordonii species were placed in the monophyletic Mitis group, whereas in our tree these two species plus the newly added species S. cristatus were found, with high support, in Anginosus group (sister lineage of the Mitis group). The second inconsistency between our tree and the 16S tree is the separation of four previously defined Mutans group species into two independent lineages. As shown in Figure 1A, the two species S. criceti and S. downei that were previously placed in the monophyletic Mutans group were now separated from this lineage and clustered in the Salivarius group.
Figure 1. Distribution of all identified htp genes among 38 streptococci in a clear phylogenetic background.
A. Phylogenetic analysis of 38 streptococci based on 530 single-copy orthologous proteins. Node labels indicate aLRT SH-like branch support values. Twenty-six species have been used in the previous phylogeny analysis based on 16s rDNA, while 14 species or subspecies were newly added and labeled in bold. Group division was updated based on the phylogenetic relationship. B. Copy numbers of htp genes within each genome are indicated with complete genes plus pseudogenes.
Identification of htp Genes in Streptococcus
By performing BLAST and HMM searches, we were able to identify a total of 240 putative htp genes from 84 out of 105 surveyed streptococcal genomes, covering 25 out of 38 species/subspecies. No htp gene was found from the rest 21 surveyed genomes representing thirteen streptococcal species.
The length of those identified sequences was highly variable. The shortest sequence found in S. pneumoniae comprised only 138 nucleotides, with only one HTP domain found in its deduced amino acid sequence. In contrast, the longest gene found in S. mitis codes for a protein of 1327 amino acids in length and has nine HTP domains. Also, many short genes appeared in tandem with very short intervals. As described in several previously published literatures [19], [24], htp genes usually encode proteins with more than 800 amino acids. Therefore, we speculated that these short genes are likely mis-annotated. According to a previous study [37], the rate of automated annotation error is about 36% in a plant genome. One important obstacle to automated annotation is reading frame shift, caused by either spontaneous mutation or sequence error. Automated annotation would not identify the sequence as a pseudogene on the occasion of reading frame shift, but divide the sequence into two coding exons by insetting an intron in eukaryotic genomes or factitiously generating two genes that actually do not exist. Considering this, we undertook manual re-annotation and analysis for all identified genes shorter than 2 kb. For the genes that appeared in pair, both of the two fragments were subjected to Blastn search against a local database comprised of genes longer than 2 kb. If the two genes both had high similarity to one long gene but localized in different regions, they were regarded as one pseudogene. For other short genes, ∼500 bp of both the 5′ and 3′ flanking sequences were analyzed to evaluate whether they are long htp genes. If reading frame shifts or non-sense mutations were observed, then the gene was also identified as a pseudogene. A total of 190 intact htp genes were finally obtained.
The number of intact htp genes found from each surveyed genome is listed in Table S1. From this table, one could tell that the copy number of htp genes was largely consistent among different isolates of a streptococcal species. For example, two conserved htp genes were consistently found in all 14 S. pyogenes isolates surveyed and three copies were always detected in 13 S. suis isolates. However, differently in S. pneumoniae and S. agalactiae, the intact htp gene copy number was variable among different isolates, ranging from two to four and two to three copies, respectively.
To avoid redundant calculations and to maximally represent the diversity of streptococcal htp genes for further analyses, we selected one genome with maximal copy of htp gene to represent each species/subspecies (Table S1). For S. pneumoniae and S. agalactiae, we selected the isolates S. pneumoniae R6 and S. agalactiae A909, since they contain the maximal number of htp genes in the two species. As a result, from a total of 38 representative genomes, we finally identified 46 intact htp genes as well as 12 pseudo ones (Figure 1), exhibiting a concise picture for htp gene distributions in Streptococcus.
Variant htp Genes in Streptococcal Species
As shown in Figure 1, the 46 functional htp genes were identified from 25 representative streptococcal genomes (representing Mitis, Anginosus, Pyogenic groups and also S. suis), but the htp gene was not found in the other thirteen species (representing Bovis, Mutans and Salivarius groups); this indicated an unequal distribution of htp genes among the different groups. We also found that among the 25 species with htp genes, the maximal copy number of htp genes was variable, ranging from one to four copies. After locating the htp genes on the chromosome of each species (Figure S1), we found that a majority of htp genes was maintained in one of the two conserved gene contexts: in the first case, many htp genes formed an operon with an upstream laminin-binding protein gene (lmb, also termed lbp) [18], [19], [24]. Among the 46 htp genes, 26 appeared in the lmb-htp operon structure (Figure S1). In the second case, some htp genes were located between two conserved housekeeping genes, and interestingly, these htp genes all had a LRR domain at the C-terminus, forming an htp-lrr gene structure (Figure S1). Also, this htp-lrr type gene appears to mainly exist in Pyogenic group species, as it was missing from species of the Mitis and Anginosus groups. In addition, there were also some remaining htp genes located in non-conserved regions; and some were accompanied by transposons (Figure S1).
