Abstract
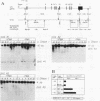
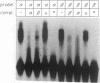
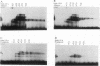
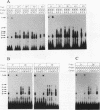
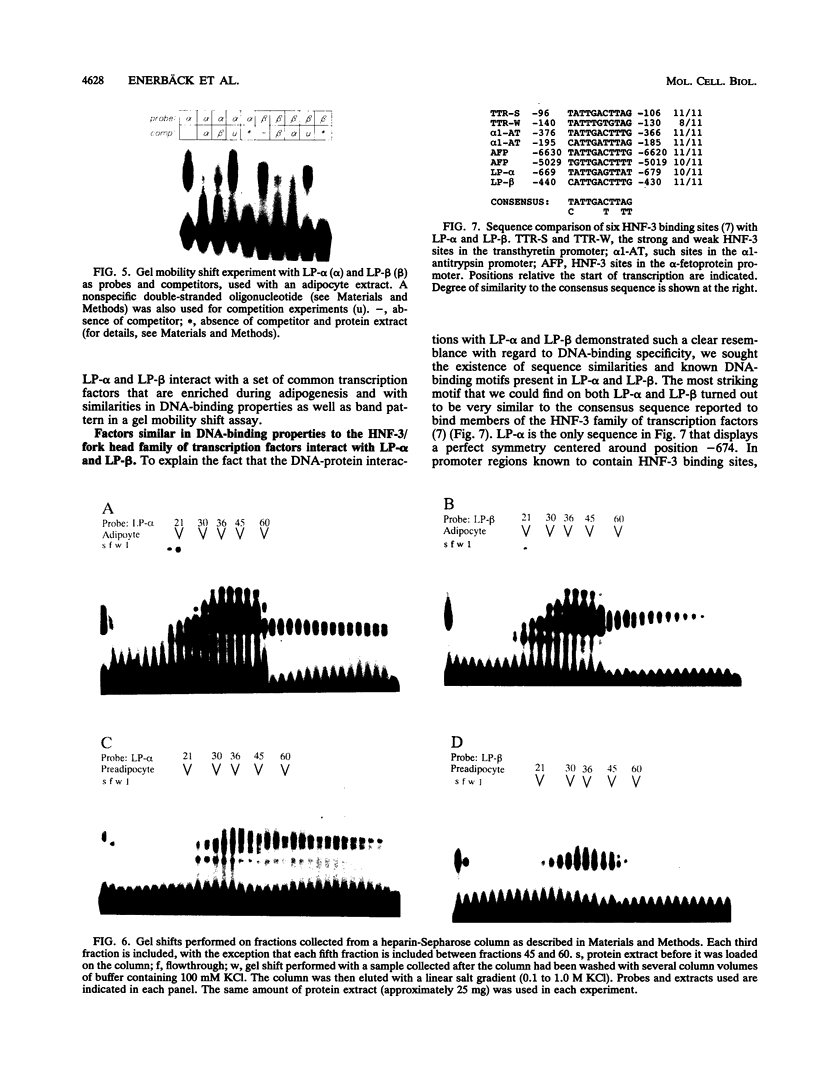
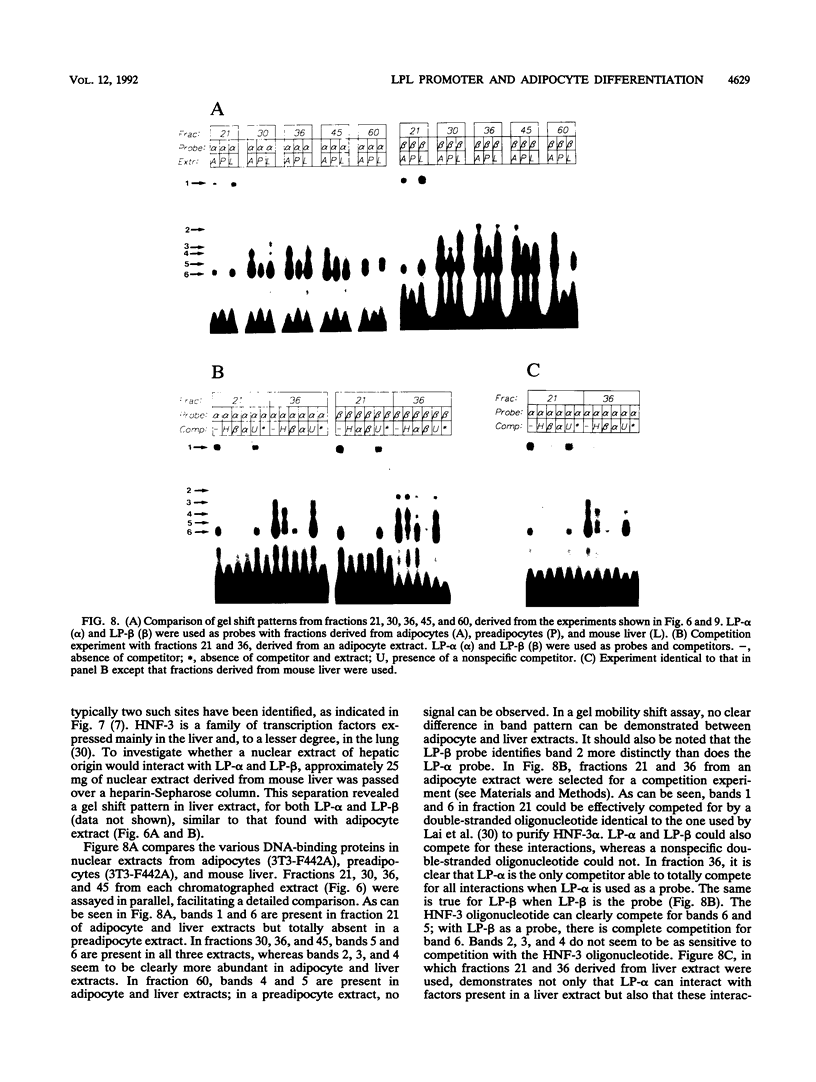
When preadipocytes differentiate into adipocytes, several differentiation-linked genes are activated. Lipoprotein lipase (LPL) is one of the first genes induced during this process. To investigate early events in adipocyte development, we have focused on the transcriptional activation of the LPL gene. For this purpose, we have cloned and fused different parts of intragenic and flanking sequences with a chloramphenicol acetyltransferase reporter gene. Transient transfection experiments and DNase I hypersensitivity assays indicate that several positive as well as negative elements contribute to transcriptional regulation of the LPL gene. When reporter gene constructs were stably introduced into preadipocytes, we were able to monitor and compare the activation patterns of different promoter deletion mutants at selected time points representing the process of adipocyte development. We could delimit two cis-regulatory elements important for gradual activation of the LPL gene during adipocyte development in vitro. These elements, LP-alpha (-702 to -666) and LP-beta (-468 to -430), contain a striking similarity to a consensus sequence known to bind the transcription factors HNF-3 and fork head. Results of gel mobility shift assays and DNase I and exonuclease III in vitro protection assays indicate that factors with DNA-binding properties similar to those of the HNF-3/fork head family of transcription factors are present in adipocytes and interact with LP-alpha and LP-beta. We also demonstrate that LP-alpha and LP-beta were both capable of conferring a differentiation-linked expression pattern to a heterolog promoter, thus mimicking the expression of the endogenous LPL gene during adipocyte differentiation. These findings indicate that interactions with LP-alpha and LP-beta could be a part of a differentiation switch governing induction of the LPL gene during adipocyte differentiation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amri E. Z., Dani C., Doglio A., Grimaldi P., Ailhaud G. Coupling of growth arrest and expression of early markers during adipose conversion of preadipocyte cell lines. Biochem Biophys Res Commun. 1986 Jun 13;137(2):903–910. doi: 10.1016/0006-291x(86)91165-4. [DOI] [PubMed] [Google Scholar]
