Abstract
Background
Matrix metalloproteinase-1 (MMP-1) plays an important role during the destruction of periodontal tissue. Although multiple studies had focused on the association between MMP-1 g.-1607dupG and periodontitis susceptibility, the results remained inconclusive. The purpose of this meta-analysis was to explore its role in the development of periodontitis.
Methods
Retrieved studies from Pubmed, Web of Science, Medline and Google Scholar Search regarding MMP-1 g.-1607dupG and periodontitis susceptibility were included into the final analysis with definite selection and exclusion criteria. Overall and stratified analyses based on disease type, severity, ethnicity and smoking status were performed. Odds ratio (OR) and 95% confidence interval (CI) were used to evaluate the association between MMP-1 g.-1607dupG and periodontitis susceptibility, while Q test and Egger’s test were adopted respectively to assess heterogeneity among studies and publication bias.
Results
A total of 1580 periodontitis cases and 1386 controls in 11 case-control studies were included in the meta-analysis. The pooled results showed significant association between periodontitis susceptibility and MMP-1 g.-1607dupG polymorphism in homozygote (2G/2G versus 1G/1G, OR = 1.50, 95% CI = 1.02–2.20) and dominant model analysis (2G/2G+2G/1G versus 1G/1G, OR = 1.28, 95% CI = 1.04–1.57). For subgroups by type of periodontitis, increased risk of chronic periodontitis was observed on heterozygote (2G/1G versus 1G/1G, OR = 2.01, 95% CI = 1.58–2.56) and dominant model (OR = 1.27, 95% CI = 1.03–1.57). Furthermore, similar association was also detected in severe chronic periodontitis (2G/2G versus 1G/1G, OR = 2.15, 95% CI = 1.35–3.43; 2G/2G+2G/1G versus 1G/1G, OR = 1.64, 95% CI = 1.12–2.39; 2G/2G versus 2G/1G+1G/1G, OR = 1.86, 95% CI = 1.31–2.64).
Conclusions
Our meta-analysis demonstrated that MMP-1 g.-1607dupG polymorphism was associated with chronic periodontitis, especially the severity of the disease condition.
Introduction
Periodontitis are of the most common oral diseases around the world with high prevalence of 10%–15% [1], constituted by two major types: chronic periodontitis (CP) and aggressive periodontitis (AgP). As kinds of inflammatory diseases, they could not only cause great periodontium damage by interaction between pathogens challenge and host immunological reaction [2], [3], but also contribute to tooth loosening and loss. Furthermore, their potential adverse effects on systemic health [4], such as adverse pregnancy outcome [5], diabetes mellitus [6], cardiovascular disease [7], [8] and some other general diseases [9], [10], should also be paid much attention to.
Matrix metalloproteinases (MMPs), a series of proteolytic enzymes responsible for the degradation of extracellular matrix and basement membranes in the beginning and developing courses of a wide range of diseases [11]–[13], have been verified to be involved in the pathogenesis of periodontitis [14], [15]. Among them, MMP-1 is the most abundant component of the periodontal tissue matrix [16], [17], regulating the degradation of native interstitial collagens [18]. It is worth noting that fibrillar collagens types I and III, the predominant types of interstitial collagens in periodontium which are resistant to most proteinases, can be degraded by MMP-1 [19]. Consequently, tissue inhibitors of metalloproteinases (TIMPs) have been used as the hypurgia for human periodontitis to control MMP-mediated extracellular matrix breakdown [16].
Owing to the important role of MMP-1 in the pathogenesis of periodontitis, a variety of molecular epidemiological studies have been conducted to explore the association between MMP-1 polymorphisms and the susceptibility of periodontitis. The guanine addition at the -1607 position, the substitution of guanine for adenine at position -519 as well as the adenine to thymidine mutation at position -442 of the MMP-1 gene promoter were supposed to be the functional polymorphisms associated with periodontitis. However, polymorphism at position -1607 (MMP-1 g.-1607dupG, rs1799750) was the most extensive studied locus. During the past few years, various studies from different ethnic groups were conducted to test its relevance with periodontitis susceptibility. Nevertheless, these results still remained inconsistent, which warranted us to perform a meta-analysis to further clarify its role in the pathogenesis of periodontitis.