Identification of htp Genes from Bacteria Outside Streptococcus
Previously, it was speculated that htp genes only exist in the genus Streptococcus [38]. To test this hypothesis, we performed an online Blastp search against over 1800 non-streptococcal bacterial genomes in the Genbank database with the E-value set to 10. After manually checking and subjecting the hits to Pfam analysis, a total of 10 htp genes were surprisingly found to be in nine other bacteria. The genome of the Granulicatella elegans strain ATCC 700633 contained two htp genes, whereas the other eight genomes including Gemella sanguinis M325, Gemella haemolysans M341, Gemella haemolysans ATCC 10379, Slackia exigua ATCC 700122, Granulicatella adiacens ATCC 49175, Catonella morbi ATCC 51271, Aerococcus urinae ACS120VCol10a and Facklamia languida CCUG 37842 each had one htp gene. Among these species, seven of them (like Streptococcus), belong to the class Bacilli within the phylum Firmicutes; the remaining two species Catonella morbi and Slackia exigua belong to class Clostridia within the phyla Firmicutes and Actinobacteria, respectively. Among these htp genes identified from non-streptococcal species, two appeared in lmb-htp formats; and the one in Slackia exigua was located downstream of adcA, which is a homolog of lmb. The rest of the seven htp genes were not found in a conserved gene context compared with Streptococcus.
Inferring the Evolutionary History of htp Genes
A typical character of the htp gene is that it encodes one to multiple HTP domains. However in some htp genes, the LRR domains were found at the C-terminus; which has been previously reported in S. agalactiae and S. pyogenes [22], [23]. Our data revealed that among the 46 functional htp genes in Streptococcus, ten showed this chimeric structure (Figure S1). Furthermore, the htp-lrr type gene was also found in G. haemolysans, a non-streptococcal species.
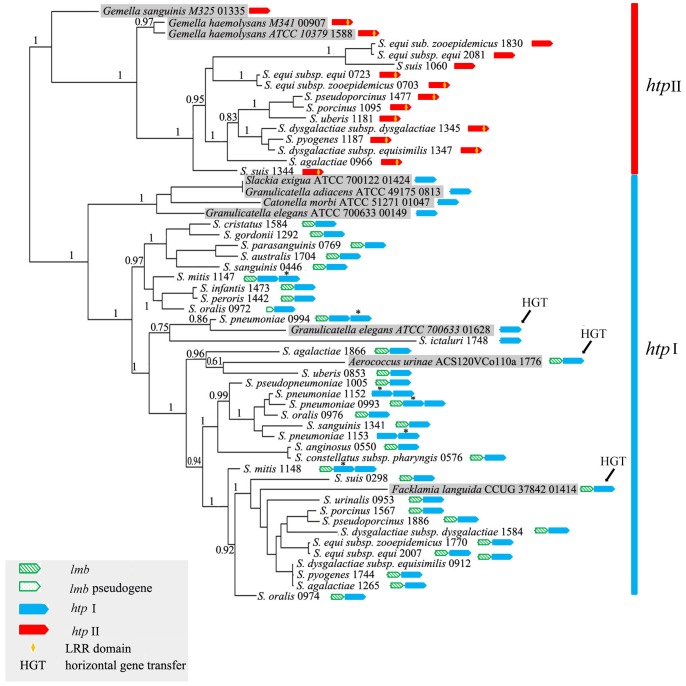
In order to determine whether htp genes obtained in non-streptococcal species were recently acquired from streptococcal species by horizontal transfer, and to understand the origin and evolutionary history of htp genes, two phylogenetic trees were reconstructed based on either CDS or amino acid sequences of 56 htp genes identified from the representative streptococcal genomes and other bacteria. These two trees showed very similar topologies and are supportive of the theory that htp genes formed into two clades or subfamilies (Figures 2 and S2). Sixteen genes formed a small subfamily, with all htp-lrr type genes included; whereas the other 40 htp sequences formed a large subfamily and none possessed a LRR domain. We designated the large subfamily as subfamily I (htp I), and the small subfamily that contains htp-lrr type genes as subfamily II (htp II).
Figure 2. The phylogenetic tree of htp genes based on CDS.
Node labels indicate aLRT SH-like branch support values. The htp genes found from non-streptococcal species are shaded. For each gene, the CDS number in its genome is found behind the species name. Arrows in different colors are used to represent htp I or htp II genes. Asterisks (*) are used to indicate the represented gene when a species has tandem duplicated htp genes.
Importantly, both subfamilies included sequences from non-streptococcal species. In subfamily II, three sequences from G. sanguinis and G. haemolysans formed the basal lineages of this clade, while four sequences from S. exigua, G. elegans, G. adiacens and C. morbi also forming the basal lineages in subfamily I (Figure 2). This topology strongly indicated that the division of the two subfamilies must have occurred earlier than did the genus Streptococcus. Also within subfamily I, three htp genes in F. languida, A. urinae and G. elegans were closely related with streptococcal htp genes, suggesting three horizontal gene transfer events.
