Abstract
Non-compaction of ventricular myocardium (NVM) is a rare clinical entity. It has been reported more frequently in recent years because of continuous improvements in imaging techniques and resolution. Although apical region of the left ventricle is the most commonly involved site, biventricular involvement has also been reported in the published literature. This abnormality is often associated with other congenital cardiac and extracardiac anomalies. We describe such a case, incidentally detected and documented by the combination of echocardiography and multidetector CT coronary angiography. In our case, NVM was associated with Cor-triatriutum, ventricular septal defect, persistent left superior vena cava and an anomalous extracardiac vessel. Synchronous association of all these anomalies in a child has never been reported in the literature.
Background
Non-compaction of ventricular myocardium (NVM), also called spongiform cardiomyopathy, is a rare congenital cardiomyopathy that affects both children and adults. The condition is believed to be caused by the arrest of normal embryogenesis in the endocardium and myocardium. Clinical manifestations are highly variable, ranging from no symptoms to disabling congestive heart failure, arrhythmias and systemic thromboemboli. It is also associated with other cardiac and extracardiac abnormalities. Herein, we present a rare clinical entity in the form of synchronous occurrence of NVM with Cor-triatriutum, persistent left superior vena cava (PLSVC), ventricular septal defect (VSD) and an anomalous extracardiac vessel connecting left hepatic vein with coronary sinus.
Case presentation
A 12-year-old normotensive girl presented with progressively worsening chest pain and exertional dyspnoea with negative family history of sudden cardiac death. Physical examination yielded a resting heart rate of 78 beats/min and blood pressure of 116/72 mm Hg along with irregular heart sound and diastolic murmur at the mitral area. ECG revealed atrial fibrillation and T wave inversions in the V4–6 precordial leads and in the II, III and aVF limb leads. Chest x-ray revealed clear lung fields and no cardiomegaly. Cardiac enzymes were found to be normal, and the laboratory data did not indicate inflammation.
Investigations
Transthoracic two-dimensional echocardiography (ECHO) revealed prominent trabeculations and deep intertrabecular recesses in the apical region of left ventricle (LV; figure 1A) with focal hypokinesia consistent with NVM. A membrane (figure 1B) dividing the left atrium into a lower chamber communicating with mitral valve and an upper chamber receiving the pulmonary veins was noted which is diagnostic of Cor-triatriatum. Dilatation of coronary sinus (CS) (figure 2A) and VSD was also noted. Saline microbubble echocardiography (figure 2B) revealed rapid filling of coronary sinus followed by filling of right atrium, suggestive of PLSVC.
Figure 1.
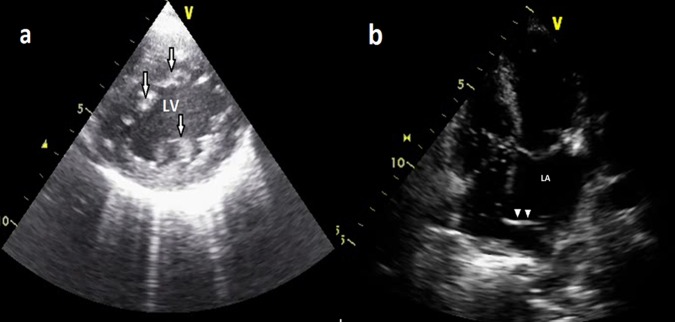
(A) Transthoracic two-dimensional echo short axis view showing arrows—prominent trabeculations and deep intertrabecular recesses in the left ventricle. (B) Transthoracic two-dimensional echo subcostal view showing arrow heads—membrane dividing the left atrium into two chambers, one communicating with mitral valve and other receiving the pulmonary veins.
Figure 2.
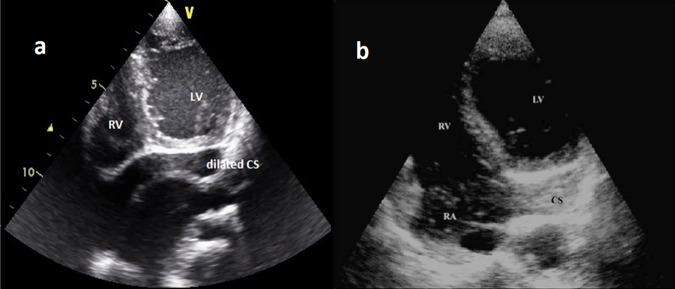
(A) Transthoracic two-dimensional echo showing dilated coronary sinus. (B) Contrast transthoracic two-dimensional echo with saline microbubbles showing opacification of coronary sinus followed by that of RA.
Based on these findings, multidetector row CT (MDCT; Philips Extended Brilliance Workspace, The Netherlands)) was performed for confirmation of the findings and to rule out associated coronary artery anomalies. Retrospective ECG-gated imaging was performed using 60 ml of non-ionic contrast (iohexol: 375 mg I/ml) followed by 20-ml saline chase. The imaging parameters were set at 80 kV tube potential, ECG-modulated tube current of 200–500 mA, 64×0.625 mm configuration; 0.40 s gantry rotation time and small field of view with approximate DLP 152 mGy×cm. Multiplanar reformations and three-dimensional (3D) volume rendered reconstructions in the diastolic phase were used for a detailed morphological assessment.