- Aronow B., Lattier D., Silbiger R., Dusing M., Hutton J., Jones G., Stock J., McNeish J., Potter S., Witte D. Evidence for a complex regulatory array in the first intron of the human adenosine deaminase gene. Genes Dev. 1989 Sep;3(9):1384–1400. doi: 10.1101/gad.3.9.1384. [DOI] [PubMed] [Google Scholar]
- Auwerx J. H., Deeb S., Brunzell J. D., Wolfbauer G., Chait A. Lipoprotein lipase gene expression in THP-1 cells. Biochemistry. 1989 May 30;28(11):4563–4567. doi: 10.1021/bi00437a009. [DOI] [PubMed] [Google Scholar]
- Cao Z., Umek R. M., McKnight S. L. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991 Sep;5(9):1538–1552. doi: 10.1101/gad.5.9.1538. [DOI] [PubMed] [Google Scholar]
- Carlsson P., Bjursell G. Negative and positive promoter elements contribute to tissue specificity of apolipoprotein B expression. Gene. 1989 Apr 15;77(1):113–121. doi: 10.1016/0378-1119(89)90365-x. [DOI] [PubMed] [Google Scholar]
- Christy R. J., Yang V. W., Ntambi J. M., Geiman D. E., Landschulz W. H., Friedman A. D., Nakabeppu Y., Kelly T. J., Lane M. D. Differentiation-induced gene expression in 3T3-L1 preadipocytes: CCAAT/enhancer binding protein interacts with and activates the promoters of two adipocyte-specific genes. Genes Dev. 1989 Sep;3(9):1323–1335. doi: 10.1101/gad.3.9.1323. [DOI] [PubMed] [Google Scholar]
- Costa R. H., Grayson D. R., Darnell J. E., Jr Multiple hepatocyte-enriched nuclear factors function in the regulation of transthyretin and alpha 1-antitrypsin genes. Mol Cell Biol. 1989 Apr;9(4):1415–1425. doi: 10.1128/mcb.9.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani C., Amri E. Z., Bertrand B., Enerback S., Bjursell G., Grimaldi P., Ailhaud G. Expression and regulation of pOb24 and lipoprotein lipase genes during adipose conversion. J Cell Biochem. 1990 Jun;43(2):103–110. doi: 10.1002/jcb.240430202. [DOI] [PubMed] [Google Scholar]
- Dawson P. A., Hofmann S. L., van der Westhuyzen D. R., Südhof T. C., Brown M. S., Goldstein J. L. Sterol-dependent repression of low density lipoprotein receptor promoter mediated by 16-base pair sequence adjacent to binding site for transcription factor Sp1. J Biol Chem. 1988 Mar 5;263(7):3372–3379. [PubMed] [Google Scholar]
- Deeb S. S., Peng R. L. Structure of the human lipoprotein lipase gene. Biochemistry. 1989 May 16;28(10):4131–4135. doi: 10.1021/bi00436a001. [DOI] [PubMed] [Google Scholar]