Ten Streptococcus species were found to have both htp I and II copies, including nine species of the Pyogenic group and also S. suis (Figures 2 and S1). In the htp II clade, two subspecies of S.equi and species S. suis were found to each possess two copies of htp II genes, with one copy manifesting LRR domain while the other did not. Seven other streptococcal species have only one copy of the htp II gene, which contains the LRR domain (Figure 2). Moreover, one non-streptococcal species, G. haemolysans (two strains), was found to have an htp-lrr type gene. This suggests to us that LRR domain was lost from the second copy of the htp II gene in subspecies of S.equi and the species S. suis.
Previously, we found that the functional htp-lrr type gene was lacking in both the Mitis and Anginosus groups. However, by checking pseudogenes we actually found one each in S. anginosus and S. constellatus subspecies pharyngis showing high sequence identity to htp II genes (Figure S1). Additionally, one functional copy of the htp-lrr gene was found in the species S. suis (which evolutionarily separated early); and in Figure 2, this S. suis htp II gene was indeed located outside of the Pyogenic group htp II genes, suggesting a consistency between gene tree and species tree. Considering these findings, we argue that one functional htp II gene (with LRR) likely exists in the common ancestor of all streptococcal species; however, it was pseudogenized in Anginosus group and totally lost from the Mitis group.
Frequent Recombination and Fast Rate of Evolution of htp Genes
Although bacteria are haploid, recombination could happen among multi-copy genes. On the other hand, horizontal transfer of DNA fragment also provides an opportunity for recombination events to occur. To study how frequently recombination occurred among htp genes in Streptococcus, we performed recombination analysis with RDP software for 46 streptococcal htp genes. As shown in Figure S3, a total of 66 recombination events were detected, with many more events observed in htp I (58/66) than in the htp II subfamily (8/66). Among all the 66 recombination events, the parent sequences of 46 events were clearly detected, whereas the parent sequences of 20 other recombination events could not be distinguished unambiguously.
To evaluate the rate of evolution for htp genes, the synonymous substitution rates of htp genes within each subfamily were compared with those of neighboring lmb genes and eight housekeeping genes. The average Ks values for htp I (1.92±0.05) and htp II (2.06±0.1) were similar to that of lmb (2.13±0.06), but all were higher than the Ks of housekeeping genes (1.58±0.03); indicating that htp genes and the lmb-htp operon evolved faster than did housekeeping genes. Interestingly, the Ks of HTP and LRR domains (1.52±0.09, 1.24±0.11) were consistently lower than those of the overall Ks value of the entire gene, and lower even than the housekeeping gene, indicating strong mutation constraints on the evolution of these domains.
Conservation of Functional Domains in htp Genes
In order to provide higher resolution of the nucleotide substitution patterns among different regions of htp genes, and especially to elucidate whether certain sites are under positive selection, a sliding Ka/Ks window analysis was carried out for two htp subfamilies using Dnasp v5.0 [39]. In the htp I subfamily, a high Ka/Ks ratio (>1) was detected throughout the coding regions (Figure 3A). However, the scattered htp domains showed much lower Ka/Ks ratios (<1, Figure 3A), indicating a purifying selection on these domains. Similarly, for htp II subfamily, all HTP domains and most LRR domains showed low Ka/Ks ratios (<1); however, regions outside of these domains often showed large Ka/Ks (>1, Figure 3B). We also detected regions in the LRR domain showing Ka/Ks ratios larger than 1 (Figure 3B). Currently it is unknown whether positive selection occurring in this LRR region would make HTP II proteins change their ligand-binding abilities so as to adapt to different hosts or tissues. This deserves further experimental study.
Figure 3. Sliding-window analysis for Ka/Ks values of two subfamilies of htp genes.
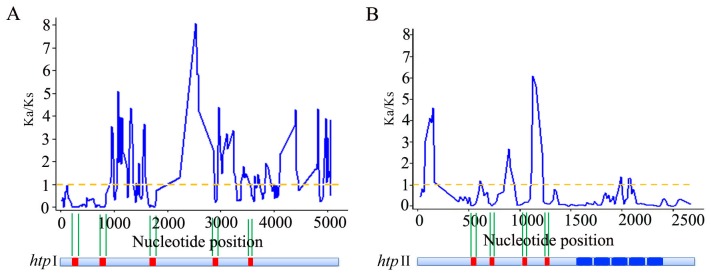
Ka/Ks ratios were calculated for A) htp I subfamily and B) htp II subfamily using DnaSP with a sliding window of 60 bases and a 15-base step size.
Discussion
The Early Origin and Separation of the Two htp gene Subfamilies
The first HTP protein was found in S. pneumoniae in 2001 [19], and during the next decade, additional HTP proteins were found in several pathogenic streptococci [18], [24]. However, the origin and evolution of this novel family have not been evaluated. In this study, a maximum of 46 complete htp genes was identified among 25 streptococcal species/subspecies. These htp genes could be divided into htp I and htp II subfamilies based upon their locations in a phylogenetic tree (Figure 2). Over the past ten years, it has been hypothesized that this family protein may be restricted only in the genus Streptococcus [38]. In this study, we are able to identify 10 htp genes from nine non-streptococcal bacteria. Because all of these bacteria can infect humans, we determined whether the htp genes found in their genomes were a result of recent horizontal gene transfer events. Our phylogenetic analysis revealed that although three of the htp genes were indeed acquired from some streptococci by horizontal gene transfer, others fell outside of the streptococcal htp genes for both the htp I and htp II clades, precluding the possibility of recent lateral acquisition of htp genes in these seven non-streptococcal species via horizontal gene transfer. These findings offer strong evidence to support the notion that htp genes originated earlier than did the Streptococcus genus. The separation of htp I and htp II genes for both streptococcal and non-streptococcal species also indicates that both htp I and II genes appeared in the common ancestor of Streptococcus.