MDCT showed segmental thickening of the left ventricular myocardial wall consisting of two layers: a thin, compacted epicardial layer and an extremely thickened endocardial layer with prominent trabeculations and deep recesses (figure 3A) confirming the diagnosis of ventricular non-compaction. VSD (figure 3B) and PLSVC (figure 4) were also confirmed but Cor-triatriatum could not be demonstrated, possibly owing to the presence of a very thin membrane and mixing of blood. An additional and very rare finding detected was the presence of an anomalous vein draining left hepatic vein into CS (figure 4).
Figure 3.
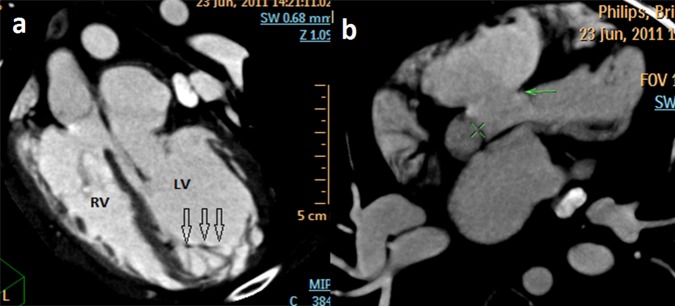
(A) Multidetector row CT oblique coronal maximum intensity projection image showing arrows—prominent trabeculations with deep intertrabecular recesses in the apical region of left ventricle. (B) Multidetector row CT axial maximum intensity projection image showing arrow—ventricular septal defect. LV, left ventricle; RV, right ventricle.
Figure 4.
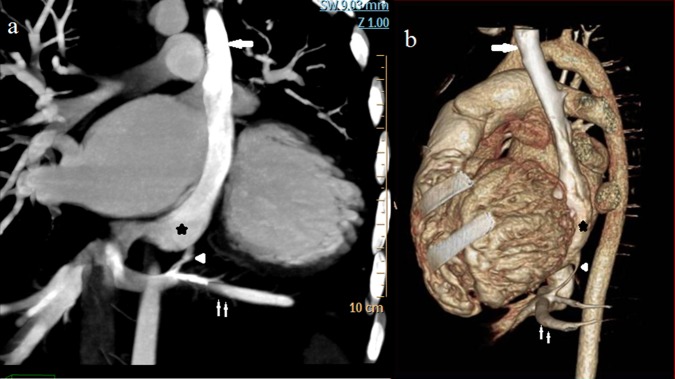
Multidetector row CT coronal maximum intensity projection (A) and sagittal volume rendered (B) image showing arrow—persistent left superior vena cava, small arrow—left hepatic vein, arrow head—anomalous vein draining left hepatic vein into coronary sinus, star—dilated coronary sinus.
Combining the data of ECHO and MDCT, we conclusively reached a diagnosis of NVM with Cor-triatriatum, PLSVC, VSD and anomalous hepatic venous drainage. Such an anatomical variant has not been described to the best of literature search. Supportive care and treatment was prescribed for the ventricular cardiomyopathy.
Discussion
The normal ventricular walls are made up of a compacted layer of myocardial fibres in a matrix of supporting connective tissue. In the normal human hearts, the luminal surfaces of the ventricles show trabeculations that are particularly prominent at the ventricular apices.1 The morphological LV has up to three prominent trabeculations, and it is less trabeculated as compared with RV. The proportion of the normal ventricular wall formed by trabeculations is always lesser than the thickness of the compacted layer. NVM, first described in 1932, was classified as a genetic cardiomyopathy by American Heart Association in 2006.2 The mutation in LDB3 and α-dystrobrevin 14 genes and 1q21.1 deletion syndrome are associated with this condition. The salient features of this entity are (1) increased thickness of the non-compacted myocardium as compared with the abnormally thinned out compacted layer; (2) a disproportionately increased number of conspicuous ventricular trabeculations; (3) deep intertrabecular recesses that do not communicate with the epicardial coronary artery system and receive flow from the ventricular cavity.3 The rate of diagnosis of NVM has been steadily increasing because of continuous improvements in imaging techniques and resolution quality. This abnormality is often associated with other congenital cardiac anomalies including dextrocardia, atrial septal defect (ASD), VSD, patent ductus arteriosus, coronary arterioventricular fistulae and a left coronary artery originating from the pulmonary artery. Its association with extracardiac anomalies particularly neuromuscular disorders has also been reported.4
Cor-triatriatum is a rare congenital anomaly, first described by Church in 1868 and accounts for 0.1% of congenital heart diseases. It is characterised by a fenestrated fibro-muscular membrane separating the common pulmonary venous chamber from the true left atrium. Most commonly associated anomalies include ASD, mitral regurgitation and partial anomalous pulmonary venous return. Generally, patients are asymptomatic owing to small size of the perforation. Symptoms may appear at a later stage owing to fibrosis and calcification in the orifice of the membrane, development of mitral regurgitation and the onset of atrial flutter or fibrillations. The diagnosis is usually established by transthoracic or transesophageal echocardiography or cardiac catheterisation. Recent studies mention the utility of MDCT with 3D reconstructed images in the demonstration of atrial membrane, evaluation of the size and number of fenestrations and in finding associated congenital cardiovascular anomalies.5
Persistent left superior vena cava, though an uncommon variant is the most common congenital thoracic venous anomaly with a prevalence of 0.3–0.5% in general population.6 It results when the left superior cardinal vein caudal to the innominate vein fails to regress. It is most commonly observed in isolation but can be associated with other cardiovascular abnormalities. The most common subtype of PLSVC results in the presence of both left and right superior vena cavas (SVCs). Rarely, the caudal right superior cardinal vein regress leading to an absent right SVC with PLSVC becoming the draining channel of blood from cranial aspect of the body, as seen in our case. Variations have also been reported in the insertion of left SVC. In 80–90% of individuals, the PLSVC drains into the right atrium via the coronary sinus and is of no clinical significance. In the remaining cases, it may drain in left atrium resulting in a right-to-left-sided shunt. PLSVC has various practical implications as it can create complications when left subclavian approach is used for vascular access to the right side of the heart or pulmonary vasculature.