- Dobson D. E., Groves D. L., Spiegelman B. M. Nucleotide sequence and hormonal regulation of mouse glycerophosphate dehydrogenase mRNA during adipocyte and muscle cell differentiation. J Biol Chem. 1987 Feb 5;262(4):1804–1809. [PubMed] [Google Scholar]
- Edlund T., Walker M. D., Barr P. J., Rutter W. J. Cell-specific expression of the rat insulin gene: evidence for role of two distinct 5' flanking elements. Science. 1985 Nov 22;230(4728):912–916. doi: 10.1126/science.3904002. [DOI] [PubMed] [Google Scholar]
- Enerbäck S., Semb H., Bengtsson-Olivecrona G., Carlsson P., Hermansson M. L., Olivecrona T., Bjursell G. Molecular cloning and sequence analysis of cDNA encoding lipoprotein lipase of guinea pig. Gene. 1987;58(1):1–12. doi: 10.1016/0378-1119(87)90023-0. [DOI] [PubMed] [Google Scholar]
- Enerbäck S., Semb H., Tavernier J., Bjursell G., Olivecrona T. Tissue-specific regulation of guinea pig lipoprotein lipase; effects of nutritional state and of tumor necrosis factor on mRNA levels in adipose tissue, heart and liver. Gene. 1988 Apr 15;64(1):97–106. doi: 10.1016/0378-1119(88)90484-2. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimble J. M., Levens D., Max E. E. B-cell nuclear proteins binding in vitro to the human immunoglobulin kappa enhancer: localization by exonuclease protection. Mol Cell Biol. 1987 May;7(5):1815–1822. doi: 10.1128/mcb.7.5.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimble J. M., Max E. E. Human immunoglobulin kappa gene enhancer: chromatin structure analysis at high resolution. Mol Cell Biol. 1987 Jan;7(1):15–25. doi: 10.1128/mcb.7.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski K., Carneiro M., Schibler U. Tissue-specific in vitro transcription from the mouse albumin promoter. Cell. 1986 Dec 5;47(5):767–776. doi: 10.1016/0092-8674(86)90519-2. [DOI] [PubMed] [Google Scholar]
- Green H., Kehinde O. Formation of normally differentiated subcutaneous fat pads by an established preadipose cell line. J Cell Physiol. 1979 Oct;101(1):169–171. doi: 10.1002/jcp.1041010119. [DOI] [PubMed] [Google Scholar]
- Green H., Kehinde O. Spontaneous heritable changes leading to increased adipose conversion in 3T3 cells. Cell. 1976 Jan;7(1):105–113. doi: 10.1016/0092-8674(76)90260-9. [DOI] [PubMed] [Google Scholar]
- Gross D. S., Garrard W. T. Nuclease hypersensitive sites in chromatin. Annu Rev Biochem. 1988;57:159–197. doi: 10.1146/annurev.bi.57.070188.001111. [DOI] [PubMed] [Google Scholar]