Methods
Search Strategy and Data Extraction
To systematically retrieve all the case-control studies related to the association between MMP-1 g.-1607dupG and periodontitis risks, databases of PubMed, Web of Science, Medline and Google Scholar Search were searched (by May 30, 2012) with the key words “periodontitis”, “MMP-1” (or “matrix metalloproteinase-1”) and “polymorphism” (or “variant”). The references of all identified publications were manually searched for additional studies. In the search results, only English articles were taken in. All selected studies complied with the two main criteria: (1) independent case-control study evaluating the association between MMP-1 polymorphism and periodontitis susceptibility; (2) the number or frequency of genotype given in detail. Studies with insufficient information (e.g. neither the frequency nor the number of genotype was given) were excluded. Two investigators (Li and Pan) independently extracted the data and reached a consensus in order to minimize the bias and improve the reliability. Then information including the first author’s name, year of publication, country of origin, ethnicity, type of periodontitis, source of control, number of cases and health controls, genotyping method, Hardy-Weinberg Equilibrium among controls and the main result of each publication was picked up. Different ethnic descents were classified as Caucasian, Asian or Mixed (derived from an admixture of different ethnic groups).
Statistical Analysis
Hardy-Weinberg equilibrium of the genotype distributions among controls were estimated by a goodness-of-fit χ2 test. The association between MMP-1 g.-1607dupG and susceptibility of periodontitis was estimated by odds ratio (OR) and 95% confidence interval (CI). In addition to the overall analysis, stratified analyses based on disease type, severity, ethnicity and smoking status were performed respectively. The 2G/2G+2G/1G versus 1G/1G and 2G/2G versus 2G/1G+1G/1G comparison were estimated to assume dominant and recessive effects of the variant 2G allele respectively. The statistical significance of pooled ORs was determined by Z test. Q test based on P and I2 value was used to assess heterogeneity among studies. I2 was a value that could describe the percentage of variation across studies. The bigger I2 value, the stronger heterogeneity is. P>0.05 for the Q-test indicated no significant heterogeneity across studies, and the fixed-effects model (the Mantel-Haenszel method) was applied; if not, the random-effects model was used (the DerSimonian and Laird method) [20].
Publication bias was evaluated with the linear regression asymmetry test by Egger et al [21]. P<0.05 was used as an indication for the presence of potential publication bias. All analyses were done with STATA software (version 11; StataCorp LP, College Station, TX, USA), and the P values were all two-sided.
Results
Characteristics of Studies
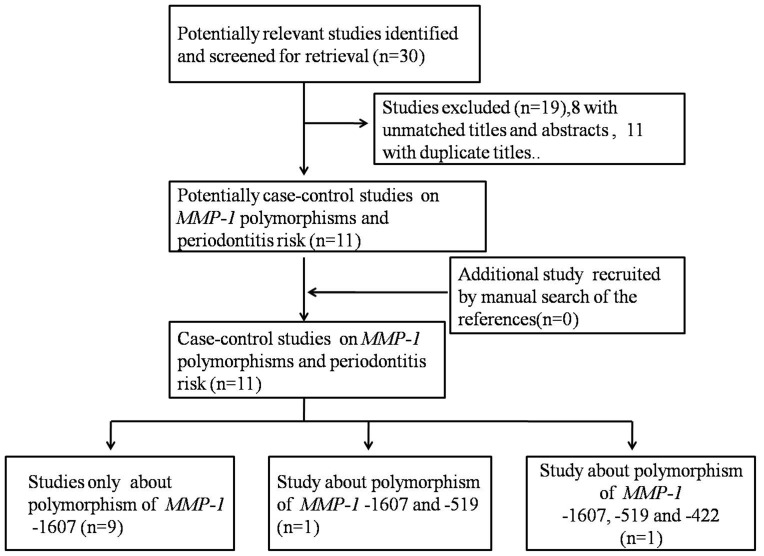
11 relevant papers with 1580 cases and 1386 controls about MMP-1 g.-1607dupG polymorphism were recruited and put into the final meta-analyses [22]–[32] (Figure 1). All studies were case-control studies, including nine studies [22], [23], [26]–[32] for CP, one study [25] for AgP and one [24] for both. There were four studies of Asian descent [24]–[26], [31], four studies of European descent [22], [23], [28], [29] and three studies of mixed ethnicity descent [27], [30], [32]. The distribution of genotypes in the controls was not in agreement with HWE for three studies [28], [30], [31]. (Table 1).