Few case reports of anomalous drainage of hepatic veins into coronary sinus, right and left atria are available in literature,7 but the presence of an anomalous vein connecting left hepatic vein (LHV) to coronary sinus has not been mentioned. Drainage into CS and RA are clinically insignificant but drainage into left atrium may result in clinically significant right-to-left shunts. The occurrence of LHV draining into the RA may be explained owing to the persistence of the left vitelline connection with the left sinus horn but embryological development and clinical significance of anomalous vein has not been described earlier.
Development of non-invasive cross-sectional cardiovascular imaging techniques (MDCT and MRI) has considerably changed the diagnosis and follow-up of patients with congenital heart disease. Multiplanar reformations and 3D volume-rendered imaging allows accurate diagnosis and detailed anatomical evaluation of heart, vasculature and other anomalies. High spatial and temporal resolution makes MDCT a fast, easy and efficient technique, especially in paediatric age group. Although ionising radiation is a major drawback, it can be minimised using paediatric protocol and tube-current modulation techniques.8 MRI is important for the localisation and extent of non-compaction and identification of associated congenital anomalies. Contrast-enhanced cardiac MRI has been shown to demonstrate areas of fibrosis in the non-compacted myocardium that may prove to be the substrate for potentially lethal arrhythmias. Disadvantages are mainly the accessibility, need for sedation or general anaesthesia, especially in children, longer scanning time and poor spatial resolution. Besides, adequate breath hold is also an essential prerequisite. These techniques are complementary and depend on the age, the clinical condition and the diagnosis of the patient. Since our patient was dyspnoeic, instead of MRI, CT was chosen for evaluation and provided important clinical information with morphological assessment.
Learning points.
Clinical presentation mimics dilated and restrictive cardiomyopathies, so patients should be thoroughly investigated with appropriate imaging modalities.
Multidetector row CT is capable of showing the abnormal architecture of the ventricular walls in non-compaction of ventricular myocardium (NVM) and can exclude coronary artery anomalies or significant stenosis, which is usually not possible with MRI or echocardiography.
Prognosis of NVM is poor with progression to severe heart failure and death. In severely symptomatic patients, cardiac transplantation is an option if medical therapy fails to stabilise the condition.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Boyd MT, Seward JB, Tajik AJ, et al. Frequency and location of prominent left ventricular trabeculations at autopsy in 474 normal human hearts: implications for evaluation of mural thrombi by two-dimensional echocardiography. J Am Coll Cardiol 1987;9:323–6 [DOI] [PubMed] [Google Scholar]
- 2.Maron BJ, Towbin JA, Thiene G, et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 2006;113:1807–16 [DOI] [PubMed] [Google Scholar]
- 3.Jenni R, Oechslin E, Schneider J, et al. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: a step towards classification as a distinct cardiomyopathy. Heart 2001;86:666–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feldt RH, Rahimtoola SH, Davis GD, et al. Anomalous ventricular myocardial patterns in a child with complex congenital heart disease. Am J Cardiol 1969;23:732–4 [DOI] [PubMed] [Google Scholar]
- 5.Su CS, Tsai IC, Lin WW, et al. Usefulness of multidetector-row computed tomography in evaluating adult cor triatriatum. Tex Heart Inst J 2008; 35:349–51 [PMC free article] [PubMed] [Google Scholar]
- 6.Wood P. Diseases of heart and circulation. Philadelphia: JB Lippincott, 1956 [Google Scholar]
- 7.Nagai I, Oda Y, Kawada K, et al. (1966) Case of communication of the left atrium and the coronary sinus complicated by anomalous hepatic vein return. Kyobu Geka 1966;19:363–6 [PubMed] [Google Scholar]
- 8.Sigal-Cinqualbre A, Lambert V, Ronhean A, et al. Role of MSCT and MRI in the diagnosis of congenital heart disease. Arch Pediatr 2011;18:617–27 [DOI] [PubMed] [Google Scholar]