- Herrera R., Ro H. S., Robinson G. S., Xanthopoulos K. G., Spiegelman B. M. A direct role for C/EBP and the AP-I-binding site in gene expression linked to adipocyte differentiation. Mol Cell Biol. 1989 Dec;9(12):5331–5339. doi: 10.1128/mcb.9.12.5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X. X., Enerbäck S., Hudson J., Youkhana K., Gimble J. M. Cloning and characterization of the promoter of the murine lipoprotein lipase-encoding gene: structural and functional analysis. Gene. 1991 Nov 15;107(2):247–258. doi: 10.1016/0378-1119(91)90325-6. [DOI] [PubMed] [Google Scholar]
- Jones K. A., Yamamoto K. R., Tjian R. Two distinct transcription factors bind to the HSV thymidine kinase promoter in vitro. Cell. 1985 Sep;42(2):559–572. doi: 10.1016/0092-8674(85)90113-8. [DOI] [PubMed] [Google Scholar]
- Kaestner K. H., Christy R. J., Lane M. D. Mouse insulin-responsive glucose transporter gene: characterization of the gene and trans-activation by the CCAAT/enhancer binding protein. Proc Natl Acad Sci U S A. 1990 Jan;87(1):251–255. doi: 10.1073/pnas.87.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchgessner T. G., Chuat J. C., Heinzmann C., Etienne J., Guilhot S., Svenson K., Ameis D., Pilon C., d'Auriol L., Andalibi A. Organization of the human lipoprotein lipase gene and evolution of the lipase gene family. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9647–9651. doi: 10.1073/pnas.86.24.9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai E., Prezioso V. R., Tao W. F., Chen W. S., Darnell J. E., Jr Hepatocyte nuclear factor 3 alpha belongs to a gene family in mammals that is homologous to the Drosophila homeotic gene fork head. Genes Dev. 1991 Mar;5(3):416–427. doi: 10.1101/gad.5.3.416. [DOI] [PubMed] [Google Scholar]
- Previato L., Parrott C. L., Santamarina-Fojo S., Brewer H. B., Jr Transcriptional regulation of the human lipoprotein lipase gene in 3T3-L1 adipocytes. J Biol Chem. 1991 Oct 5;266(28):18958–18963. [PubMed] [Google Scholar]
- Samuelsson L., Strömberg K., Vikman K., Bjursell G., Enerbäck S. The CCAAT/enhancer binding protein and its role in adipocyte differentiation: evidence for direct involvement in terminal adipocyte development. EMBO J. 1991 Dec;10(12):3787–3793. doi: 10.1002/j.1460-2075.1991.tb04948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. J., Wise L. S., Berkowitz R., Wan C., Rubin C. S. Insulin-like growth factor-I is an essential regulator of the differentiation of 3T3-L1 adipocytes. J Biol Chem. 1988 Jul 5;263(19):9402–9408. [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Tsuchiya S., Yamabe M., Yamaguchi Y., Kobayashi Y., Konno T., Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int J Cancer. 