Figure 1. Studies identification diagram.
Table 1. Relevant literatures concerning relationships between MMP-1 polymorphisms and periodontitis.
| Author | Year | Country | Ethnicity | Type of disease | Source of control | Sample size ofcase/control | genotyping methods | HWE among controls | Result |
| MMP1 -1607dupG | |||||||||
| de Souza [22] | 2003 | Brazil | Caucasian | CP | HB | 50/37 | PCR-RFLP | 0.87 | +a |
| Holla [23] | 2004 | Czech | Caucasian | CP | PB | 133/196 | PCR-RFLP | 0.52 | +a |
| Itagaki [24] | 2004 | Japan | Asian | AgP | HB | 37/142 | Taqman | 0.47 | − |
| CP | HB | 205/142 | Taqman | 0.47 | − | ||||
| Cao [25] | 2005 | China | Asian | AgP | HB | 40/52 | PCR-RFLP | 0.77 | + |
| Cao [26] | 2006 | China | Asian | CP | HB | 60/50 | PCR-RFLP | 0.99 | +b |
| Astolfi [27] | 2006 | Brazil | Mixed | CP | PB | 114/109 | PCR-RFLP | 0.68 | − |
| Pirhan [28] | 2008 | Turkey | Caucasian | CP | HB | 101/97 | PCR-RFLP | 0.009 | − |
| Ustun [29] | 2008 | Turkey | Caucasian | CP | HB | 126/54 | PCR-RFLP | 0.75 | − |
| Repeke [30] | 2009 | Brazil | Mix | CP | PB | 178/190 | PCR-RFLP | 0.0005 | − |
| Loo [31] | 2011 | China | Asian | CP | PB | 280/250 | PCR-RFLP | 1.36E−39 | + |
| Luczyszyn [32] | 2012 | Brazil | Mix | CP | HB | 60/67 | PCR-RFLP | 0.07 | − |
| MMP1 -519 A>G | |||||||||
| Holla [23] | 2004 | Czech | Caucasian | CP | PB | 133/196 | PCR-RFLP | 0.52 | − |
| Pirhan [28] | 2008 | Turkey | Caucasian | CP | HB | 102/97 | PCR-RFLP | 0.009 | − |
| MMP1 -422 A>T | |||||||||
| Holla [23] | 2004 | Czech | Caucasian | CP | PB | 133/196 | PCR-RFLP | 0.52 | +c |
+The significant relevance between MMP-1 polymorphism and the risk of periodontitis was picked up in this article.
−No association between MMP-1 polymorphism and the risk of periodontitis was picked up in this article.
only association with severe chronic periodontitis in non-smoking population.
only association with severe chronic periodontitis.
only association with severe chronic periodontitis in smoking population.
There were only 2 studies for MMP-1-519 A/G, neither found any relationship with periodontitis [23], [28]. Meanwhile, MMP1 -422 A/T was only mentioned in one literature [23] (Table 1). Therefore, meta-analyses for the latter two SNPs were not performed in the present study.
Overall Analysis
In general, the MMP-1 -1607 2G/2G homozygote was significantly associated with an increased risk of periodontitis compared with wild-type homozygote (1G/1G) (OR = 1.50, 95% CI = 1.02–2.20). Significant association was also found in the dominant genetic model (OR = 1.28, 95%CI = 1.04–1.57), but neither in recessive model (OR = 1.31, 95%CI = 0.95–1.79) nor in heterozygote comparison (OR = 1.23, 95%CI = 0.98–1.53).