As for htp gene location, an interesting finding was that many htp I genes were present in lmb-htp operon and many htp II genes were located between two conserved housekeeping genes. Such conservation in gene context further suggested to us the ancientness of the two htp subfamilies. We conjecture that in the common ancestor of Streptococcus, one copy of the htp I gene was involved in the lmb-htp operon with its own promoter region lost evolutionarily; while the other copy of the htp II gene fused with a LRR domain, as the out-group species G. haemolysans demonstrated.
The Evolutionary History of htp Genes in Streptococcus
Although the origin and separation of htp genes appeared to have occurred earlier than did Streptococcus, both htp subfamily genes were evidently lost later in three groups of Streptococcus (Bovis, Mutans and Salivarius). Since both htp I and II subfamilies are present in later-derived Pyogenic group and also in the individual species S. suis, the absence of htp genes in these groups is likely due to independent gene-loss events that occurred during lineage-specific evolution. Likewise, the htp II gene appeared to be totally absent among eight Mitis group species, whereas two htp II pseudogenes were still found in two of five closely related Anginosus group species (Figure S1). This strongly supports the concept that the htp II subfamily was lost from the Mitis group after separating from the Anginosus group.
Gene duplication and deletion were also frequently observed in the Streptococcus pyogenic group. However, both subfamily genes are well maintained in their genomes, which indicates that both types of genes are functionally important to these bacteria. Indeed, previous studies have shown that both subfamily genes play important roles in the survival and/or pathogenic processes of these bacteria [18], [22], [23], [40]. Knocking out either the htp I subfamily gene htpA or the htp II subfamily gene slr significantly decreased the pathogenicity of S. pyogenes [22]. However, the mechanism(s) underlining this phenomenon is still unclear. Some investigators have proposed that the decreased pathogenicity of mutated S. pyogenes results from a reduced capability of zinc absorption [18]. Other recent studies carried out by Bober et al. [31] demonstrated that Slr could adhere to collagen I with high affinity, which is critical for colonization and infection of S. pyogenes. Functional differentiation between htp I and htp II subfamilies may result from the absence or presence of LRR domains in their C-terminus.
Phylogenetic and chromosomal synteny analysis revealed that gene birth and death processes frequently occur across species. Tandem or segmental duplication has contributed to the increase in copy number in many species. As shown in Figure S2, although it does not possess an htp II gene, S. pneumoniae contains four functional htp I genes and one htp I pseudogene (incorrectly annotated as five tandem ORFs). By comparing S. pneumoniae with its closely related species within the same group, we observed that htp genes have experienced several rounds of gene duplications. These duplicated genes likely play redundant roles in S. pneumoniae, because variation of htp gene copy number was found among S. pneumoniae isolates (Table S1). Furthermore, it was also found that independently knocking out three of the four functional htp genes did not affect its pathogenicity [16]. The dynamic birth and death evolution of htp genes of this lineage could potentially provide opportunities for newer functional innovations.
Sophisticated Evolutionary Strategies Drive HTP Proteins to Escape from Host Immune System Recognition
The synonymous substitution rates of both htp I and htp II genes were greater than those for housekeeping genes, suggesting a fast rate of evolution for htp gene. However, the Ks value of the HTP and LRR domains were even lower than those of housekeeping gene, reflecting strict mutation constraints on these domains. Increase evidence supports the hypothesis that synonymous substitutions suffer selections from codon usage or order bias [41], [42]; however, whether this inconsistent substitution rate within the same gene is caused by selection or mutation bias is unknown. HTP proteins are involved in several important functions of Streptococcus, including zinc absorption, binding of complement H and adhesion to extracellular matrix. Although gene structures between the two htp subfamilies are different, they both share the HTP domain. Crystal structure analysis revealed that the property of zinc binding is subserved by the HTP domain [28]. Recent study by Loisel et al. [29] found that zinc-binding capability is positively correlated with the total number of HTP domains. In addition, a conserved AdcR binding element was found in promoter regions of almost all identified htp genes. As shown in Figure 1, many pathogenic species such as S. pneumoniae, S. agalactiae and S. pyogenes possessed at least one htp gene, whereas a complete loss of the htp gene was only observed in some commensal and non-pathogenic streptococcal species, such as S. salivarius and S. thermophilus. This correlation indicates a role of htp genes in Streptococcus infection.