1980 Aug;26(2):171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- Umek R. M., Friedman A. D., McKnight S. L. CCAAT-enhancer binding protein: a component of a differentiation switch. Science. 1991 Jan 18;251(4991):288–292. doi: 10.1126/science.1987644. [DOI] [PubMed] [Google Scholar]
- Vilaró S., Llobera M., Bengtsson-Olivecrona G., Olivecrona T. Synthesis of lipoprotein lipase in the liver of newborn rats and localization of the enzyme by immunofluorescence. Biochem J. 1988 Jan 15;249(2):549–556. doi: 10.1042/bj2490549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel D., Jäckle H. The fork head domain: a novel DNA binding motif of eukaryotic transcription factors? Cell. 1990 Nov 2;63(3):455–456. doi: 10.1016/0092-8674(90)90439-l. [DOI] [PubMed] [Google Scholar]
- Weigel D., Jürgens G., Küttner F., Seifert E., Jäckle H. The homeotic gene fork head encodes a nuclear protein and is expressed in the terminal regions of the Drosophila embryo. Cell. 1989 May 19;57(4):645–658. doi: 10.1016/0092-8674(89)90133-5. [DOI] [PubMed] [Google Scholar]
- Whitaker J. R., Granum P. E. An absolute method for protein determination based on difference in absorbance at 235 and 280 nm. Anal Biochem. 1980 Nov 15;109(1):156–159. doi: 10.1016/0003-2697(80)90024-x. [DOI] [PubMed] [Google Scholar]
- Wion K. L., Kirchgessner T. G., Lusis A. J., Schotz M. C., Lawn R. M. Human lipoprotein lipase complementary DNA sequence. Science. 1987 Mar 27;235(4796):1638–1641. doi: 10.1126/science.3823907. [DOI] [PubMed] [Google Scholar]
- Wise L. S., Green H. Studies of lipoprotein lipase during the adipose conversion of 3T3 cells. Cell. 1978 Feb;13(2):233–242. doi: 10.1016/0092-8674(78)90192-7. [DOI] [PubMed] [Google Scholar]
- Wood T. G., McGeady M. L., Baroudy B. M., Blair D. G., Vande Woude G. F. Mouse c-mos oncogene activation is prevented by upstream sequences. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7817–7821. doi: 10.1073/pnas.81.24.7817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. An exonuclease protection assay reveals heat-shock element and TATA box DNA-binding proteins in crude nuclear extracts. Nature. 1985 Sep 5;317(6032):84–87. doi: 10.1038/317084a0. [DOI] [PubMed] [Google Scholar]