Stratified Analysis by Type of Disease
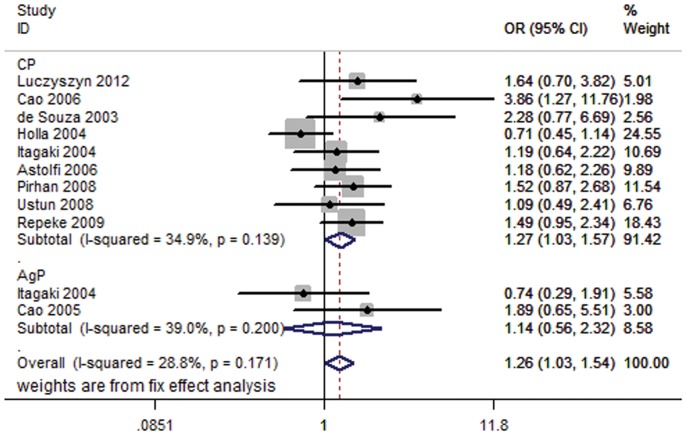
Appreciable differences were identified in the etiology feature between CP and AgP [33], implicating that there might be different genetic mechanism between them. Subgroup analysis showed that individuals were more susceptible to CP (OR = 1.27, 95% CI = 1.03–1.57) rather than AgP (OR = 1.14, 95% CI = 0.56–2.32) under the dominant model (Figure 2). The significantly elevated risk of CP was also observed in heterozygote comparison (OR = 2.01, 95% CI = 1.58–2.56) (Table 2).
Figure 2. Forest plot of periodontitis risk associated with MMP-1 g.-1607dupG by type of disease under dominant model (2G/2G+1G/2G vs. 1G/1G).
Fixed-effects model was used.
Table 2. Meta-analysis for MMP-1 g.-1607dupG polymorphism and periodontitis risk.
| Variables | Na | 2G/2G versus1G/1G | 2G/1G versus1G/1G | 2G/2G+2G/1Gversus 1G/1G | 2G/2G versus2G/1G+1G/1G | 2G versus 1G | |||||
| OR (95%CI) | P b | OR (95%CI) | P b | OR (95%CI) | P b | OR (95%CI) | P b | OR (95%CI) | P b | ||
| Total | 10 | 1.50 (1.02–2.20) | 0.023 | 1.23(0.98–1.53) | 0.522 | 1.28 (1.04–1.57) | 0.162 | 1.31(0.95–1.79) | 0.012 | 1.28 (1.03–1.59) | 0.040 |
| Type of disease | |||||||||||
| CP | 9 | 1.41(0.96–2.07) | 0.040 | 2.01 (1.58–2.56) | 0.368 | 1.27 (1.03–1.57) | 0.139 | 1.17(0.96–1.43) | 0.059 | 1.23(0.99–1.51) | 0.012 |
| AgP | 2 | 1.54(0.28–8.55) | 0.031 | 1.30(0.59–2.84) | 0.222 | 1.14(0.56–2.32) | 0.200 | 1.64(0.35–7.70) | 0.008 | 1.34(0.48–3.73) | 0.010 |
| Severity of CP | |||||||||||
| moderate | 4 | 1.31(0.74–2.32) | 0.312 | 1.25(0.73–2.16) | 0.531 | 1.29(0.77–2.15) | 0.409 | 1.14(0.76–1.70) | 0.559 | 1.14(0.87–1.51) | 0.306 |
| Severe | 5 | 2.15 (1.35–3.43) | 0.191 | 1.35(0.89–2.05) | 0.753 | 1.64 (1.12–2.39) | 0.444 | 1.86 (1.31–2.64) | 0.187 | 1.38 (1.16–1.65) | 0.101 |
| Ethnicityc | |||||||||||
| Caucasian | 4 | 1.22(0.59–2.51) | 0.047 | 1.06(0.75–1.48) | 0.363 | 1.06(0.78–1.45) | 0.099 | 1.01(0.71–1.44) | 0.160 | 1.04(0.86–1.25) | 0.058 |
| Asian | 2 | 2.79(0.56–13.98) | 0.020 | 1.31(0.74–2.32) | 0.242 | 1.61(0.95–2.73) | 0.071 | 2.01(0.72–5.61) | 0.027 | 1.75(0.77–3.95) | 0.011 |
| Mixed | 3 | 1.33(0.89–1.98) | 0.976 | 1.48 (1.03–2.14) | 0.474 | 1.42 (1.01–1.99) | 0.793 | 1.05(0.77–1.45) | 0.472 | 1.20(0.95–1.50) | 0.094 |
| Smoking statusc | |||||||||||
| Smoking | 3 | 1.14(0.43–3.02) | 0.222 | 1.12(0.54–2.34) | 0.976 | 1.12(0.55–2.29) | 0.682 | 1.19(0.53–2.65) | 0.140 | 1.19(0.81–1.76) | 0.415 |
| non–smoking | 7 | 1.36(0.80–2.32) | 0.012 | 1.17(0.89–1.54) | 0.497 | 1.25(0.84–1.87) | 0.046 | 1.20(0.96–1.50) | 0.014 | 1.21(0.90–1.65) | 0.001 |
Number of comparisons.