Proteins secreted to the cell wall of bacteria are easily detected by the immune system, and are therefore likely to encounter positive selection. A recent study in S. pneumoniae found that genes encoding surface antigens are often under diversifying selection so as to escape targeting by the human immune system [43]. This phenomenon has also been shown for other animal and plant pathogens [44]–[49]. A study on the surface exposed toxin protein VacA of Helicobacter pylori found that all sites under positive selection of this protein were located on the surface of its tertiary structure, and speculated that these residues are subjected to antibody recognition [50]. Our data revealed that the htp gene is also under positive selection. Sliding window of Ka/Ks analysis demonstrated that in htp genes, regions outside the HTP or LRR domains are often subject to strong positive selection (Figure 3); while HTP or LRR domains often exhibit low Ka/Ks values. These opposite evolutionary patterns observed within and outside functional domains of htp genes likely reflect a sophisticated strategy in the evolution of these bacterial cell surface-exposed proteins. These findings from our study and from others suggested that positive selection with respect to surface proteins is not a rare occurrence, but is rather ubiquitous in bacterial pathogens. A comprehensive study published recently by Nogueira et al. [51] reported that in bacteria cell-exposed and secreted proteins generally have a fast rate of evolution and are often under positive selection. Additionally, the frequent recombination events observed in this study may also be due to a selective advantage that further increases the sequence divergences. Collectively, two opposite forces are coordinated in shaping the evolution of htp genes: negative selection pressure that maintain the conserved functions (such as Zinc absorption, adhesion, etc.) of HTP and/or LRR domains; and positive selection pressure which comes from host immune system and drive the quick evolution of non-essential regions to change their amino acids; this would then assist the bacteria in escaping recognition by the host immune system.
Materials and Methods
Databases
Both the genomic sequences and annotation information used in this study were downloaded from the Pathosystems Resouce Integration Center (PATRIC, http://www.patricbrc.org) [36] on March 8, 2012. The detailed information of these genomes is listed in Table S1.
Reconstruction of the Streptococcal Species Tree
As did in previous study [7], we select Lactococcus lactis as an out-group species to reconstruct the Streptococcus phylogeny. To build a super multi-gene matrix for a better resolution of phylogeny, we used the Proteinortho v4.26 [52] to identify a maximal number of orthologous genes that are shared by 38 streptococcal genomes and Lactococcus lactis. A total of 530 single-copy protein-coding genes were finally obtained. For each gene, its amino acid sequences extracted from the 39 genomes were concatenated and aligned by using the ClustalW program. The maximum likelihood (ML) analysis was then performed using PhyML v3.0.1 [53] to reconstruct the species tree. The reliability of internal nodes of the tree was assessed by aLRT SH-like branch support.
Searching for Sequences that Encode Histidine Triad Motifs
To identify genes containing HTP domains in Streptococcus, both BLAST and HMM (hidden Markov model) searching methods were adopted. The amino acid sequence of the HTP domain in PhtD was first used as query sequence to Blast against the protein database of all streptococcal genomes. The amino acid sequences of hits obtained were then used one by one to Blast against all genomes for any undetected homologs. The threshold for the E-value was set to 1.0 to find a maximal number of candidate genes. In the HMM procedure, the HTP domain model (PF04270.8) was downloaded from the Pfam database and served as the search query. The hits obtained from the two methods were consolidated together to remove redundant hits. All remaining non-redundant genes were tested by Pfam analysis to remove sequences that did not contain the HTP domain.
Alignment and Phylogenetic Analysis of htp Genes
Multiple alignments of amino acid sequences were performed using the ClustalW program integrated in mega 5.0 [54], [55] with default parameters. The resulting amino acid sequence alignments were then used to guide the alignments of nucleotide coding sequences. Phylogenetic trees were constructed based on either the alignments of CDS or amino acid sequences by using PhyML v3.0.1 [53]. The reliability of internal nodes was assessed by aLRT SH-like branch support.
Calculation of Nucleotide Substitution Rate and Recombination Tests
To evaluate the relative rate of evolution of the two htp subfamilies, the synonymous substitution rates (Ks) for the full-length htp genes, their neighboring lmb genes and housekeeping genes were calculated using mega 5.0 with the Nei and Gojobori model [55]. Eight housekeeping genes were selected in this study, including dnaE (encoding DNA polymerase III), gltX (encoding glutamyl-tRNA synthetase), pyrE (encoding orotate phosphoribosyltransferase), recA (encoding recombinase A protein), rpoB (encoding the DNA-directed RNA polymerase beta chain), secA (encoding preprotein translocase subunit SecA), secY (encoding preprotein translocase subunit SecY) and sodA (encoding superoxide dismutase). All of these genes have been separately or collectively used in phylogenetic analysis of Streptococcus [7], [10], [56], [57]. To detect the evolutionary constraints on the functional domains, the Ks of HTP and LRR domains were also calculated. A sliding-window analysis of the ratio of non-synonymous to synonymous substitution rates (Ka/Ks) was performed on the sequences of the two subfamilies using DnaSP (http://www.ub.edu/dnasp) [39], with a window size of 60 bases and a step size of 15 bases. Recombinant events among htp genes were detected with the RDP v3.34 software [58] using four automated recombination detection methods, including RDP, Genconv, Chimaera and Maximum Chi Square, with default parameters.