P value of Q-test for heterogeneity test. Random-effects model was used when P value for heterogeneity test <0.05; otherwise, fix-effects model was used.
AgP individuals are not included.
Stratified Analysis by Severity
As showed in Table 2, significant association between the variation and severe CP was found in almost all types of comparisons. However, no significant relationship was found in moderate periodontitis (Table 2).
Stratified Analysis by Ethnicity and Smoking Status
No significant association was found in Asians or Caucasians under any genetic model when analyzing the association between MMP-1 g.-1607dupG and CP by ethnicity. Similar effects were also observed in stratified analysis by smoking status.
Heterogeneity Analysis
Heterogeneity among studies was observed in MMP-1 g.-1607dupG recessive model (I2 = 57.3%, P = 0.012) and homozygote comparison (I2 = 53.3%, P = 0.023). Z test of subgroup analysis indicated that type of periodontitis (P z = 0.024) and smoking condition (P z = 0.048) may be the main source of heterogeneity by homozygote comparison.
Sensitive Analysis
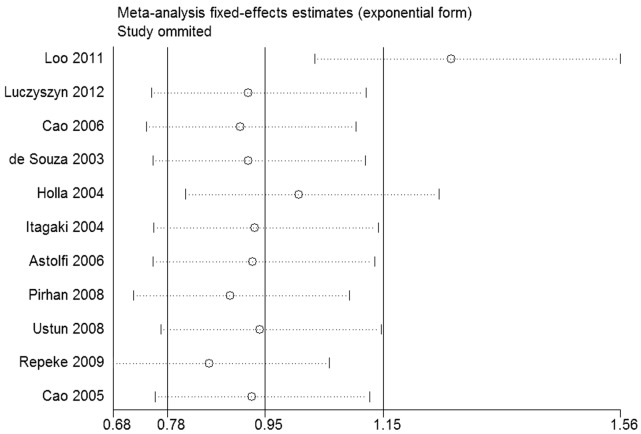
There are three of the 11 studies at variance with Hardy-Weinberg Equilibrium [28], [30], [31]. Among them, the study of Loo et al. [31] was found to be the major source of heterogeneity by sensitive analysis (Figure 3), and heterogeneity was effectively removed after excluding this study under the dominant model (I2 = 30.9%, P = 0.162). In addition, the pooled ORs and 95% CI under the dominant model in overall comparison was obviously influenced (OR = 1.28, 95% CI = 1.04–1.57). (Before excluding, OR = 1.10, 95% CI = 0.60–2.03). The other two studies were reserved because they did not substantially affect the heterogeneity and the results (Figure 3). Then the statistical process was limited to ten articles left.
Figure 3. The result of sensitive analysis under the dominant model (2G/2G+1G/2G vs. 1G/1G).
Fix effect model was used.
Publication Bias Analysis
Egger’s test was conducted to assess the publication bias of the included studies. No publication bias was found in any genetic models. (t = −1.46, P = 0.183 for 2G/2G+2G/1G versus 1G/1G).
Discussion
Since 2003, there have been 11 studies focusing on the relationship between the SNP at position -1607 of MMP-1 and periodontitis susceptibility. De Souza et al. found that subjects carrying -1607 2G allele tended to be more susceptible to severe CP in non-smoking Brazilian subjects [22]. Similar results were then observed in Czech [23] and Chinese subjects [26]. Elevated risk of CP and AgP was also found in 2G carriers of Asian origins by Loo [31] and Cao [25] et al. The other studies, however, obtained negative results [24], [27], [28]–[30], [32]. In sum, the definite role of MMP-1 g.-1607dupG in the development of periodontitis remains controversy.
By recruiting all of the above studies, we conducted the current meta-analysis and finally found that this variant could contribute to increased risk of periodontitis. In stratified analysis by type of disease, its association with susceptibility of CP rather than AgP was observed. Furthermore, its association with elevated risk of severe CP was also obtained. However, we did not find any meaningful associations in stratified analysis by ethnic and smoking status, both of which were considered to be the relevant factors of periodontitis.
MMP-1, playing a crucial role in paradentium destruction, was suggested to be an important risk factor of CP. It was previously demonstrated that 2G allele instead of 1G allel at MMP-1 -1607 created a new 5′-GGA-3′ core recognition sequence for members of the erythroblast transformation specific family as the binding site, causing increased transcriptional activity, systemically accelerate MMP-1 gene transcription and protein over-expression, expounding the molecular basis of a anabatic matrix degradation. [34]. In addition, some scholars demonstrated that MMP1-1607 2G allele was associated with increased MMP-1 mRNA expression in vivo [30]. Therefore, it was biologically plausible that individuals carrying MMP1-1607 2G allele were associated with over-expression of MMP-1, consequently contributing to more susceptibility to CP.
On the other hand, lack of association with AgP was found in the current study, not only implicating that MMP-1 might not been entirely activated in the pathogenesis of AgP, but also providing further evidence that AgP was different from CP in some aspects. Some scholars considered that AgP and CP shared some susceptibility genes, but not all [35]. Similarly, effect of some genetic variants had also been proved to be different [36] or even contract [37] on CP and AgP. Therefore, the role MMP-1 played in the development of AgP, if any, may not be as important as it did in CP. However, the negative association should be carefully interpreted because of the limited sample size.
Several limitations should be addressed. Firstly, the sample size is still a formidable problem. Based on the current sample size, we only had 63% power at a 0.05 or smaller with level to detect an OR of 1.2 or greater and 0.83 or smaller with an exposure frequency of 30%. Secondly, gene-environment interactions which may modulate the periodontitis susceptibility were limited owing to the lack of the origin data in the including studies.
Stated thus, our meta-analysis suggested that MMP-1 g.-1607dupG contribute to the elevated risk of CP. Further studies with large sample size and detailed information are needed to validate these results.
Supporting Information
PRISMA 2009 checklist.
(DOC)
PRISMA 2009 Flow Diagram.
(DOC)
Funding Statement
This study was supported in part by the National Natural Science Foundation of China (30973361, 81170981 and 81000457), Natural Science Foundation of JiangSu Province (81000457), Natural Science Foundation for Colleges and Universities in JiangSu Province (10kJB320004), Project of State Key Laboratory of Oral Diseases (SKLODSCU2009KF05) and Project Funded by the Priority Academic Program Development of JiangSu Higher Education Institutions (PAPD). The funders had no role in study design,data collection and analysis,decision to publish,or preparation of the manuscript.