Supporting Information
Physical map of all identified htp genes in 38 streptococcal genomes.
(TIF)
The phylogenetic tree of htp genes based on amino acid sequences.
(TIF)
Schematic representation of the recombination events occurring among htp genes identified from streptococcal species. Each line represents a recombinant sequence, and the red boxes below indicate the sequences exchanged from homologs. The name of the putative parental species for each fragment is indicated.
(TIF)
Detailed information and htp copy numbers for all genomes used in this study.
(XLS)
Acknowledgments
We thank the three anonymous reviewers and the PLOS One editor for their critical reading of our paper.
Funding Statement
This work was supported by the National Natural Science Foundation of China (30930008, 31000105, 31170210 and 8117294), National Postdoctoral Science Foundation of China (20090461092, 201003570), Nanjing University Start Grant, the Natural Science Foundation of Jiangsu Province (BK2010113) and Postgraduate Students Innovation Project of Jiangsu Province (CXZZ11_0038). http://www.nsfc.gov.cn/; http://210.79.234.200/V1/Manage/Login.aspx; www.nju.edu.cn/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kawamura Y, Hou XG, Sultana F, Miura H, Ezaki T (1995) Determination of 16S rRNA sequences of Streptococcus mitis and Streptococcus gordonii and phylogenetic relationships among members of the genus Streptococcus. Int J Syst Bacteriol 45: 406–408. [DOI] [PubMed] [Google Scholar]
- 2. Jarva H, Jokiranta TS, Wurzner R, Meri S (2003) Complement resistance mechanisms of streptococci. Mol Immunol 40: 95–107. [DOI] [PubMed] [Google Scholar]
- 3. Soriani M, Telford JL (2010) Relevance of pili in pathogenic streptococci pathogenesis and vaccine development. Future Microbiol 5: 735–747. [DOI] [PubMed] [Google Scholar]
- 4. Anisimova M, Bielawski J, Dunn K, Yang Z (2007) Phylogenomic analysis of natural selection pressure in Streptococcus genomes. BMC Evol Biol 7: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bessen DE (2009) Population biology of the human restricted pathogen, Streptococcus pyogenes. Infect Genet Evol 9: 581–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lefebure T, Stanhope MJ (2007) Evolution of the core and pan-genome of Streptococcus: positive selection, recombination, and genome composition. Genome Biol 8: R71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marri PR, Hao W, Golding GB (2006) Gene gain and gene loss in streptococcus: is it driven by habitat? Mol Biol Evol 23: 2379–2391. [DOI] [PubMed] [Google Scholar]
- 8. Brochet M, Rusniok C, Couve E, Dramsi S, Poyart C, et al. (2008) Shaping a bacterial genome by large chromosomal replacements, the evolutionary history of Streptococcus agalactiae . Proc Natl Acad Sci U S A 105: 15961–15966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gogarten JP, Townsend JP (2005) Horizontal gene transfer, genome innovation and evolution. Nat Rev Microbiol 3: 679–687. [DOI] [PubMed] [Google Scholar]
- 10. Boggs JM, South AH, Hughes AL (2012) Phylogenetic analysis supports horizontal gene transfer of L-amino acid oxidase gene in Streptococcus oligofermentans. Infect Genet Evol 12: 1005–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lindahl G, Stalhammar-Carlemalm M, Areschoug T (2005) Surface proteins of Streptococcus agalactiae and related proteins in other bacterial pathogens. Clin Microbiol Rev 18: 102–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Richards VP, Lang P, Bitar PD, Lefebure T, Schukken YH, et al. (2011) Comparative genomics and the role of lateral gene transfer in the evolution of bovine adapted Streptococcus agalactiae . Infect Genet Evol 11: 1263–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nobbs AH, Lamont RJ, Jenkinson HF (2009) Streptococcus adherence and colonization. Microbiol Mol Biol Rev 73: 407–450, Table of Contents. [DOI] [PMC free article] [PubMed]
- 14. Kadioglu A, Weiser JN, Paton JC, Andrew PW (2008) The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat Rev Microbiol 6: 288–301. [DOI] [PubMed] [Google Scholar]
- 15. Mitchell TJ (2003) The pathogenesis of streptococcal infections: from tooth decay to meningitis. Nat Rev Microbiol 1: 219–230. [DOI] [PubMed] [Google Scholar]
- 16. Ogunniyi AD, Grabowicz M, Mahdi LK, Cook J, Gordon DL, et al. (2009) Pneumococcal histidine triad proteins are regulated by the Zn2+-dependent repressor AdcR and inhibit complement deposition through the recruitment of complement factor H. FASEB J. 23: 731–738. [DOI] [PubMed] [Google Scholar]
- 17. Maruvada R, Prasadarao NV, Rubens CE (2009) Acquisition of factor H by a novel surface protein on group B Streptococcus promotes complement degradation. FASEB J 23: 3967–3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kunitomo E, Terao Y, Okamoto S, Rikimaru T, Hamada S, et al. (2008) Molecular and biological characterization of histidine triad protein in group A streptococci. Microbes Infect 10: 414–423. [DOI] [PubMed] [Google Scholar]