References
- 1. Albandar JM, Rams TE (2002) Global epidemiology of periodontal diseases: an overview. Periodontol 2000 29: 7–10. [DOI] [PubMed] [Google Scholar]
- 2. Page RC, Offenbacher S, Schroeder HE, Seymour GJ, Kornman KS (1997) Advances in the pathogenesis of periodontitis: summary of developments, clinical implications and future directions. Periodontol 2000 14: 216–248. [DOI] [PubMed] [Google Scholar]
- 3. Silva N, Dutzan N, Hernandez M, Dezerega A, Rivera O, et al. (2008) Characterization of progressive periodontal lesions in chronic periodontitis patients: levels of chemokines, cytokines, matrix metalloproteinase-13, periodontal pathogens and inflammatory cells. J Clin Periodontol 35: 206–214. [DOI] [PubMed] [Google Scholar]
- 4. Mealey BL (1999) Influence of periodontal infections on systemic health. Periodontol 2000 21: 197–209. [DOI] [PubMed] [Google Scholar]
- 5. Dasanayake AP, Russell S, Boyd D, Madianos PN, Forster T, et al. (2003) Preterm low birth weight and periodontal disease among African Americans. Dental Clinics of North America 47: 115. [DOI] [PubMed] [Google Scholar]
- 6. Mealey BL, Rethman MP (2003) Periodontal disease and diabetes mellitus. Bidirectional relationship. Dent Today 22: 107–113. [PubMed] [Google Scholar]
- 7. Desvarieux M, Demmer RT, Rundek T, Boden-Albala B, Jacobs DR, et al. (2003) Relationship between periodontal disease, tooth loss, and carotid artery plaque. Stroke 34: 2120–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yamazaki K, Honda T, Oda T, Ueki-Maruyama K, Nakajima T, et al. (2005) Effect of periodontal treatment on the C-reactive protein and proinflammatory cytokine levels in Japanese periodontitis patients. Journal of periodontal research 40: 53–58. [DOI] [PubMed] [Google Scholar]
- 9. Scannapieco F, Rethman M (2003) The relationship between periodontal diseases and respiratory diseases. Dentistry today 22: 79. [PubMed] [Google Scholar]
- 10. Contreras A, Slots J (2001) Typing of herpes simplex virus from human periodontium. Oral microbiology and immunology 16: 63–64. [DOI] [PubMed] [Google Scholar]
- 11. Ravanti L, Kahari VM (2000) Matrix metalloproteinases in wound repair (review). Int J Mol Med 6: 391–407. [PubMed] [Google Scholar]
- 12. Nagase H, Woessner JF Jr (1999) Matrix metalloproteinases. J Biol Chem 274: 21491–21494. [DOI] [PubMed] [Google Scholar]
- 13. Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, et al. (1993) Matrix metalloproteinases: a review. Crit Rev Oral Biol Med 4: 197–250. [DOI] [PubMed] [Google Scholar]
- 14. Lee W, Aitken S, Sodek J, McCulloch C (1995) Evidence of a direct relationship between neutrophil collagenase activity and periodontal tissue destruction in vivo: role of active enzyme in human periodontitis. Journal of periodontal research 30: 23–33. [DOI] [PubMed] [Google Scholar]
- 15. Sorsa T, Tjaderhane L, Salo T (2004) Matrix metalloproteinases (MMPs) in oral diseases. Oral Dis 10: 311–318. [DOI] [PubMed] [Google Scholar]
- 16. Verstappen J, Von den Hoff J (2006) Tissue inhibitors of metalloproteinases (TIMPs): their biological functions and involvement in oral disease. Journal of dental research 85: 1074–1084. [DOI] [PubMed] [Google Scholar]
- 17. Hannas AR, Pereira JC, Granjeiro JM, Tjaderhane L (2007) The role of matrix metalloproteinases in the oral environment. Acta Odontol Scand 65: 1–13. [DOI] [PubMed] [Google Scholar]
- 18. Ejeil AL, Igondjo-Tchen S, Ghomrasseni S, Pellat B, Godeau G, et al. (2003) Expression of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) in healthy and diseased human gingiva. Journal of periodontology 74: 188–195. [DOI] [PubMed] [Google Scholar]
- 19. Visse R, Nagase H (2003) Matrix metalloproteinases and tissue inhibitors of metalloproteinases. Circulation research 92: 827–839. [DOI] [PubMed] [Google Scholar]
- 20. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 21. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Souza AP, Trevilatto PC, Scarel-Caminaga RM, Brito RB, Line SR (2003) MMP-1 promoter polymorphism: association with chronic periodontitis severity in a Brazilian population. J Clin Periodontol 30: 154–158. [DOI] [PubMed] [Google Scholar]