- 19. Adamou JE, Heinrichs JH, Erwin AL, Walsh W, Gayle T, et al. (2001) Identification and characterization of a novel family of pneumococcal proteins that are protective against sepsis. Infect Immun 69: 949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang Y, Masi AW, Barniak V, Mountzouros K, Hostetter MK, et al. (2001) Recombinant PhpA protein, a unique histidine motif-containing protein from Streptococcus pneumoniae, protects mice against intranasal pneumococcal challenge. Infect Immun 69: 3827–3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hamel J, Charland N, Pineau I, Ouellet C, Rioux S, et al. (2004) Prevention of pneumococcal disease in mice immunized with conserved surface-accessible proteins. Infect Immun 72: 2659–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reid SD, Montgomery AG, Voyich JM, DeLeo FR, Lei B, et al. (2003) Characterization of an extracellular virulence factor made by group A Streptococcus with homology to the Listeria monocytogenes internalin family of proteins. Infect Immun 71: 7043–7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Waldemarsson J, Areschoug T, Lindahl G, Johnsson E (2006) The streptococcal Blr and Slr proteins define a family of surface proteins with leucine-rich repeats: camouflaging by other surface structures. J Bacteriol 188: 378–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shao Z, Pan X, Li X, Liu W, Han M, et al. (2011) HtpS, a novel immunogenic cell surface-exposed protein of Streptococcus suis, confers protection in mice. FEMS Microbiol Lett 314: 174–182. [DOI] [PubMed] [Google Scholar]
- 25. Aranda J, Garrido ME, Fittipaldi N, Cortes P, Llagostera M, et al. (2009) Protective capacities of cell surface-associated proteins of Streptococcus suis mutants deficient in divalent cation-uptake regulators. Microbiology 155: 1580–1587. [DOI] [PubMed] [Google Scholar]
- 26. Plumptre CD, Ogunniyi AD, Paton JC (2012) Polyhistidine triad proteins of pathogenic streptococci. Trends Microbiol 20: 485–493. [DOI] [PubMed] [Google Scholar]
- 27. Riboldi-Tunnicliffe A, Bent CJ, Isaacs NW, Mitchell TJ (2004) Expression, purification and X-ray characterization of residues 18–230 from the pneumococcal histidine triad protein A (PhtA) from Streptococcus pneumoniae. Acta Crystallogr D Biol Crystallogr 60: 926–928. [DOI] [PubMed] [Google Scholar]
- 28. Riboldi-Tunnicliffe A, Isaacs NW, Mitchell TJ (2005) 1.2 Angstroms crystal structure of the S. pneumoniae PhtA histidine triad domain a novel zinc binding fold. FEBS Lett 579: 5353–5360. [DOI] [PubMed] [Google Scholar]
- 29. Loisel E, Chimalapati S, Bougault C, Imberty A, Gallet B, et al. (2011) Biochemical characterization of the histidine triad protein PhtD as a cell surface zinc-binding protein of pneumococcus. Biochemistry 50: 3551–3558. [DOI] [PubMed] [Google Scholar]
- 30. Panina EM, Mironov AA, Gelfand MS (2003) Comparative genomics of bacterial zinc regulons: enhanced ion transport, pathogenesis, and rearrangement of ribosomal proteins. Proc Natl Acad Sci U S A 100: 9912–9917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bober M, Morgelin M, Olin AI, von Pawel-Rammingen U, Collin M (2011) The membrane bound LRR lipoprotein Slr, and the cell wall-anchored M1 protein from Streptococcus pyogenes both interact with type I collagen. PLoS One 6: e20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Simell B, Ahokas P, Lahdenkari M, Poolman J, Henckaerts I, et al. (2009) Pneumococcal carriage and acute otitis media induce serum antibodies to pneumococcal surface proteins CbpA and PhtD in children. Vaccine 27: 4615–4621. [DOI] [PubMed] [Google Scholar]
- 33. Holmlund E, Quiambao B, Ollgren J, Jaakkola T, Neyt C, et al. (2009) Antibodies to pneumococcal proteins PhtD, CbpA, and LytC in Filipino pregnant women and their infants in relation to pneumococcal carriage. Clin Vaccine Immunol 16: 916–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lebon A, Verkaik NJ, Labout JA, de Vogel CP, Hooijkaas H, et al. (2011) Natural antibodies against several pneumococcal virulence proteins in children during the pre-pneumococcal-vaccine era: the generation R study. Infect Immun 79: 1680–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Denoel P, Philipp MT, Doyle L, Martin D, Carletti G, et al. (2011) A protein-based pneumococcal vaccine protects rhesus macaques from pneumonia after experimental infection with Streptococcus pneumoniae . Vaccine 29: 5495–5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gillespie JJ, Wattam AR, Cammer SA, Gabbard JL, Shukla MP, et al. (2011) PATRIC: the comprehensive bacterial bioinformatics resource with a focus on human pathogenic species. Infect Immun 79: 4286–4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Meyers BC, Kozik A, Griego A, Kuang H, Michelmore RW (2003) Genome-wide analysis of NBS-LRR-encoding genes in Arabidopsis. Plant Cell 15: 809–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rioux S, Neyt C, Di Paolo E, Turpin L, Charland N, et al. (2011) Transcriptional regulation, occurrence and putative role of the Pht family of Streptococcus pneumoniae . Microbiology 157: 336–348. [DOI] [PubMed] [Google Scholar]