- 23. Izakovičová Hollá L, Jurajda M, Fassmann A, Dvorakova N, Znojil V, et al. (2004) Genetic variations in the matrix metalloproteinase-1 promoter and risk of susceptibility and/or severity of chronic periodontitis in the Czech population. Journal of clinical periodontology 31: 685–690. [DOI] [PubMed] [Google Scholar]
- 24. Itagaki M, Kubota T, Tai H, Shimada Y, Morozumi T, et al. (2004) Matrix metalloproteinase-1 and -3 gene promoter polymorphisms in Japanese patients with periodontitis. J Clin Periodontol 31: 764–769. [DOI] [PubMed] [Google Scholar]
- 25. Cao Z, Li C, Jin L, Corbet EF (2005) Association of matrix metalloproteinase-1 promoter polymorphism with generalized aggressive periodontitis in a Chinese population. J Periodontal Res 40: 427–431. [DOI] [PubMed] [Google Scholar]
- 26. Cao Z, Li C, Zhu G (2006) MMP-1 promoter gene polymorphism and susceptibility to chronic periodontitis in a Chinese population. Tissue Antigens 68: 38–43. [DOI] [PubMed] [Google Scholar]
- 27. Astolfi CM, Shinohara AL, da Silva RA, Santos MC, Line SR, et al. (2006) Genetic polymorphisms in the MMP-1 and MMP-3 gene may contribute to chronic periodontitis in a Brazilian population. J Clin Periodontol 33: 699–703. [DOI] [PubMed] [Google Scholar]
- 28. Pirhan D, Atilla G, Emingil G, Sorsa T, Tervahartiala T, et al. (2008) Effect of MMP-1 promoter polymorphisms on GCF MMP-1 levels and outcome of periodontal therapy in patients with severe chronic periodontitis. J Clin Periodontol 35: 862–870. [DOI] [PubMed] [Google Scholar]
- 29. Ustun K, Alptekin NO, Hakki SS, Hakki EE (2008) Investigation of matrix metalloproteinase-1–1607 1G/2G polymorphism in a Turkish population with periodontitis. J Clin Periodontol 35: 1013–1019. [DOI] [PubMed] [Google Scholar]
- 30. Repeke CE, Trombone AP, Ferreira SB Jr, Cardoso CR, Silveira EM, et al. (2009) Strong and persistent microbial and inflammatory stimuli overcome the genetic predisposition to higher matrix metalloproteinase-1 (MMP-1) expression: a mechanistic explanation for the lack of association of MMP1–1607 single-nucleotide polymorphism genotypes with MMP-1 expression in chronic periodontitis lesions. J Clin Periodontol 36: 726–738. [DOI] [PubMed] [Google Scholar]
- 31.Loo WTY, Wang M, Jin L, Cheung MNB, Li G (2011) Association of matrix metalloproteinase (MMP-1, MMP-3 and MMP-9) and cyclooxygenase-2 gene polymorphisms and their proteins with chronic periodontitis. Archives of Oral Biology. [DOI] [PubMed] [Google Scholar]
- 32.Luczyszyn SM, de Souza CM, Braosi AP, Dirschnabel AJ, Claudino M, et al.. (2012) Analysis of the association of an MMP1 promoter polymorphism and transcript levels with chronic periodontitis and end-stage renal disease in a Brazilian population. Arch Oral Biol. [DOI] [PubMed] [Google Scholar]
- 33. Benoist HM, Seck-Diallo A, Diouf A, Yabbre S, Sembene M, et al. (2011) Profile of chronic and aggressive periodontitis among Senegalese. J Periodontal Implant Sci 41: 279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rutter JL, Mitchell TI, Buttice G, Meyers J, Gusella JF, et al. (1998) A single nucleotide polymorphism in the matrix metalloproteinase-1 promoter creates an Ets binding site and augments transcription. Cancer Res 58: 5321–5325. [PubMed] [Google Scholar]
- 35. Yoshie H, Kobayashi T, Tai H, Galicia JC (2007) The role of genetic polymorphisms in periodontitis. Periodontology 2000 43: 102–132. [DOI] [PubMed] [Google Scholar]
- 36. Deng H, Liu F, Pan Y, Jin X, Wang H, et al. (2011) BsmI, TaqI, ApaI, and FokI polymorphisms in the vitamin D receptor gene and periodontitis: a meta-analysis of 15 studies including 1338 cases and 1302 controls. J Clin Periodontol 38: 199–207. [DOI] [PubMed] [Google Scholar]
- 37. Ding C, Zhao L, Sun Y, Li L, Xu Y (2012) Interleukin-1 receptor antagonist polymorphism (rs2234663) and periodontitis susceptibility: A meta-analysis. Arch Oral Biol 57: 585–593. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA 2009 checklist.
(DOC)
PRISMA 2009 Flow Diagram.
(DOC)