- 39. Librado P, Rozas J (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25: 1451–1452. [DOI] [PubMed] [Google Scholar]
- 40. Aranda J, Teixido L, Fittipaldi N, Cortes P, Llagostera M, et al. (2012) Inactivation of the gene encoding zinc-binding lipoprotein 103 impairs the infectivity of Streptococcus suis . Can J Vet Res 76: 72–76. [PMC free article] [PubMed] [Google Scholar]
- 41. Shao ZQ, Zhang YM, Feng XY, Wang B, Chen JQ (2012) Synonymous codon ordering: a subtle but prevalent strategy of bacteria to improve translational efficiency. PLoS One 7: e33547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang B, Shao ZQ, Xu Y, Liu J, Liu Y, et al. (2011) Optimal codon identities in bacteria: implications from the conflicting results of two different methods. PLoS One 6: e22714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Croucher NJ, Harris SR, Fraser C, Quail MA, Burton J, et al. (2011) Rapid pneumococcal evolution in response to clinical interventions. Science 331: 430–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kakizawa S, Oshima K, Jung HY, Suzuki S, Nishigawa H, et al. (2006) Positive selection acting on a surface membrane protein of the plant-pathogenic phytoplasmas. J Bacteriol 188: 3424–3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Petersen L, Bollback JP, Dimmic M, Hubisz M, Nielsen R (2007) Genes under positive selection in Escherichia coli . Genome Res 17: 1336–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fabre A, Danet JL, Foissac X (2011) The stolbur phytoplasma antigenic membrane protein gene stamp is submitted to diversifying positive selection. Gene 472: 37–41. [DOI] [PubMed] [Google Scholar]
- 47. Xu Z, Chen H, Zhou R (2011) Genome-wide evidence for positive selection and recombination in Actinobacillus pleuropneumoniae. BMC Evol Biol 11: 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Andrews TD, Gojobori T (2004) Strong positive selection and recombination drive the antigenic variation of the PilE protein of the human pathogen Neisseria meningitidis . Genetics 166: 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu C, Chen Z, Tan C, Liu W, Xu Z, et al. (2012) Immunogenic characterization of outer membrane porins OmpC and OmpF of porcine extraintestinal pathogenic Escherichia coli . FEMS Microbiol Lett 337: 104–111. [DOI] [PubMed] [Google Scholar]
- 50. Gangwer KA, Shaffer CL, Suerbaum S, Lacy DB, Cover TL, et al. (2010) Molecular evolution of the Helicobacter pylori vacuolating toxin gene vacA. J Bacteriol 192: 6126–6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nogueira T, Touchon M, Rocha EP (2012) Rapid evolution of the sequences and gene repertoires of secreted proteins in bacteria. PLoS One 7: e49403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lechner M, Findeiss S, Steiner L, Marz M, Stadler PF, et al. (2011) Proteinortho: detection of (co-)orthologs in large-scale analysis. BMC Bioinformatics 12: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Criscuolo A (2011) morePhyML: improving the phylogenetic tree space exploration with PhyML 3. Mol Phylogenet Evol 61: 944–948. [DOI] [PubMed] [Google Scholar]
- 54.Thompson JD, Gibson TJ, Higgins DG (2002) Multiple sequence alignment using ClustalW and ClustalX. Curr Protoc Bioinformatics Chapter 2: Unit 2 3. [DOI] [PubMed]
- 55. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, et al. (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Delorme C, Bartholini C, Bolotine A, Ehrlich SD, Renault P (2010) Emergence of a cell wall protease in the Streptococcus thermophilus population. Appl Environ Microbiol 76: 451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Delorme C, Poyart C, Ehrlich SD, Renault P (2007) Extent of horizontal gene transfer in evolution of Streptococci of the salivarius group. J Bacteriol 189: 1330–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Martin DP, Lemey P, Lott M, Moulton V, Posada D, et al. (2010) RDP3: a flexible and fast computer program for analyzing recombination. Bioinformatics 26: 2462–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Physical map of all identified htp genes in 38 streptococcal genomes.
(TIF)
The phylogenetic tree of htp genes based on amino acid sequences.
(TIF)
Schematic representation of the recombination events occurring among htp genes identified from streptococcal species. Each line represents a recombinant sequence, and the red boxes below indicate the sequences exchanged from homologs. The name of the putative parental species for each fragment is indicated.
(TIF)
Detailed information and htp copy numbers for all genomes used in this study.
(XLS)